Abstract
The median eminence at the base of the hypothalamus serves as an interface between the neural and peripheral endocrine systems. It is the site where hypothalamic releasing hormones are released into the portal capillary bed to be transported to the anterior pituitary, which provides further signals to target endocrine systems. Of specific relevance to reproduction, a group of about 1000 neurons in mammals release the gonadotropin-releasing hormone (GnRH) peptide from neuroterminals in the median eminence. During the life cycle, there are dramatic changes in reproductive demands, and we focus this review on how GnRH terminals in the median eminence change during reproductive senescence. We discuss morphological and functional properties of the median eminence, and how relationships among GnRH terminals and their microenvironment of nerve terminals, glial cells, and the portal capillary vasculature determine the ability of GnRH peptide to be secreted and to reach its target in the anterior pituitary gland.
Keywords: median eminence, hypothalamus, GnRH, glia, tanycyte, reproductive aging
Overview of the median eminence
The median eminence is the structure at the base of the hypothalamus where hypothalamic-releasing and –inhibiting hormones converge onto the portal capillary system that vascularizes the anterior pituitary gland. This region acts as a key interface between the neural and endocrine systems involved in hypothalamic-adenohypophysial regulation of reproduction, stress, lactation, growth, and thyroid and metabolic systems. The median eminence is a very unusual neural structure: although it contains nerve terminals and glial cells, it is virtually devoid of synapses and it has structural properties that distinguish it from other brain regions. In this article, we provide information about the molecular, anatomical and physiological features of the median eminence, and their age-related changes. Our focus will be on the hypothalamic system controlling reproduction through the release of the gonadotropin-releasing hormone (GnRH) peptide from nerve terminals in the median eminence. The hypothalamic-pituitary-gonadal (HPG) axis of females includes GnRH cells and terminals in hypothalamus; the pituitary gonadotropes which produce the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH); and the ovary, which produces steroid (particularly estradiol and progesterone) and protein hormones (Figure 1). All of the HPG levels must function normally for reproduction to occur, and during aging, each of these levels undergoes changes that may conribute to reproductive dysfunction and ultimately failure. Here we will discuss age-related changes in the hypothalamic median eminence in general, and in GnRH terminals in particular, and provide speculation about how this may influence reproductive senescence in females.
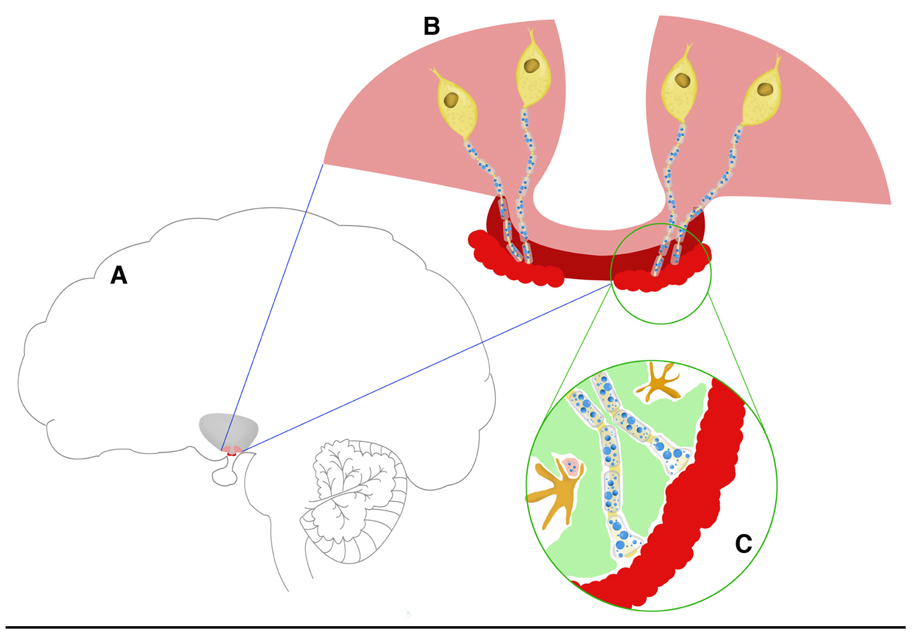
Figure 1.
Schematic representation of the hypothalamic-pituitary axis components that are involved in the control of reproduction. Panel A: The locations of the base of the hypothalamus (pink) and median eminence (dark red) are shown in reference to the brain as a whole. At the next layer of magnification (panel B), four GnRH cell bodies are depicted in yellow, with their nerve terminals projecting through the median eminence (dark red) to the portal capillaries (light red). Panel C is the highest magnification of the GnRH axons terminating at the portal capillaries. Tanycytes (green) are interspersed between GnRH and other terminals, and microglia (dark yellow) are shown. The blue circles in the axons and terminals in panels B and C represent large and small secretory vesicles. As the organization of the median eminence in this schematic is linear and the portal capillary vasculature basal lamina border is regular, this drawing depicts the situation in young rats. In aged rats (not shown) these relationships become disorganized.
The median eminence is one of eight circumventricular organs – regions surrounding the cerebral ventricles - in the central nervous system.1 As such, these brain regions contain porous blood-brain barriers, postulated to enable the circumventricular organs to sense and respond to chemical signals conveyed by the circulatory system within these limited regions in the brain. Two circumventricular organs, the median eminence (ME) and the organum vasculosum of the lamina terminalis (OVLT), are located within the hypothalamus and are involved in neuroendocrine regulation. The median eminence in particular also sends neuronal signals to regulate the peripheral endocrine organs through the hypophyseal portal system, which is the only portal system in the brain (Figure 1).2
The median eminence is one of three portal systems in the human body (the hepatic and renal portal system being the other two), and as the brain’s only portal system, it has unique structural and functional properties. Unlike the general capillary system that drains the blood into the heart directly through a vein, a portal capillary system drains blood into another capillary system through veins. The portal system in the median eminence provides a way for the hypothalamus to communicate with the peripheral endocrine system by both sending and receiving signals through the portal vasculature. It also provides a selective barrier of three layers: the fenestrated endothelial processes, the basal lamina and glial endfeet.3, 4
Another unique feature of the median eminence is the presence of specialized glial cells, tanycytes, which form a bridge between the portal system and the third ventricle.3 Tanycytes provide an interconnection between the bloodstream and other parts of the brain through the cerebrospinal fluid (CSF).5 Thus, the median eminence should be considered to be the communication center from the brain to the rest of the body, via endocrine signals; the route for the body to communicate to the brain, via the leaky fenestrated capillary system; and a route of communication among other brain regions, via the CSF.
Finally, the median eminence, while a neural structure at the base of the hypothalamus, is largely devoid of neural perikarya, dendrites and synaptic contacts.6, 7 Despite this unusual organization, the nerve terminals, glial cells and the portal vasculature of the median eminence are able to communicate with each other, as evidenced by the presence of many different types of neurotransmitter receptors in this region (reviewed in 8). The lack of synapses and somatic neural bodies suggests that cell-to-cell communication in the median eminence is probably mainly through volume transmission.9
Although the median eminence clearly exhibits important and unique features and roles, it is a surprisingly under-studied brain region, a gap that needs to be filled for better appreciating the mechanisms for neuroendocrine control during the life cycle.
The ultrastructure of the median eminence
The ultrastructure of the median eminence has been carefully studied by light, transmission and scanning electron microscopy. Four distinctive zones can be discerned from dorsal to ventral, as shown in Figure 2: the third ventricular zone, myelinated axon zone, neural profile zone and capillary zone.7, 10 Under the electron microscope, numerous protrusions (blebs) are distributed on the floor of the third ventricle showing different functions of this area compared to the lateral portion of the third ventricle, which are covered by cilia. In the ventricular zone, ependymal cells and tanycytes line the walls of the third ventricle.11 In the myelinated axon zone, myelin-covered oxytocin and vasopressin neural axons travel through the median eminence to the posterior pituitary. The neural profile zone contains unmyelinated neuronal processes targeting the portal vasculature in the basal median eminence – these are the hypothalamic releasing and inhibiting hormone neural processes. Finally, the capillary zone is characterized by the presence of large neuroterminals, extensive extracellular space and fenestrated capillaries. The extended long processes of tanycytes are seen in all four zones, with tanycytic endfeet in contact with the basal lamina of the fenestrated portal capillary.
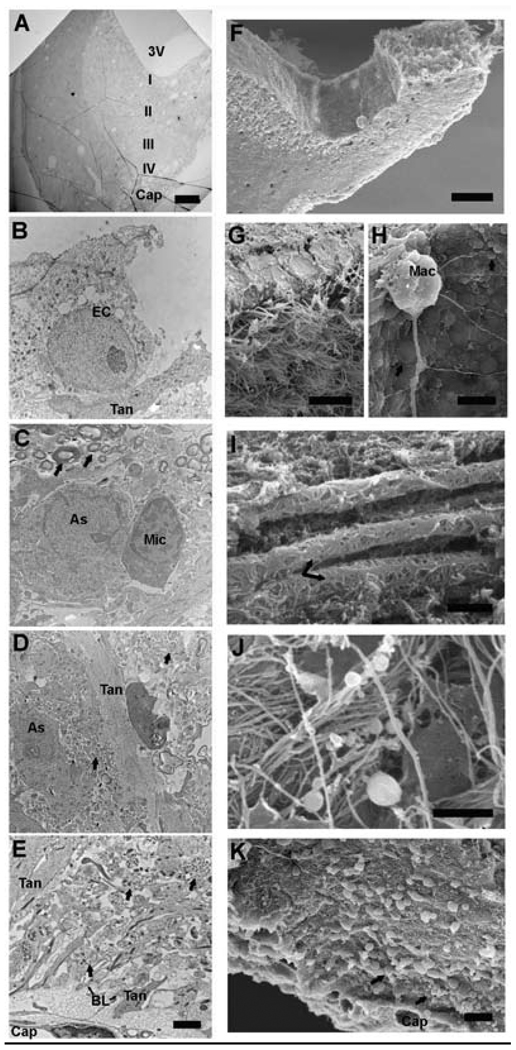
Figure 2.
Electron micrographs of the median eminence are shown, with transmission EM (TEM, A-E left) and scanning EM (SEM, F-K right). A) Half of the median eminence is shown in a low magnification TEM section, oriented with a slight angle to show the structures from the ventral 3rd ventricle (3V) to the dorsal portal capillary (Cap). Distinctive cellular structures of the four zones (I, II, III, IV) from the 3rd ventricle to the portal capillary are show in higher magnification in panels B, C, D and E, respectively. The corresponding SEM micrograph (F) shows the median eminence on a 100 µm coronal section. Tissue is oriented at a slight angle to show the floor of the 3rd ventricle and the cryo-fractured lateral median eminence. Cellular structures from the 3rd ventricle to the portal capillary in the lateral median eminence are shown in panels G-K. Tanycyte cell bodies (Tan) and ependymal cells (EC) are shown by TEM (B) and SEM (G). In G, ependymal cells line the lateral walls of the lateral 3rd ventricle. In H, the floor of the 3rd ventricle is covered by small and large bleb like structures (arrow). A Kolmer cell (also called an epiplexus macrophage) is shown with long projections extending from its body (Mac). Panels C and I show the myelinated axon zone by TEM and SEM, respectively. In C, an astrocyte (Ast) and microglia (Mic) cell are indicated. Myelinated axons (arrows) are indicated in C and I. Panels D and J show the neural profile zone by TEM and SEM, respectively. In D, Neural profiles (arrow), astrocyte (Ast) and tanycyte (Tan) processes are seen. In J, numerous neural profile fibers with varicosities (puncta) are seen. Panels E and K show the pericapillary zone of the median eminence. In E, neuroterminals (arrow) containing large dense-core vesicles are in close proximity to tanycyte processes (Tan). Tanycyte endfeet, basal lamina (BL) and capillary epithelial cell form a selective barrier in the portal system. In K, neuroterminals (arrow) on the surface of the cryo-fracture are shown to close to the portal capillaries (Cap). Scale bar A = 100 µm, B – E = 2 µm, F = 100 µm, G = 2 µm, H = 20 µm, I – K = 2 µm. Abbreviations: As = astrocyte, EC = Ependymal cell, BL = basal lamina, Cap = portal capillaries, I = third ventricular zone, II = myelinated axon zone, III = neural profile zone, IV = portal capillary zone, Mac = Macrophage (Kolmer cell), Mic = microglia, Tan = tanycyte, 3V = third ventricle. Scanning EM micrographs courtesy of John Mendenhall.
The axons of hypothalamic releasing hormone neurons such as GnRH exhibit punctate immunolabeling. For example, GnRH terminals are described as “varicosities” or “puncta”8, 10 because of the discontinuous appearance of the axons that project to the median eminence. Punctae along the neuronal profiles contain abundant large secretory vesicles and small vesicles that are probably on transit to terminals at the portal capillary system (Figure 2).
GnRH in the median eminence
The median eminence contains neuroterminals that release the hypothalamic-adenohypophysial releasing/inhibiting hormones, among which is GnRH. Although neurons that synthesize these hormones have terminals in the median eminence, the synthesis of these hypothalamic adenohypophysial hormones occurs in neural cell bodies found in different subdivisions of the hypothalamus. In the case of GnRH, cell bodies are distributed in the preoptic area and medial basal hypothalamus, with some variability across species. The synthesis of GnRH begins with the transcription of its gene to an RNA primary transcript in the nucleus. Following further processing, the mature mRNA is translocated to the cytoplasm. Translation of mRNA occurs in the endoplasmic reticulum and Golgi apparatus, followed by budding of large secretory vesicles, which contain the precursor peptide. Prohormone peptide splicing continues by enzymes in the large secretory vesicles and results in the production of the decapeptide GnRH, which is transported from perikarya to axon to terminal, and stored and released from secretory vesicles in the neuroterminals into the portal vasculature bed in the median eminence12 (Figure 1).
Thus, the median eminence is the region where release of GnRH and other hypothalamic neuropeptides is controlled, as synthesis occurs elsewhere in the hypothalamus. The microenvironment of the median eminence, including hypothalamic neuroterminals, the surrounding glial cells, and the extracellular fluid that surrounds these structures and the portal vasculature, enables communication among these elements. Considering that the release of GnRH occurs in discrete pulses on the order of approximately hourly intervals in mammals, the coordination of pulses likely depends upon physical and chemical interactions in the median eminence. Indeed, studies showing that explanted median eminence has the capability of releasing GnRH in pulses suggest that there is an intrinsic ability of this region to coordinate this process.13, 14
GnRH release and reproductive aging
In young adults, the GnRH peptide is released at a frequency of approximately 30–60 minutes depending upon the species and the study.13, 15, 16 This pulsatile pattern of release is critical to the development and maintenance of reproductive capacity. In females of those species with spontaneous ovulatory cycles, such as primates and rodents, the amplitude and frequency of GnRH pulses changes across reproductive cycles to control folliculogenesis and ovulation. Although it is now well-accepted that GnRH is the primary regulatory hormone of the HPG reproductive axis in adulthood, the role for GnRH in reproductive senescence is less clear. Some of the lack of clarity is due to species differences. In rodents, reproductive failure occurs in mid-life, a period during which the ovaries continue to have the capacity to produce follicles and ovulate (reviewed in 17, 18). Thus, reproductive senescence in rodents is likely attributable to a neuroendocrine change. However, it is probably not due to changes in the numbers or morphology of GnRH perikarya, which vary relatively little with age.19, 20 Rather, we believe these differences in function are due to a combination of factors that regulate GnRH cell bodies and dendrites (e.g., neurotransmitters), and potential changes at the level of GnRH terminals in the median eminence, as the latter is the most immediate site for release of the decapeptide. These possibilities are not mutually exclusive. Central nervous system (CNS) inputs to GnRH perikarya undergo documented changes with aging – for example, glutamatergic stimulation of GnRH neurons decreases with age, and GABAergic inhibition increases with age (21; reviewed in 22). The summation of these and other CNS regulatory inputs to GnRH is manifested in rodents as a decline in pulsatile release of GnRH, together with a loss of the surge mode of GnRH/LH release that precedes ovulation. At the level of nerve terminals, there may also be differences in age-related regulatory factors, as the median eminence is rich in neurotransmitters and receptors, but we are not aware of published studies addressing this specific issue.
The diminution of hypothalamic drive with age also relates to the potential decline in both negative and positive feedback regulation of GnRH release by steroid hormones.23 However, experiments on feedback to GnRH neurons have focused at the level of GnRH perikarya – not the nerve terminals in the median eminence. An important future area of research is to determine whether membrane hormone receptors such as GPR30 may be expressed on GnRH cells, and if so, whether the GnRH terminals express this receptor. This would enable feedback from estradiol to act directly and rapidly upon GnRH release. It was recently reported using a fetal primate GnRH cell culture that GPR30 is co-expressed in GnRH perikarya.24 Our laboratory has ongoing experiments designed to evaluate and quantify the co-expression of GPR30 on GnRH terminals in the median eminence of aging rats using double-label immunofluorescence and confocal microscopy. Our preliminary data show that 1) GnRH terminals co-express GPR30; 2) overall, there is a significant age-related increase in this co-expression; and 3) estradiol up-regulates GPR30 co-expression in GnRH terminals in young but not aging rats (M. Noel, S. Ng, K. Resendiz, A.C. Gore, unpublished). These data remain to be confirmed, but they provide tantalizing evidence for a direct site of regulation of estradiol on GnRH release at the level of the median eminence, and for an age-related loss of responsiveness.
As already mentioned, there are species differences in the aging of the hypothalamic GnRH system. Studies in women and non-human primates show that the proximal event for menopause is the loss of ovarian follicles through a process involving accelerated follicular atresia. However, neuroendocrine markers do change in primates, and in some cases, precede the period of exponential follicular atresia in a manner that has some parallels to rodents. For example, prior to or during the perimenopause, an erosion of positive feedback effects of estradiol25, an elevation of FSH26, a decrease in serum anti-Mullerian hormone26, and other physiological changes have been reported (reviewed in 23). In addition, a loss of negative feedback during the perimenopause may not initially be observed in perimenopausal women, but it develops gradually with years post-menopause, suggesting an eventual hypothalamic/pituitary dysregulation.27, 28
It is extremely difficult to measure GnRH directly from nerve terminals in the median eminence, due to an inability to detect GnRH in peripheral serum, together with the obvious limitations of measuring the release of a hormone from the brain. This has deterred the ability to measure how GnRH release changes with aging. To our knowledge, there are two studies that directly measured GnRH neurosecretion with aging, one in rats and one in non-human primates. In the rat, Rubin & Bridges performed push-pull perfusion on GnRH release from the medial basal hypothalamus of ovariectomized rats given steroid hormones to induce positive feedback.29 They showed a significant decline in GnRH release of the older rats, a finding that mirrored previous work on the diminution of gonadotropin release with aging in rodent species.
The other study that directly measured GnRH release also utilized push-pull perfusion of the median eminence in young adult and perimenopausal (ovarian intact) rhesus monkeys.16 We demonstrated a robust increase in pulsatile GnRH release. Mean GnRH release was significantly increased, and although GnRH pulse amplitude was elevated, this latter effect was not significant (probably due to a small sample size, as it was very difficult to obtain very aged female rhesus monkeys). Nevertheless, we observed in three out of four of our perimenopausal monkeys some very elevated GnRH pulses that exceeded even preovulatory GnRH levels.16 This report of an age-related increase in GnRH has parallels to studies showing increases in LH in rhesus monkeys and women,30, 31 and increases in gonadotropin free-alpha subunit in postmenopausal women.31 Although there are differences in exactly which parameters of GnRH release increase with age (i.e., mean levels, pulse amplitude, pulse frequency), these are relatively minor compared to the consensus for an age-related up-regulation of these neuroendocrine hormones. We interpret these data to mean that a loss of steroid hormone feedback with menopause in primates initially results in an up-regulation of GnRH, and subsequently gonadotropin, secretion. However, with greater time post-menopause, the negative feedback effect on GnRH/LH release diminishes,27 bringing the primate model back in parallel with the rodent model.
Glial cells in the median eminence
Thus far, this article has focused on the hypothalamic GnRH terminals in the median eminence. However, glial cells constitute a significant part of the median eminence, and they interact extensively with adenohypophysial neurons and the portal capillary system.10, 32 It is important to consider the expression of glia in the median eminence, and further, to understand how they change with aging in their intrinsic properties and in their inter-relationships with GnRH terminals.
The median eminence expresses three glial cell types - astrocytes, microglia, and tanycytes – as illustrated in Figure 3. For these micrographs, we used hypothalamic tissues from transgenic mice, kindly provided by Dr. Wesley Thompson’s laboratory (University of Texas at Austin; protocols were approved by the Institutional Animal Care and Use Committee).33 These transgenic mice had the green fluorescent protein (GFP) marker driven by promoters for different glial cell type (astrocyte: S100; microglia: S100; and tanycyte: nestin). Notably, when S100 was used to drive expression of GFP, the glial cells differentiated into either astrocytes or microglia, which could be distinguished by other specific astrocyte and microglia markers and which had very different and easily differentiated morphological properties; see 33 for details). Tissues from each transgenic mouse line were immunolabeled for GnRH using the mouse monoclonal antibody, HU11b.34 The distribution of the three glial cell types, and their close relationship with the GnRH neuroterminals, can be clearly seen in the median eminence (Figure 3). GnRH axons and terminals are in close proximity to astrocytes, tanycytes and microglia cells. The microglia and astrocyte signals are evenly distributed in the lateral portion of the median eminence. In the central portion of the median eminence, astrocytes show long extended processes in the myelinated axon zone with some processes extending to the portal capillary zone. Interestingly, nestin-GFP signal was observed in the tanycytes, similar to earlier reports of nestin immunohistochemistry in human hypothalamus.35 Nestin is an intermediate filament protein that had originally been identified as a marker of neuroepithelial stem/progenitor cells.36 We observe nestin-GFP signal in the mouse hypothalamus with the same pattern as that described for tanycytes and ependymal cells10 (Figure 3). These transgenic mice models provide us with excellent resolution of the interaction between GnRH neuroterminals and glia (Figure 4).
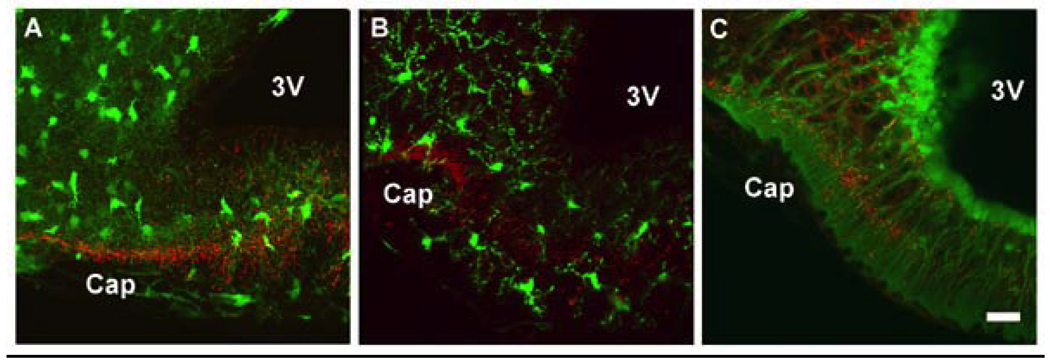
Figure 3.
Confocal fluorescence images are shown for transgenic mice with green fluorescent protein (GFP) labeling of astrocytes (A), microglia (B) and tanycytes (C). In all three panels, tissues were subjected to fluorescence immunohistochemistry with a GnRH mouse monoclonal primary antibody (HU11b34), detected with an anti-mouse secondary antibody linked to the fluorophore Texas red. Immunoreactive GnRH neuroterminals (red) are seen in close proximity to all three glial cell types. For astrocytes (A) and microglia (B), these cells are scattered fairly evenly through the median eminence. Tanycyte cell bodies (C) are aligned along the 3rd ventricle, and they extend long processes to the base of the brain at the portal capillary system. Scale bar = 100 µm, Cap = portal capillary region, 3V = 3rd ventricle.
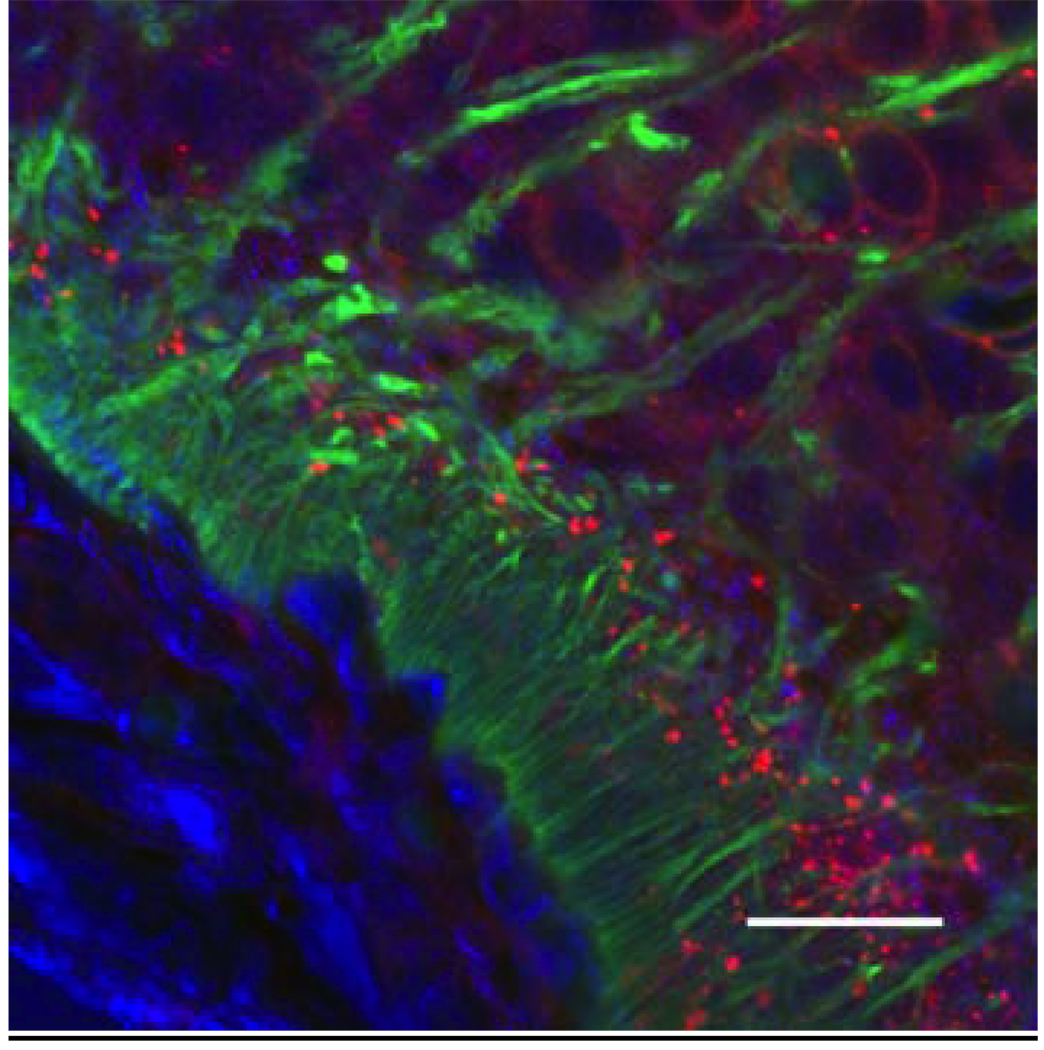
Figure 4.
Confocal microscopic image showing the network of GnRH neuroterminals, tanycytes and their relationship to the portal capillary vasculature of a representative young male nestin-green fluorescent protein (GFP) mouse (a marker for tanycytes). Tanycytic endfeet (seen in the green GFP channel) separate GnRH processes (seen as punctate red immunofluorescence, labeled by Texas red) from the portal capillary bed (DIC signal, pseudocolored in blue). Scale bar = 20 µm.
Glial cells and the aging median eminence
Tanycytes are believed to be involved in the transport and release of hormones in the median eminence.37 It has been proposed that the regulation of GnRH terminals may result from ensheathment by tanycytes,38 which may allow GnRH terminals access to the portal vasculature or to other neuronal processes. Tanycytes appear to play a role in the remodeling of the aged basal hypothalamus in male and female rats through phagocytotic actions on degenerating neurons.39, 40 Light and electron microscopic studies show that tanycytes undergo morphological modifications with the increased presence of lipid droplets in the cytoplasm of aging male and female rats compared to their younger counterparts.39 However, relatively little is known about how glial cells in the median eminence undergo age-related changes, and even less about the more specific relationships between GnRH terminals and their glial environment with aging.
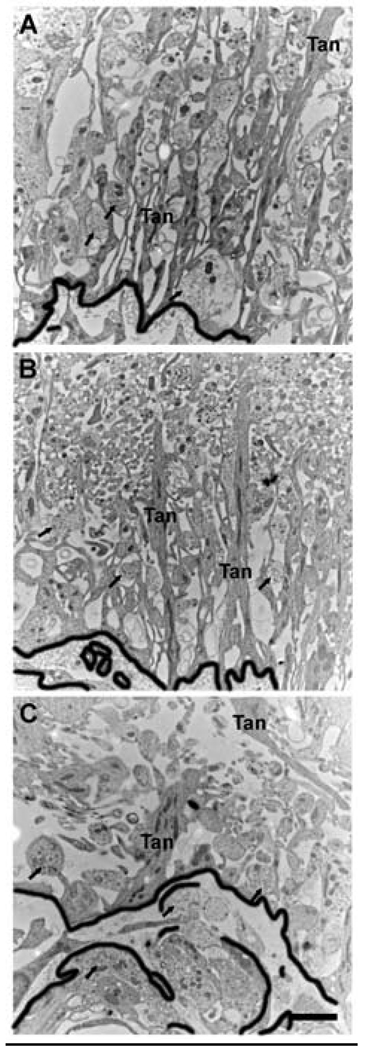
We have recently undertaken studies in our laboratory to determine effects of aging on the organization of the median eminence. Using ultramicrotome sections of the median eminence of young, middle-aged and old female rats, we observed significant age-related changes in the structure of the median eminence, and more specifically, substantial changes to the morphology and anatomy of tanycytes.41 In young female rats, tanycytic cell bodies are lined up along the third ventricle and extend a long process across the median eminence to the portal capillary vasculature (e.g., Figure 2, 5). With aging, tanycyte processes become thicker and disorganized in the pericapillary zone, with a loss of perpendicular orientation (Figure 5). We also noted that the relationships among tanycytes, neural terminals, and the basal lamina of the portal capillary system undergo a progressive disorganization.41, 43 In young rats, the basal laminar boundary is easy to discern, but with aging, the boundary between the neuroterminals of the pericapillary zone and the portal capillaries becomes convoluted (Figure 5). These structural changes to the organization of the median eminence are likely to alter the function of the selective barrier of the portal system, and alter the access of neuropeptides such as GnRH to the portal blood vessels. As the neural-glial relationship is considered to be important for the maintenance of neuroendocrine function,11, 42 and it undergoes age-related changes, we speculate that there is an alteration in the ability of GnRH (and other) terminals to release their neuropeptide effectively. Further research is necessary to ascertain how these processes occur.
Figure 5.
Transmission electron microscopic images showing the organization of the pericapillary zone of the lateral median eminence from a representative young (A), middle-aged (B) and old (C) ovariectomized rat. In young rats, tanycyte processes (Tan) are long an narrow, and organized linearly from top (towards the 3rd ventricle) to bottom (towards the portal capillaries, delinated with the thick black line). With aging, tanycyte processes become larger, wider and disorganized, with a distinct loss of perpendicular orientation. In addition, the boundary between the neuroterminals (arrow) of the pericapillary zone and the portal capillaries (outlined in thick black) becomes very convoluted with age. Scale bar = 2 µm.
Links among GnRH terminals and glia in the aging median eminence
The microenvironment of the median eminence maintains the ability of GnRH neuroterminals to release the decapeptide. Our laboratory performed cryo-embedding immunogold electron microscopy for GnRH using ultrathin sections collected from the caudal median eminence of aging rats. Using this technique, we were able to identify GnRH immunopositive terminals and compare their ultrastructural differences, as well as to study the changes in their surrounding microenvironment with aging41. We quantified several properties of the GnRH immunopositive terminals including the size of terminals, the density of secretory vesicles (large dense-core vesicles LDCV) within the terminals, the density of immunogold-labeled GnRH peptide and the area fraction of mitochondria in our rats.43 Our ultrastructural data suggest that the major change to GnRH immunopositive terminals with age is an increased density of large dense-core secretory vesicles in the terminals. We believe this may reflect a buildup of vesicles due to the loss of ability to release the peptide, consistent with reports of decreased GnRH/LH release with age.29
Previously, GnRH terminal and glial interactions have been investigated in young rats, with differences detected in the distance between GnRH terminals and the basal lamina, the ensheathment of GnRH terminals by tanycytes, and the proximity of GnRH terminals to portal capillaries in animals of differing developmental age and hormonal status.44 Additional TEM studies from our laboratory using postembedding tissues immunolabeled for GnRH provide ultrastructural evidence that the ensheathment of GnRH neuroterminal by glia decreased in old female rats indicating a decreased GnRH-glia interaction during the aging process.41, 43
Conclusions
A greater understanding of the structure of the median eminence is necessary to fully appreciate the regulation of the release of neurohormones such as GnRH. The morphology of nerve terminals and glia, particularly tanycytes, undergoes dramatic age-related changes. There are also changes in subcellular properties of GnRH terminals such as decreased tanycytic ensheathment and an increase in the density of secretory vesicles with aging. Further, the linear organization of tanycytes in the median eminence becomes lost in the aging median eminence. These findings are interpreted to mean that GnRH release in aging rats is impeded by structural organizational changes. By studying ultrastructural properties of the median eminence, and relating them to reproductive status, we are increasing our understanding of the potential role of the hypothalamus in reproductive senescence in female mammals.
Acknowledgments
We thank John Mendenhall (Institute of Cellular and Molecular Biology microscopy facility, University of Texas at Austin) for providing the scanning electron microscopy images shown in Figure 2. We are grateful to Dr. Wesley Thompson and Dr. Yi Zuo for providing glia-GFP mice tissues to study the hypothalamus. Sharon Kim provided expert assistance in the preparation of mouse GFP tissues. Work described in this report was funded by the NIH (AG16765, AG028051 to ACG).
REFERENCES
- 1.Johnson A. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 2.Green J, Harris G. Observation of the hypophysio-portal vessels of the living rat. J. Physiol. 1949;108:359–361. [PubMed] [Google Scholar]
- 3.Rodriguez EM, et al. Hypothalamic tanycytes: A key component of brain-endocrine interaction. Int. Rev. Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- 4.Jennes L, Stumpf WE. Gonadotropin-releasing hormone immunoreactive neurons with access to fenestrated capillaries in mouse brain. Neuroscience. 1986;18:403–416. doi: 10.1016/0306-4522(86)90162-4. [DOI] [PubMed] [Google Scholar]
- 5.Kozlowski GP, Coates PW. Ependymoneuronal specializations between LHRH fibers and cells of the cerebroventricular system. Cell Tissue Res. 1985;242:301–311. doi: 10.1007/BF00214542. [DOI] [PubMed] [Google Scholar]
- 6.Durrant A, Plant T. A study of the gonadotropin releasing hormone neuronal network in the median eminence of the rhesus monkey (Macaca mulatta) using a post-embedding immunolabelling procedure. J. Neuroendocrinol. 1999;11:813–821. doi: 10.1046/j.1365-2826.1999.00397.x. [DOI] [PubMed] [Google Scholar]
- 7.Yin W, et al. Novel localization of NMDA receptors within neuroendocrine gonadotroping-releasing hormone terminals. Exp. Biol. Med. 2007;232:662–673. [PubMed] [Google Scholar]
- 8.Yin W, Gore AC. Neuroendocrine control of reproduction aging: roles of GnRH neurons. Reproduction. 2006;131:403–414. doi: 10.1530/rep.1.00617. [DOI] [PubMed] [Google Scholar]
- 9.Agnati LF, et al. Intercellular communication in the brain: Wiring versus volume transmission. Neuroscience. 1995;69:711–726. doi: 10.1016/0306-4522(95)00308-6. [DOI] [PubMed] [Google Scholar]
- 10.Ojeda S, Lomzicai A, Sandau U. Glial-gonadotrophin hormone (GnRH) neurone interactions in the median eminence and the control of GnRH secretion. J. Neuroendocrinol. 2008;20:732–742. doi: 10.1111/j.1365-2826.2008.01712.x. [DOI] [PubMed] [Google Scholar]
- 11.Wittkowski W. Tanycytes and pituicytes: morphological and functional aspects of neuroglial interaction. Microscopy Res. Tech. 1998;41:29–42. doi: 10.1002/(SICI)1097-0029(19980401)41:1<29::AID-JEMT4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.King J, et al. LHRH immunopositive cells and their projections to the median eminence and organum vasculosum of the lamina terminalis. J. Comp. Neurol. 1982;209:287–300. doi: 10.1002/cne.902090307. [DOI] [PubMed] [Google Scholar]
- 13.Terasawa E. Luteinizing hormone-releasing hormone (LHRH) neurons: mechanism of pulsatile LHRH release. Vitamin Horm. 2001;63:91–129. doi: 10.1016/s0083-6729(01)63004-8. [DOI] [PubMed] [Google Scholar]
- 14.Purnelle G, et al. Pulsatile secretion of gonadotropin-releasing hormone by rat hypothalamic explants without cell bodies of GnRH neurons. Neuroendocrinol. 1997;66:305–312. doi: 10.1159/000127253. [DOI] [PubMed] [Google Scholar]
- 15.Scarbrough K, Wise PM. Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinology. 1990;126:884–890. doi: 10.1210/endo-126-2-884. [DOI] [PubMed] [Google Scholar]
- 16.Gore AC, Windsor-Engnell B, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta) Endocrinology. 2004;145:4653–4659. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- 17.Maffucci JA, Gore AC. Age-related changes in hormone and their receptors in animal models of female reproductive senescence. In: Conn PM, editor. Handbook of Models for Human Aging. Academic Press, Inc.; 2006. pp. 533–552. [Google Scholar]
- 18.Finch CE, et al. Ovarian and steroidal influences on neuroendocrine aging processes in female rodents. Endocrine Rev. 1984;5:467–497. doi: 10.1210/edrv-5-4-467. [DOI] [PubMed] [Google Scholar]
- 19.Bestetti GE, et al. Functional and morphological changes in the hypothalamopituitary-gonadal axis of aged female rats. Biol. Reprod. 1991;45:221–228. doi: 10.1095/biolreprod45.2.221. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman GE, Sladek JJ. Age-related changes in dopamine, LHRH and somatostatin in the rat hypothalamus. Neurobiol. Aging. 1980;1:27–37. doi: 10.1016/0197-4580(80)90021-4. [DOI] [PubMed] [Google Scholar]
- 21.Neal-Perry GS, et al. Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area. Biol. Reprod. 2008;79:878–888. doi: 10.1095/biolreprod.108.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maffucci JA, Gore AC. Hypothalamic neural systems controlling the female reproductive life cycle: gonadotropin-releasing hormone, GABA, and glutamate. Int. Rev. Cell and Mol. Biol. 2009;274:69–127. doi: 10.1016/S1937-6448(08)02002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wise PM, et al. Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog. Horm. Res. 2002;57:235–256. doi: 10.1210/rp.57.1.235. [DOI] [PubMed] [Google Scholar]
- 24.Noel SD, et al. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol. Endocrinol. 2009;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss G, et al. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- 26.Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol. Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- 27.Rossmanith WG. Gonadotropin secretion during aging in women: Review article. Exp. Gerontol. 1995;30:369–381. doi: 10.1016/0531-5565(94)00030-7. [DOI] [PubMed] [Google Scholar]
- 28.Gill S, et al. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J. Clin. Endocrinol. Metab. 2002;87:2297–2302. doi: 10.1210/jcem.87.5.8510. [DOI] [PubMed] [Google Scholar]
- 29.Rubin BS, Bridges RS. Alterations in luteinizing hormone-releasing hormone release from the mediobasal hypothalamus of ovariectomized, steroid-primed middle-aged rats as measured by push-pull perfusion. Neuroendocrinol. 1989;49:225–232. doi: 10.1159/000125121. [DOI] [PubMed] [Google Scholar]
- 30.Woller MJ, et al. Aging-related changes in release of growth hormone and luteinizing hormone in female rhesus monkeys. J. Clin. Endocrinol. Metab. 2002;87:5160–5167. doi: 10.1210/jc.2002-020659. [DOI] [PubMed] [Google Scholar]
- 31.Gill S, et al. Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J. Clin. Endocrinol. Metab. 2002;87:2290–2296. doi: 10.1210/jcem.87.5.8508. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Segura L, McCarthy M. Minireview: Role of glia in neuroendocrine function. Endocrinology. 2004;145:1082–1086. doi: 10.1210/en.2003-1383. [DOI] [PubMed] [Google Scholar]
- 33.Zuo Y, et al. Fluorescent proteins expressed in mouse transgenic lines mark subsets of glia, neurons, macrophages, and dendritic cells for vital examination. J. Neurosci. 2004;24:10999–11009. doi: 10.1523/JNEUROSCI.3934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbanski HF. Monoclonal antibodies to luteinizing hormone-releasing hormone: Production, characterization, and immunocytochemical application. Biol. Reprod. 1991;44:681–686. doi: 10.1095/biolreprod44.4.681. [DOI] [PubMed] [Google Scholar]
- 35.Baroncini M, et al. Morphological evidence for direct interaction between gonadotrophin-releasing hormone neurones and astroglial cells in the human hypothalamus. Journal of Neuroendocrinology. 2007;19:691–702. doi: 10.1111/j.1365-2826.2007.01576.x. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerman L, et al. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 37.Akmayev I, Fidelina O. Tanycytes and their relation to the hypophyseal gonadotrophic function. Brain Res. 1981;210:253. doi: 10.1016/0006-8993(81)90898-2. [DOI] [PubMed] [Google Scholar]
- 38.Flament-Durand J, Brion J. Tanycytes: morphology and functions: a review. Int. Rev. Cytol. 1985;96:121–155. doi: 10.1016/s0074-7696(08)60596-3. [DOI] [PubMed] [Google Scholar]
- 39.Brawer J, Walsh R. Response of tanycytes to aging in the median eminence of the rat. Am. J. Anat. 1982;163:247–256. doi: 10.1002/aja.1001630305. [DOI] [PubMed] [Google Scholar]
- 40.Zoli M, et al. Age-related alterations in tanycytes of the mediobasal hypothalamus of the male rat. Neurobiol. Aging. 1995;16:77–83. doi: 10.1016/0197-4580(95)80010-o. [DOI] [PubMed] [Google Scholar]
- 41.Yin W, et al. Three-dimensional properties of GnRH neuroterminals in the median eminence of young and old rats. J. Comp. Neurol. 2009;20:284–295. doi: 10.1002/cne.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez E, et al. Hypothalamic tanycytes: a key component of brain-endocrine interaction. International Review of Cytology. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- 43.Yin W, et al. GnRH neuroterminals and their microenvironment in the median eminence: Effects of aging and estradiol treatment. Endocrinology. 2009 doi: 10.1210/en.2009-0679. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King JC, Rubin BS. Dynamic changes in LHRH neurovascular terminals with various endocrine conditions in adults. Horm. Behav. 1994;28:349–356. doi: 10.1006/hbeh.1994.1031. [DOI] [PubMed] [Google Scholar]