Abstract
BACKGROUND
Thymosin beta 4 (Tβ4) has been shown to be associated with tumor metastasis and angiogenesis; however, its role in pancreatic cancer has not been understood. In the current study, we examined the expression of Tβ4 in pancreatic cancer cells, and determined the effect of exogenous Tβ4 on cytokine secretion, and signal transduction in human pancreatic cancer cells.
METHODS
The mRNA levels of Tβ4 were determined by real-time RT PCR. Phosphorylation of JNK in pancreatic cancer cells was determined using Bio-Plex phosphoprotein assay. The expression of cytokines in human pancreatic cancer cell lines was determined with Bio-Plex cytokine assay.
RESULTS
Pancreatic cancer cell lines expressed higher amount of Tβ4 mRNA than normal human pancreatic ductal epithelium (HPDE) cells. Exogenous Tβ4 increased the secretion of proinflammatory cytokines IL-6, IL-8 and MCP-1 in Panc-1 cells. In addition, Tβ4 activated Jun N-terminal Kinase (JNK) signaling pathways in pancreatic cancer cells.
CONCLUSIONS
Tβ4 might be involved in stimulating human pancreatic cancer progression by promoting proinflammatory cytokine environment and activating JNK signaling pathway. Targeting Tβ4 and related molecules may be a novel therapeutic strategy for pancreatic cancer.
Keywords: Thymosin beta 4, overexpression, tumor tissue, cytokine, proinflammation, JNK activation, pancreatic cancer
Introduction
Thymosin β4 (Tβ4) is a 43 amino-acid small peptide originally isolated from bovine thymus and thought to be a thymocite maturation factor 1. It was subsequently shown that Tβ4 interacts with G-actin and functions as a major actin-sequestering protein in almost all cell types. Tβ4 also serves as an important regulator of angiogenesis, wound healing, and cell motility 2-4. Multiple studies have indicated that Tβ4 was overexpressed in various tumor tissues and may play an important role in the carcinogenesis of several cancers, such as malignant renal tumors and non-small cell lung cancer (NSCLC) patients 5, 6, particularly by facilitating tumor metastasis. Those studies suggest that Tβ4 might be a prognostic marker in metastatic tumors.
Previous studies have indicated the role of Tβ4 in cell proliferation, migration, angiogenesis, and metastasis in many cancers such as colon cancer, renal cell carcinoma, rat osteosarcomas, and murine fibrosarcomas 6-10. SW 480 colon cancer cells overexpressing Tβ4 were more resistant to apoptosis induced by T cells and chemotherapeutic agents. The acquired resistance to apoptosis by colon cancer cells through Tβ4 overexpression might facilitate their survival during metastasis and chemotherapy 7. The expression of Tβ4 in mouse fibrosarcoma cells was also found to correlate with tumorigenicity and metastatic potential. Up-regulation of Tβ4 in weakly tumorigenic and nonmetastatic QR-32 cells (32-S) converted the cells to develop tumors and formed lung metastases in mice. In contrast, antisense Tβ4 cDNA-transfected malignant QRsP-30 (30-AS) cells significantly reduced tumor formation and metastases. It was also indicated that Tβ4 regulated fibrosarcoma cell tumorigenicity and metastasis through actin-based cytoskeletal organization 8.
Tβ4 normally serves as an important mediator for wound angiogenesis by binding to fibrin clots by factor IIIa, and directs revascularization by acting as a chemo-attractant for epithelial cells which results in increased vascular endothelial growth factor (VEGF) 11, 12. This correlates with findings describing a direct relationship between the level of Tβ4 and the metastatic potential of the melanoma cell line F16-10. Analysis of lung metastases from a highly malignant variant of F16-10 cells showed a high expression of Tβ4, which was nearly absent in the parental cells 11. F16-10 cells infected with an adenovirus overexpressing Tβ4 resulted in significantly larger tumors, in which a greater than four fold increase in blood vessel formation was seen compared with the control group 11.
Pancreatic cancer is a deadly disease without effective treatments, and ranks number one in mortality rate of all cancers. It aggressively invades surrounding tissues and metastasizes to distant sites, and is highly resistant to standard adjuvant therapies. The role of Tβ4 in pancreatic cancer remains unknown. In this study, we examined the expression of Tβ4 in human pancreatic cancer cell lines and clinical specimens, and investigated the effect of Tβ4 in inducing proinflammatory cytokine production in pancreatic cancer cells. We also studied the signaling pathway induced by Tβ4 in pancreatic cancer.
Materials and Methods
Chemicals and reagents
Human Tβ4 was purchased from ALPCO Diagnostics (Windham, NH). The Ambion “RNAqueous-4PCR” kit and DNA removing kit were obtained from Ambion (Austin, Texas). The iQ SYBR Green supermix, Bioplex phosphoprotein and cytokine kits were purchased from Bio-Rad (Hercules, CA). Rabbit anti-Tβ4 Ab was purchased from Biodesign International (Cincinnati, OH).
Cell culture and human tissue specimens
Human pancreatic cancer cell lines (Panc-1, MIA PaCa-2, BxPC-3, Hs766T, ASPC-1, Capan-1, Capan-2, HPAF-II, PL45, and Panc 03.27) were obtained from the American Type Culture Collection (ATCC, Rockville, MD). The human pancreatic ductal epithelium (HPDE) cells were provided as a generous gift from Dr. Ming-Sound Tsao from the University of Toronto, Canada 13, 14. All cells were cultured as previously described 15, 16. Human pancreatic adenocarcinoma specimens and normal surrounding tissues were collected from patients who underwent surgery according to an approved human protocol (H-16215) at Baylor College of Medicine (Houston, TX).
RNA Extraction and real-time RT-PCR
Total RNA was extracted from pancreatic cancer cell lines, as well as HPDE cells, using an Ambion “RNAqueous-4PCR” kit following the manufacture’s instruction (Austin, Texas) as described previously 15, 16. Briefly, cells were lysed by Ambion lysis buffer and then transferred to an Ambion mini-column, and centrifuged at 10,000 ×g for 1 min. The column was washed three times with wash buffer and eluted in 100 μl of elution buffer. RNA solution was treated with DNAse I to remove any trace amounts of genomic DNA contamination by using an Ambion DNA removing kit. Specific primers for Tβ4 were designed with the Beacon Designer 5.1 software (PREMIER Biosoft International, Palo Alto, CA). The homology between different subtypes and the template secondary structure were carefully examined and the primers were chosen to avoid the homologies. The primer sequences for Tβ4 are: sense, 5′ GAACTTGCATGTTGGTGAAGGAAG 3′; antisense, 5′ GGCCAGCTTGGTTTTACTCTAGAT 3′.
The mRNA levels for Tβ4 were analyzed by real-time RT-PCR using iCycler system (Bio-Rad, Hercules, CA). The mRNA was reverse-transcribed into cDNAs using the iScript cDNA synthesis kit (Bio-Rad). PCR reaction included the following components: 100 nM each primer, diluted cDNA templates and iQ SYBR Green supermix, and running for 40 cycles at 95°C for 20 sec and 60°C for 1 min. Each cDNA sample was run as triplicates and the corresponding no-reverse transcriptase (RT) mRNA sample was included as a negative control. The β-actin primer was included in every plate to avoid sample variations. The mRNA level of each sample for each gene was normalized to that of the β-actin mRNA. The relative mRNA level was presented as unit values of 2^[Ct(β-actin) – Ct(gene of interest)].
Immunohistochemical Staining
Clinical human pancreatic adenocarcinoma and surrounding normal tissues were collected and processed into 5 μm slices. For immunohistochemical analysis, fixed tissue slides were incubated with anti-Tβ4 Ab for 30 min at 4°C. After washing with PBS, the section was incubated with biotinylated secondary antibody for 30 min. An avidin-biotin reaction using peroxidase enzyme was used for protein detection (ABC kit; Vector Laboratories, Burlingham, CA). Immune complexes were detected with diaminobenzidine (DAB) under a phase contrast microscope (Olympus USA, Melville, NY). Images were captured with an attached SPOT-RT digital camera (Diagnostic Instruments, Sterling Heights, MI).
Bio-Plex Cytokine Assay
Panc-1 cells were treated with 100 ng/mL of Tβ4 for 24 h, and the supernatant was collected. Cytokine concentrations were determined using the Bioplex multiplex Human Cytokine Assay kit (Bio-Rad) and the Cytokine Reagent kit (Bio-Rad) according to the manufacturer’s protocol as described previously 17. Briefly, 50 μl of culture supernatants or cytokines standards were plated in a 96-well filter plate coated with a multiplex of antibodies against a panel of cytokines and incubated on a platform shaker at 300 rpm at room temperature. Data from the reaction was then acquired and analyzed using the Bio-Plex suspension array system (Luminex 100 system) from Bio-Rad Laboratories.
Bio-Plex Phosphoprotein Assay
Cells at 1.5 × 105/ml were treated with 100 ng/mL of Tβ4 for 5, 10, 30, or 60 min. Protein lysates were prepared using Cell lysis kit (Bio-Rad) on samples collected at each indicated time point. The presence of p-JNK was detected by Bio-Plex 4-plex phosphoprotein assay kit (Bio-Rad) and the Phosphoprotein Testing Reagent kit (Bio-Rad) according to the manufacturer’s protocol as described previously 17. Briefly, 50 μl of cell lysate (adjusted to a concentration of 100-450 μg/ml of protein) was plated in the 96-well filter plate coated with anti-p-JNK antibodies and incubated overnight on a platform shaker at 300 rpm at room temperature. After a series of washes to remove the unbound proteins, a mixture of biotinylated detection antibodies, each specific for a different epitope, was added to the reaction resulting in the formation of sandwiches of antibodies around the target proteins. Streptavidin-phycoerythrin (streptavidin-PE) was then added to bind to the biotinylated detection antibodies on the bead surface. Data from the reaction was then acquired and analyzed using the Bio-Plex suspension array system (Luminex 100 system) from Bio-Rad Laboratories. The total proteins for JNK were tested using the Bio-plex 4-plex total protein assay kit (Bio-Rad).
Statistical analysis
Data from real-time PCR, and bioplex assay were expressed as mean±SEM. Significant differences were determined by paired Student’s t-test (two tails).
Results
Tβ4 is overexpressed in human pancreatic cancer cell lines and tissues specimens
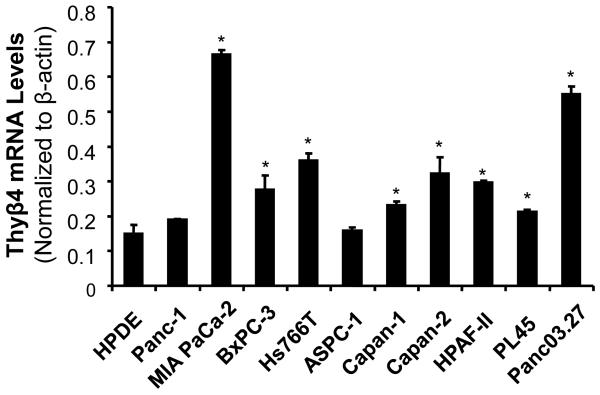
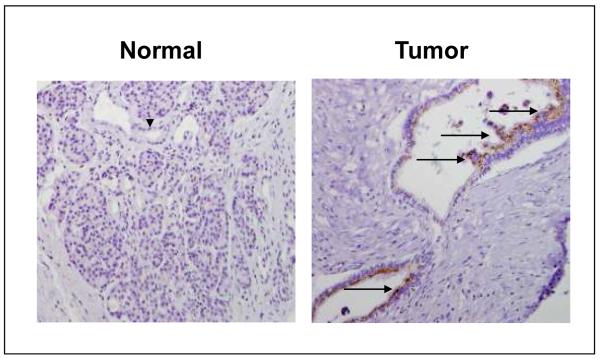
We examined Tβ4 mRNA expression in ten human pancreatic cancer cell lines and HPDE cells. Tβ4 was differentially overexpressed in all ten cell lines we tested, especially in MIA PaCa-2 and Panc03.27 cells, in which the Tβ4 mRNA was 4.5 and 3.7-fold of that in HPDE cells (Fig. 1). To investigate the expression of Tβ4 in human pancreatic cancer tissues, 10 pairs of clinical pancreatic adenocarcinoma specimens and their surrounding normal pancreatic tissues were collected from the operating room under an approved IRB protocol. Tβ4 protein levels were examined by immunohistochemical staining using specific antibodies against Tβ4. Strong immunoreactivity was observed in tumor tissues, whereas negative or very weak positive staining was observed in surrounding benign tissues. A representative tissue staining was shown in Fig. 2. Therefore, human pancreatic cancer is associated with increased expression of Tβ4.
Fig. 1.
Expression of Tβ4 mRNA in human pancreatic cancer cells and HPDE cells. The mRNA level of each sample was normalized to that of β-actin. Relative mRNA levels were presented as 2^[Ct(β-actin)-Ct(gene of interest)]. All data shown are the mean±SEM of three separate experiments. *p < 0.05 as compared to HPDE cells.
Fig. 2.
Immunostaining of Tβ4 in pancreatic adenocarcinoma tissues. Normal pancreatic tissue composed of acinar cells and ductal epithelial cells with negative immunostaining of Tβ4. Representative pancreatic ductal adenocarcinoma showed positive immunostaining of Tβ4. Dark brown color represents positive staining of Tβ4. Arrow head, normal ductal epithelial cells; arrow, tumor epithelial cells.
Tβ4 increases secretion of multiple cytokines in pancreatic cancer cells
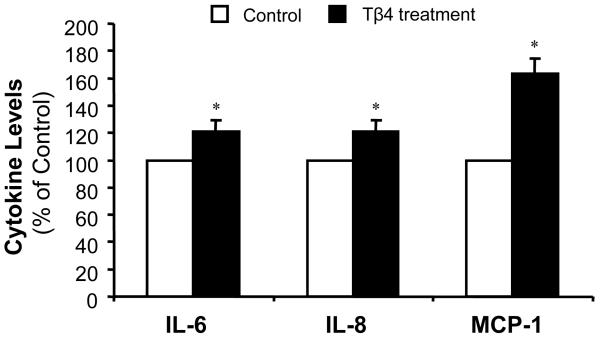
Cytokines play important roles in pancreatic cancer pathogenesis and progression, and interact with many signaling pathways in pancreatic cancer cells. In order to study the interaction of Tβ4 with cytokine secretion in pancreatic cancer, we examined the effect of exogenous Tβ4 treatment on cytokine production by Panc-1 cells. As shown in Fig. 3, treatment with Tβ4 caused a significant increase of proinflammatory cytokines, interleukin-6 (IL-6) and interleukin-8 (IL-8), and monocyte chemotactic protein-1 (MCP-1). A 21% and 20% increase of IL-6 and IL-8 were observed in Panc-1 cells, respectively, after treating with exogenous Tβ4. The MCP-1 level was increased by 63%. We could not find any elevated Th1 type of cytokines, including IL-2 and interferon-γ, by Tβ4 treatment. In addition, we also detected a 16% increase of Th2 type cytokine IL-10 in Panc-1 cells treated with Tβ4. These data indicate that Tβ4 stimulates secretion of proinflammatory cytokines from pancreatic cancer cells, and consequently, may lead to increased pancreatic cancer progression.
Fig. 3.
Effect of Tβ4 on cytokine secretion in pancreatic cancer cells. Supernatants from Tβ4-treated Panc-1 cells were collected after 24 h. Cytokine levels were determined by Bio-plex cytokine assay. The values plotted are the percentage increase to the non-treated cells. Data are expressed as mean±SEM of duplicate values from two separate experiments. *p < 0.05 as compared to Tβ4-untreated controls.
Tβ4 activates JNK signaling pathway
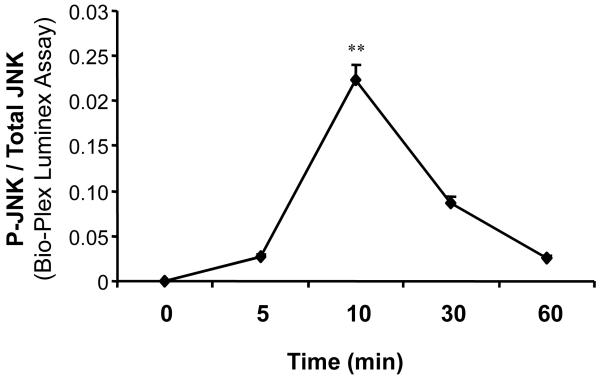
To examine the signaling transduction molecules involved in the Tβ4 pathway in pancreatic cancer, we investigated the phosphorylation of JNK in Panc-1 cells treated by Tβ4. Panc-1 cells were stimulated with 100 ng/mL of Tβ4 for 0, 5, 10, 30, or 60 minutes. As shown in Fig. 4, the addition of exogenous Tβ4 dramatically increased the phosphorylation of JNK in Panc-1 cells, which peaked at 10 minutes before dropping off towards baseline levels, indicating the involvement of JNK pathway in Tβ4 mediated pancreatic cancer progression.
Fig. 4.
Effect of Tβ4 on phosphorylation of JNK. Panc-1 cells were treated with 100 ng/mL Tβ4 for 5, 10, 30, or 60 min. Panc-1 cells without Tβ4 served as controls. Proteins extracted from samples collected at the indicated time points were tested for the presence of phosphorylated and total JNK by a Bio-Plex assay kit. The values plotted show the percentage of controls for the ratio of phosphoprotein to total protein. Data are expressed as mean±SEM of triplicate values from two separate experiments. **p < 0.01 as compared to Tβ4-untreated controls.
Discussion
Our data have shown that Tβ4 expression was elevated in pancreatic cancer cell lines and clinical tissue specimens, compared with that in the control HPDE cells and surrounding normal pancreatic tissues. Exogenous Tβ4 treatment significantly increased multiple cytokines production, especially proinflammatory cytokines such as IL-6 and IL-8, and MCP-1. We have also shown that Tβ4 activated JNK pathway in Panc-1 cells. This is the first evidence indicating the aberrant expression of Tβ4 in human pancreatic cancer, and the important role of Tβ4 in pancreatic cancer pathogenesis. This adds to the previous body of literature showing that Tβ4 is differentially regulated in various cancers 6, 8-11, 18, 19. The specificity of Tβ4 in malignant cancer cells makes it a promising tumor marker or a targeting molecule for detecting tumor metastasis. Therapies that block Tβ4 are worth considering and more detailed in vitro and in vivo studies on Tβ4 are warranted for further investigation.
Previous studies have shown that Tβ4 was overexpressed in various tumor tissues. Higher levels of Tβ4 mRNA were expressed in malignant renal tumors, while only low levels of Tβ4 mRNA were observed in normal tissues, indicating that Tβ4 may be a new molecular marker for renal-cell carcinoma as well as other malignancies 6. Tβ4 was also demonstrated to be significantly associated with metastasis in early-stage non-small cell lung cancer (NSCLC) patients (n=70). The association of Tβ4 with metastasis was stage and histology specific, suggesting that Tβ4 might be a prognostic marker for patient survival in stage I NSCLC 5. Our data strongly suggest that Tβ4 was overexpressed in ten pancreatic cancer cell lines we tested compared with that in the control HPDE cells. Majority of the pancreatic cancer cells expressed Tβ4 mRNA from 143% (PL45) to 447% (MIA PaCa-2) of that in HPDE control cells, which indicated the critical role of Tβ4 in human pancreatic cancer cells.
Pancreatic cancer is a malignancy characterized by secreting multiple proinflammatory cytokines and Th2 type cytokines, which promotes the progression of pancreatic cancer by providing a more favorable microenvironment for tumor growth and suppressing the anti-cancer immunity. We found that in pancreatic cancer cells, exogenous Tβ4 upregulated the expression of proinflammatory cytokines IL-6 and IL-8. IL-6 is a well known pro-inflammatory cytokine associated with various malignancies. It is a poor prognostic factor in several solid tumors, and has been shown to be a prominent cachexic factor, affecting tumor-related weight loss and decreasing albumin in cancer patients in several studies including pancreatic cancer 20-23. Serum IL-6 level is directly associated with decreased performance and increased mortality rates in pancreatic cancer. IL-8 is another inflammatory cytokine upregulated in both cancer and chronic inflammatory diseases of the pancreas 24. It is linked to pancreatic cancer tumorigenesis primarily through its regulation of angiogenesis and metastasis 25. The increase of IL-6 and IL-8 levels in pancreatic cancer cells by Tβ4 may cause increased cell proliferation and metastasis in pancreatic cancer, and therefore, enhance the pancreatic cancer pathogenesis and progression.
MCP-1 belongs to a small inducible gene (SIG) family, and plays an important role in the recruitment of monocytes to the sites of injury and infection. MPC-1 has also been found elevated in the urine of lupus patients as a sign of inflammation in the kidney. IL-8 and MCP-1 secretion were often seen in pancreatic cancer cells, which suggest that these factors may contribute to the accumulation of tumor-associated immune cells. In addition, the transcriptional activation of those chemokine genes in pancreatic cancer cells may involve NF-κB activation 26. In this study we have shown that MCP-1 level was significantly increased by 63% in pancreatic cancer cells upon exogenous Tβ4 treatment, indicating a possible role of inflammation and chemoattraction in pancreatic cancer. IL-10 is a Th2 type cytokine with a potent immunosuppressive activity 27, and is thought to be a tumor-promoting cytokine. Its increase (16%) caused by Tβ4 suggests a strategy to evade the host immune surveillance in pancreatic cancer. The increase of IL-6, IL-8, MCP-1, and IL-10 by Tβ4 suggests a combined effect of the cytokines for the tumor to evade the host immune response.
Our data also revealed that Tβ4 treatment caused phosphorylation of Jun N-terminal Kinase (JNK), also known as Stress Activated Protein Kinase (SAPK), which belongs to the family of MAP kinases. The MAPK pathway is important for cell growth and survival, and its increase has been shown to be a prominent feature of pro-oncogenic peptides. However, it is still unknown if Tβ4 acts through other pathways in pancreatic cancer, or if the pattern of activation is altered in vivo. We hope that further studies will clarify these questions. We have also examined other signaling pathways such as IκBα, ERK1/2, and p38MAPK, and we did not find any significant difference between control group and Tβ4 treatment group, indicating that JNK pathway may be specifically activated by exogenous Tβ4. How the activation of JNK resulted in overexpression of proinflammation cytokines is not completely understood, but previous studies have shown that MAPKs are involved in upregulating inflammation cytokine (such as IL-8) upon stimulation from chemokines and other cytokines. Our study also showed that Tβ4 can activate JNK pathway, and increase the expression of proinflammation cytokines. Further studies are needed to elucidate the detailed pathway involved in Tβ4 induced JNK activation and inflammation cytokine upregulation.
In conclusion, we have found that Tβ4, a known stimulator of invasion and metastasis, is overexpressed in both pancreatic cancer cell lines and in malignant tissue samples from human patients. Exogenous Tβ4 caused increased secretion of proinflammatory cytokines IL-6, IL-8, and MCP-1. Furthermore, we found Tβ4 acts through JNK signaling pathway, which is well known to promote survival and proliferation in pancreatic adenocarcinoma. We thus suggest that this cytokine may be an important determinant of prognosis and survival, that it may have clinical applications as a tumor marker in pancreatic cancer.
ACKNOWLEDGEMENTS
This work was supported in part by the American Cancer Society Grant #IRG-93-034-09, and the MacDonald Research Fund 06RDM013, (M. Li), by National Institutes of Health (NIH) Grants HL065916, HL072716, EB002436, HL08347 (C. Chen), RO1 DE15543, and R21 AT003094 (Q. Yao).
References
- 1.Low TL, Hu SK, Goldstein AL. Complete amino acid sequence of bovine thymosin beta 4: a thymic hormone that induces terminal deoxynucleotidyl transferase activity in thymocyte populations. Proc Natl Acad Sci U S A. 1981;78:1162–6. doi: 10.1073/pnas.78.2.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malinda KM, Goldstein AL, Kleinman HK. Thymosin beta 4 stimulates directional migration of human umbilical vein endothelial cells. Faseb J. 1997;11:474–81. doi: 10.1096/fasebj.11.6.9194528. [DOI] [PubMed] [Google Scholar]
- 3.Malinda KM, Sidhu GS, Mani H, Banaudha K, Maheshwari RK, Goldstein AL, Kleinman HK. Thymosin beta4 accelerates wound healing. J Invest Dermatol. 1999;113:364–8. doi: 10.1046/j.1523-1747.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- 4.Safer D, Golla R, Nachmias VT. Isolation of a 5-kilodalton actin-sequestering peptide from human blood platelets. Proc Natl Acad Sci U S A. 1990;87:2536–40. doi: 10.1073/pnas.87.7.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Muller-Tidow C. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–41. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 6.Hall AK. Differential expression of thymosin genes in human tumors and in the developing human kidney. Int J Cancer. 1991;48:672–7. doi: 10.1002/ijc.2910480507. [DOI] [PubMed] [Google Scholar]
- 7.Hsiao HL, Wang WS, Chen PM, Su Y. Overexpression of thymosin beta-4 renders SW480 colon carcinoma cells more resistant to apoptosis triggered by FasL and two topoisomerase II inhibitors via downregulating Fas and upregulating Survivin expression, respectively. Carcinogenesis. 2006;27:936–44. doi: 10.1093/carcin/bgi316. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T, Okada F, Fujii N, Tomita N, Ito S, Tazawa H, Aoyama T, Choi SK, Shibata T, Fujita H, Hosokawa M. Thymosin-beta4 regulates motility and metastasis of malignant mouse fibrosarcoma cells. Am J Pathol. 2002;160:869–82. doi: 10.1016/s0002-9440(10)64910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkinson MJ, Freeman MW, Kronenberg HM. Thymosin beta 4 is expressed in ROS 17/2.8 osteosarcoma cells in a regulated manner. Mol Endocrinol. 1990;4:69–74. doi: 10.1210/mend-4-1-69. [DOI] [PubMed] [Google Scholar]
- 10.Nummela P, Yin M, Kielosto M, Leaner V, Birrer MJ, Holtta E. Thymosin beta4 is a determinant of the transformed phenotype and invasiveness of S-adenosylmethionine decarboxylase-transfected fibroblasts. Cancer Res. 2006;66:701–12. doi: 10.1158/0008-5472.CAN-05-2421. [DOI] [PubMed] [Google Scholar]
- 11.Cha HJ, Jeong MJ, Kleinman HK. Role of thymosin beta4 in tumor metastasis and angiogenesis. J Natl Cancer Inst. 2003;95:1674–80. doi: 10.1093/jnci/djg100. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein AL. Thymosin beta4: a new molecular target for antitumor strategies. J Natl Cancer Inst. 2003;95:1646–7. doi: 10.1093/jnci/djg126. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa T, Duguid WP, Rosenberg L, Viallet J, Galloway DA, Tsao MS. Long-term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am J Pathol. 1996;148:1763–70. [PMC free article] [PubMed] [Google Scholar]
- 14.Ouyang H, Mou L, Luk C, Liu N, Karaskova J, Squire J, Tsao MS. Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am J Pathol. 2000;157:1623–31. doi: 10.1016/S0002-9440(10)64800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Yang H, Chai H, Fisher WE, Wang X, Brunicardi FC, Yao Q, Chen C. Pancreatic carcinoma cells express neuropilins and vascular endothelial growth factor, but not vascular endothelial growth factor receptors. Cancer. 2004;101:2341–50. doi: 10.1002/cncr.20634. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Zhai Q, Bharadwaj U, Wang H, Li F, Fisher WE, Chen C, Yao Q. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer. 2006;106:2284–94. doi: 10.1002/cncr.21862. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Feurino LW, Li F, Wang H, Zhai Q, Fisher WE, Chen C, Yao Q. Thymosinalpha1 stimulates cell proliferation by activating ERK1/2, JNK, and increasing cytokine secretion in human pancreatic cancer cells. Cancer Lett. 2006 doi: 10.1016/j.canlet.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Wang WS, Chen PM, Hsiao HL, Ju SY, Su Y. Overexpression of the thymosin beta-4 gene is associated with malignant progression of SW480 colon cancer cells. Oncogene. 2003;22:3297–306. doi: 10.1038/sj.onc.1206404. [DOI] [PubMed] [Google Scholar]
- 19.Wang WS, Chen PM, Hsiao HL, Wang HS, Liang WY, Su Y. Overexpression of the thymosin beta-4 gene is associated with increased invasion of SW480 colon carcinoma cells and the distant metastasis of human colorectal carcinoma. Oncogene. 2004;23:6666–71. doi: 10.1038/sj.onc.1207888. [DOI] [PubMed] [Google Scholar]
- 20.Feurino LW, Zhang Y, Bharadwaj U, Zhang R, Li F, Fisher WE, Brunicardi FC, Chen C, Yao Q, Li M. IL-6 Stimulates Th2 Type Cytokine Secretion and Upregulates VEGF and NRP-1 Expression in Pancreatic Cancer Cells. Cancer Biol Ther. 2007;6 doi: 10.4161/cbt.6.7.4328. [DOI] [PubMed] [Google Scholar]
- 21.Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–36. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 22.Friess H, Guo XZ, Nan BC, Kleeff O, Buchler MW. Growth factors and cytokines in pancreatic carcinogenesis. Ann N Y Acad Sci. 1999;880:110–21. doi: 10.1111/j.1749-6632.1999.tb09515.x. [DOI] [PubMed] [Google Scholar]
- 23.Martignoni ME, Kunze P, Hildebrandt W, Kunzli B, Berberat P, Giese T, Kloters O, Hammer J, Buchler MW, Giese NA, Friess H. Role of mononuclear cells and inflammatory cytokines in pancreatic cancer-related cachexia. Clin Cancer Res. 2005;11:5802–8. doi: 10.1158/1078-0432.CCR-05-0185. [DOI] [PubMed] [Google Scholar]
- 24.Farrow B, Sugiyama Y, Chen A, Uffort E, Nealon W, Mark Evers B. Inflammatory mechanisms contributing to pancreatic cancer development. Ann Surg. 2004;239:763–9. doi: 10.1097/01.sla.0000128681.76786.07. discussion 9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–91. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 26.Takaya H, Andoh A, Shimada M, Hata K, Fujiyama Y, Bamba T. The expression of chemokine genes correlates with nuclear factor-kappaB activation in human pancreatic cancer cell lines. Pancreas. 2000;21:32–40. doi: 10.1097/00006676-200007000-00049. [DOI] [PubMed] [Google Scholar]
- 27.Urosevic M, Dummer R. HLA-G and IL-10 expression in human cancer--different stories with the same message. Semin Cancer Biol. 2003;13:337–42. doi: 10.1016/s1044-579x(03)00024-5. [DOI] [PubMed] [Google Scholar]