Abstract
Polycystic Liver Disease may complicate ADPKD, a disease caused by mutations in polycystins, proteins that regulate signaling, morphogenesis and differentiation in epithelial cells. The cystic biliary epithelium (LCE) secretes VEGF, which promotes liver cyst growth via autocrine and paracrine mechanisms. Expression of IGF-1, IGF1-R, phospho-mTOR and the PKA-dependent phosphorylation of ERK1/2 are also upregulated in LCE. We have hypothesized that mTOR represents a common pathway for the regulation of HIF1α-dependent VEGF secretion by IGF-1 and ERK1/2.
Methods
Conditional polycystin-2-defective (Pkd2KO) mice were used for in vivo studies and to isolate cystic cholangiocytes (LCEC). Expression of phospho-mTOR, VEGF, cleaved-caspase-3 (CC3), PCNA, IGF-1, IGF-1R, pERK, p-P70S6K, HIF1α and VEGF in LCE, LCEC and WT-cholangiocytes was studied by immunohistochemistry, Western blot, or ELISA. Cystic area was measured by computer-assisted morphometry of panCK-stained sections. Cell proliferation in vitro was studied by MTS and BrdU assays.
Results
Treatment of Pkd2KO mice with the mTOR inhibitor Rapamycin significantly reduced liver cysts area, liver/body weight ratio, pericystic microvascular density, and PCNA expression, while increasing expression of CC3. Rapamycin inhibited IGF-1-stimulated HIF1α accumulation and VEGF secretion in LCEC. IGF-1-stimulated LCEC proliferation was inhibited by Rapamycin and SU5416 (a VEGFR2 inhibitor). Phosphorylation of the mTOR-dependent kinase P70S6K was significantly reduced by the PKA inhibitor PKI and by the MEK inhibitor U1026.
Conclusions
These data demonstrate that PKA-dependent up-regulation of mTOR has a central role in the proliferative, anti-apoptotic and pro-angiogenic effects of IGF-1 and VEGF, in polycystin-2-defective mice. This study also highlights a mechanistic link between PKA, ERK, mTOR and HIF-1α-mediated VEGF secretion and provides a proof of concept for the potential use of mTOR inhibitors in ADPKD and conditions with aberrant cholangiocyte proliferation.
Keywords: Cholangiocytes, ADPKD, VEGF, Rapamycin, ERK1/2, PI3K/AKT
INTRODUCTION
Polycystic Liver Disease may complicate Autosomal Dominant Polycystic Kidney Disease (ADPKD)1. Liver cysts originating from the biliary epithelium progressively enlarge and eventually cause complications related to mass effects, hemorrhages, infection or rupture. Some patients may require cyst fenestration, liver resection and even liver transplantation.
ADPKD is caused by mutations in the PKD1 or PKD2 genes2. These encode, respectively, for polycystin-1 (PC1) and polycystin-2 (PC2), two proteins involved in epithelial cell proliferation, morphogenesis and differentiation2. In the liver, PC1 and PC2 are expressed by biliary epithelial cells and are located in the primary cilium of the cell. PC2, a non-selective membrane Ca2+ channel, is located also in the endoplasmic reticulum, where it contributes to Ca2+ regulation3, 4.
Cholangiocytes lining the liver cysts in patients with ADPKD show phenotypic and functional peculiarities, such as a marked over-expression of VEGF-A, angiopoietins, IGF-1, and of their cognate receptors, VEGFR2, Tie-2 and IGF-1R5, 6. Using mice with conditional defects of PC2, we have shown that the cystic epithelium of PC2-defective mice over-expresses VEGF which, in turn, stimulates cholangiocyte proliferation and that blockade of VEGFR-2 signaling, inhibits liver cyst growth in vivo7. We also shown that PKA-mediated up-regulation of ERK1/2 sustains the increased secretion of VEGF, as well as its proliferative effects.
Insulin-like growth factor-1 (IGF-1) is concentrated in the fluid drained from liver cysts of ADPKD patients and IGF-1 and his main receptor IGF1-R8 along with phospho-AKT and p-mTOR are over-expressed in the cystic epithelium of human ADPKD5. AKT is an inhibitor of tuberin, and thereby an activator of mTOR, through which it controls the hypoxia-inducible factor-1 alfa (HIF1α), the major transcriptional factor for VEGF9. Through similar mechanisms, IGF1 up-regulates the expression of VEGF in colon cancer cells10.
Expression of mTOR is increased in the cystic epithelium of the kidney cells11, suggesting that the mTOR pathway may play a crucial role in the growth of kidney cysts. Rapamycin is an mTOR inhibitor which has been approved for use in humans because of its immune-suppressive properties. Rapamycin slows renal cysts development and renal function deterioration in rodent models of polycystic kidney disease11–13. A recent retrospective study showed a reduction in liver cysts volume in ADPKD patients that received sirolimus as immunosuppressive therapy after kidney transplantation14.
Recent experimental work has showed that the mTOR inhibitor rapamycin is a potent inhibitor of angiogenesis and proliferation of endothelial, cancer and stromal cells15, 16. We hypothesized that inhibition of mTOR by rapamycin could block the progression of polycystic liver disease by interfering with IGF1 and VEGF autocrine signaling. We tested this hypothesis in conditional PC2 knock-out mice, which over-expresses VEGF, VEGFR-2 and IGF1 in a manner similar to human ADPKD.
MATERIAL AND METHODS
Reagents
All reagents were purchased from Sigma Chemical Co. (St. Louis, MO), unless otherwise indicated. Further information can be found in the on-line supplementary material.
Animals and experimental protocol
The study was conducted in normal WT mice and ADPKD mouse models, generated by an inducible defect in polycystin 2 (Pkd2flox/−:pCxCreER™), targeted through a Cre system, fused to the ligand-binding domain of a mutated estrogen receptor, as previously described17 (S. Somlo, manuscript submitted). Both males and females were used. Twenty-eight days old mice were treated with tamoxifen (0.2 mg/g/day) for five days to induce the PC2 gene-excision. These mice develop a liver phenotype resembling human ADPKD. To inhibit mTOR signaling, animals were treated for 8 weeks, beginning from the fifth week of age with Rapamycin (LC laboratories, Woburn, MA) 1.5 mg/kg/day i.p. (n=10), or vehicle, DMSO (n=10). All experiments were performed according to protocols approved by the Yale University Institutional Animal Care and Use Committee.
Immunohistochemical studies
Paraffin-fixed liver sections (5 µm thick) were deparaffinised and stained by H&E. A pancytokeratin antibody (56kDa and 64kDa keratins, DAKO; Carpinteria, CA; 1:300) was used to identify the biliary cysts. To detect the antigen of interest, serial liver tissue sections were immunostained as described6. The source of antibodies used is detailed in the on line supplementary material. For all immunoreactions, negative controls were also included and showed no staining.
Morphometric analysis of cystic area and pancytokeratin-positive structures
Serial sections of the two main liver lobes treated as above were mounted on 0.1% poly-L-lysine-coated glass slides and immunostained with a pancytokeratin antibody to allow a correct discrimination of the biliary cysts structures from the vessels. The relative area covered by the biliary cysts (5 random non-overlapping fields per each main liver lobe) was recorded by a digital camera, at 10× magnification, (in total 10-fields per mouse). In each field the cystic areas was measured by two investigators blinded to the treatment modality, using an Image-J software (NIH, Bethesda, MD)7. On the same liver sections we also calculated the percentage of the whole liver lobe area occupied by pancytokeratin positive cells using a motorized stage system able to scan the whole liver lobes. Images taken at a 4× magnification were analyzed by the Metamorph software (Molecular devices, Downington, PA, USA) (see supplementary material).
Quantification of proliferation, apoptosis and pERK-positive structures in liver tissue sections
Liver sections were immunostained with anti-PCNA antibody (1:200) (Santa Cruz Biotechnology; Santa Cruz, Inc., CA) to measure the percent of cystic cholangiocytes entering the cell cycle. Immunodetection of the cleaved form of Caspase-3 (1:50) (R&D Systems, Minneapolis, MN) was used to detect cells undergoing apoptosis, and immunodetection of the phosphorylated form of ERK (pERK) (Cell Signaling Technology, Danvers, MA) (1:100), was used to assess the activation of the ERK pathway. The amount of pERK and cleaved caspase-3 positive structures was estimated by a computer-assisted morphometric analysis, as described above.
Isolation and characterization of cholangiocytes
Mouse cholangiocytes and cystic epithelial cells were isolated and cultured from WT and Pkd2KO mice essentially as previously described6, 7. Microdissected intrahepatic bile ducts were used to obtain WT cholangiocytes, while in the case of conditional KO mice cells were isolated from microdissected cysts, as described6, 7.WT and PKD2KO mouse cholangiocytes were maintained in 25cm2 tissue culture flasks in medium enriched with 10% FBS at 37°C in a humidified, 5% CO2 atmosphere. For a detailed characterization of the cultured cells, see the online material. The biliary phenotype and maintenance of the normal polarity was confirmed by staining for cytokeratin-19 (CK-19), for acetylated α-tubulin and by measuring transepithelial resistance and by electron microscopy, as previously described7.
Determination of HIF1α in cultured cells
Cells were incubated in presence of the prolyl 4-hydroxylases inhibitor, the 2-oxoglutarate analogs dimethyloxaloylglycine (DMOG; 3mM, 18 hrs) or IGF-1 (10ng/ml), with or without the PI3K inhibitor LY294002 (10 µM, with 10min pre-treatment) or Rapamycin (10nM with 10min pretreatment) and compared with control cells. The nuclear fraction of each sample was isolated using a nuclear extraction kit (NE-PER; Pierce Biotechnology, Rockford, IL) (for the purity of the nuclear extract see supplementay fig. 1). The concentration of protein was determined by the Bradford method (Pierce Biotechnology, Rockford, IL). The amount of HIF-1α was measured using an HIF1α kit (R&D Systems, Minneapolis, MN) by Duoset-enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s protocol. The amount of HIF1α was then normalized to the amount of nuclear protein.
Measurement of VEGF secretion
An ELISA assay (Biosource International) was used to quantify VEGF in culture medium collected from cholangiocytes isolated from polycystic and controls mice, as we previously described7. A VEGF standard curve was generated for each individual experiment. Readings were normalized for the total protein in the well.
Determination of Cell Proliferation
Cells were plated into 96-multiwells plates (5000 cells/well) and serum-starved7. After 24 hours, cells were supplemented with IGF1 (10 ng/ml) alone, and with Rapamycin (10nM) or a competitive VEGFR-2 inhibitor, SU5416 (5µM), as shown in the result section. Cell proliferation was measured using: a) the CellTiter 96 AQueous One Solution (Promega Italia, Milan, Italy), which exploits the MTS tetrazolium compound colorimetric bioreduction by the cells; b) the Biotrak™ ELISA system (GE Healthcare, Piscataway NJ) which measures the incorporation of the pyrimidine analogue 5-bromo-2’-deoxyuridine during DNA synthesis in proliferating cells.
Western Blots
Methodologic details of Western blots can be found in the on-line supplementary material.
Microvascular density
To study the changes in pericystic microvascular density induced by treatment with rapamycin, liver sections were stained with rat anti-CD3418 and counterstained with pancytokeratin7. The biliary and vascular areas were calculated as reported in the supplementary material.
Statistical analysis
Results are shown as mean±standard deviation. Statistical comparisons were made using one-way ANOVA, or the Wilcoxon-Mann-Whitney two-sample rank-sum test, where appropriate. In the latter, the p-value was obtained from the exact permutation null distribution. The statistical analysis was performed using SAS software (SAS, Cary,NC). p values <0.05 were considered as significant.
RESULTS
Phospho-mTOR, IGF1 and IGF1-R are over-expressed in Pkd2KO cystic cholangiocytes
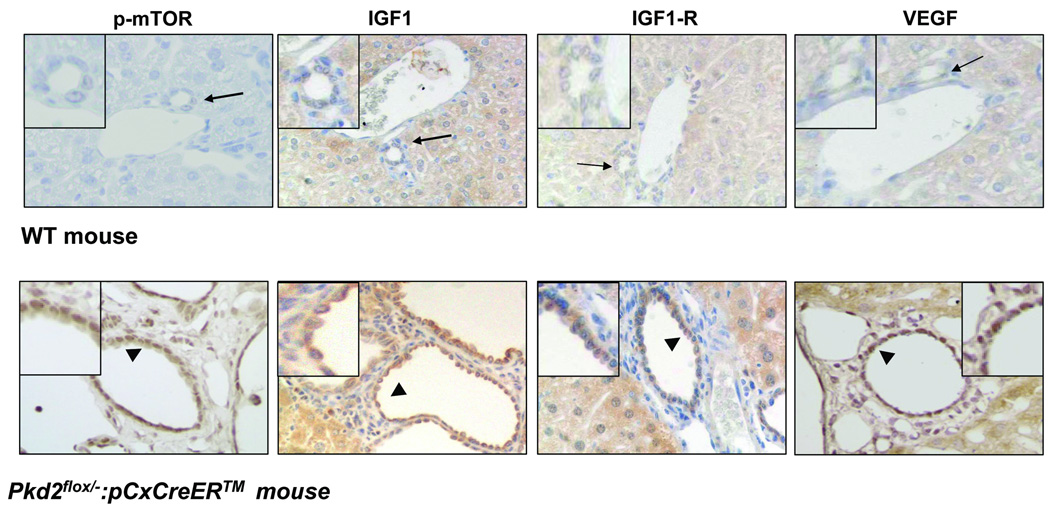
Pkd2KO mice developed a liver phenotype similar to human ADPKD 7. VEGF, VEGFR-2, phospho-mTOR (the phosphorylated, active, form of mTOR), IGF1 and its receptor IGF1-R were expressed in the cystic epithelium (n=3) by immunohistochemistry (fig.1). These findings are consistent with previous reports showing over-expression of mTOR, VEGF, VEGFR-2, IGF1 and IGF1-R in liver cysts of patients with ADPKD5, 6 and establish that the Pkd2KO mouse is an adequate model to study the role of mTOR, VEGF and IGF1 in liver cyst growth.
Figure 1. Expression of p-mTOR, IGF1, IGF1-R and VEGF in cystic cholangiocytes of Pkd2KO mice.
Paraffin embedded liver sections (5µM) of WT and Pkd2flox/−:pCxCreERTM (Pkd2KO) mice were labeled with specific antibodies against the phosphorylated form of mTOR, IGF1, IGF1-R and VEGF. Immunoreactivity for p-mTOR IGF1, IGF1-R and VEGF (arrowhead) is up-regulated on the biliary epithelium in Pkd2KO mice, as respect to control mice (arrow). Magnification: 40×
Rapamycin inhibits liver cysts growth in conditional polycystin-2 KO mice
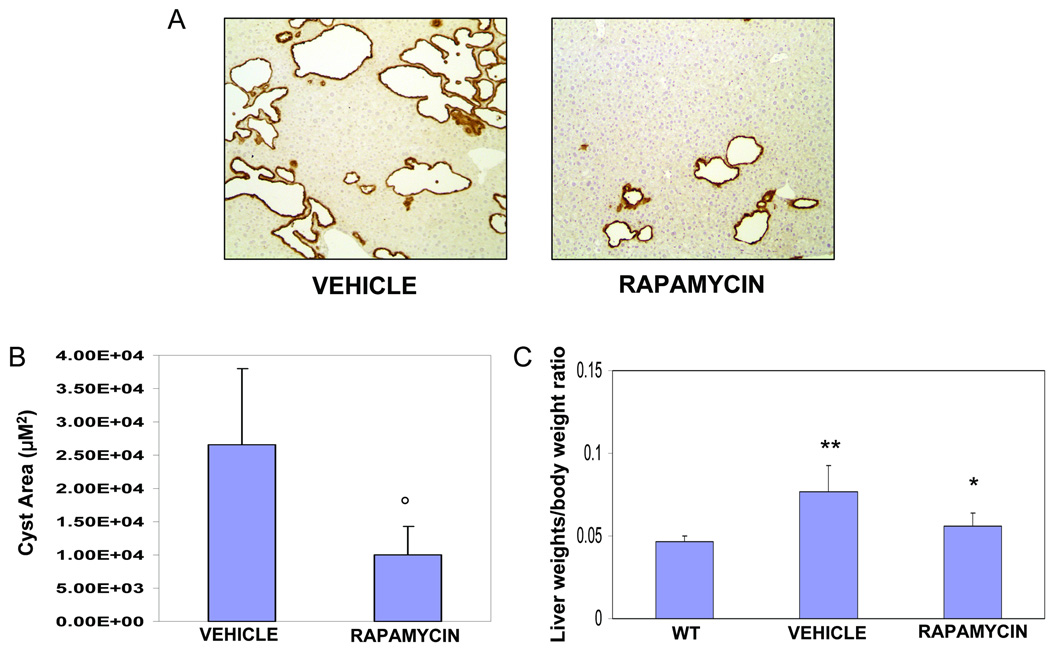
To understand the pathophysiologic relevance of increased p-mTOR expression, we treated Pkd2KO mice with the mTOR inhibitor rapamycin. Preliminary experiments using rapamycin at the dose of 5 mg/kg/day11, encountered significant toxicity (2 out of 3 mice died before completing the 8 weeks treatment). The dose of 1.5 mg/kg/daily i.p. for 8 weeks11, 13 was well tolerated, without mortality, or liver toxicity, (supplementary Table 1). After 8 weeks of treatment, mice were sacrificed. Pancytokeratin staining revealed a significant reduction in cystic area in rapamycin-treated, as compared to untreated Pkd2KO mice (Cystic area: Pkd2KO control= 27027± 10810µM2 vs 10810± 4324 µM2 in treated mice, n=10, p<0.001 (fig. 2). Similarly, the percent amount of the total area of the lobe covered by cytokeratin-positive structures was lower in rapamycin-treated mice (Pkd2KO control mice: 4.5±1.3% vs 2.2±1.1% in rapamycin-treated mice; n=10,p<0.001)(not shown). As a result of the reduction in liver cysts, treatment with rapamycin decreased the liver weight/body weight ratio (liver/bwt) of Pkd2KO mice (rapamycin treated mice=0.056±0.008, vs 0.0777±0.016 in untreated Pkd2KO mice, n=10 p<0.01; in wild type mice used as controls liver/bwt was 0.039± 0.002 n=6) (fig.2 and supplementary fig.2). Cystic area was higher in female mice; both females and males responded to rapamycin treatment (supplementary fig. 3). Furthermore, as confirm that rapamycin treatment was active we found a significant reduction in the P70S6K activation (supplementary fig 4). Furthermore, consistent with an inhibitory effect on angiogenic factors pericystic microvascular density(fig. 3A), and VEGF (supplementary fig 4) were significantly reduced.
Figure 2. Rapamycin reduces cystic area and liver weight/BW percentage in Pkd2KO mice.
(A) Micrographs are representative of vehicle (left) and rapamycin (1.5 mg/Kg/day) treated mice (right). (B) As shown in the bar graph, a significant reduction in cystic area was observed in Pkd2KO treated animals (°=p<0.001, n=10). (C) The decrease in cysts growth is reflected also in the significant reduction of liver weight/body weight ratio. In fact, liver weight/body weight ratio was higher in mice treated with vehicle (n=10) compared to wild type mice (**p<0.001, n=6) and was significantly reduced in rapamycin treated mice (n=10) (*=p<0.01).
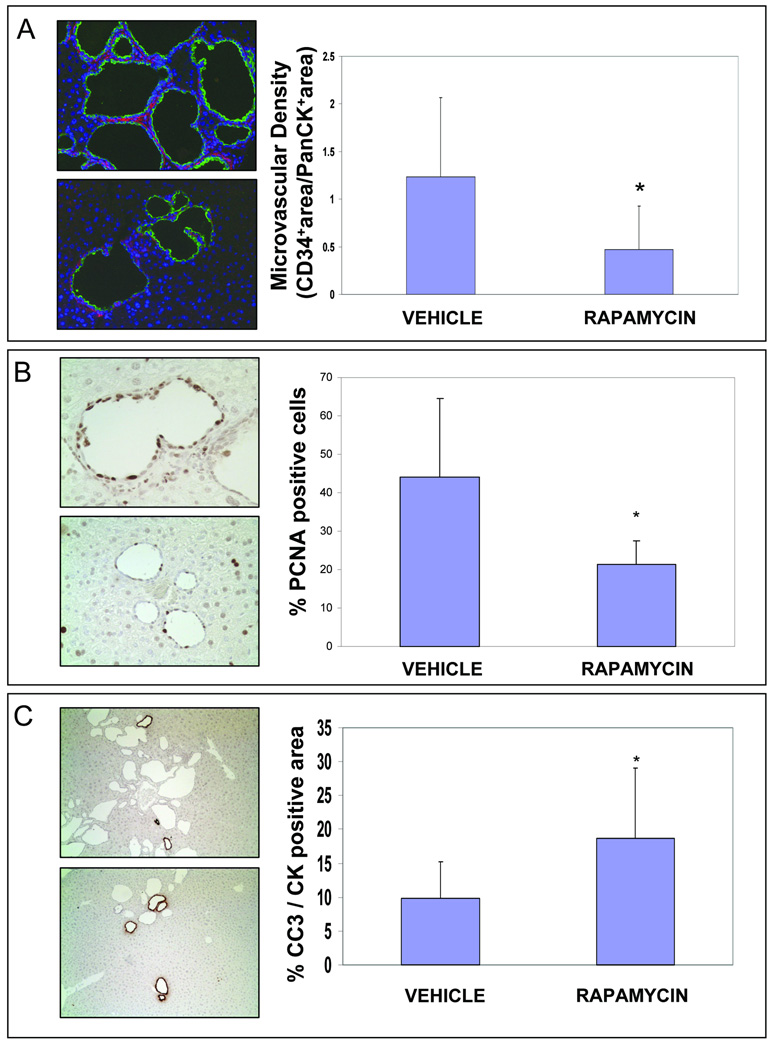
Figure 3. Rapamycin reduces microvascular density (CD34) and cell proliferation (PCNA), while increases apoptosis (CC3 expression) and in Pkd2KO mice.
Each couple of micrographs is representative of vehicle (above) and rapamycin treated mice (below). For quantitative analysis five random non-overlapping fields taken at 40× magnification per each slide were recorded by a digital camera connected to Spot Advanced imaging software (version 3.5) by an observer who was blinded to the treatment modality. (A) Rapamycin treatment reduced microvascular density in Pkd2KO mice. Paraffin embedded liver sections (5µM) were stained with an anti-CD34 and with an anti-Cow Cytokeratin Wide Spectrum (PanCK). To calculate the vascular and biliary areas, two different thresholds were set out for CD34 (red fluorescence) and PanCK (green fluorescence) positive structures respectively, and then expressed as percentage of pixel above the threshold per field. As shown in the bar graphs treatment with rapamycin significantly reduced the microvascular density. (# = p<0.05 vs vehicle;). (B) Cystic cholangiocytes showed strong proliferative activity (PCNA staining). As shown in the bar graph, a significant reduction in PCNA expression, assessed by morphometric analysis, was observed in Pkd2KO treated animals (*=p<0.001 n=10). An average of 1000 nuclei was counted per each mouse and the percentage of PCNA positive nuclei was then calculated. Only strongly positive immunostained nuclei were considered as PCNA positive. (B) To account for the decrease in liver cysts in rapamycin-treated mice, we quantified the amount of apoptosis (Cleaved Caspase-3 staining) in cystic cells. The bar graph show a significant increase in CC3 expression in Pkd2KO treated animals (*=p<0.01, n=10). CC3-positive area was expressed as percent of the cytokeratin-positive area.
Rapamycin treatment affects cell proliferation/apoptosis rate in PKD2KO mice
The proliferative activity of cystic cholangiocytes is increased as respect to controls 7 In this study we show that, after 8 weeks of treatment, PCNA immunostaining was lower in rapamycin-treated (21.4±6.1%, of cyst nuclei were positive for PCNA) as compared to vehicle-treated Pkd2KO mice (44±20.6%, PCNA-positive) (n=10,p<0.01), suggesting that rapamycin reduces the proliferation of liver cyst cells (fig. 3B).
Given the role of mTOR in cell survival, we hypothesized that rapamycin could also increase apoptosis in cystic cholangiocytes. Apoptosis in vivo was analyzed, from the immunohistochemical expression of the cleaved, activated, form of caspase-3 (CC3), using computer-assisted morphometry, in the same liver specimens used for PCNA staining. As shown in fig 3C, the CC3-positive area, was 18.7±10.3% of the cytokeratin-positive area in rapamycin-treated mice, vs 9.8±5.4% in untreated mice (n=10,p<0.05). These data suggest that mTOR inhibition reduces cysts growth through the combined reduction of proliferation and the increase in apoptosis rate in the cystic epithelium.
Rapamycin inhibits IGF1-induced HIF1α accumulation and VEGF secretion in cultured cholangiocytes
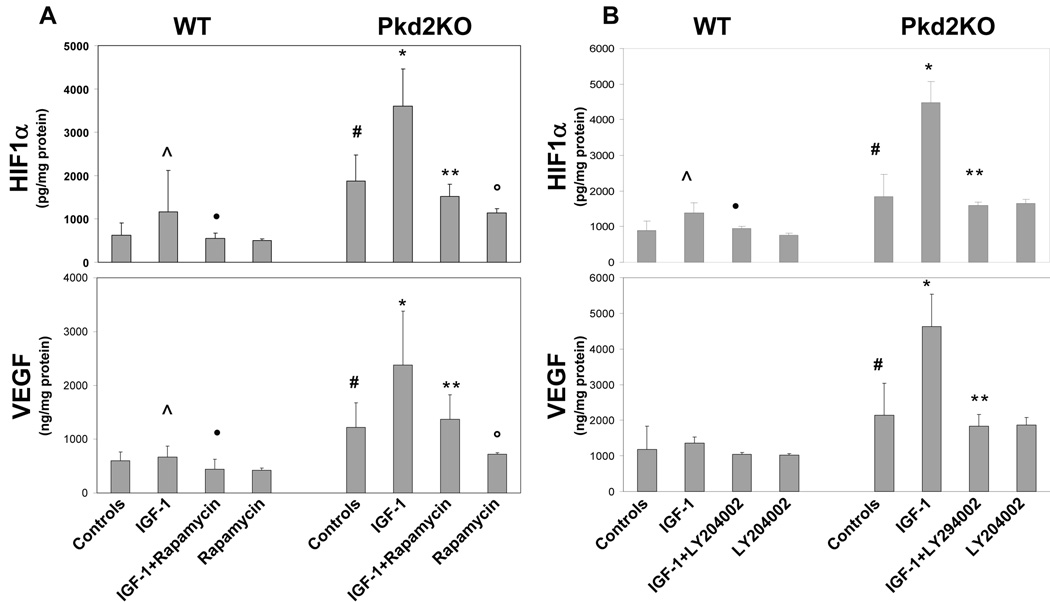
We have previously shown that VEGF/VEGFR-2 regulate cyst growth and cholangiocyte proliferation in Pkd2KO mice 7. Therefore, we studied the effect of rapamycin on VEGF secretion and on the nuclear expression of its main transcription factor, HIF1α, in cystic cholangiocytes cultured from Pkd2KO mice (n=8 isolations) and in cholangiocytes cultured from WT mice (n=5 isolations). Preliminary experiments showed that rapamycin significantly inhibited HIF1α and VEGF after administration of 3mM DMOG (2-oxoglutarate analogs dimethyloxaloylglycine), an inhibitor that blocks prolyl 4-hydroxylase-dependent HIF1α degradation, indicating that mTOR controls HIF1α-dependent VEGF secretion in cystic cholangiocytes (not shown). We next studied the effects of IGF-1 (10ng/ml for 18 hrs) in the presence or the absence of rapamycin. As shown in Fig.4A and supplementary table 2, nuclear expression of HIF1α was significantly higher in Pkd2KO, than in WT cells, both at baseline (1875±605 pg/mg of protein vs 625 ± 286 pg/mg in WT cells, n=15, p<0.001) and after IGF1 administration (3598 ± 860 pg/mg vs 1167 ± 959 pg/mg in WT cells after IGF1 stimulation, n=7, p<0.001. Rapamycin significantly inhibited IGF1-induced HIF1α accumulation in Pkd2KO cholangiocytes (1516 ± 288 ng/mg n=4, p<0.001). Similarly, VEGF released in the culture medium was significantly higher in Pkd2KO than in WT cholangiocytes, both at baseline (1440 ± 52 ng/mg of protein in cells vs 596 ± 167 ng/mg in WT cells, n=15, p<0.001) and after IGF1 administration (2381± 997 ng/mg in Pkd2KO cells vs 665 ± 205 ng/mg in WT cells after IGF1 stimulation, n=7, p<0.001). In Pkd2KO cholangiocytes, rapamycin significantly decreased VEGF secretion after stimulation with IGF1 (1368 ± 462 ng/mg n=4, p<0.05). These results indicate that IGF-1 stimulates HIF1α and VEGF via the mTOR pathway and that inhibition of mTOR by rapamycin inhibits HIF1α-dependent VEGF secretion in cystic cholangiocytes.
Figure 4. Rapamycin and LY294002 inhibited IGF1-induced HIF1α accumulation and VEGF secretion.
The effect of rapamycin on VEGF secretion and on the nuclear expression of its main transcription factor, HIF1α, in primary cultures of cystic cholangiocytes cultured from Pkd2KO mice was assessed by ELISA. (A) In Pkd2KO cultured cholangiocytes, HIF-1α accumulation and VEGF secretion induced by IGF1 are significantly higher as respect to WT cholangiocytes. This effect was completely blunted in cells treated with rapamycin (5 µM) (n=4) and in cells treated with a PI3K inhibitor, LY204002 (10 µM) (n=3) (B). (#= p<0.005 vs WT C), (^= p<0.05 vs WT C), (•= p<0.05 vs WT+IGF-1), (*= p<0.001 vs Pkd2KO C), (**= p<0.001 vs Pkd2KO+IGF-1)
PI3 kinase inhibition blocks IGF1-induced HIF1α accumulation and VEGF secretion
To better understand the relationship between the PI3K/pAKT/mTOR pathway and VEGF production in cystic cholangiocytes, we studied the effects of the PI3K inhibitor LY294002 (10 µM), on HIF1α nuclear expression and on VEGF secretion in the presence of IGF1 (10 ng/ml). Figure 4B and supplementary table 3 show that both HIF1α and VEGF production are significantly reduced in Pkd2KO-cholangiocytes treated with LY294002 (HIF1α=4483±586 pg/mg of protein after IGF1 stimulation vs 1589 ± 95 pg/mg after IGF1 and LY24002 treatment n=3, p<0.001) (VEGF=4629 ± 304 ng/mg of protein after IGF1 stimulation vs 1838 ± 313 ng/mg after IGF1 and LY24002 treatment n=3, p<0.01) This is consistent with the hypothesis that IGF1 stimulates HIF1α-dependent VEGF production through the PI3K/AKT/mTOR pathway.
IGF1-induced cell proliferation in cystic cholangiocytes is mediated by mTOR and inhibited by the blockade of VEGF autocrine signaling
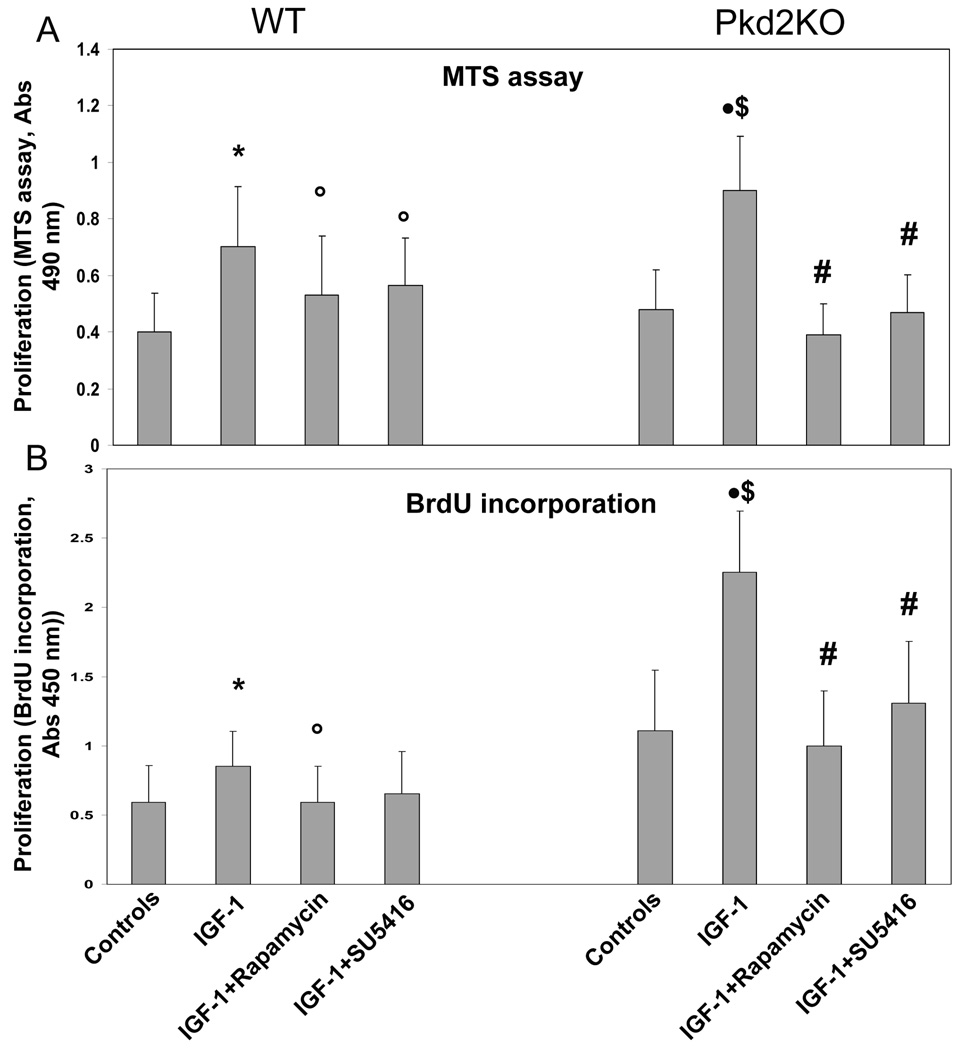
We have previously shown that administration of VEGF increased proliferation of cystic cholangiocytes through VEGFR-2 stimulation7. IGF1 (10 ng/ml) increased cell proliferation, as assessed by MTS assay and by BrDU incorporation, in both normal and cystic cholangiocytes (fig. 5). Administration of rapamycin (10 nM) to IGF1-treated Pkd2KO- and WT-cholangiocytes significantly inhibited IGF1-induced cell proliferation, as measured by BrdU incorporation and MTS assay (Fig. 5). These data indicate that the PI3K/AKT/mTOR pathway mediates IGF1R signaling in cholangiocytes, and, to a larger extent, in cystic cholangiocytes. Interestingly, the VEGFR-2 inhibitor SU5416 (5µM), significantly decreased IGF1-induced proliferation of cystic cholangiocytes by 50%, suggesting that IGF1 proliferative effects in cholangiocytes may be in part mediated through the increased secretion of VEGF.
Figure 5. Rapamycin and SU5614 inhibit IGF-1-induced cell proliferation in cultured Pkd2KO cystic cholangiocytes.
Using two different cell proliferation assays similar results were obtained. MTS assay results are shown in panel A, and BrdU incorporation is shown in panel B. In both WT and Pdk2KO cholangiocytes, IGF1 significantly enhanced cell proliferation compared to untreated cells (*=p<0.05 with respect to WT controls cholangiocytes. •=p<0.01 with respect to control Pkd2KO cholangiocytes.) The increase in cell proliferation stimulated by Pkd2KO cholangiocytes was significantly higher in Pkd2KO cholangiocytes (&=p<0.05) with respect to WT cholangiocytes exposed to IGF-1). IGF1-induced cell proliferation was significantly inhibited by treatment with rapamycin (5 µM) or with SU5416, a VEGFR-2 inhibitor; °=p<0.05 in WT cholangiocytes treated with IGF-1 plus rapamycin or SU5416, as respect to IGF-1-treated WT cholangiocytes; #=p<0.01 in Pkd2KO cholangiocytes treated with IGF-1 plus rapamycin or SU5416, as respect to IGF-1-treated Pkd2KO cholangiocytes;) (n=3).
Cross-talk between ERK1/2 and mTOR in cystic cholangiocytes
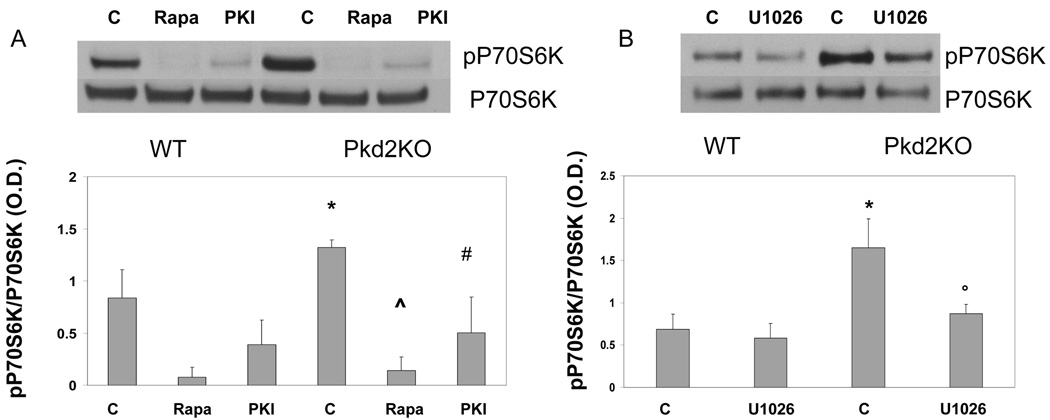
Our data show that IGF1, through PI3K/pAKT/mTOR, increases HIF1α-dependent VEGF secretion and cell proliferation. A recent paper suggested that polycystins controls mTOR activity by inhibiting ERK19. We have previously shown that PKA-mediated phosphorylation of pERK1/2 is increased in cystic cholangiocytes, and correlates with increased secretion of VEGF and response to VEGFR2 stimulation7. To better understand the relationships between mTOR activation and PKA-mediated phosphorylation of ERK in cystic cholangiocytes, we measured the phosphorylation of P70S6K, a kinase activated by mTOR, after inhibition of PKA with PKI (1 µM, n=3) and after inhibition of ERK pathway using the MEK inhibitor (U1026, 10 µM). As shown in fig 6, phosphorylation of P70S6K was increased in Pkd2KO cholangiocytes and was inhibited by PKI, and by U1026, suggesting that the PKA/ERK pathway activates mTOR19.
Figure 6. Phosphorylation of the mTOR-activated P70S6K is PKA- and ERK-mediated.
A) In Pkd2KO cystic cholangiocytes the ratio in phospho-P70S6K/P70S6K is higher with respect to WT cells, it was completely inhibited by rapamycin, as expected, and significantly inhibited by the PKA inhibitor PKI (*=p<0.05 with respect to WT untreated cholangiocytes; ^=p<0.001 with respect to Pkd2KO untreated cholangiocytes; #=p<0.01 with respect to Pkd2KO untreated cholangiocytes). B) In Pkd2KO cholangiocytes the ratio in phospho-P70S6K/P70S6K was significantly inhibited by the MEK inhibitor U1026 (10µM). (*=p<0.05 with respect to WT untreated cholangiocytes; °=p<0.05 with respect to Pkd2KO untreated cholangiocytes)
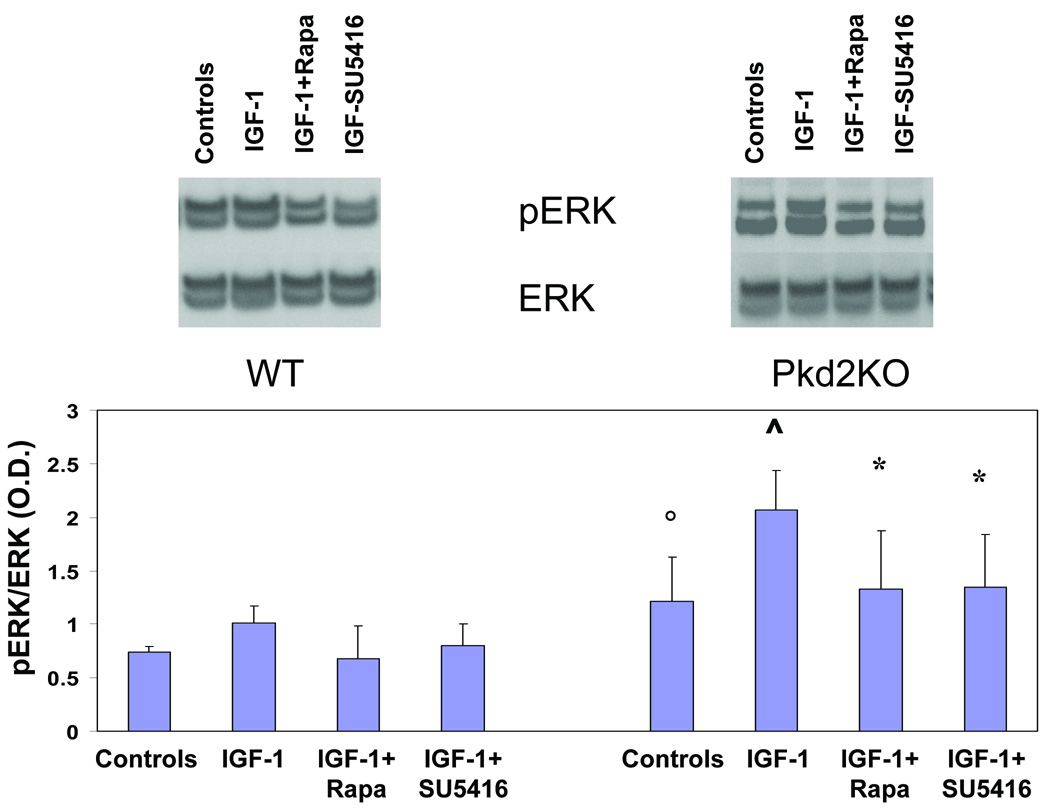
Conversely, to understand if mTOR affects ERK1/2 activity, we studied phospho-ERK1/2 expression after administration of IGF1 with or without rapamycin or the VEGFR2 inhibitor SU5416. As shown in fig.7 IGF1-induced ERK1/2 phosphorylation, was significantly inhibited by treatment with rapamycin (5 µM), and also by the VEGFR-2 inhibitor SU5416, (pERK/ERK ratio in control Pkd2KO cells, was 1,21 ± 0,4, versus 2,1 ± 0,4 after IGF1 administration (p<0.05). The pERK/ERK ratio was reduced to 1,34 ± 0,5 after IGF1 and rapamycin (p<0.05) (n=5) and to 1,34 ± 0,5 after IGF1 and SU5416 (p<0.05) (n=5). As shown in the supplementary fig. 5, SU5416 had no inhibitory effects on IGFR-1, therefore these findings suggest that mTOR does not directly activates pERK1/2, but, rather, the increased secretion of VEGF caused by IGF1 via the mTOR pathway, activates the MEK/ERK1/2 pathway downstream of VEGFR-2.
Figure 7. Rapamycin and SU5614 inhibit IGF-1-induced ERK phosphorylation in cultured Pkd2KO cystic cholangiocytes.
IGF1 significantly enhances ERK phosphorylation. IGF1-induced ERK phosphorylation was significantly inhibited by treatment with rapamycin (5 µM) or with SU5416, a VEGFR-2 inhibitor; the bar graph illustrates the ratio in phosphorylated ERK/ERK as assessed by densitometry (°=p<0.05 as respect to WT cells) (^=p<0.05 as respect to Pkd2KO controls) (*p<0.05 as respect to Pkd2KO controls) (n=4).
DISCUSSION
The progressive growth of liver cysts can cause significant morbidity in patients with ADPKD1. Understanding the mechanisms by which liver cysts enlarge may lead to novel treatment paradigms. Liver cysts are not connected to the biliary tree and their growth is dependent on the autocrine effect of cytokines and growth factors produced by the cystic epithelium. Among these factors, VEGF and IGF-1, along with their cognate receptors, are expressed by cystic cholangiocytes and capable of autocrine stimulation of the liver cyst epithelium5, 6.
In this study, using mice with conditional inactivation of polycystin-2, we demonstrate that mTOR plays a central role in cyst growth. Furthermore we show that IGF-1 and VEGF signaling are linked through the PI3K/AKT/mTOR pathway, that there is significant cross-talk between mTOR and ERK1/2 and that the mTOR inhibitor rapamycin reduces the growth of liver cysts in vivo through the repression of VEGF secretion, with reduced cell proliferation and increased apoptosis.
Mammalian-TOR is a signaling molecule that integrates a broad spectrum of signals, including growth factors20. Hormones and growth factors activate several downstream transduction pathways that include the PI3K/AKT and the Ras/MEK/ERK pathways. Both pathways converge to activate mTOR by inhibiting the activity of its negative regulator tuberin (more specifically the tumor suppressor complex 2 or TSC2)21. It has been shown that AKT and ERK may directly phosphorylate different serine residues on TSC2, thereby inhibiting its activity22, 23. A number of functions modulated by mTOR are potentially relevant for liver cyst growth. Among them, mTOR stimulates HIF1α, a main transcription factor for VEGF24.
Rapamycin, an inhibitor of mTOR, commonly used as an anti-rejection agent has shown promising oncologic applications, because of its ability to promote chemotherapy-induced apoptosis, and inhibit angiogenesis16. Previous studies in animal models of polycystic kidney diseases, non orthologous to polycystin defects, such as the Han:Sprd rats25, orpk and bpk mice11 reported that treatment with rapamycin reduced kidney cysts and improved kidney function. Retrospective studies showed a reduction in kidney and liver cysts in patients with advanced-stage ADPKD who received a renal transplant and were treated with a rapamycin-containing anti-rejection regimen14.
We show that administration of rapamycin significantly decreased the cystic area of the liver and the liver/body-weight ratio in Pkd2KO mice. At a dose of 1.5 mg/kg/daily rapamycin was well tolerated with no significant changes in liver function test compared to untreated controls. Treatment with rapamycin decreased the PCNA index of liver cysts, while increasing the expression of cleaved caspase-3, suggesting that rapamycin alters the balance between proliferation and apoptosis by reducing the number of proliferating cells and enhancing cyst apoptosis in vivo.
Because of the role of VEGF on polycystic liver disease progression and the reported antiangiogenic effects of rapamycin in cancer, we studied the effects of rapamycin on VEGF production in cystic cholangiocytes cultured from PC2-defective mice. We found that rapamycin suppressed the increased HIF1α nuclear expression and VEGF production typical of PC2-defective cells. This indicates that VEGF production in cystic cholangiocytes is controlled by mTOR and that the inhibitory effects of rapamycin on liver cysts could be, in part explained by the inhibition of VEGF expression.
IGF1 is a cholangiocyte growth factor able to stimulate the PI3K/AKT pathway. IGF1 is over-expressed by the cystic epithelium and reaches a high concentration into the fluid of hepatic cystic in ADPKD patients5. The IGF1-receptor (IGF1-R) is over-expressed in human cholangiopathies, including cholangiocarcinoma, and human liver ADPKD5, 26. Here we show that administration of IGF1 significantly increased HIF-1α and VEGF in cystic cholangiocytes, as respect to WT cholangiocytes. Stimulation of IGF1-R is known to activate different common transduction pathways that modulate proliferation/survival27. Consistent with earlier studies, showing that, in cholangiocytes, IGF1 activates mainly the PI3K/AKT/mTOR pathway5, 27, 28, we have found that IGF1-stimulated, HIF-1α-dependent, VEGF expression was inhibited by rapamycin. The significant inhibitory effect of the PI3K inhibitor LY294002 on IGF1-induced, HIF1α̃-dependent, VEGF secretion is consistent with a major role of PI3K/AKT in mediating IGF-1 signaling in cholangiocytes.
Consistent with the above data, IGF-1 stimulated cell proliferation in PC2 defective cells (fig.5) and this effect was significantly inhibited by rapamycin. However, IGF1 stimulates secretion of VEGF, also a strong mitogen for cystic cholangiocytes. As shown in fig. 5, IGF1-induced cell proliferation in cystic cholangiocytes was significantly inhibited by the VEGF-R2 inhibitor SU5416. SU5416 did not affect the phosphorylation of the IGF1 receptor, on the contrary to the specific IGF1-R inhibitor AG102429, used as positive control, indicating that SU5416 effects on VEGFR2 are specific (supplementary fig. 5). These data strongly argue for the presence of an autocrine loop in which IGF-1-stimulates PI3/AKT/mTOR, mTOR-stimulates HIF1a-dependent secretion of VEGF, that in turn, interacting with his VEGFR-2 receptor activates a MEK/ERK1/2-dependent proliferative effect. Consistent with this interpretation, both rapamycin and SU5416 inhibited the increase in p-ERK expression induced by IGF-1 in cystic cholangiocytes. Further indication of this autocrine loop involving IGF, mTOR and VEGF secretion is the strong reduction in pericystic microvascular density in mice treated with rapamycin (fig. 3A).
An open question is the mechanistic relationships between the above mechanisms and the polycystin defect. Schillingford et al11 and Distefano et al19 proposed that PC1 acts as an inhibitor of TSC2, a mechanism that would be lost in ADPKD, thereby leading to an increased activity of mTOR. These data represent an important clue; however they have been generated by over-expressing PC1. In our study we have instead used a strategy involving the loss of function of PC2. In addition to its functional relationships with PC1, PC2 participates in the cellular and ER homeostasis of calcium 3, 4. Lower cellular and ER calcium stimulates PKA and ERK phopsphorylation30–33. We have recently shown that in PC2-defective cholangiocytes, the increase in pERK1/2 is PKA-dependent7. We here provide evidence that baseline p-mTOR activation in PC2 defective cholangiocytes (as judged by its downstream kinase P70S6), is PKA- and ERK-dependent, and thus linked to the altered calcium homeostasis.
We can only speculate as to why the cystic epithelium produces this vast array of growth factors. The mechanisms are unclear, but this is akin to what happens to wild type biliary epithelium during liver repair. Thus, cystic cholangiocytes resembles “activated” cholangiocytes, in terms of their ability to generate autocrine and paracrine growth factors34. This property may be a consequence of a relative lack of terminal differentiation, as we suggested earlier, or a response needed to cope with cellular stress. In this study we did not explore the relationship between these mechanisms and estrogens; however they must be taken into account in the overall scenario, as also shown by the more severe phenotype of female mice. A number of clinical observations35 indicate that estrogens play a role in polycystic liver diseases. The estrogen receptor-β is up-regulated in liver cysts of ADPKD patients and 17-β estradiol stimulates the proliferation of cystic cholangiocytes obtained from patients with ADPKD have also shown that ADPKD epithelium is sensitive to the proliferative effects of estrogens and IGF15. Estrogens also promote the synthesis and release of growth factors, including IGF-1, from the cyst epithelium5.
In conclusion our study demonstrates that mTOR plays a central role in liver cyst growth in mice with defective polycystin-2 (fig. 8). The mTOR pathway regulates HIF1α-dependent VEGF-secretion and appears central to the proliferative, anti-apoptotic and pro-angiogenic effects of IGF-1, one of the major factors generated by the cystic epithelium. The mTOR inhibitor rapamycin inhibits VEGF secretion and signaling and significantly reduces liver cysts growth, by reducing proliferation and increasing apoptosis of the cystic epithelium. This study also reveals a mechanistic link between mTOR and ERK, and HIF-1α-mediated VEGF secretion and provides a proof of concept for the potential use of mTOR inhibitors in polycystic liver disease, as well as in conditions with aberrant cholangiocyte proliferation.
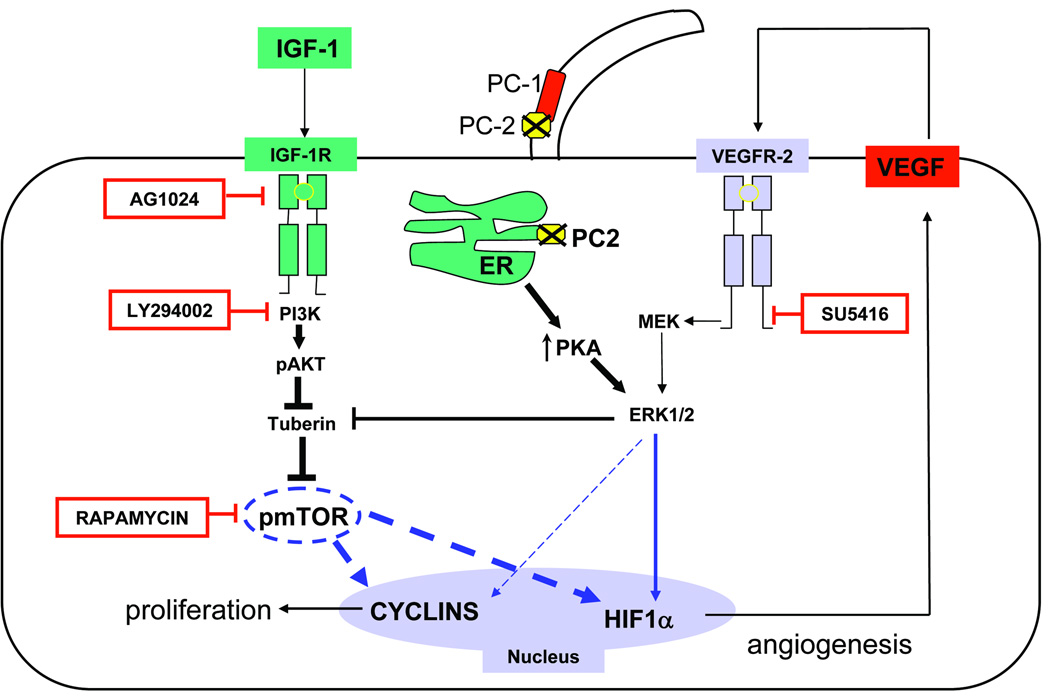
Figure 8. Working Model to explain cyst growth in PC2-defective cholangiocytes.
The cartoon summarizes the main findings of this study, in the contest of the most recent literature7, 19. Cells with defective PC2 are characterized by increased PKA production and ERK1/2 phosphorylation7. Erk 1/2 stimulates HIF1α-dependent VEGF secretion directly and by inhibiting tuberin, a negative regulator of mTOR23. mTOR has a central role in IGF-1-stimulated proliferation of cystic cholangiocytes. IGF-1, a growth factor secreted by the cystic epithelium and by cholangiocyte under stress, binds to its receptor IGF1-R and activates the PI3K/AKT/mTOR pathway; mTOR stimulates proliferation through cyclins and through a HIF1α/VEGF-dependent autocrine loop.
Rapamycin is an mTOR inhibitor; LY294002 is a PI3K inhibitor; AG1024 is an IGF-1R inhibitor; SU5416 is a VEGFR-2 inhibitor
Supplementary Material
Acknowledgments
Financial support:
Grant Support: supported by NIH DK079005, and PKD Foundation to MS and by Yale University Liver Center (NIH DK34989) to MS and CS and by NIH DK51041 and DK54053 to SS. CS is a recipient of an ALF/AASLD Liver Scholar Award. SO was a recipient of a fellowship from FADE (Fondazione Amici della Epatologia) and from AISF (Associazione Italiana Studio del Fegato). The support of Fondazione S. Martino, Bergamo is also gratefully acknowledged.
List of abbreviations
- ADPKD
Autosomal Dominant Polycystic Kidney Disease
- cAMP
cyclic AMP
- CC3
cleaved caspse 3
- DMOG
2-oxoglutarate analogs dimethyloxaloylglycine
- ERK
Extracellular signal-regulated kinase
- HIF
Hypoxia Inducible Factor
- IGF
Insulin like Growth Factor
- LCE
liver cystic epithelium
- LCEC
liver cystic epithelial cells
- MEK
mitogen signal-regulated kinase
- mTOR
mammalian target of rapamycin
- PC1
Polycystin-1
- PC2
Polycystin-2
- PI3K
Phospho-Inositol 3 Kinase
- PCNA
Proliferating Cell Nuclear Antigen
- PKA
Protein Kinase A
- PKI
PKA inhibitor 14–22 Amide
- VEGF
Vascular Endothelial Growth Factor-A
Footnotes
All Authors state there are no conflicts of interest to disclose
Contributor Information
Carlo Spirli, Email: carlo.spirli@yale.edu.
Stefano Okolicsanyi, Email: s.okolicsanyi@campus.unimib.it.
Romina Fiorotto, Email: romina.fiorotto@yale.edu.
Luca Fabris, Email: luca.fabris@unipd.it.
Massimiliano Cadamuro, Email: maxcada@libero.it.
Silvia Lecchi, Email: silvia.lecchi@gmail.com.
Xin Tian, Email: xin.tian@yale.edu.
Stefan Somlo, Email: stefan.somlo@yale.edu.
Mario Strazzabosco, Email: mario.strazzabosco@yale.edu.
REFERENCES
- 1.Bae KT, Zhu F, Chapman AB, Torres VE, Grantham JJ, Guay-Woodford LM, et al. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol. 2006;1:64–69. doi: 10.2215/CJN.00080605. Epub 2005 Oct 26. [DOI] [PubMed] [Google Scholar]
- 2.Torres VE, Harris PC. Mechanisms of Disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol. 2006;2:40–55. doi: 10.1038/ncpneph0070. quiz 55. [DOI] [PubMed] [Google Scholar]
- 3.Anyatonwu GI, Estrada M, Tian X, Somlo S, Ehrlich BE. Regulation of ryanodine receptor-dependent calcium signaling by polycystin-2. Proc Natl Acad Sci U S A. 2007;104:6454–6459. doi: 10.1073/pnas.0610324104. Epub 2007 Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Wright JM, Qian F, Germino GG, Guggino WB. Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J Biol Chem. 2005;280:41298–41306. doi: 10.1074/jbc.M510082200. Epub 2005 Oct 13. [DOI] [PubMed] [Google Scholar]
- 5.Alvaro D, Onori P, Alpini G, Franchitto A, Jefferson DM, Torrice A, et al. Morphological and functional features of hepatic cyst epithelium in autosomal dominant polycystic kidney disease. Am J Pathol. 2008;172:321–332. doi: 10.2353/ajpath.2008.070293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabris L, Cadamuro M, Fiorotto R, Roskams T, Spirli C, Melero S, et al. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology. 2006;43:1001–1012. doi: 10.1002/hep.21143. [DOI] [PubMed] [Google Scholar]
- 7.Spirli C, Okolicsanyi S, Fiorotto R, Fabris L, Cadamuro M, Lecchi S, et al. Erk1/2-Dependent Vascular Endothelial Growth Factor Signaling Sustains Cyst Growth in Polycystin-2 Defective Mice. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.09.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shelton JG, Steelman LS, White ER, McCubrey JA. Synergy between PI3K/Akt and Raf/MEK/ERK pathways in IGF-1R mediated cell cycle progression and prevention of apoptosis in hematopoietic cells. Cell Cycle. 2004;3:372–379. [PubMed] [Google Scholar]
- 9.Frost P, Shi Y, Hoang B, Lichtenstein A. AKT activity regulates the ability of mTOR inhibitors to prevent angiogenesis and VEGF expression in multiple myeloma cells. Oncogene. 2007;26:2255–2262. doi: 10.1038/sj.onc.1210019. [DOI] [PubMed] [Google Scholar]
- 10.Treins C, Giorgetti-Peraldi S, Murdaca J, Monthouel-Kartmann MN, Van Obberghen E. Regulation of hypoxia-inducible factor (HIF)-1 activity and expression of HIF hydroxylases in response to insulin-like growth factor I. Mol Endocrinol. 2005;19:1304–1317. doi: 10.1210/me.2004-0239. [DOI] [PubMed] [Google Scholar]
- 11.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao Y, Kim J, Schrier RW, Edelstein CL. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol. 2005;16:46–51. doi: 10.1681/ASN.2004080660. [DOI] [PubMed] [Google Scholar]
- 13.Wu M, Wahl PR, Le Hir M, Wackerle-Men Y, Wuthrich RP, Serra AL. Everolimus retards cyst growth and preserves kidney function in a rodent model for polycystic kidney disease. Kidney Blood Press Res. 2007;30:253–259. doi: 10.1159/000104818. [DOI] [PubMed] [Google Scholar]
- 14.Qian Q, Du H, King BF, Kumar S, Dean PG, Cosio FG, et al. Sirolimus reduces polycystic liver volume in ADPKD patients. J Am Soc Nephrol. 2008;19:631–638. doi: 10.1681/ASN.2007050626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 16.Hidalgo M, Rowinsky EK. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene. 2000;19:6680–6686. doi: 10.1038/sj.onc.1204091. [DOI] [PubMed] [Google Scholar]
- 17.Guo C, Yang W, Lobe CG. A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis. 2002;32:8–18. doi: 10.1002/gene.10021. [DOI] [PubMed] [Google Scholar]
- 18.Jebreel A, England J, Bedford K, Murphy J, Karsai L, Atkin S. Vascular endothelial growth factor (VEGF), VEGF receptors expression and microvascular density in benign and malignant thyroid diseases. Int J Exp Pathol. 2007;88:271–277. doi: 10.1111/j.1365-2613.2007.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Distefano G, Boca M, Rowe I, Wodarczyk C, Ma L, Piontek KB, et al. Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol Cell Biol. 2009;29:2359–2371. doi: 10.1128/MCB.01259-08. Epub 2009 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. Epub 2004 Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carriere A, Ray H, Blenis J, Roux PP. The RSK factors of activating the Ras/MAPK signaling cascade. Front Biosci. 2008;13:4258–4275. doi: 10.2741/3003. [DOI] [PubMed] [Google Scholar]
- 23.Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, et al. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–14064. doi: 10.1074/jbc.M700906200. Epub 2007 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Jia WD, Xu GL, Wang ZH, Li JS, Ma JL, et al. Antitumoral Activity of Rapamycin Mediated Through Inhibition of HIF-1alpha and VEGF in Hepatocellular Carcinoma. Dig Dis Sci. 2008 doi: 10.1007/s10620-008-0605-3. [DOI] [PubMed] [Google Scholar]
- 25.Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wuthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2006;21:598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 26.Alvaro D, Macarri G, Mancino MG, Marzioni M, Bragazzi M, Onori P, et al. Serum and biliary insulin-like growth factor I and vascular endothelial growth factor in determining the cause of obstructive cholestasis. Ann Intern Med. 2007;147:451–459. doi: 10.7326/0003-4819-147-7-200710020-00003. [DOI] [PubMed] [Google Scholar]
- 27.Onori P, Alvaro D, Floreani AR, Mancino MG, Franchitto A, Guido M, et al. Activation of the IGF1 system characterizes cholangiocyte survival during progression of primary biliary cirrhosis. J Histochem Cytochem. 2007;55:327–334. doi: 10.1369/jhc.6R7125.2006. [DOI] [PubMed] [Google Scholar]
- 28.Alvaro D, Metalli VD, Alpini G, Onori P, Franchitto A, Barbaro B, et al. The intrahepatic biliary epithelium is a target of the growth hormone/insulin-like growth factor 1 axis. J Hepatol. 2005;43:875–883. doi: 10.1016/j.jhep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Kisielewska J, Ligeza J, Klein A. The effect of tyrosine kinase inhibitors, tyrphostins: AG1024 and SU1498, on autocrine growth of prostate cancer cells (DU145) Folia Histochem Cytobiol. 2008;46:185–191. doi: 10.2478/v10042-008-0028-1. [DOI] [PubMed] [Google Scholar]
- 30.Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, et al. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci U S A. 2007;104:19138–19143. doi: 10.1073/pnas.0705964104. Epub 2007 Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM. Store-operated cyclic AMP signalling mediated by STIM1. Nat Cell Biol. 2009;11:433–442. doi: 10.1038/ncb1850. Epub 2009 Mar 15. [DOI] [PubMed] [Google Scholar]
- 32.Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, et al. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003;63:1983–1994. doi: 10.1046/j.1523-1755.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 34.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90–S102. doi: 10.1097/01.mcg.0000155549.29643.ad. [DOI] [PubMed] [Google Scholar]
- 35.Alvaro D, Mancino MG, Onori P, Franchitto A, Alpini G, Francis H, et al. Estrogens and the pathophysiology of the biliary tree. World J Gastroenterol. 2006;12:3537–3545. doi: 10.3748/wjg.v12.i22.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.