Abstract
Interleukin‐8 (IL‐8) is associated with tumorigenesis by promoting angiogenesis and metastasis. Although up‐regulation of IL‐8 is indicated in many cancers, its function in pancreatic cancer has not been well characterized. In this study we examined the expression of IL‐8 on pancreatic cancer cells and clinical tissue specimens, and investigated the effect of exogenous IL‐8 on gene expression, and signaling in human pancreatic cancer cells. We found that pancreatic cancer cells expressed higher amount of IL‐8 mRNA than normal human pancreatic ductal epithelium cells. IL‐8 mRNA was also substantially overexpressed in 11 of 14 (79%) clinical pancreatic‐adenocarcinoma samples compared with that in their surrounding normal tissues. Exogenous IL‐8 up‐regulated the expression of vascular endothelial growth factor165, and neuropilin (NRP)‐2 in BxPC‐3 cells, one of human pancreatic cancer cell lines. IL‐8 expression was inducible by hypoxia mimicking reagent cobalt chloride. In addition, IL‐8 activated extracellular signal‐regulated kinase (ERK)1/2 signaling pathway in BxPC‐3 cells. Our studies suggest that IL‐8 might be a malignant factor in human pancreatic cancer by induction of vascular endothelial growth factor and NRP‐2 expression and ERK activation. Targeting IL‐8 along with other antiangiogenesis therapy could be an effective treatment for this malignancy. (Cancer Sci 2008, 99: 733–737)
Interleukin‐8 (IL‐8) is an inflammatory cytokine up‐regulated in both cancer and chronic inflammatory diseases of the pancreas.( 1 ) It is linked to pancreatic cancer tumorigenesis primarily through its regulation of angiogenesis and metastasis.( 2 ) Human umbilical vein endothelial cells proliferation and angiogenesis were both increased when cocultured with pancreatic cancer cells, or cultured with exogenous IL‐8. The increase of cell proliferation and angiogenesis of human umbilical vein endothelial cells can be blocked by IL‐8 neutralizing antibodies.( 3 ) IL‐8 and vascular endothelial growth factor (VEGF) expressions were found to be up‐regulated in a highly metastatic pancreatic cancer cell line Colo357L3.6 pL compared with the parental cell Colo357FG, likely mediated through a mitogen activated protein kinase (MAPK) pathway.( 4 ) IL‐8 transfection also made non‐metastatic human melanoma cells metastatic.( 5 )
Previous studies have shown that IL‐8 is overexpressed in most human pancreatic cancer tissues and established cell lines.( 2 ) Higher IL‐8 levels in pancreatic cancer patient serum were associated with significant weight loss, but not with performance status.( 6 ) Additionally, the expression levels of IL‐8 appear to correlate with their tumorigenic and metastatic potential in an orthotopic xenograft model.( 2 , 7 , 8 , 9 , 10 ) Furthermore, treatment with exogenous IL‐8 increased the invasiveness of human pancreatic cancer cell line Panc‐1 cells in a matrigel assay, while blocking IL‐8 inhibited the growth of another human pancreatic cancer cell line Capan‐1 cells.( 11 , 12 ) Blocking IL‐8 in pancreatic cancer cell Hup‐T4 cells decreased their growth and their ability to attach to endothelial cells, suggesting that IL‐8 is an autocrine mitogenic factor important for metastasis.( 13 )
The expression of IL‐8 can be induced by many stimuli including lipopolysaccharide, phorbol 12‐myristate 13‐acetate, IL‐1, and tumor necrosis factor. Several stress factors, such as hypoxia, acidosis, nitric oxide (NO), and cell density, also significantly influenced the production of IL‐8 in human pancreatic cancer cells.( 7 ) IL‐8 expression is shown to be regulated by NO secreted from endothelial cells. Cells modified to produce physiologic levels of NO showed increased IL‐8 production compared to low NO producing cells, and the NO synthase inhibitor NG‐monomethyl‐L‐arginine blocked this effect.( 14 ) IL‐8 has also been shown to be involved in cancer hypoxia pathway, in which IL‐8 expression is regulated by hypoxia inducible factor‐1 (HIF‐1), Nuclear factor‐kB (NF‐kB), and v‐Ki‐ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).( 10 , 15 )
In this study we investigated the expression of IL‐8 in a number of pancreatic cancer cell lines, culture medium, and surgical tissue samples. We also studied the effects of IL‐8 in regulating other key molecules in pancreatic cancer growth and angiogenesis, and the signaling pathway induced by IL‐8 in pancreatic cancer.
Materials and Methods
Chemicals and reagents. Human IL‐8 and cobalt chloride (CoCl2) were purchased from Sigma (St. Louis, MO, USA). Endothelial Cell Medium‐2 Bullet kit, trypsin‐ethylenediaminetetraacetic acid and trypsin neutralization solution were purchased from Clonetics (Walkersville, MD, USA). The Ambion ‘RNAqueous‐4PCR’ kit and DNA removing kit were obtained from Ambion (Austin, TX, USA). The iQ SYBR Green supermix, Bioplex phosphoprotein and cytokine kits were purchased from Bio‐Rad Laboratories (Hercules, CA, USA).
Cell culture and human tissue specimens. Human pancreatic cancer cell lines (Panc‐1, MIA PaCa‐2, BxPC‐3, Capan‐2, HPAF‐II, and Panc03.27) were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). The human pancreatic ductal epithelium (HPDE) cells were provided as a generous gift from Dr Ming‐Sound Tsao from the University of Toronto, Canada.( 16 , 17 ) Panc‐1 cells were cultured in Dulbecco's modification of eagle's medium with 10% fetal bovine serum (FBS) at 37°C with 5% CO2. MIA PaCa‐2 cells were cultured in Dulbecco's modification of eagle's medium with 10% FBS and 2.5% horse serum at 37°C with 5% CO2. BxPC‐3 and Panc03.27 cells were cultured in Roswell Park Memorial Institute 1640 medium with 10% FBS at 37°C with 5% CO2. HPAF‐II cells were cultured in Eagle's minimal essential medium (MEM) with 10% FBS at 37°C with 5% CO2. Capan‐2 cells were cultured in McCoy's medium with 10% FBS at 37°C with 5% CO2. HPDE cells were cultured in keratinocyte serum‐free medium supplied with 5 ng/mL epidermal growth factor (EGF) and 50 µg/mL bovine pituitary extract (Invitrogen, Carlsbad, CA, USA). Human pancreatic adenocarcinoma specimens were collected from patients who underwent surgery according to an approved human protocol (H‐16215) at the Baylor College of Medicine (Houston, TX, USA).
Real‐time reverse transcriptase PCR (RT‐PCR). Total RNA was extracted from six pancreatic cancer cell lines (Panc‐1, MIA PaCa‐2, BxPC‐3, Capan‐2, HPAF‐II, and Panc03.27 cells), HPDE cells, and clinical specimens using an Ambion ‘RNAqueous‐4PCR’ kit following the manufacture's instruction (Austin, TX, USA) as described previously.( 18 ) Specific primers for IL‐8 were designed with the Beacon Designer 5.1 software (PREMIER Biosoft International, Palo Alto, CA, USA). The primer sequences for human IL‐8 gene are: sense 5′GGCACAAACTTTCAGAGACAGCAG3′; and antisense 5′GTTTCTTCCTGGCTCTT GTCCTAG3′.
The mRNA for IL‐8 was analyzed by real‐time RT PCR using iCycler system (Bio‐Rad Laboratories, Hercules, CA, USA). PCR reaction included the following components: 100 nM each primer, diluted cDNA templates and iQ SYBR Green supermix, and running for 40 cycles at 95°C for 20 s and 60°C for 1 min. Each cDNA sample was run as triplicates and the corresponding no‐RT mRNA sample was included as a negative control. The β‐actin primer was included in every plate to avoid sample variations. The mRNA level of each sample for each gene was normalized to that of the β‐actin mRNA. The relative mRNA level was presented as unit values of 2^[Ct(β‐actin) – Ct(gene of interest)].( 18 )
For IL‐8 and hypoxia treatment, subconfluent pancreatic cancer cells were treated with IL‐8 (100 ng/mL) or different concentrations of CoCl2 for 24 h. Total RNA was extracted and the mRNA levels for VEGF165, VEGF receptors neuropilin‐1 (NRP‐1), neuropilin‐2 (NRP‐2), and IL‐8 were analyzed by real‐time RT PCR as above. The primer sequences for human VEGF165 gene are: sense 5′CCAGCAGAAAGAGGAAAGAGGTAG3′; and antisense 5′CCCCAAAAGCAGGTCACTCAC‐3′. The primer sequences for human NRP‐1 gene are: sense 5′‐AAGGTTTCTCAGCAAACTACAGT G‐3′; and antisense 5′‐GGGAAGAAGCTGTGATCTGGTC‐3′. The primer sequences for human NRP‐2 gene are: sense 5′‐GATTCGGGATGGGGACAGTGA‐3′; and antisense 5′‐GGTGAACTTGATGTAGAGCATGGA‐3′.( 18 )
Bio‐Plex phosphoprotein assay. Pancreatic cancer cells at 1.5 × 105/mL were treated with IL‐8 (100 ng/mL) for 0, 5, 15, 30, or 60 min. Protein lysates were prepared using cell lysis kit (Bio‐Rad Laboratories, Hercules, CA, USA) on samples collected at each indicated time point. The presence of p‐ERK1/2 was detected by Bio‐Plex phosphoprotein assay kit (Bio‐Rad Laboratories) and the Phosphoprotein Testing Reagent kit (Bio‐Rad Laboratories) according to the manufacturer's protocol as described previously.( 19 ) Briefly, 50 µL of cell lysate (100–450 µg protein/mL) was plated in the 96‐well filter plate coated with antip‐ERK1/2 antibody and incubated overnight on a platform shaker at 300 r.p.m. at room temperature. After incubation with detection antibodies, data from the reaction was then acquired and analyzed using the Bio‐Plex suspension array system (Luminex 100 system) from Bio‐Rad Laboratories. The total proteins for ERK were tested using the Bio‐Plex total protein assay kit (Bio‐Rad Laboratories).
Bio Plex cytokine assay. Panc‐1, MIA PaCa‐2, BxPC‐3, and HPDE cells were treated with IL‐8 (100 ng/mL) for 24 h, and the supernatant was collected. Cytokine concentrations were determined using the Bio‐Plex multiplex Human Cytokine Assay kit (Bio‐Rad Laboratories) and the Cytokine Reagent kit (Bio‐Rad Laboratories) according to the manufacturer's protocol and was described previously.( 19 , 20 ) Briefly, 50 µL of culture supernatants or cytokines standards were plated in a 96‐well filter plate coated with a multiplex of antibodies against the above mentioned cytokines and incubated overnight on a platform shaker at 300 r.p.m. at room temperature. Data from the reaction was then acquired and analyzed using the Bio‐Plex suspension array system (Luminex 100 system) from Bio‐Rad Laboratories as described above.
Statistical analysis. Data from real‐time PCR, and bioplex assay were expressed as mean ± standard error of the mean. Significant differences were determined by paired Student's t‐test (two tails).
Results
IL‐8 was overexpressed in human pancreatic cancer cell lines and tissues. We examined IL‐8 mRNA expression in six human pancreatic cancer cell lines and HPDE cells. IL‐8 was differentially increased in five cell lines (MIA PaCa‐2, BxPC‐3, Capan‐2, HPAF‐II, and Panc03.27) compared with that in HPDE cells (Fig. 1a). Bio‐plex cytokine assay was used to determine the amount of IL‐8 protein in the culture media from Panc‐1, MIA PaCa‐2, and BxPC‐3 cell lines. The culture media from the HPDE cell line was included as a control. As shown in Figure 1b, the IL‐8 protein levels in MIA PaCa‐2 and BxPC‐3 cells were increased by 900%, and 304%, respectively, as compared to that in HPDE cells, which is consistent with the real‐time PCR results.
Figure 1.
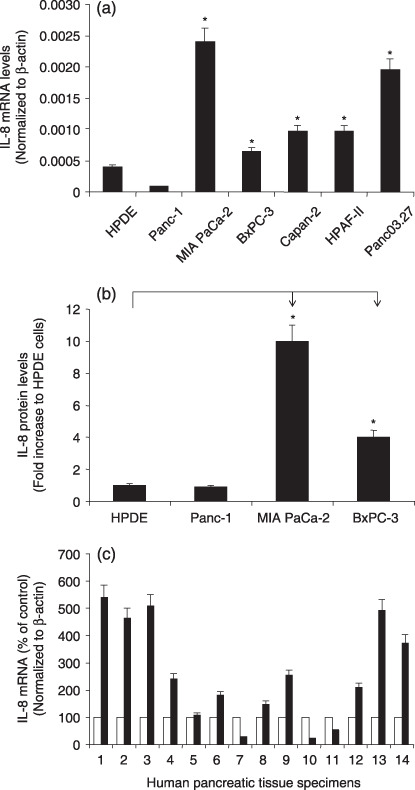
Expression of IL‐8 mRNA in human pancreatic cancer cells and tissues. (a) The IL‐8 mRNA expression in six human pancreatic cancer cells, and control HPDE cells were determined by real‐time RT PCR. (b) The IL‐8 protein expression in three human pancreatic cancer cells, and control human pancreatic ductal epithelium (HPDE) cells were determined by Bio‐plex cytokine assay. (c) The IL‐8 mRNA expression in 14 pairs of human pancreatic cancer specimens and surrounding normal tissues were determined by real‐time RT PCR. Normal ( ), Tumor (
), Tumor ( ). All data shown are the mean ± SEM of three separate experiments. *P < 0.05 as compared to control HPDE cells.
). All data shown are the mean ± SEM of three separate experiments. *P < 0.05 as compared to control HPDE cells.
We also examined the IL‐8 expression in 14 pairs of human pancreatic cancer tissues with the surrounding normal tissues. IL‐8 mRNA was substantially overexpressed in 11 of 14 (79%) clinical pancreatic‐adenocarcinoma samples compared with that in their surrounding normal tissues (Fig. 1c). Overall fold increase of IL‐8 mRNA expression in pancreatic cancer tissue was 2.6 times that in the surrounding normal tissues. Therefore, human pancreatic cancer is associated with increased expression of IL‐8.
IL‐8 up‐regulated the expression of key molecules for angiogenesis and cell proliferation. To investigate the effect of IL‐8 on the expression of key molecules controlling angiogenesis and cell proliferation in pancreatic cancer, we examined the mRNA levels of VEGF165, NRP‐1, and NRP‐2 in BxPC‐3 cells upon treatment with 100 ng/mL of IL‐8. VEGF165 is a critical molecule for promoting angiogenesis, and in pancreatic cancer, the neuropilins (NRP‐1 and NRP‐2) may act as alternate receptors for VEGF165. ( 18 ) As shown in Figure 2, VEGF165 and NRP‐2 mRNAs were significantly increased by IL‐8 treatment in BxPC‐3 cells. Therefore, IL‐8 may be involved in up‐regulation of VEGF and NRPs in pancreatic cancer.
Figure 2.
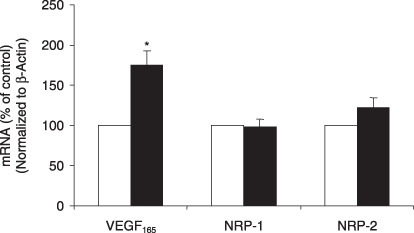
Effect of IL‐8 on the mRNA levels of vascular endothelial growth factor (VEGF)165 and NRPs in pancreatic cancer cells. Serum‐starved BxPC‐3 cells were treated with 100 ng/mL of IL‐8 for 24 h. Relative mRNA levels of VEGF165 and NRPs were presented as 2^[Ct(β actin) – Ct(gene)] (n = 10). *P < 0.05 as compared to IL‐8‐untreated controls. Control ( ), IL‐8 treatment(
), IL‐8 treatment( ).
).
IL‐8 was induced under hypoxia conditions in pancreatic cancer. To determine if IL‐8 is involved in response to hypoxia in pancreatic cancer, and if there is correlation between IL‐8 and VEGF under hypoxia conditions, we treated MIA PaCa‐2 cells with different doses of CoCl2, the hypoxia mimicking reagent, and found that IL‐8 mRNA level was increased by hypoxia in a dose‐dependent manner. At 200 µM of CoCl2, IL‐8 mRNA was increased by 203% (Fig. 3). Similarly, VEGF165 mRNA level was also increased by CoCl2 in a dose‐dependent manner as described before( 21 ) which indicates that IL‐8 is inducible by hypoxia, and the levels of IL‐8 and VEGF might correlate under hypoxia conditions in pancreatic cancer cells.
Figure 3.
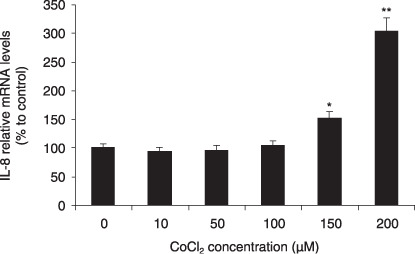
Effect of CoCl2 on IL‐8 expression in pancreatic cancer cells. MIA PaCa‐2 cells were treated with 10, 50, 100, 150, and 200 µM of CoCl2 for 24 h. Relative mRNA levels of IL‐8 was determined (n = 10). *P < 0.05 and **P < 0.01 as compared to CoCl2‐untreated controls.
IL‐8 activates ERK signaling pathway. To examine the signal molecules involved in the IL‐8 pathway in pancreatic cancer, we investigated the phosphorylation of ERK in pancreatic cancer cells treated by IL‐8. BxPC‐3 cells were stimulated with 100 ng/mL of IL‐8 for 0, 5, 15, 30, or 60 min. The percentage of phosphorylated ERK versus the total ERK proteins was then measured using a Bio‐Plex phosphoprotein assay. As shown in Figure 4, the addition of exogenous IL‐8 dramatically increased the phosphorylation of ERK in BxPC‐3 cells, which peaked at 15 min before dropping off towards baseline levels at 60 minute.
Figure 4.
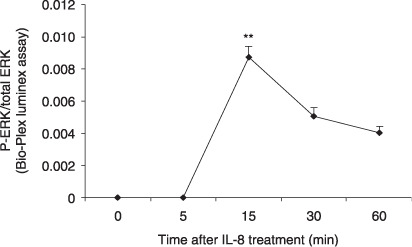
IL‐8 induces phosphorylation of ERK. BxPC‐3 cells were treated with 100 ng/mL IL‐8 for 5, 15, 30, or 60 min and tested for the presence of phosphorylated and total ERK by Bio‐Plex phosphoprotein assay. The values plotted show the ratio of phosphoprotein to total protein. Data are expressed as mean ± SEM of triplicate values from two separate experiments. **P < 0.001 as compared to untreated control (0 min).
Discussion
These data demonstrated that IL‐8 is overexpressed in human pancreatic cancer cell lines and clinical specimens. IL‐8 protein was also elevated in the cultured medium of human pancreatic cancer cells compared to HPDE cells. Exogenous IL‐8 caused the up‐regulation of VEGF165, and NRP‐2 in pancreatic cancer cells. IL‐8 mRNA was found to be increased under hypoxia condition. Furthermore, we have found that IL‐8 activates the MAPK pathway, with significant increased phosphorylation of ERK observed in pancreatic cancer cells treated by IL‐8. Our findings reinforce the hypothesis that IL‐8 might serve as a prognostic factor for pancreatic cancer, and is an important target for potential therapeutic intervention.
Previous studies have shown that IL‐8 was overexpressed in pancreatic cancer, caused increased MMP‐2 activity and played an important role in the invasiveness of human pancreatic cancer.( 11 , 12 , 22 ) The immunohistochemical analysis of 40 surgically resected human pancreatic cancer demonstrated that positive staining for IL‐8 was 50%, and positive staining for both IL‐8 and IL‐8 receptors was 40%.( 11 ) In contrast, immunoreactivity for IL‐8 was significantly suppressed in normal pancreatic tissues. Our data is consistent with the literature, showing that IL‐8 protein levels in MIA PaCa‐2 and BxPC‐3 cells were increased by 900%, and 304%, respectively, as compared with that in HPDE cells. IL‐8 mRNA was also found to be substantially overexpressed in 11 of 14 (79%) clinical pancreatic‐adenocarcinoma samples compared with that in their surrounding normal tissues with an overall 2.6‐fold increase. Therefore, human pancreatic cancer is associated with increased expression of IL‐8. IL‐8 expression has also been found in various human cancers, including brain tumors, breast cancer, gastric cancer, lung cancer, and prostate cancer( 2 , 23 , 24 , 25 , 26 , 27 ) indicating a critical role of IL‐8 in cancer pathogenesis.
We also showed that IL‐8 increased the expression of VEGF165 and NRP‐2. As indicated in our previous study, VEGF165 is an important angiogenic factor, while NRPs serves as receptors for VEGF in pancreatic cancer cells.( 18 ) It has been shown that IL‐8 promotes the metastasis of pancreatic cancer through increased angiogenesis, particularly involving VEGF.( 3 , 4 , 10 , 13 , 28 , 29 ) Our data further implicate IL‐8 as a pro‐angiogenic cytokine that helps the spread of distant metastasis by inducing neovascularization, and possibly promoting the survival of the tumor mass in general by maintaining a rich capillary network to accommodate the heavy nutrient requirements of this aggressive cancer. Other studies have reported a direct effect on tumor growth, in which decreased IL‐8 level by IL‐8 antisense oligonucleotide caused decrease in the growth of a pancreatic cancer cell FG cells in mice after intrapancreatic implantation, which correlated with decreased tumor angiogenesis.( 7 ) IL‐8 is also a powerful inflammatory cytokine associated with chronic diseases of the pancreas.( 1 ) This study, along with others, strongly indicates that IL‐8 plays a critical role in angiogenesis and pancreatic cancer progression by influencing several aspects of creating an environment conducive to the development and spread of pancreatic adenocarcinoma. Blocking IL‐8 and IL‐8 receptor CXCR2 significantly inhibited angiogenesis.( 3 , 28 )
Both IL‐8 and VEGF are important components in cellular response to hypoxia, a common event in cancer, including human melanoma, colon cancer and pancreatic cancer.( 2 ) Mizukami et al. found that besides the preserved expression of VEGF, IL‐8 was induced by hypoxia in a colon cancer cell line DLD‐1HIF–kDa, in which the HIF‐1 was knocked out, but not in HIF‐1 wild type DLD‐1HIF–wt cells. This induction was mediated by activation of NF‐κB and KRAS. A neutralizing antibody to IL‐8 significantly inhibited angiogenesis and tumor growth in DLD‐1HIF– kDa but not DLD‐1HIF–wt xenografts, indicating the functional significance of IL‐8 in the antiangiogenesis and hypoxia therapy of solid tumor. Strategies that inhibit hypoxia pathway which is mostly mediated by HIF‐1 may be more effective when IL‐8 is simultaneously targeted.( 15 ) In our study, we found that IL‐8 was up‐regulated in a dose‐dependent manner in response to hypoxia mimicking reagent CoCl2 in pancreatic cancer cells. Our previous study also showed that both VEGF165 and another pro‐inflammatory cytokine IL‐6 were inducible by hypoxia.( 21 ) The correlation of IL‐8 and VEGF under hypoxia conditions warrants further investigation. The interaction between IL‐8, VEGF, HIF‐1, and maybe other cytokines such as IL‐6 are critical in response to hypoxia in solid tumors, and may become targets for combinational cancer therapy.
It has been suggested that the MAPK pathway is involved in IL‐8 and VEGF activation.( 4 ) We have also examined possible cellular mechanisms of IL‐8 in pancreatic cancer cells. We found that ERK was activated after treatment with exogenous IL‐8 in BxPC‐3 cells. The MAPK pathway plays important role for cell growth and survival, and its activation has been shown to be associated with tumorigenesis.( 20 , 30 , 31 ) Previous studies have shown that constitutive activation of several signaling pathways in tumor cells is associated with increased IL‐8 expression.( 8 , 10 ) Several molecules have been reported to stimulate IL‐8 expression in tumor cell lines through MAPK cascades such as IL‐1α, which up‐regulates the secretion of IL‐8 in human pancreatic cancer cells via activation of ERK‐1/2, p38 MAPK, AP‐1, and NF‐κB.( 3 , 32 ) It has also been shown that activation of Src and its downstream pathways ERK‐1/2 and p38 MAPKs played important roles in up‐regulating IL‐8 secretion in pancreatic cancer cells.( 33 ) Inflammatory cytokines and chemokines including IL‐8 can also activate ERK pathway possibly through protein kinase C. IL‐8 binds to two different receptors on cell surface, CXCR1 and CXCR2. We found that in the six pancreatic cancer cells we examined (Panc‐1, MIA PaCa‐2, BxPC‐3, Capan‐2, HPAF‐II, and Panc03.27), CXCR1 was highly expressed in five of them (except for in MIA PaCa‐2 cells) compared with HPDE cells, but CXCR2 expression was low in all six pancreatic cancer cell lines. A few other studies have also shown various expressions of IL‐8 receptors in different pancreatic cancer cell lines such as Capan‐1 and SUIT‐2 cells.( 12 , 34 ) Upon IL‐8 binding, CXCR1 and CXCR2 are phosphorylated, and have been shown to mediate MAPK activation.( 35 ) This autocrine loop, in which IL‐8 exerts a positive feedback for ERK/MAPK activation might be important for pancreatic cancer progression. Therefore, our data suggest that IL‐8 act as a direct growth and survival factor on pancreatic cancer cells. This complements our picture of IL‐8 as a multifaceted regulator of gene expression that can regulate multiple pathways including angiogenesis, metastasis, and response to hypoxia in pancreatic cancer. Further investigation is needed to elute more clearly the cellular mechanisms employed by IL‐8.
In conclusion, we have found that IL‐8, a multifunctional inflammatory cytokine, is over‐expressed in both pancreatic cancer cell lines and in malignant tissue samples from pancreatic cancer patients. We have also demonstrated IL‐8 was inducible under hypoxia condition and was able to up‐regulate key molecules known to promote angiogenesis and metastasis possibly through MAPK pathway. Our study indicates that this cytokine remains an important focus in pancreatic cancer and suggests the need for further research to investigate the role and mechanism of IL‐8, and for therapeutic intervention with potent combinational treatment which includes both antiangiogenesis and suppressing pro‐oncogenic cytokines.
Acknowledgments
This work was supported in part by the American Cancer Society Grant #IRG‐93‐034‐09, and the St Luke's Episcopal Hospital Roderick D. MacDonald Research Fund 06RDM013 (M. Li), by National Institutes of Health (NIH) Grants NIH EB002436, HL08347 (C. Chen), NIH RO1 DE15543, and R21 AT003094 (Q. Yao).
References
- 1. Farrow B, Sugiyama Y, Chen A, Uffort E, Nealon W, Mark Evers B. Inflammatory mechanisms contributing to pancreatic cancer development. Ann Surg 2004; 239: 763–9; discussion 9–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xie K. Interleukin‐8 and human cancer biology. Cytokine Growth Factor Rev 2001; 12: 375–91. [DOI] [PubMed] [Google Scholar]
- 3. Matsuo Y, Sawai H, Funahashi H et al . Enhanced angiogenesis due to inflammatory cytokines from pancreatic cancer cell lines and relation to metastatic potential. Pancreas 2004; 28: 344–52. [DOI] [PubMed] [Google Scholar]
- 4. Sclabas GM, Fujioka S, Schmidt C et al . Overexpression of tropomysin‐related kinase B in metastatic human pancreatic cancer cells. Clin Cancer Res 2005; 11: 440–9. [PubMed] [Google Scholar]
- 5. Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar‐Eli M. Expression of interleukin‐8 by human melanoma cells up‐regulates MMP‐2 activity and increases tumor growth and metastasis. Am J Pathol 1997; 151: 1105–13. [PMC free article] [PubMed] [Google Scholar]
- 6. Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma. correlation with phenotypic characteristics and prognosis. Cancer 2004; 101: 2727–36. [DOI] [PubMed] [Google Scholar]
- 7. Shi Q, Abbruzzese JL, Huang S, Fidler IJ, Xiong Q, Xie K. Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin Cancer Res 1999; 5: 3711–21. [PubMed] [Google Scholar]
- 8. Shi Q, Le X, Abbruzzese JL et al . Cooperation between transcription factor AP‐1 and NF‐kappaB in the induction of interleukin‐8 in human pancreatic adenocarcinoma cells by hypoxia. J Interferon Cytokine Res 1999; 19: 1363–71. [DOI] [PubMed] [Google Scholar]
- 9. Shi Q, Le X, Wang B, Xiong Q, Abbruzzese JL, Xie K. Regulation of interleukin‐8 expression by cellular pH in human pancreatic adenocarcinoma cells. J Interferon Cytokine Res 2000; 20: 1023–8. [DOI] [PubMed] [Google Scholar]
- 10. Le X, Shi Q, Wang B et al . Molecular regulation of constitutive expression of interleukin‐8 in human pancreatic adenocarcinoma. J Interferon Cytokine Res 2000; 20: 935–46. [DOI] [PubMed] [Google Scholar]
- 11. Kuwada Y, Sasaki T, Morinaka K, Kitadai Y, Mukaida N, Chayama K. Potential involvement of IL‐8 and its receptors in the invasiveness of pancreatic cancer cells. Int J Oncol 2003; 22: 765–71. [PubMed] [Google Scholar]
- 12. Takamori H, Oades ZG, Hoch OC, Burger M, Schraufstatter IU. Autocrine growth effect of IL‐8 and GROalpha on a human pancreatic cancer cell line, Capan‐1. Pancreas 2000; 21: 52–6. [DOI] [PubMed] [Google Scholar]
- 13. Miyamoto M, Shimizu Y, Okada K, Kashii Y, Higuchi K, Watanabe A. Effect of interleukin‐8 on production of tumor‐associated substances and autocrine growth of human liver and pancreatic cancer cells. Cancer Immunol Immunother 1998; 47: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiong Q, Shi Q, Le X, Wang B, Xie K. Regulation of interleukin‐8 expression by nitric oxide in human pancreatic adenocarcinoma. J Interferon Cytokine Res 2001; 21: 529–37. [DOI] [PubMed] [Google Scholar]
- 15. Mizukami Y, Jo WS, Duerr EM et al . Induction of interleukin‐8 preserves the angiogenic response in HIF‐1alpha‐deficient colon cancer cells. Nat Med 2005; 11: 992–7. [DOI] [PubMed] [Google Scholar]
- 16. Furukawa T, Duguid WP, Rosenberg L, Viallet J, Galloway DA, Tsao MS. Long‐term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am J Pathol 1996; 148: 1763–70. [PMC free article] [PubMed] [Google Scholar]
- 17. Ouyang H, Mou L, Luk C et al . Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am J Pathol 2000; 157: 1623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li M, Yang H, Chai H et al . Pancreatic carcinoma cells express neuropilins and vascular endothelial growth factor, but not vascular endothelial growth factor receptors. Cancer 2004; 101: 2341–50. [DOI] [PubMed] [Google Scholar]
- 19. Li M, Feurino LW, Li F et al . Thymosinalpha1 stimulates cell proliferation by activating ERK1/2, JNK, and increasing cytokine secretion in human pancreatic cancer cells. Cancer Lett 2007; 248: 58–67. [DOI] [PubMed] [Google Scholar]
- 20. Li M, Zhai Q, Bharadwaj U et al . Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer 2006; 106: 2284–94. [DOI] [PubMed] [Google Scholar]
- 21. Feurino LW, Zhang Y, Bharadwaj U et al . IL‐6 stimulates Th2 type cytokine secretion and upregulates VEGF and NRP‐1 expression in pancreatic cancer cells. Cancer Biol Ther 2007; 6: 1096–100. [DOI] [PubMed] [Google Scholar]
- 22. Feijoo E, Alfaro C, Mazzolini G et al . Dendritic cells delivered inside human carcinomas are sequestered by interleukin‐8. Int J Cancer 2005; 116: 275–81. [DOI] [PubMed] [Google Scholar]
- 23. Morita M, Kasahara T, Mukaida N et al . Induction and regulation of IL‐8 and MCAF production in human brain tumor cell lines and brain tumor tissues. Eur Cytokine Netw 1993; 4: 351–8. [PubMed] [Google Scholar]
- 24. Green AR, Green VL, White MC, Speirs V. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin‐8 as a potential regulatory factor in breast tumours. Int J Cancer 1997; 72: 937–41. [DOI] [PubMed] [Google Scholar]
- 25. Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP‐1 and NF‐kB‐like binding sites of the interleukin 8 gene. J Biol Chem 1992; 267: 22506–11. [PubMed] [Google Scholar]
- 26. Smith DR, Polverini PJ, Kunkel SL et al . Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med 1994; 179: 1409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reiland J, Furcht LT, McCarthy JB. CXC‐chemokines stimulate invasion and chemotaxis in prostate carcinoma cells through the CXCR2 receptor. Prostate 1999; 41: 78–88. [DOI] [PubMed] [Google Scholar]
- 28. Wente MN, Keane MP, Burdick MD et al . Blockade of the chemokine receptor CXCR2 inhibits pancreatic cancer cell‐induced angiogenesis. Cancer Lett 2006; 241: 221–7. [DOI] [PubMed] [Google Scholar]
- 29. Ikeda O, Egami H, Ishiko T et al . Signal of proteinase‐activated receptor‐2 contributes to highly malignant potential of human pancreatic cancer by up‐regulation of interleukin‐8 release. Int J Oncol 2006; 28: 939–46. [PubMed] [Google Scholar]
- 30. Li M, Becnel LS, Li W, Fisher WE, Chen C, Yao Q. Signal transduction in human pancreatic cancer: roles of transforming growth factor beta, somatostatin receptors, and other signal intermediates. Arch Immunol Ther Exp (Warsz) 2005; 53: 381–7. [PubMed] [Google Scholar]
- 31. Li M, Wang H, Li F, Fisher WE, Chen C, Yao Q. Effect of cyclophilin A on gene expression in human pancreatic cancer cells. Am J Surg 2005; 190: 739–45. [DOI] [PubMed] [Google Scholar]
- 32. Kida Y, Kobayashi M, Suzuki T et al . Interleukin‐1 stimulates cytokines, prostaglandin E2 and matrix metalloproteinase‐1 production via activation of MAPK/AP‐1 and NF‐kappaB in human gingival fibroblasts. Cytokine 2005; 29: 159–68. [DOI] [PubMed] [Google Scholar]
- 33. Trevino JG, Summy JM, Gray MJ et al . Expression and activity of SRC regulate interleukin‐8 expression in pancreatic adenocarcinoma cells: implications for angiogenesis. Cancer Res 2005; 65: 7214–22. [DOI] [PubMed] [Google Scholar]
- 34. Hidaka H, Ishiko T, Furuhashi T et al . Curcumin inhibits interleukin 8 production and enhances interleukin 8 receptor expression on the cell surface: impact on human pancreatic carcinoma cell growth by autocrine regulation. Cancer 2002; 95: 1206–14. [DOI] [PubMed] [Google Scholar]
- 35. Nasser MW, Raghuwanshi SK, Malloy KM et al . CXCR1 and CXCR2 activation and regulation. Role of aspartate 199 of the second extracellular loop of CXCR2 in CXCL8‐mediated rapid receptor internalization. J Biol Chem 2007; 282: 6906–15. [DOI] [PubMed] [Google Scholar]


