Abstract
Avian influenza virus (AIV) specific CD8+ T lymphocyte responses stimulated by intramuscular administration of an adenovirus (Ad) vector expressing either HA or NP were evaluated in chickens following ex vivo stimulation by non-professional antigen presenting cells. The CD8+ T lymphocyte responses were AIV specific, MHC-I restricted, and cross-reacted with heterologousH7N2 AIV strain. Specific effector responses, at 10 days post-inoculation (p.i.), were undetectable at 2 weeks p.i., and memory responses were detected from 3 to 8 weeks p.i. Effector memory responses, detected 1 week following a booster inoculation, were significantly greater than the primary responses and, within 7 days, declined to undetectable levels. Inoculation of an Ad-vector expressing human NP resulted in significantly greater MHC restricted, activation of CD8+ T cell responses specific for AIV. Decreases in all responses with time were most dramatic with maximum activation of T cells as observed following effector and effector memory responses.
Keywords: Avian influenza virus, Chicken T lymphocytes, Adenovirus vectors, Influenza hemagglutinin, Influenza nucleocapsid, Memory T lymphocytes, Effector T lymphocytes
Introduction
Since 1996, the zoonotic threat of avian influenza viruses (AIV) has been exemplified by the direct transmission of the H5N1 from poultry to humans in many countries throughout the world, including Asia, Africa and Europe (Alexander, 2006; Capua and Alexander, 2002; Fauci, 2006; Melidou, 2009). The AIV are classified as type A influenza viruses in the Orthomyxoviridae family. Although shore birds and waterfowl, such as ducks, swans, geese, waders and terns, are considered the original hosts, AIV strains have also been isolated from pheasants, quails and poultry (Alexander, 2006; Causey and Edwards, 2008; Collisson et al., 2008). In poultry, the virulence of AIV determines its classification as either low pathogenic or highly pathogenic virus. Infection with low pathogenic AIV strains produces asymptomatic to mild respiratory and enteric disease, while infection with the highly pathogenic strains results in clinical illness and systemic disease (Fauci, 2006; Krauss et al., 2007; Olsen et al., 2006). Furthermore, poultry are considered a critical intermediate host for adaptation of the AIV strains from wild birds to mammals, including swine and humans (Collisson et al., 2008; Perdue and Swayne, 2005). Zoonotic infections of the H5N1 strains of AIV have resulted in fatality in 60% of the cases reported, although human-to-human transmission of the H5N1 is rare (Alexander, 2006). The segmented nature of these negative strand RNA viruses facilitates reassortment events that result in human strains with gene segments of avian origin (Capua and Alexander, 2002; Ungchusak et al., 2005; Webster et al., 1992). The influenza pandemics of 1918, 1957 and 1968 were caused by human influenza viruses encoding genes of avian, swine and human origins (Alexander, 2006; Capua and Alexander, 2002; Causey and Edwards, 2008). The genome of the current “swine-like” H1N1 of 2009 also carries two genes from AIV (Babakir-Mina et al., 2009; Garten et al., 2009).
Both the zoonotic nature of AIV which provides a potential mechanism for emergence of new human strains, and the economic losses sustained by the poultry industry from AIV outbreaks justify efforts to develop more efficacious, safe vaccines (Capua and Alexander, 2002; Davison et al., 2003). While only inactivated, whole AIV and fowlpox vectored vaccines are available commercially, AIV vaccines are discouraged or prohibited in many countries, such as the United States and many countries of the European Union (Capua and Marangon, 2003; Davison et al., 2003; Hall, 2004; Suarez et al., 2006). The use of inactivated whole virus vaccine as a control strategy for AIV is limited because of the inability to distinguish infected from vaccinated animals (DIVA), and pre-existing immunity against fowlpox virus can prevent the development of optimum protective immunity against AIV(Davison et al., 2003; Suarez et al., 2006; Swayne et al., 2000a,b). Incomplete protection allows AIV strains to survive and circulate in flocks and to potentially mutate into highly pathogenic strains (Suarez et al., 2006; Swayne et al., 2000b). The mass slaughter policy applied in the event of highly pathogenic AIV outbreaks in poultry contributes to extensive economic losses (Capua and Marangon, 2003; Davison et al., 2003). Highly pathogenic strains with an increase in infectious viral load within the bird are considered a risk to both birds and mammals.
In both mice and chickens, the efficacy of a non-replicating adenovirus serotype 5 (Ad5) based vector, expressing HA of influenza virus, to protect against a challenge of H5N1 virus has been demonstrated (Gao et al., 2006; Hoelscher et al., 2007). Toro et al. (2007) have shown that in ovo inoculation of the non-replicating (replication competent (RCA) free) human adenovirus vectored vaccine encoding HA transgene of H5N9/Tur/Wis/68 AIV strain induced neutralizing anti-HA antibody in chickens. Although well-characterized, humoral immunity protects against the viral strains expressing homologous HA protein by neutralization of the virus, its efficacy is limited against variant or heterologous viruses (Lee et al., 2008; Rimmelzwaan et al., 2007; Seo and Collisson, 1997). The ability of the non-replicating adenovirus vectored vaccine to induce HA-specific CD8+ T lymphocyte responses in chickens has not been determined.
The induction of CD8+ T lymphocyte immunity against infections of respiratory viruses, such as influenza and infectious bronchitis viruses, can greatly diminish the clinical disease by clearing virally infected cells (Lee et al., 2008; Pei et al., 2003; Rimmelzwaan et al., 2007; Seo and Collisson, 1997, 1998; Seo et al., 2002; Seo and Webster, 2001; Seo et al., 2000; Tamura et al., 2005). Additionally, the CD8+ T lymphocytes target more conserved epitopes than neutralizing antibodies and thus can confer protection against a broader range of viruses with distinct glycoproteins (Haghighi et al., 2009; Lee et al., 2008; Seo et al., 2002; Seo and Webster, 2001; Swain et al., 2004). The studies in this manuscript demonstrate that viral specific, MHC-I restricted effector and memory CD8+ T lymphocytes are generated in chickens following administration of the RCA-free human Ad5 vector encoding AIV HA and to an even greater extent, nucleocapsid protein (NP). Furthermore the T lymphocyte responses do cross-react with heterologous AIV and administration of a booster dose induces a more robust secondary humoral and cellular immune response against AIV.
Results
HA-specific, humoral immune responses following primary and booster inoculation with a non-replicating adenovirus HA vector
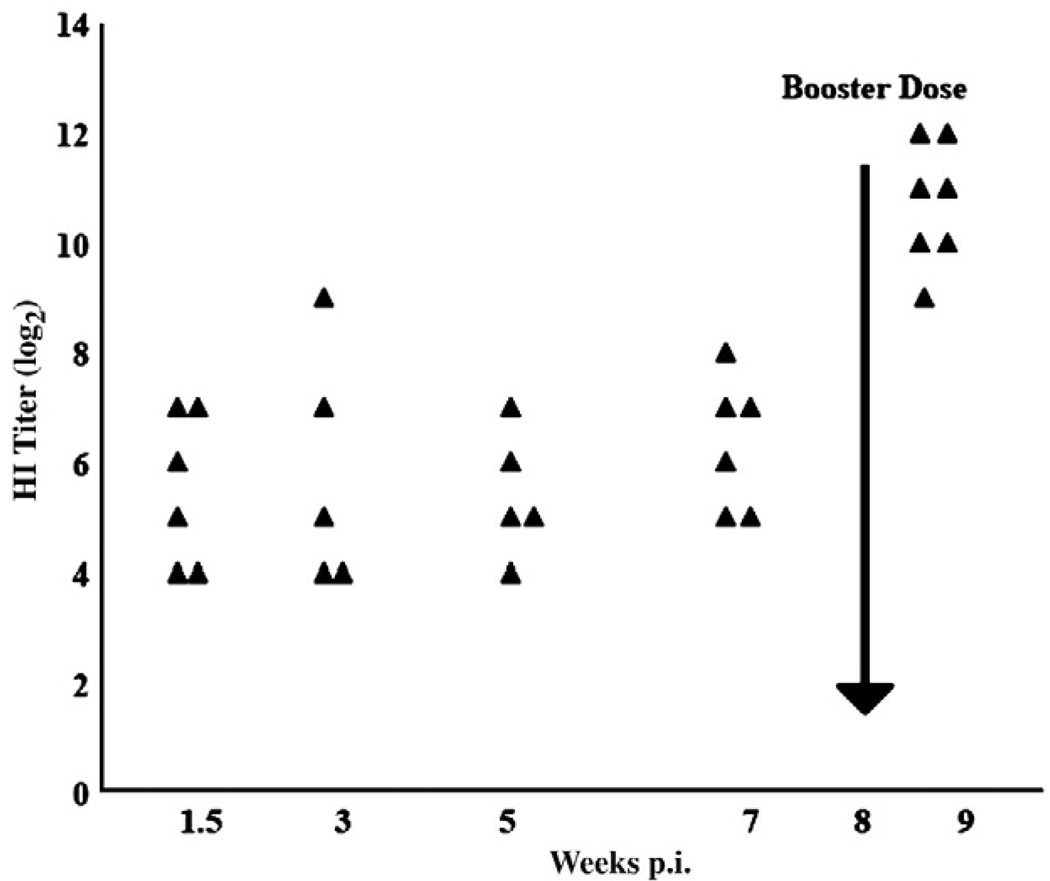
Three-week-old chicks were given a primary inoculation i.m. with 1 × 108 ifu of the adenovirus vector expressing H5 protein of H5N9 (A/Turkey/Wis/68) (Ad-HA) or with an equivalent amount of the empty vector (AdE) (Toro et al., 2007). Eight weeks later, they were given i.m. a secondary, booster inoculation. The HI titers of specific antibody for the H5N9 AIV strain from chickens after primary inoculation with the Ad-HA vector ranged from 4 to 8 log2 (GMT) (Fig. 1). Greater than a 16-fold increase in the HI titers was observed 1 week post-boosting (p.b.). No detectable HI activity was observed against a heterologous H7N2 AIV strain. The sera from AdE and PBS inoculated chickens were also negative for any HI activity (data not shown).
Fig. 1.
Kinetics of the humoral immune response induced following i.m. inoculation with Ad-HA. Serum HI antibody titers of individual chickens were evaluated, expressed as log2 of the reciprocal of the greatest dilution of serum inhibiting agglutination of 1% chicken RBCs by 4 HA units of the H5N9/Turkey/Wis/68 AIV. Results shown were derived from two separate experiments.
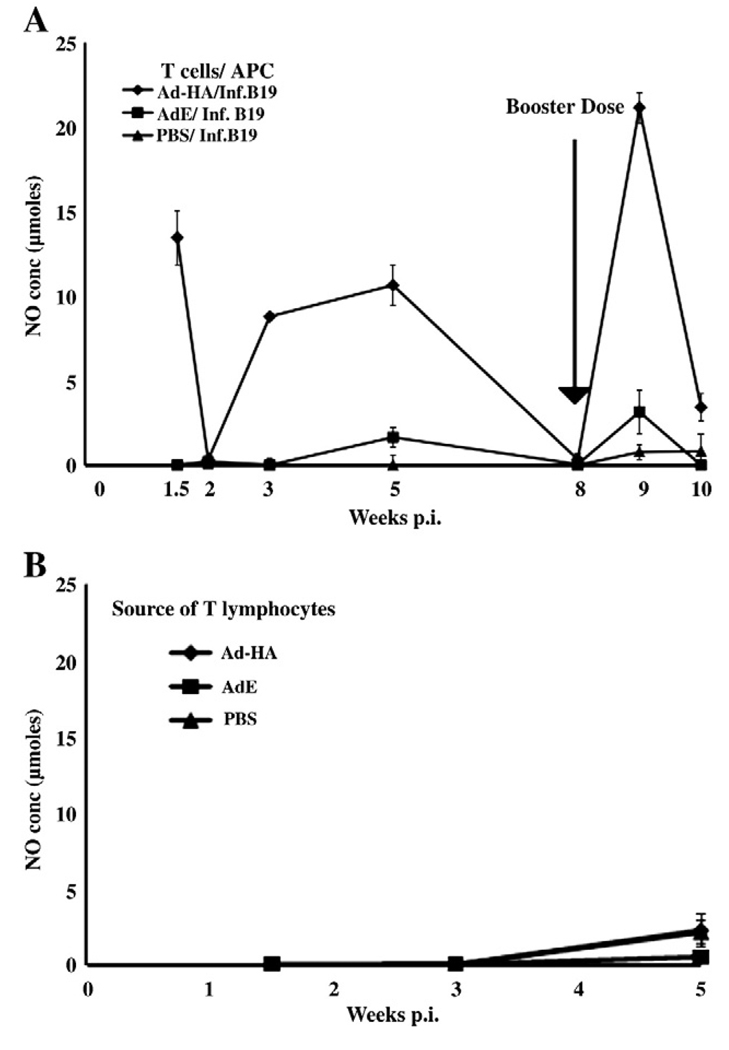
The Ad-HA vector induces AIV specific, effector, memory and effector memory T lymphocyte responses
Specific T lymphocyte responses were also evaluated following the i.m. inoculation of 3-week-old B19/B19 MHC haplotype chickens with Ad-HA or AdE vectors with 1 × 108 ifu. An indirect IFN-γ assay relying on the production of NO by the HD-11 chicken macrophage cell line was used to demonstrate AIV specific, ex vivo activation of T lymphocytes by infected APCs. An effector T cell response to the HA, detected at 10 days after administration of the Ad-HA, declined to basal levels by 16 days p.i. Memory T lymphocyte responses were detected by 3 weeks p.i. and maximum stimulation of viral specific T lymphocytes derived from Ad-HA inoculated chickens was detected at 5 weeks p.i. By 8 weeks p.i., T cell responses had declined to undetectable levels.
Following the decline of memory responses of peripheral blood T lymphocytes, 4 birds were boosted with a second, 1 × 108 ifu dose of Ad-HA or AdE given i.m. at 8 weeks after the primary inoculation. At 1 week p.b. with Ad-HA, the memory effector T cell responses detected following ex vivo stimulation of T lymphocytes were significantly greater for the T cells isolated from Ad-HA inoculated chickens than for T lymphocytes from AdE or PBS inoculated chickens. This secondary effector response also markedly declined at 2 weeks p.b., similar to the decline observed following the primary effector response.
Antigen specific activation of T lymphocytes from Ad-HA inoculated chickens following culturing with AIV infected APCs was demonstrated (Fig. 2A). Furthermore, the induction of the B19/B19 derived T lymphocytes by AIV infected B19/B19 APCs was MHC-I restricted since B2/B2 infected CKC expressing MHC-I did not activate these T lymphocytes (Fig. 2B). Lymphocytes from chickens inoculated with empty AdE stimulated only basal levels of NO.
Fig. 2.
Kinetics of the AIV specific T lymphocyte responses from B19/B19, MHC haplotype chickens following primary and booster inoculations with 1 × 108 ifu of RCA-free human Ad5 adenovirus vector expressing AIV HA (Ad-HA). T lymphocytes isolated from birds inoculated with Ad-HA were ex vivo stimulated with H5N9 AIV infected MHC matched B19/B19 APCs before evaluating induction of the T lymphocyte response. Activation was quantified as IFNγ secretion from T cells determined through production of NO by an HD11 macrophage cell line. Results are expressed as the average (±S.E.) of two experiments (n=6 or 7 birds). Each ex vivo stimulation assay is denoted by the adenovirus vector inoculated animal source of T lymphocytes and virus infected MHC-I APCs. (A) The APCs were kidney cells derived from B19/B19 chicks. (B) The APCs were derived from mismatched B2/B2 chicks. The difference in the responses of T lymphocytes from Ad-HA inoculated birds and AdE inoculated control birds at 10 days and at 3 and 5 weeks p.i. were significant (p≤0.001).
Direct IFNγ ELISA confirmed the generation of HA-specific T lymphocyte responses
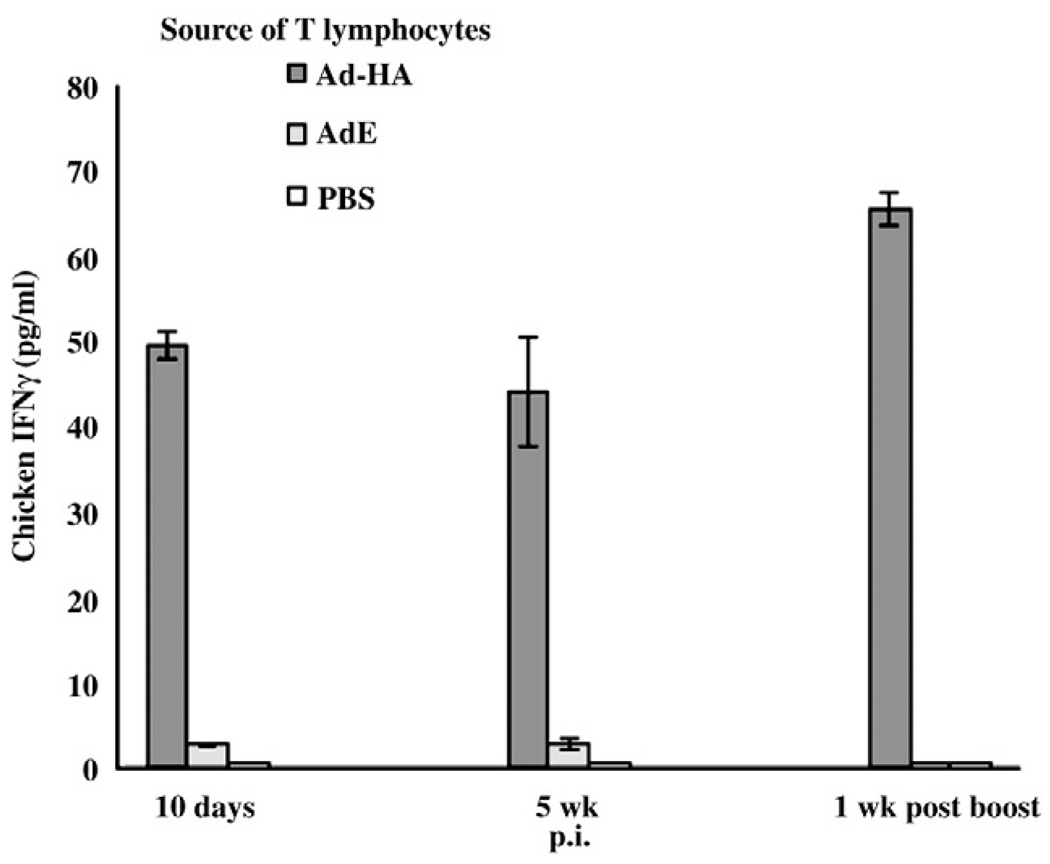
In addition to the NO production a commercial ELISA kit (Invitrogen, La Jolla, CA) directly evaluating the IFNγ production by activated T lymphocytes confirmed the AIV specific response of T lymphocytes at 10 days and 5 weeks p.i. and 1 week p.b (9 weeks p.i.) from chickens receiving Ad-HA, with no response from those given AdE or PBS. The mean amount of IFNγ produced by the primary effector T lymphocytes at 10 days p.i. from Ad-HA inoculated chickens was 49.5 pg/ml which was nearly 24-fold greater than that secreted from lymphocytes of chickens inoculated with AdE (Fig. 3). The memory T lymphocytes collected from chickens, 5 weeks p.i. with the HA adenovirus vector secreted an average of 44 pg/ml of IFNγ which was more than 20-fold greater than that produced by the T lymphocytes derived from chickens receiving the empty AdE control. The responses evaluated by the ELISA were also MHC restricted responses at 10 days, as well as 5 weeks p.i. At 5 weeks p.i., IFNγ secreted by the B19/B19 infected APC-stimulated T lymphocytes from Ad-HA inoculated birds was more than 44-fold greater than the IFNγ secreted by the lymphocytes stimulated by mismatched B2/B2 infected APCs (data not shown). The IFNγ production from the memory effector cells as detected by the ELISA 1 week p.b. also confirmed greater AIV specific and MHC-I restricted secondary effector responses. The results obtained by ELISA were in corroboration with the results from NO production assay for CD8+ T lymphocyte responses at 10 days and 5 weeks p.i. and 1 week p.b.
Fig. 3.
An IFNγ ELISA confirmed induction of specific T lymphocyte responses by primary and booster administration of Ad-HA. Concentration of IFNγ secreted in the supernatant of ex vivo APC-stimulated T lymphocytes was determined using a commercial ELISA (Invitrogen, La Jolla, CA). Results are expressed as the average (± S.E.) of 3 birds. Each ex vivo stimulation assay, using AIV infected B19/B19 derived APCs, is denoted by the adenovirus vector inoculated animal source of T lymphocyte and virus infected MHC-I APCs. Uninfected APCs did not elicit a response. The difference between IFNγ secretion by activation of T lymphocytes derived from Ad-HA and AdE inoculated chickens was significant (p≤0.001).
AIV specific memory T lymphocytes are mostly CD8+
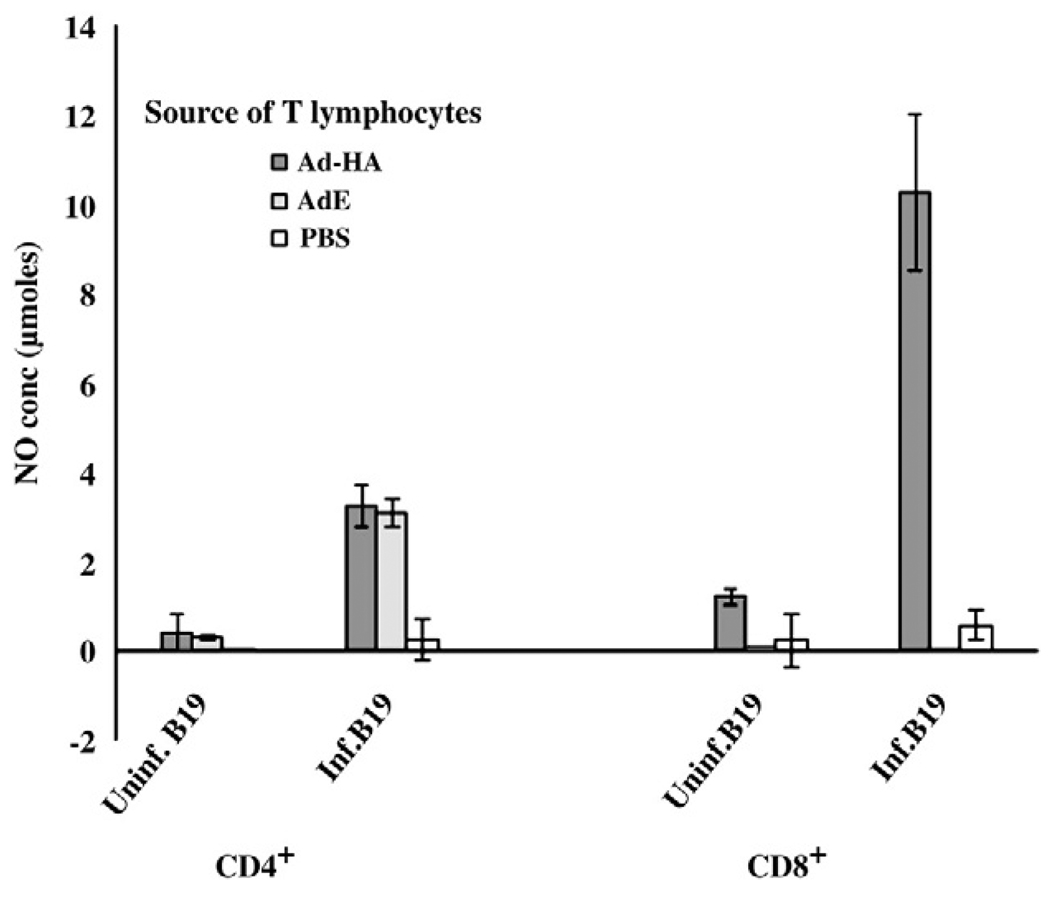
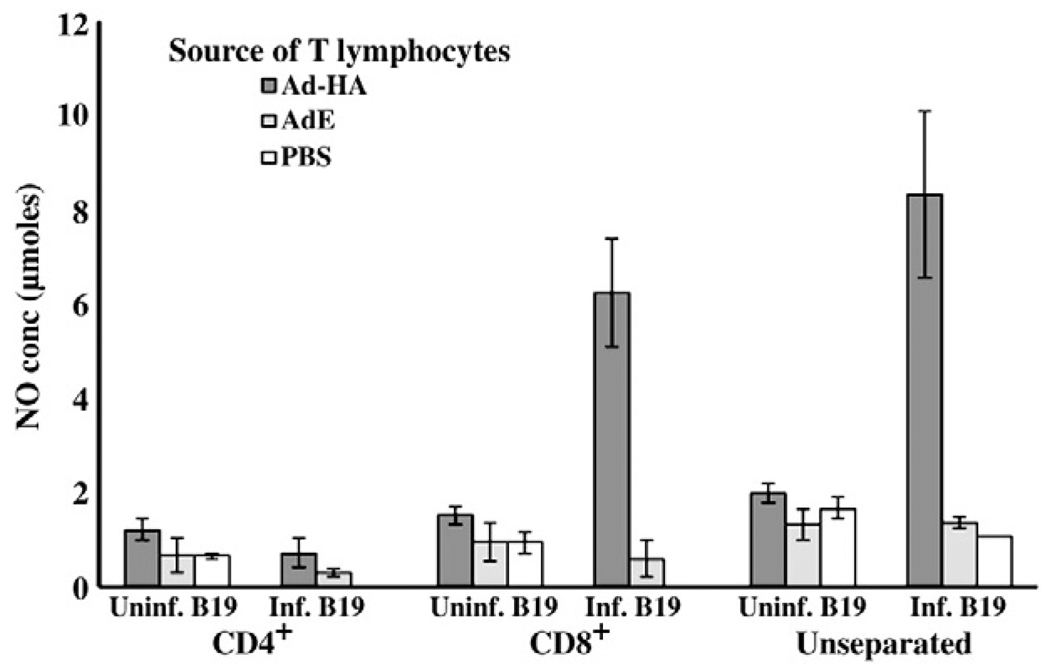
To determine the phenotype of the responding memory T lymphocytes reacting to AIV infected APCs, T lymphocyte subpopulations expressing either CD8 or CD4 were enriched by negative selection using specific antibody and magnetic beads (28) prior to ex vivo stimulation with the B19/B19 (MHC matched) haplotype APCs. Either CD4+ or CD8+ cells were enriched from T lymphocytes prepared from PBMC collected at 6 weeks p.i. The T lymphocytes enriched for the CD8+ phenotype were primarily responsible for the AIV specific, MHC restricted T cell responses (Fig. 4). The marginal stimulation of CD4+ lymphocytes from chickens given the AdE without HA by AIV infected APCs was not reproducible in repeated experiments.
Fig. 4.
T lymphocytes from Ad-HA inoculated chickens responsible for the AIV specific, MHC restricted responses were CD8+. At 6 weeks p.i., the T lymphocytes from the peripheral blood mononuclear cells were separated into CD4+ and CD8+ subpopulations prior to stimulation with AIV infected and uninfected B19 APCs. The activation of the T lymphocytes was measured by indirect stimulation of NO production. Results are expressed as average NO production (±S.E) of an n of 3 birds.
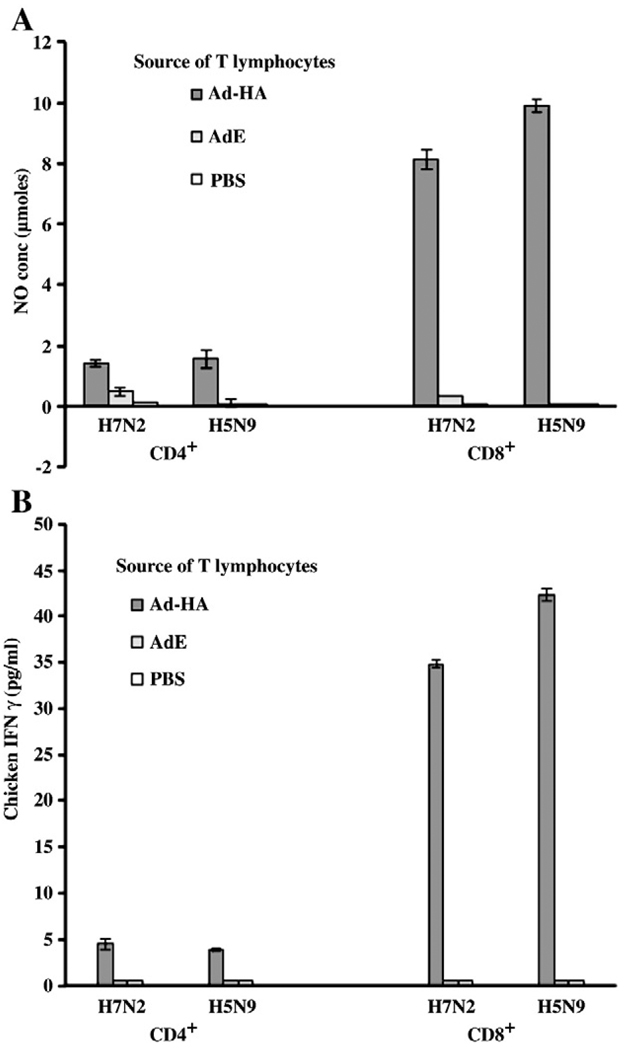
H5 specific, CD8+ T lymphocytes were activated by heterologous H7 infected APCs
The potential for T lymphocytes from H5 Ad-HA to cross-react with H7 was determined at 7 weeks p.i., when the responses remained detectable. Using H7N2 infected APCs for ex vivo stimulation of T lymphocytes, activation was detected by secretion of IFNγ using the macrophage secretion of NO and the ELISA (Invitrogen, La Jolla, CA). The responses of T cells from Ad-HA inoculated birds were not significantly different whether the APCs were infected with the H5 or the H7 virus, although the response to H5 virus infected APCs was slightly greater (Fig. 5). Using either the indirect NO production assay (Fig. 5A) or direct ELISA (Fig. 5B) to detect T cell secretion of IFNγ, the responses of CD8+ T lymphocytes to APCs infected with homologous H5 virus were 10 fold greater than responses of CD4+ lymphocytes. Responses of CD8+ T cells to APCs infected with heterologous H7 virus were 7 fold greater than responses of CD4+ lymphocytes (p≤0.001–0.0001). Stimulation of either CD8+ or CD4+ T lymphocyte populations from AdE or PBS inoculated chickens was either very weak or absent.
Fig. 5.
CD8+ T lymphocytes from B19 birds inoculated with Ad-HA respond to APCs infected with the homologous (H5N9) or a heterologous (H7N2) virus. At 7 weeks p.i., purified CD4+ and CD8+ T lymphocytes from chickens (n = 3) inoculated with Ad-HA were stimulated by B19 derived APCs infected with either the H5N9 or H7N2 strain. Assays represent the mean (± S.E.) of T lymphocytes of 3 birds; (A) The T lymphocyte responses as determined by the indirect IFNγ assay, and (B) The T lymphocyte responses of the same birds as determined by the direct IFNγ ELISA. Responses of CD8+ T lymphocytes were significantly greater than those of the CD4+ T lymphocytes (p≤0.01 and p≤0.001, respectively, as determined by the two assays).
AIV specific memory responses were detected in spleen derived T lymphocytes
Memory T lymphocyte subpopulations in the spleens were evaluated 4 weeks p.b., when the study was terminated. Whole T lymphocyte populations and the purified CD4+ and CD8+ T lymphocyte subpopulations were stimulated ex vivo with AIV infected and uninfected B19/B19 APCs. The stimulation of the T lymphocytes from the spleens of the Ad-HA inoculated chickens was AIV specific and MHC-I restricted. The secondary splenic T lymphocyte response, which was lower in comparison to the peripheral lymphocyte secondary effector response at 7 weeks, was mediated primarily by CD8+ lymphocytes (Fig. 6). In contrast, the activation of the CD4+ T lymphocytes was 7 fold lower (p≤0.003) and non-specific as indicated by stimulation with uninfected APCs.
Fig. 6.
AIV specific CD8+ T lymphocytes were detected from spleens of Ad-HA inoculated chickens 4 weeks p.b. Unseparated or the antibody enriched CD4+ and CD8+ splenic T lymphocytes isolated from chickens inoculated with adenovirus vector were stimulated ex vivo with MHC matched B19/B19 APCs infected with homologous H5N9 virus. Production of NO by macrophages induced by secretion of IFNγ from stimulated T lymphocytes was used to quantify the activation of the lymphocytes. Results are expressed as the average (± S.E.) of 4 birds. Each ex vivo stimulation assay is denoted by the adenovirus vector inoculated animal source of T lymphocyte and virus infected MHC-I APCs. AIV specific memory CD8+ T lymphocyte responses in the spleens were significantly greater than the CD4+ T lymphocyte responses (p≤0.003), but not significantly different from the response of the unseparated lymphocytes.
Non-replicating adenovirus NP expression vector stimulated greater T lymphocyte responses than the HA vector
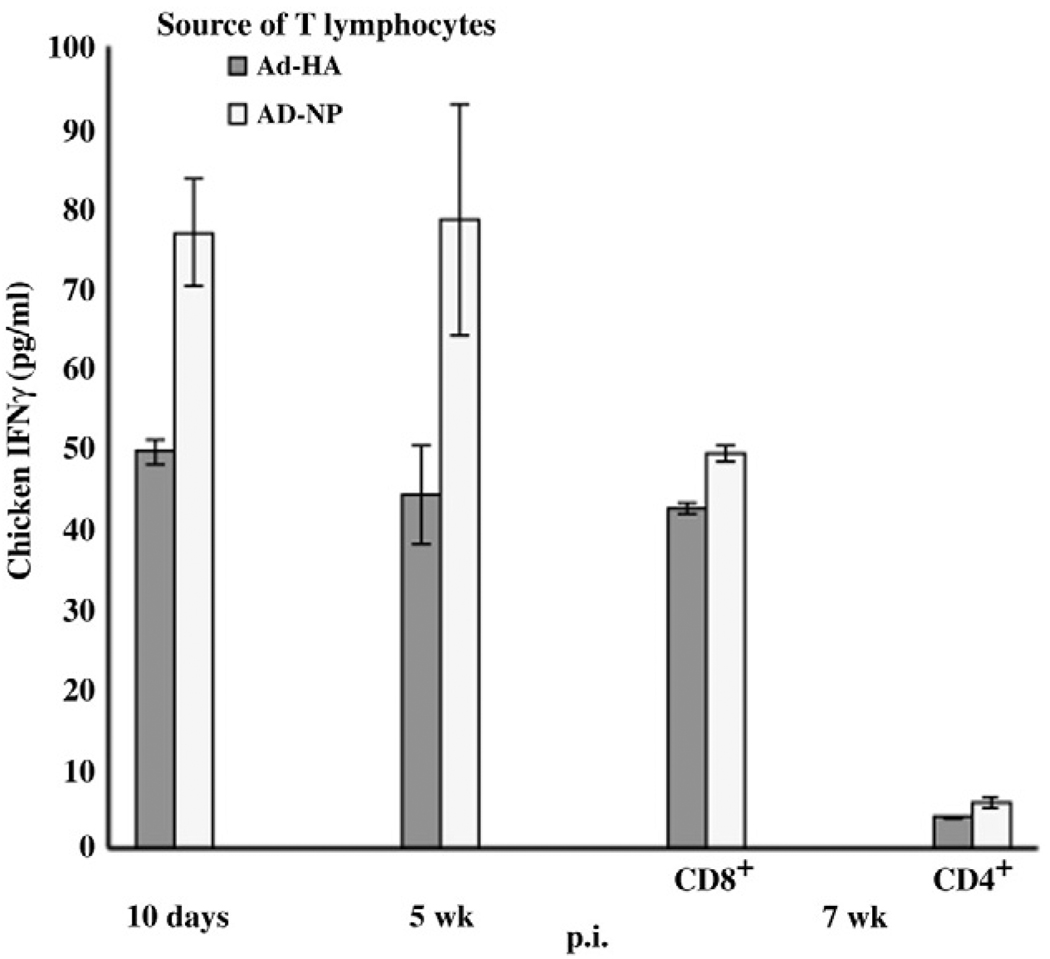
Studies using plasmid expression vectors indicated that in chickens the CD8+ T lymphocyte response directed against AIV NP may be more vigorous than that against HA (Singh et al., 2010). Therefore, the T lymphocyte responses following inoculation of the RCA-free Ad5 human adenovirus vector expressing NP (Ad-NP) were compared with the Ad-HA (Fig. 7). Although the origin of the NP was a human H1N1/PR8 (GenBank accession EF190983.1), the NP protein has 93% amino acid identity with the H5N9 AIV NP. Six 3-week-old B19 haplotype chickens were inoculated with 1 × 108 ifu of Ad-NP or Ad-HA. The AIV specific responses of T lymphocytes prepared from PBMCs collected at 10 days, 5 weeks and 7 weeks p.i. were evaluated by ex vivo activation using H5N9 infected APCs or the uninfected APCs. The activation of lymphocytes was determined with the direct ELISA. The 10 day p.i. effector and 5 week p.i. memory responses of T lymphocytes collected from the Ad-NP inoculated chickens were greater than those from HA inoculated chickens (p≤0.02 and p≤0.09, respectively).Moreover, the T lymphocytes collected from Ad-NP inoculated chickens also were induced by heterologous virus, H5N9/Turkey/Wis/68 infected APCs. At 7 weeks p.i., the separation of T lymphocytes into CD4+ and CD8+ subpopulations indicated a 48-fold greater activation of CD8+ T lymphocytes compared to CD4+ T lymphocytes. The responses of T lymphocytes specific for either NP or HA declined by 7 weeks. The decline in the NP was far greater than the decline of the response to HA narrowing the difference between responses to NP and HA. However, the NP appeared to remain a significantly (p≤0.006) better CD8+ T cell antigen than HA even at 7 weeks p.i.
Fig. 7.
CD8+ lymphocyte responses of birds receiving the Ad-NP vector were greater than those from birds receiving the Ad-HA vector. T lymphocytes, prepared from B19/B19 chickens inoculated with either vector (n = 3), were ex vivo stimulated with H5N9 AIV infected B19 APCs. The responses of the T lymphocytes were determined using an IFNγ ELISA. Results are expressed as the average (± S.E.) of 3 birds. Each ex vivo stimulation assay is denoted by the adenovirus vector inoculated animal source of T lymphocytes and virus infected MHC-I APCs.
Discussion
The increased incidence of outbreaks of highly pathogenic AIV infections in poultry throughout the world and the threat of emergence of a zoonotic pandemic are compelling reasons for the development of more efficacious vaccines for these viruses (Capua and Marangon, 2003; Collisson et al., 2008; Fauci, 2006; Swayne and Kapczynski, 2008). Although humoral immunity provides protection against a specific AIV HA type or subtype, protection against serologically distinct HA variants is limited (Lee et al., 2008; Rimmelzwaan et al., 2007). In contrast, the immune responses mediated by CD8+ T lymphocytes have broad application for antigenically distinct strains (Haghighi et al., 2009; Lee et al., 2008; Seo et al., 2002; Seo and Webster, 2001). In the current study, avian CD8+ T lymphocyte responses to AIV were identified following the inoculation of RCA-free Ad5 vectors expressing AIV HA or influenza virus NP. Our studies demonstrated that T lymphocytes induced by RCA-free Ad5 vectors expressing either the HA or NP were specific for both the autologous AIV H5N9 and a heterologous H7N2 AIV strain. The two HA proteins share only 41% amino acid sequence identity while the NP expressed by this vector shares 93% identity with the NP of the H5N9 virus used to infect the APCs. The vigorous AIV specific CD8+T lymphocyte responses induced by the RCA-free human Ad5 vectors expressing either the HA or the NP were AIV specific and MHC-I restricted. The Ad-HA studies further provide the first quantification of a viral specific secondary or booster T cell response in chickens.
The capacity of adenovirus vectored vaccines expressing influenza viral proteins to stimulate CD8+ T lymphocytes has been demonstrated in mice (Hoelscher et al., 2007; Holman et al., 2008; Singh et al., 2008). A replication competent (an E1/E3 deleted) adenovirus vectored AIV vaccine was shown to protect chickens from challenge with homologous virus (Gao et al., 2006). Toro et al. (2007) showed that the RCA-free human Ad5 vector expressing H5N9 AIV HA used in this current study protected chickens against highly pathogenic AIV H5N1 and H5N2 variants. Although inoculation of chickens with these Ad-HA vectored adenoviruses resulted in production of anti-HA antibodies and HI of homologous virus, antibody responses in our study failed to inhibit hemagglutination mediated by the heterologous, H7N2, AIV strain. In addition, the activation of CD4+ T lymphocytes from Ad-HA inoculated chickens following stimulation with both AIV infected and uninfected APCs could not be consistently demonstrated, for example at week 7 post-primary inoculation and at week 4 post-booster inoculation with the Ad-HA. The lack of CD4+T lymphocyte response can be attributed to the absence of MHC-II expression on the surface of APCs.
With comprehensive time course studies, the persistent control of each T cell response can be appreciated. Declines were observed following the effector, memory and memory effector responses. The current studies further established the transient nature of the responding AIV specific, CD8+ T lymphocytes following primary and booster administration of Ad-HA. Similar to the MHC-I restricted, T lymphocyte responses stimulated by infection with whole IBV or AIV or inoculation with recombinant fowlpox vector expressing AIV HA, the primary effector CD8+ T lymphocyte response was observed at 10 days p.i. (Haghighi et al., 2009; Seo et al., 2000, 2002). However at 16 days p.i., the responses of the T lymphocytes to IBV or the Ad-HA were undetectable. The AIV specific memory T lymphocyte response induced by Ad-HA could be observed between 3 weeks and 7 weeks p.i. Although the magnitude of the memory response was greatest at 5 weeks p.i., it remained lower than the primary effector response observed at 10 days p.i. or the booster effector response. The response of the memory T lymphocytes declined by 7 weeks after the primary inoculation of the vector and was reduced to nearly undetectable levels at 8 weeks p.i. Seo and Webster observed that in chickens inoculated with H9N2 AIV, the protection against a challenge with a variant virus had greatly declined by 10 weeks p.i. (Seo and Webster, 2001; Seo et al., 2002). Similarly, a decline in the human memory T lymphocyte responses has been reported with time (McMichael et al., 1983). The mechanism for this apparent regulation of CD8+ T lymphocyte activity is not know although increased apoptosis was associated with the decrease in the response to IBV following the effector response (Pei et al., 2003).
The impact of booster, secondary inoculation of the vectors on the chicken T lymphocyte mediated responses was also determined in the current studies. Induction of mice and non-human primate immune responses to adenovirus vaccine vectors following primary inoculation have been shown to be an impediment to their efficacy for further homologous challenge with the same vector (Liu et al., 2008; Schirmbeck et al., 2008; Tatsis et al., 2009). However, in contrast, the induction of a robust chicken T lymphocyte response with a 16-fold increase in anti-HA antibody titers 1 week post-boosting with the same Ad-HA vector, demonstrates the efficacy for challenge infection. Toro et al. (2008) have also established that in ovo vaccination of chickens with H5 Ad vectored vaccine did not impair the development of protective immunity induced by the post-hatched vaccination with the same Ad-vector expressing different HA. The presence of pre-existing immunity against the Ad-vector and HA did not inhibit the efficacy of this vaccine to stimulate a secondary cellular immune response against HA in chickens. This could be attributed to the non-replicating nature of the vector, establishing a weaker immune response against the adenovirus vector itself (Toro et al., 2008).
Additionally, the present study established that the non-replicating Ad-vector encoding an NP originating from a human H1N1/PR8 influenza virus has the ability to induce chicken T lymphocyte mediated responses that can cross-react with an AIV derived NP. Studies with mice have also demonstrated the immunodominance of NP in induction of CD8+ T lymphocytes (Lee et al., 2008; Vitiello et al., 1996; Yewdell et al., 1985). Our previous studies using plasmid DNA expressing HA or NP further indicated that the NP induced a greater, MHC-I restricted CD8+ T lymphocyte response than HA in chickens (Singh et al., 2010). The presence of memory T lymphocytes in the peripheral blood specific for both viral proteins is detectable only for 7 weeks p.i. of the primary inoculation. The presence of responding memory T lymphocytes in the spleens 4 weeks p.b. indicated that circulating memory lymphocytes also localize to central immune organs in chickens. IBV specific, MHC restricted, splenic T cells from chickens were also identified following primary infection (Pei et al., 2003; Seo and Collisson, 1998; Seo et al., 2000).
This study conclusively demonstrates that RCA-free Ad5 human adenovirus vectored vaccine expressing HA has the potential to induce both cell mediated and humoral immunity against AIV HA in chickens. The CD8+ T lymphocyte response induced by this RCA-free Ad5 vector has the ability to effectively cross-react with heterologous AIV strains. Additionally, this study distinctly establishes that booster or secondary administration of an HA expressing human adenoviral vector stimulates an efficacious secondary CD8+ T lymphocyte response in chickens. Therefore, the RCA-free Ad-HA vector may also be applicable in an avian homologous prime-boost vaccination program.
Materials and methods
Viruses
Viral stocks of low pathogenic AIVs, H5N9 (A/Turkey/Wis/68) and H7N2 (A/Turkey/Virginia/158512/02), were propagated in the allantoic sacs of 10-day-old, embryonated chicken eggs (ECE) for 48 h and virus was identified by the hemagglutination HA assay performed according to OIE guidelines (http://www.oie.int/eng/normes/mmanual/2008/pdf/2.03.04_AI.pdf). Virus titers were determined in embryonated eggs and expressed as embryo infectious doses 50% (EID50) (Beard, 1989).
Vector
An RCA-free E1/E3 deleted human adenovirus serotype 5 (Ad5) vector encoding HA gene from H5N9 (A/Turkey/Wis/68) AIV, (Ad-HA) was synthesized as described previously (Toro et al., 2007). The NP fragment used to generate the adenovirus NP vector (Ad-NP) was amplified by PCR from H1N1/PR8 NP-encoding plasmid pVIJ-NP using a primer pair creating unique EcoRI sites at both ends of the NP fragments. The PCR-amplified NP product was subsequently cloned into the EcoRI site of shuttle plasmid pAC-SR to create the plasmid pAC-NP. The adenovirus Ad-NP was generated by co-transfecting pAC-NP and Ad5 backbone plasmid pJM17 in human 293 cells as described previously by Shi et al. (2001). The seed Ad-NP vector stock was plaque selected and validated by PCR analysis. Virus titers were determined by the Adeno-X rapid titer kit (BD Clontech, Mountain View, CA), according to the manufacturer's protocol, and expressed as infectious units (ifu) per ml. The amino acids of the NP from H1N1 and NP from H5N9 AIV were 93% identical.
Experimental animals
Embryonated eggs of B19/B19 MHC-defined chicken lines were obtained from Dr. Briles’ laboratory at Northern Illinois University (DeKalb, IL). Post-hatching, the chicks were housed in a specific, pathogen free environment at the vivarium facility of Western University of Health Sciences, Pomona, CA. All procedures involving the use of chickens were approved by and conducted according to guidelines established by the Institutional Animal Care and Use Committee of Western University of Health Sciences. At 3 and 9 weeks of age, chickens with the B19/B19 MHC haplotype were inoculated intramuscularly (i.m.) with 0.3 ml (1 × 108 ifu total) of Ad-HA or Ad-NP vectors. Control birds were inoculated with either AdE, the empty vector without AIV genes, or PBS only.
Determination of HA-specific antibodies
Serum samples were prepared from blood collected from the jugular vein of chickens at various times post-inoculation (p.i.) to evaluate the humoral responses. The hemagglutination inhibition (HI) assay was used to determine titers of anti-HA antibodies specific to H5N9 virus (A/Turkey/Wis/68) HA and H7N2 (A/Turkey/Virginia/158512/02) and expressed as geometric mean titers (GMT) (http://www.oie.int/eng/normes/mmanual/2008/pdf/2.03.04_AI.pdf).
Generation of antigen presenting cells (APCs)
Primary chicken kidney cell (CKC) lines were established from 10-day-old chicks of B19/B19 and B2/B2 MHC haplotypes as described previously (Seo and Collisson, 1997). Cells from the tenth passage were used as non-professional antigen presenting cells (APCs) for the stimulation of CD8+ T lymphocytes. Flow cytometric analysis of CKCs labeled with mouse anti-chicken MHC-I and MHC-II monoclonal antibodies (Mab) indicated that these CKCs expressed surface MHC-I but not detectable MHC-II (data not shown).
T lymphocyte preparation
Peripheral blood mononuclear cells (PBMC) or splenocytes of the inoculated birds were the source of ex vivo stimulated effector T lymphocytes (Seo and Collisson, 1997). Briefly, blood was collected from the jugular vein of chicks, and mononuclear cells were prepared by Ficoll-histopaque (Histopaque-1077, Sigma-Aldrich, St. Louis, MO) density gradient centrifugation (Seo et al., 2002). Viable mononuclear cells were collected from the interface and washed twice with phosphate buffered saline (PBS, pH 7.4). Cells were resuspended in 3 ml of RPMI 1640 (Invitrogen, La Jolla, CA) supplemented with 10% fetal bovine serum (Gemini Bio-Products, West Sacramento, CA), 2 mM L-glutamine, and 0.1 mM MEM non-essential amino acids. B lymphocytes were removed by passing the cell suspension through nylon wool columns equilibrated with complete RPMI and adherent cells were removed by incubating the cell preparation in 25 cm2 tissue culture flasks as described previously (Seo and Collisson, 1997). The recovered T lymphocytes were 97% positive for the expression of CD3 on their surface as determined by flow cytometric analysis (Beckman Coulter Cytomics FC 500 Flow Cytometer, Brea, CA) of the cells labeled with fluorescent tagged mouse anti-chicken CD3 Mabs (Southern Biotech, Birmingham, AL).
Separation of CD8+ and CD4+ T lymphocytes
The CD8+ and CD4+ T lymphocytes were separated by antibody-mediated depletion using Dynabeads (Invitrogen, La Jolla, CA). The purified T lymphocytes were labeled with either mouse anti-chicken CD8 or mouse anti-chicken CD4 monoclonal antibodies (Mab) (Southern Biotech, Birmingham, AL) at a concentration of 1 µg/106 cells in PBS containing 0.1% bovine serum albumin fraction V (Sigma-Aldrich, St. Louis, MO) and incubated at 4 °C for 30 min. The unattached antibodies were removed by two washes with PBS and the cells were incubated with rat anti-mouse IgG coated Dynabeads, DynaMag-2 (Invitrogen, La Jolla, CA) according to the manufacturer's protocol, and the unlabeled cells in the supernatants were collected. The purity of the T lymphocyte subpopulations was confirmed by FACS analysis on cells labeled with fluorescent tagged, anti-chicken CD4 or CD8 Mabs (Bohls et al., 2006). The T lymphocyte subpopulations recovered after antibody mediated depletion were 95% pure for the expression of either CD4 or CD8 on the surface of the cells.
Ex vivo stimulation of T lymphocytes
T lymphocytes prepared from PBMC or spleens were stimulated ex vivo with MHC B19/B19 (matched) and B2/B2 (mismatched) APCs (Briles and Briles, 1987). APCs at a density of 1 × 105 cells/ml were incubated for 8 h at 39 °C, 5% CO2 in 96-well tissue culture plates. Each well of APCs was infected with 1 × 105 ELD50 of the H5N9 virus for 1 h followed by removal of the unattached virus by three washes with DMEM supplemented with 10% FBS. T lymphocytes (1 × 106) in complete RPMI were added 4 h after infection of the APCs. The APCs and T lymphocytes were co-cultured for 24 h at 39 °C, 5% CO2 before the media was collected and the supernatant clarified by centrifugation. Each ex vivo stimulation assay was conducted in duplicate.
Determination of IFNγ in supernatants
Activation of T lymphocytes was evaluated by either indirectly or directly determining the concentration of IFNγ in the clarified supernatants from T lymphocyte cultured with APCs. Indirect concentrations were determined using a nitric oxide detection assay following incubation with a chicken macrophage line and the direct concentrations were determined using a commercial ELISA (Invitrogen, La Jolla, CA) (Ariaans et al., 2008; Lambrecht et al., 2004). Each ex vivo stimulation assay was conducted in duplicate.
Nitric oxide secretion by IFNγ stimulated HD11 cells, a chicken macrophage cell line, was evaluated using a modification of the assays described by Karaca et al. (1996), Crippen et al. (2003) and Pei et al. (2003). Cells were incubated in individual wells of 96-well plates at a density of 105 cells/well in complete RPMI for 2 h at 39 °C, 5% CO2, prior to the addition of 100 µl of supernatants from T lymphocyte-APC cultures. After 24 h of incubation, the accumulation of nitrite from stimulated HD11 cells was measured using the Griess reagent assay according to the manufacturer's protocol (Sigma-Aldrich, St. Louis, MO). The concentration of nitrite produced was determined using sodium nitrite solutions with concentrations of 1–20 µmoles as standards. The concentration of any non-specific production of nitric oxide by soluble factors was removed by subtracting the nitrite concentration of supernatants from APCs cultured without T lymphocytes from the supernatants of the APCs cultured with T lymphocytes.
Statistical significance of differences
The nitric oxide and the ChIFN-γ concentrations were expressed as averages of three to four birds per group. ANOVA (analysis of variance) with significance of p<0.05 was used to determine statistical differences.
Acknowledgments
We thank Lisa Griggs, Victoria Hampton, and Omar Alvarado for technical assistance. We are grateful to Dr. Twani Crippen for providing us with HD11 cells. We thank Dr. Yvonne Drechsler for insightful comments and suggestions. This work was supported by USDA CSREES NRI grant award 2006-35204-16560, USDA CSREES Avian Influenza Cooperative Agriculture Project grant award 2005-35605-15388 and by NIH award #1 R43 AI068285-01.
References
- Alexander DJ. Avian influenza viruses and human health. Dev. Biol. (Basel) 2006;124:77–84. [PubMed] [Google Scholar]
- Ariaans MP, van de Haar PM, Lowenthal JW, van Eden W, Hensen EJ, Vervelde L. ELISPOT and intracellular cytokine staining: novel assays for quantifying T cell responses in the chicken. Dev. Comp. Immunol. 2008;32(11):1398–1404. doi: 10.1016/j.dci.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Babakir-Mina M, Dimonte S, Perno CF, Ciotti M. Origin of the 2009 Mexico influenza virus: a comparative phylogenetic analysis of the principal external antigens and matrix protein. Arch. Virol. 2009;154(8):1349–1352. doi: 10.1007/s00705-009-0438-1. [DOI] [PubMed] [Google Scholar]
- Beard CW. In: A laboratory manual for the isolation and identification of avian pathogens. 3rd ed. Purchase LHAHG, Domermuth CH, Pearson JE, editors. Dubuque, IA: Kendall/Hunt Publishing Co; 1989. [Google Scholar]
- Bohls RL, Smith R, Ferro PJ, Silvy NJ, Li Z, Collisson EW. The use of flow cytometry to discriminate avian lymphocytes from contaminating thrombocytes. Dev. Comp. Immunol. 2006;30(9):843–850. doi: 10.1016/j.dci.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Briles WE, Briles RW. Genetics and classification of major histocompatibility complex antigens of the chicken. Poult. Sci. 1987;66(5):776–781. doi: 10.3382/ps.0660776. [DOI] [PubMed] [Google Scholar]
- Capua I, Alexander DJ. Avian influenza and human health. Acta Trop. 2002;83(1):1–6. doi: 10.1016/s0001-706x(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Capua I, Marangon S. The use of vaccination as an option for the control of avian influenza. Avian Pathol. 2003;32(4):335–343. doi: 10.1080/0307945031000121077. [DOI] [PubMed] [Google Scholar]
- Causey D, Edwards SV. Ecology of avian influenza virus in birds. J. Infect. Dis. 2008;197 Suppl 1:S29–S33. doi: 10.1086/524991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collisson EW, Singh S, Drechsler Y. Evolving vaccine strategies for the continuously evolving avian influenza viruses: CAB Rev: Perspectives in Vet. Med., Agric. Nutr. and Nat. Resources. 2008;3:1–17. [Google Scholar]
- Crippen TL, Sheffield CL, He H, Lowry VK, Kogut MH. Differential nitric oxide production by chicken immune cells. Dev. Comp. Immunol. 2003;27(6–7):603–610. doi: 10.1016/s0145-305x(03)00035-1. [DOI] [PubMed] [Google Scholar]
- Davison S, Eckroade RJ, Ziegler AF. A review of the 1996-98 nonpathogenic H7N2 avian influenza outbreak in Pennsylvania. Avian Dis. 2003;47(3 Suppl):823–827. doi: 10.1637/0005-2086-47.s3.823. [DOI] [PubMed] [Google Scholar]
- Fauci AS. Emerging and re-emerging infectious diseases: influenza as a prototype of the host-pathogen balancing act. Cell. 2006;124(4):665–670. doi: 10.1016/j.cell.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Soloff AC, Lu X, Montecalvo A, Nguyen DC, Matsuoka Y, Robbins PD, Swayne DE, Donis RO, Katz JM, Barratt-Boyes SM, Gambotto A. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 2006;80(4):1959–1964. doi: 10.1128/JVI.80.4.1959-1964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325(5937):197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. Impact of avian influenza on U.S. poultry trade relations-2002: H5 or H7 low pathogenic avian influenza. Ann. N. Y. Acad. Sci. 2004;1026:47–53. doi: 10.1196/annals.1307.006. [DOI] [PubMed] [Google Scholar]
- Haghighi HR, Read LR, Haeryfar SM, Behboudi S, Sharif S. Identification of a dual-specific T cell epitope of the hemagglutinin antigen of an h5 avian influenza virus in chickens. PLoS ONE. 2009;4(11):e7772. doi: 10.1371/journal.pone.0007772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelscher MA, Jayashankar L, Garg S, Veguilla V, Lu X, Singh N, Katz JM, Mittal SK, Sambhara S. New pre-pandemic influenza vaccines: an egg- and adjuvant-independent human adenoviral vector strategy induces long-lasting protective immune responses in mice. Clin. Pharmacol. Ther. 2007;82(6):665–671. doi: 10.1038/sj.clpt.6100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman DH, Wang D, Raja NU, Luo M, Moore KM, Woraratanadharm J, Mytle N, Dong JY. Multi-antigen vaccines based on complex adenovirus vectors induce protective immune responses against H5N1 avian influenza viruses. Vaccine. 2008;26(21):2627–2639. doi: 10.1016/j.vaccine.2008.02.053. [DOI] [PubMed] [Google Scholar]
- Karaca K, Kim IJ, Reddy SK, Sharma JM. Nitric oxide inducing factor as a measure of antigen and mitogen-specific T cell responses in chickens. J. Immunol. Methods. 1996;192(1–2):97–103. doi: 10.1016/0022-1759(96)00026-9. [DOI] [PubMed] [Google Scholar]
- Krauss S, Obert CA, Franks J, Walker D, Jones K, Seiler P, Niles L, Pryor SP, Obenauer JC, Naeve CW, Widjaja L, Webby RJ, Webster RG. Influenza inmigratory birds and evidence of limited intercontinental virus exchange. PLoS Pathog. 2007;3(11):e167. doi: 10.1371/journal.ppat.0030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B, Gonze M, Meulemans G, van den Berg TP. Assessment of the cell-mediated immune response in chickens by detection of chicken interferon-gamma in response to mitogen and recall Newcastle disease viral antigen stimulation. Avian Pathol. 2004;33(3):343–350. doi: 10.1080/0307945042000220318. [DOI] [PubMed] [Google Scholar]
- Lee LY, Ha do LA, Simmons C, de Jong MD, Chau NV, Schumacher R, Peng YC, McMichael AJ, Farrar JJ, Smith GL, Townsend AR, Askonas BA, Rowland-Jones S, Dong T. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J. Clin. Invest. 2008;118(10):3478–3490. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ewald BA, Lynch DM, Denholtz M, Abbink P, Lemckert AA, Carville A, Mansfield KG, Havenga MJ, Goudsmit J, Barouch DH. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 2008;82(10):4844–4852. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Gotch FM, Dongworth DW, Clark A, Potter CW. Declining T-cell immunity to influenza, 1977-82. Lancet. 1983;2(8353):762–764. doi: 10.1016/s0140-6736(83)92297-3. [DOI] [PubMed] [Google Scholar]
- Melidou A. Avian influenza A(H5N1)–current situation. Euro Surveill. 2009;14(18) doi: 10.2807/ese.14.18.19199-en. [DOI] [PubMed] [Google Scholar]
- Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus AD, Fouchier RA. Global patterns of influenza a virus in wild birds. Science. 2006;312(5772):384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- Pei J, Briles WE, Collisson EW. Memory T cells protect chicks from acute infectious bronchitis virus infection. Virology. 2003;306(2):376–384. doi: 10.1016/s0042-6822(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Perdue ML, Swayne DE. Public health risk from avian influenza viruses. Avian Dis. 2005;49(3):317–327. doi: 10.1637/7390-060305R.1. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Fouchier RA, Osterhaus AD. Influenza virus-specific cytotoxic T lymphocytes: a correlate of protection and a basis for vaccine development. Curr. Opin. Biotechnol. 2007;18(6):529–536. doi: 10.1016/j.copbio.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R, Reimann J, Kochanek S, Kreppel F. The immunogenicity of adenovirus vectors limits the multispecificity of CD8 T-cell responses to vector-encoded transgenic antigens. Mol. Ther. 2008;16(9):1609–1616. doi: 10.1038/mt.2008.141. [DOI] [PubMed] [Google Scholar]
- Seo SH, Collisson EW. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J. Virol. 1997;71(7):5173–5177. doi: 10.1128/jvi.71.7.5173-5177.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SH, Collisson EW. Cytotoxic T lymphocyte responses to infectious bronchitis virus infection. Adv. Exp. Med. Biol. 1998;440:455–460. doi: 10.1007/978-1-4615-5331-1_58. [DOI] [PubMed] [Google Scholar]
- Seo SH, Webster RG. Cross-reactive, cell-mediated immunity and protection of chickens from lethal H5N1 influenza virus infection in Hong Kong poultry markets. J. Virol. 2001;75(6):2516–2525. doi: 10.1128/JVI.75.6.2516-2525.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SH, Pei J, Briles WE, Dzielawa J, Collisson EW. Adoptive transfer of infectious bronchitis virus primed alphabeta T cells bearing CD8 antigen protects chicks from acute infection. Virology. 2000;269(1):183–189. doi: 10.1006/viro.2000.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SH, Peiris M, Webster RG. Protective cross-reactive cellular immunity to lethal A/Goose/Guangdong/1/96-like H5N1 influenza virus is correlated with the proportion of pulmonary CD8(+) T cells expressing gamma interferon. J. Virol. 2002;76(10):4886–4890. doi: 10.1128/JVI.76.10.4886-4890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Zeng M, Yang G, Siegel F, Cain LJ, van Kampen KR, Elmets CA, Tang DC. Protection against tetanus by needle-free inoculation of adenovirus-vectored nasal and epicutaneous vaccines. J. Virol. 2001;75(23):11474–11482. doi: 10.1128/JVI.75.23.11474-11482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Pandey A, Jayashankar L, Mittal SK. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus. Mol. Ther. 2008;16(5):965–971. doi: 10.1038/mt.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Briles WE, Lupiani B, Collisson EW. Avian influenza viral nucleocapsid and hemagglutinin proteins induce chicken CD8(+) memory T lymphocytes. Virology. 2010;399(2):231–238. doi: 10.1016/j.virol.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez DL, Lee CW, Swayne DE. Avian influenza vaccination in North America: strategies and difficulties. Dev. Biol. (Basel) 2006;124:117–124. [PubMed] [Google Scholar]
- Swain SL, Dutton RW, Woodland DL. T cell responses to influenza virus infection: effector and memory cells. Viral Immunol. 2004;17(2):197–209. doi: 10.1089/0882824041310577. [DOI] [PubMed] [Google Scholar]
- Swayne DE, Kapczynski D. Strategies and challenges for eliciting immunity against avian influenza virus in birds. Immunol. Rev. 2008;225:314–331. doi: 10.1111/j.1600-065X.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Swayne DE, Beck JR, Kinney N. Failure of a recombinant fowl poxvirus vaccine containing an avian influenza hemagglutinin gene to provide consistent protection against influenza in chickens preimmunized with a fowl pox vaccine. Avian Dis. 2000a;44(1):132–137. [PubMed] [Google Scholar]
- Swayne DE, Garcia M, Beck JR, Kinney N, Suarez DL. Protection against diverse highly pathogenic H5 avian influenza viruses in chickens immunized with a recombinant fowlpox vaccine containing an H5 avian influenza hemagglutinin gene insert. Vaccine. 2000b;18(11–12):1088–1095. doi: 10.1016/s0264-410x(99)00369-2. [DOI] [PubMed] [Google Scholar]
- Tamura S, Tanimoto T, Kurata T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn J. Infect. Dis. 2005;58(4):195–207. [PubMed] [Google Scholar]
- Tatsis N, Lasaro MO, Lin SW, Xiang ZQ, Zhou D, Dimenna L, Li H, Bian A, Abdulla S, Li Y, Giles-Davis W, Engram J, Ratcliffe SJ, Silvestri G, Ertl HC, Betts MR. Adenovirus vector-induced immune responses in nonhuman primates: responses to prime boost regimens. J. Immunol. 2009;182(10):6587–6599. doi: 10.4049/jimmunol.0900317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro H, Tang DC, Suarez DL, Sylte MJ, Pfeiffer J, Van Kampen KR. Protective avian influenza in ovo vaccination with non-replicating human adenovirus vector. Vaccine. 2007;25(15):2886–2891. doi: 10.1016/j.vaccine.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro H, Tang DC, Suarez DL, Zhang J, Shi Z. Protection of chickens against avian influenza with non-replicating adenovirus-vectored vaccine. Vaccine. 2008;26(21):2640–2646. doi: 10.1016/j.vaccine.2008.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, Puthavathana P, Uiprasertkul M, Boonnak K, Pittayawonganon C, Cox NJ, Zaki SR, Thawatsupha P, Chittaganpitch M, Khontong R, Simmerman JM, Chunsutthiwat S. Probable person-to-person transmission of avian influenza A (H5N1) N. Engl J. Med. 2005;352(4):333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- Vitiello A, Yuan L, Chesnut RW, Sidney J, Southwood S, Farness P, Jackson MR, Peterson PA, Sette A. Immunodominance analysis of CTL responses to influenza PR8 virus reveals two new dominant and subdominant Kb-restricted epitopes. J. Immunol. 1996;157(12):5555–5562. [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992;56(1):152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell JW, Bennink JR, Smith GL, Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 1985;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]