Abstract
Glutaredoxin-1 (Glrx) is a thioltransferase that regulates protein S-glutathiolation. To elucidate the role of endogenous Glrx in cardiovascular disease, Glrx knockout (KO) mice were infused with angiotensin II (Ang II) for 6 days. After Ang II infusion, body weight and blood pressure were similar between WT and Glrx KO mice. However, compared to WT mice, Glrx KO mice demonstrated (1) less cardiac and aortic medial hypertrophy, (2) less oxidant generation in aorta assessed by dihydroethidium staining and nitrotyrosine, (3) decreased phosphorylation of Akt in the heart, and (4) less expression of inducible NOS (iNOS) in aorta and heart. In cultured embryonic fibroblasts from Glrx KO mice, S-glutathiolation of actin was enhanced and actin depolymerization was impaired after hydrogen peroxide stimulation compared with WT cells. Furthermore, oxidant generation in phorbol ester-stimulated fibroblasts and RAW 264.7 macrophage-like cells was lower with Glrx siRNA knockdown. These data indicate that Ang II-induced oxidant production and hypertrophic responses were attenuated in Glrx KO mice, which may result from impaired NADPH oxidase activation.
Keywords: Glutaredoxin, Angiotensin II, hypertrophy, superoxide, nitrotyrosine, S-glutathiolation, BioGEE
Introduction
Many proteins are susceptible to oxidative modifications that can alter their function during oxidative stress. S-glutathiolation (protein-SSG) is a reversible cysteine modification induced by reactive oxygen or nitrogen species (ROS/RNS) in the presence of glutathione (GSH). S-glutathiolation of various proteins regulates signaling pathways, transcription factors, apoptosis and cytoskeleton dynamics [14]. For instance, DNA binding of the p50 subunit of NF-κB [27] and activity of protein tyrosine phosphatase 1B (PTP1B) [4] are inhibited by S-glutathiolation. On the other hand, p21ras [1,8] and the sarcoplasmic reticulum Ca2+-ATPase [2] are activated by S-glutathiolation. This reversible cysteine modification not only may regulate protein function, but also may protect proteins from irreversible thiol oxidation (sulfonic acid formation; RSO3H).
Glutaredoxin-1 (Glrx), also known as thioltransferase, is a relatively abundant small (12 kD) cytosolic enzyme that regulates protein mixed disulfides. Glrx was originally discovered in a mutant of Escherichia coli lacking thioredoxin, but its function exceeds that of a simple backup for thioredoxin [9]. In contrast to thioredoxin, Glrx reduces mixed protein disulfides with GSH (S-glutathiolated proteins; R-SSG), but does not catalyze the reduction of sulfenic acid or intra- and intermolecular disulfides [7]. Thus, Glrx efficiently catalyzes the reduction of S-glutathiolated proteins (R-SSG) in the presence of NADPH and glutathione reductase [30]. In vitro, Glrx can restore the function of proteins that are inhibited when they are S-glutathiolated. For instance, Glrx reversed DNA binding of transcription factors [3,30], reactivated PTP1B [4,30] or inhibitory κB kinase (IKK)-β[28], and inactivated p21ras in smooth muscle cells [1] and cardiac myocytes [26]. Several lines of evidence indicate that Glrx protects cells from oxidant-induced cell death [18,22,35]. However, physiological roles of endogenous Glrx have not been well elucidated in vivo.
There are two isoforms of Glrx in mammalian cells. Glrx1 is localized mainly in the cytoplasm, and Glrx2 is found in mitochondria or the nucleus depending on splice variants. Human Glrx2 exhibits only 34-36 % identity with Glrx1 and exerts less than 10% of the specific activity of Glrx1 [10,17]. Glrx1, not Glrx2, is secreted by peripheral blood mononuclear cells and is detected in healthy human plasma [16]. Expression of Glrx1 is enhanced in human atherosclerotic lesions [25] and in the mouse airway with allergic inflammation [29], suggesting an association with oxidative stress and inflammation.
To elucidate the roles of endogenous Glrx1 in vivo in the cardiovascular system we examined Glrx1 homozygous knockout (Glrx KO) mice in an established model of cardiac hypertrophy. Unlike thioredoxin knockout mice, which are not viable [20], Glrx KO mice develop without overt abnormalities [13]. Because angiotensin (Ang II) induces hypertension as well as vascular [21,37,38] and cardiac [5] hypertrophy associated with an increase in NADPH oxidase-derived oxidants, we studied the effects of Ang II infusion in Glrx KO mice. Our results show smaller hearts, diminished aortic medial hypertrophy, and less oxidant generation in Glrx KO mice after Ang II infusion compared to wild-type mice. Pertinent to potential mechanisms, we found that knockdown of Glrx1 decreases the activation of NADPH oxidase, possibly because of altered S-glutathiolation of actin.
Materials and Methods
Glrx1 knockout mice
Targeted disruption of the Glrx-1 gene was generated in 129SVB6 and C57BL/6 hybrid background mice by Dr. Y.-S. Ho (Wayne State University, Detroit, MI, USA) [13]. Briefly, the targeting vector, in which exon 1 and 2 of the mouse Glrx1 gene was replaced with a neomycin resistance cassette, was transfected into embryonic stem cells. Chimeric mice were generated by microinjecting positive clones into blastocysts from C57BL/6 mice. Homozygous mice (Glrx-/-, KO) and genetically matched wild-type control (Glrx+/+, WT) were transferred to Boston University and the colony was expanded. Male mice of 4-5 months of age were studied.
Ang II infusion model
Ang II infusion was performed as previously described [21]. Briefly, the mice were anesthetized with inhaled isofluorane, and an incision was made in the midscapular region. Osmotic minipumps (Alzet model 1007D, Palo Alto, CA, USA) containing Ang II (Sigma, St Louis, MO, USA) dissolved in 0.15 M NaCl and 1 mM acetic acid were implanted, providing a delivery rate of 0.7 mg/kg/day for 6 days. Sham-operated mice were implanted with minipumps containing the solvent alone. Systolic blood pressure was determined by tail-cuff plethysmography (Visitec systems, Apex, NC, USA) before and at the end of Ang II infusion. The mice were euthanized by exsanguination under isoflurane anesthesia. These procedures were approved by the Boston University Medical Center Institutional Animal Care and Use Committee.
Measurement of aortic medial thickness
Aortic cross sections were obtained from the descending aorta, 3 mm distal to the left subclavian artery [21] and stained with hematoxylin and eosin and photographed at 100x magnification. Four measurements of aortic medial thickness oriented at 90° were made from each section using NIH ImageJ software.
Immunohistochemistry for nitrotyrosine and inducible nitric oxide synthase (iNOS)
Immunohistochemistry was performed as previously described [21]. Briefly, the aorta was placed in 4% formalin overnight, dehydrated and embedded in paraffin. Tissue sections were obtained from the descending thoracic aorta as mentioned above. Nonspecific binding was blocked with 10% normal goat serum in phosphate buffered saline (PBS) (pH 7.4) for 30 min before incubation with 1 μg/ml polyclonal anti-nitrotyrosine antibody (Upstate Biotechnology), polyclonal anti-iNOS antibody (Biomol) or PBS with 1% BSA overnight at 4 °C. Tissue sections were then incubated for 30 min at room temperature with biotinylated anti-rabbit IgG (1:800) and developed using Vectastain ABC kit (Vector, Burlingame, CA, USA). Specificity of anti-3-nitrotyrosine antibody was confirmed as described [21]. Semi-quantitative analysis of tissue immunoreactivity for nitrotyrosine and NOS-2 (iNOS) was done by estimating the degree of staining using an arbitrary scoring system from 1 to 4, and was performed by three observers who were blinded to the identity of each sample [21,38].
Detection of superoxide by dihydroethidium
The dye dihydroethidium, which is oxidized by superoxide to oxyethidine, was used to evaluate superoxide generation by a previously described method [21]. Mouse aortas were quickly embedded in O.C.T. compound (Miles, Elkhart, IN, USA) to freeze on dry ice and were cut into 10 μm sections. Immediately, dihydroethidium (2 × 10-6 mol/L, Molecular Probes, Eugene, OR, USA) was applied to cryostat sections at 37 °C for 30 min in the dark. Images were obtained by fluorescence microscopy (Axinvert S100TV) using an excitation wavelength of 488 nm and an emission wavelength of 610 nm. The photograph was used to evaluate semiquantitatively using the same method used to assess nitrotyrosine score as described above.
Detection of S-glutathiolated proteins in mouse embryonic fibroblasts (MEF)
MEF were prepared as described [13] and cultured in DMEM containing 10% FBS, penicillin and streptomycin. Cells were stored in liquid nitrogen and cells at passage 4-7 were used. Cells were plated at a density of 4 × 105/well in six-well plates to yield 80-90% confluence the next day. Cells were washed, incubated with Dulbecco's PBS (D-PBS; 1 g/L glucose) for 30 min, and stimulated with hydrogen peroxide (H2O2) for 10-60 min. Cells were collected in lysis buffer (Cell Signaling; No. 9803) containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and 20 mM N-ethylmaleimide (NEM) to prevent further S-glutathiolation. Protein concentration was assayed using a colorimetric reaction (Bio-Rad protein assay). Protein was separated under nonreducing conditions by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and analyzed by immunoblotting with monoclonal anti-GSH antibody (Virogen). The blots were incubated in HRP-conjugated secondary antibody and the signal was developed by enhanced chemiluminescence. Some blots were incubated with anti-mouse IRDye 800CW or anti-rabbit IRDye 700CW secondary antibodies for infrared imaging system (LI-COR).
Biotinylated GSH ester (BioGEE) and detection of S-glutathiolated actin
The method originally described [2,31] was utilized with slight modifications. Briefly, biotinylated GSH ethyl ester was made by mixing 25 mM EZ-Link sulfo-NHS-Biotin (Pierce) with 25 mM GSH ethyl ester in 50 mM NaHCO3 at pH 8.5 for 2 h at room temperature followed by adding 250 mM NH4HCO3 (pH 8.5) to quench the remaining biotinylation reagent. Semi-confluent MEF in P100 were incubated with 500 μM biotinylated GSH ethyl ester in D-PBS for 1 h, and stimulated with 5 mM H2O2 for 10 min. Cells were collected after being washed with cold PBS in lysis buffer containing 1 mM PMSF, 20 mM NEM and 100 μM diethylenetriaminepentaacetic acid. The cell lysate was passed through a PD-10 Sephadex-G25 column and an equal amount of eluted protein was mixed with streptavidin-Sepharose beads overnight at 4 °C. Beads were washed with lysis buffer four times and the final precipitate was incubated in Laemmli buffer with 5% β-mercaptoetheanol and 5M urea. GSS-actin was detected by immunoblotting with polyclonal anti-actin antibody (Cytoskeleton, Denver, CO, USA).
Immunoblotting of cardiac tissue (Akt, Glrx)
Frozen mouse hearts were homogenized in lysis buffer by Dounce glass homogenizer and centrifuged at 12,000 g for 5 min, and supernatant was analyzed for protein expression by SDS-PAGE under reducing conditions using polyclonal anti-Akt antibody and anti-phospho-Akt antibody (Upstate Biotechnology), rabbit polyclonal anti-Glrx1 antibody (BL3725, custom ordered by Bethyl Laboratories).
Assessment of actin polymerization in MEF
MEF were cultured as described above, washed, and incubated in HBSS (1 g/L glucose, HyClone) for 30 min, then stimulated with 5 mM H2O2 for 10 min. The cells were immediately collected in lysis buffer and centrifuged to analyze filamentous-to-globular (F/G) actin ratio according to manufacturer's instruction (G-actin/F-actin in vivo Assay kit, Cytoskeleton).
Knockdown of Glrx1 in RAW 264.7 macrophages by siRNA
Raw 264.7 cells (mouse macrophage cell line; ATCC) were cultured in DMEM with 10% FBS and 4.5 mg/L glucose. To knock down expression of Glrx1, approximately 5 × 105 cells were plated in six-well plates. After cells adhered (3 h), siRNA for mouse Glrx1 (Invitrogen) (sense, CCUACUGCAGAAAGACCCAAGAAAU; antisense, AUUUCUUGGGUCUUUCUGCAGUAGG) or scrambled RNA was applied at 125 nM using Lipofectamine 2000 (Invitrogen) in medium containing 10% FBS without antibiotics. After 2 days, cells were used for oxidant assay.
Measurement of oxidants by DCF assay
To measure oxidant generation 5,6-chloromethyl-2,7-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA; Molecular Probe) was used. MEF were incubated with 5 μM CM-H2DCFDA in HBSS for 30 min and stimulated with 1 μM phorbol 12-myristate 13-acetate (PMA) for another 30 min to 1 hr. Some wells were treated with cytochalasin-D (1 μM) before CM-H2DCFDA or Tiron (10 mM) before PMA. Cells were washed, medium was replaced with fresh HBSS, and fluorescent signal was scanned with a fluorescence microplate reader (Fluoroskan Ascent; Labsystems) at 485 nm excitation and 538 nm emission. Similarly Raw 264.7 macrophages treated with siRNA were replated in a 48-well plate in 1% FBS medium overnight. Then cells were incubated in HBSS with 5 μM CM-H2DCFDA followed by stimulation with PMA for 1 h. The fluorescence intensity was assessed.
Data analysis
Data are expressed as means ± SE. Statistical comparisons were performed by ANOVA and Student's t-test. Statistical significance was accepted when the p value was less than 0.05.
Results
Decreased cardiac hypertrophy in Glrx KO mice after Ang II infusion
Ang II infusion for 6 days increased blood pressure similarly in Glrx WT and Glrx KO mice (WT 149 ± 4, KO 154 ± 3, mmHg, NS). Basal blood pressure was similar (WT 115 ± 4, Glrx KO 119 ± 2 mmHg). Body weight was also similar between groups before or after Ang II infusion: Before surgery, (WT 34.0 ± 1.0, KO 34.3 ± 1.0, g), after surgery (at sacrifice), (WT 32.1 ± 1.0, KO 32.4 ± 0.9, g). However, heart weight at sacrifice after Ang II infusion was significantly lower in Glrx KO mice (WT 204 ± 5, Glrx KO 180 ± 5, mg, p < 0.01) (Figure 1). Heart weight /body weight was also significantly lower (WT 6.2 ± 0.2, KO 5.4 ± 0.1, mg/g, p < 0.01)
Figure 1. Heart weight of the mice.
Ang II was infused into WT and Glrx KO mice for 6 days as described in Materials and Methods. Heart weight was recorded at the sacrifice by exsanguination. Heart weight was significantly smaller in Glrx KO mice after Ang II infusion. (n = 17, p < 0.01)
Decreased medial hypertrophy and oxidants generation in Glrx KO mouse aorta
Ang II infusion increased aortic medial thickness as shown in our previous studies in which we demonstrated that medial hypertrophy was less when superoxide radical generation was inhibited by deletion of gp91phox or with a decrease in G6PD [21,38]. Cross-sectional medial thickness was significantly increased after Ang II infusion compared to sham controls in both WT and Glrx KO, but there was less hypertrophy in Glrx KO mice after Ang II (WT 65 ± 1.7, Glrx KO 58 ± 2.4, μm, p < 0.05, n =12) (Figure 2).
Figure 2. Aortic media thickness.
The cross-sections of mouse aorta were stained and photographed at a magnification of X100, and the medial thickness was assessed as described in Materials and Methods. The cross-sectional medial thickness was significantly increased after Ang II infusion compared to sham operation in WT mice, but there was less hypertrophy in Glrx KO mice after Ang II. WT 65 ± 1.7, Glrx KO 58 ± 2.4 μm, (n = 12, p < 0.05).
Aortas from Ang II-infused mice showed higher oxyethidine fluorescence localized primarily in medial smooth muscle compared to sham-operated mouse aorta (data not shown). Semi-quantitative analysis of DHE staining in Ang II-infused WT mouse aorta showed a greater signal than in Ang II-infused Glrx KO mouse aorta (p < 0.005, Figure 3). Furthermore, formation of 3-nitrotyrosine was examined as a marker of superoxide generation and its reaction product with nitric oxide, peroxynitrite. In Ang II-infused WT mice, immunohistochemistry for 3-nitrotyrosine on aortic cross-sections showed increased staining in the endothelium and the adventitia, and to a lesser extent in the media as shown previously [21,38]. On the other hand, 3-nitrotyrosine staining in Ang II-infused Glrx KO mouse aorta was diminished (Figure 4a). Semi-quantitative analysis showed that Ang II infusion increased nitrotyrosine staining about 3-fold compared with sham-operated WT mice, but was not increased in Glrx KO mice. After Ang II infusion the nitrotyrosine score was significantly lower in Glrx KO mice (WT 5.9 ± 0.7, Glrx KO 2.3 ± 0.3, p < 0.001) (Figure 4b). These data suggest less production of reactive oxygen/nitrogen species in Glrx KO aorta compared to WT aorta.
Figure 3. DHE staining of the mouse aorta.
a. DHE (dihydroethidium) was used to evaluate superoxide anion generation. The mouse aorta was quickly embedded in O.C.T. compound, frozen on dry ice, and cross-sectioned (10 μm). Immediately, DHE (2 × 10-6 mol/L) was applied to the cryo-sections at 37 °C for 30 min in the dark. Images were obtained by fluorescence microscopy using an excitation wavelength of 488 nm and an emission wavelength of 610 nm. The aorta from Glrx KO mice showed less fluorescence. b. DHE staining was scored by 3 observers who were blinded to the identity of the sample. The average score of 4-5 samples was significantly lower in Glrx KO mice (p < 0.005).
Figure 4. Nitrotyrosine staining of the mouse aorta.
a. Immunohistochemical staining of nitrotyrosine on cross-sections of mouse aorta is shown at a magnification of 400x. Nitrotysrosine staining was increased by Ang II infusion compared to sham-operated mice, but not increased in Glrx KO. b. Nitrotyrosine score was assessed as described in the Methods showed significant decrease in Ang II infused Glrx KO mouse aorta compared with Ang II infused WT mouse aorta (p < 0.001).
Phosphorylation of Akt in Glrx KO mouse heart
Serine-threonine kinase Akt (Protein B kinase) mediates Ang II-induced hypertrophy in smooth muscle cells, cardiomyocytes and other tissues [12,36]. Because Glrx KO mice showed smaller hearts, the phosphorylation of Ser473Akt was examined by immunoblot in mouse heart homogenates after Ang II infusion. Hearts from Glrx KO mice showed decreased phosphorylation compared to hearts from WT mice. Densitometry analysis showed a trend for lower phospho-Akt/Akt ratios in Glrx KO mouse heart vs. WT (p < 0.08, Figure 5b). Glrx-1 protein expression was not detected in Glrx KO hearts and was not changed by Ang II infusion in WT hearts (Figure 5a).
Figure 5. Protein expression in mouse heart.
a. Western blot of mouse heart homogenate showed that Glrx is not expressed in Glrx KO, and the expression was comparable between Sham (S) and Ang II infusion. Also, Cu,Zn-SOD expression was not altered by Ang II infusion or by Glrx KO. b. Western blot for phospho-Akt and total Akt was shown. Densitometric analysis showed trend of decrease in phospho-Akt in Glrx KO mouse heart compared to WT mouse heart after Ang II infusion (n = 6, p < 0.08).
Decreased inducible NOS (iNOS) induction in Glrx KO mouse aorta
Activity of NF-κB and AP-1 transcription factors can be inhibited by S-glutathiolation. The expression of one of NF-κB dependent gene product, iNOS, was examined in aortas by immunohistochemistry. Ang II infusion-induced iNOS expression was detected in the endothelial media and adventitia in WT aortas, but it was less in Glrx KO aortas. Semi-quantitative evaluation showed a significant decrease (Figure 6). The iNOS staining in hearts was also decreased in Glrx KO mice vs. WT mice (Supplemental figure 1).
Figure 6. iNOS expression in Ang II infused mouse aorta.
a. Immunohistochemistry of iNOS in Ang II infused mouse aorta showed positive staining on endothelium and media in WT but less staining in Glrx KO. Original magnification is 400x. b. Semi-quantitative score indicated significant decrease in iNOS expression in Glrx KO aorta (p < 0.05).
Glrx KO fibroblasts show enhanced glutathiolation of proteins after H2)2 exposure
Because Glrx-1 regulates S-glutathiolation, S-glutathiolation of proteins was examined by immunoblot with anti-GSH antibody. After H2O2 stimulation in MEF, some proteins showed enhanced signal with anti-GSH antibody that was more prominently in Glrx KO cells under non-reducing conditions. The enhanced bands were eliminated by dithiothreitol (DTT) (Figure 7a). The major band that was enhanced in Glrx KO cells was approximately 42 kDa. Using Licor fluorescence detection, images of proteins that bound anti-GSH and actin antibodies were superimposed at 42 kDa. The 37 kDa band overlayed with the GAPDH signal, and this was also more intense in Glrx KO compared to WT fibroblasts challenged with H2O2 (Figure 7b). Furthermore, streptavidin pull-down of proteins from cells loaded with biotinylated-GSH-ester (BioGEE) confirmed that actin formed R-SSG adducts after H2O2 exposure in Glrx KO cells (Figure 7c).
Figure 7. S-glutathiolated proteins in MEF after H2O2.
a. Immunoblot with anti-GSH antibody. MEF were treated as described in the Methods and cell lysate was analyzed by immunoblot under non-reducing condition. Some samples were analyzed in presence of 10 mM DTT. Several proteins showed increased GSH staining after H2O2 treatment in Glrx KO cells. This staining mostly disappeared after treatment with DTT. Upper panel shows the effect of dose. Lower panel shows the time course (2 mM H2O2). The most prominent band was about 42 kD, the size of actin. β-actin was shown as loading control. b. Similar experiment as a. was analyzed using infrared imaging system. Anti-GSH (monoclonal) antibody was detected by anti-mouse IgG with IRDye 800CW (green). Anti-β-actin (polyclonal, Cytoskeleton) and anti-GAPDH (polyclonal) antibody were detected by anti-rabbit IgG with IRDye 700CW (red). Overlapping two colors (yellow) demonstrate GSH adduct on actin and GAPDH. c. Streptavidin pull-down study of BioGEE-labeled proteins was performed after H2O2 (5 mM, 10 min) exposure as described in the Methods. Then, the blot was incubated with anti-β-actin antibody. Representative experiment of three similar results showed enhanced S-glutathiolation of β-actin in Glrx KO cells after exposure to oxidants. WT: MEF from wild type mice. KO: MEF from Glrx KO mice. C: control. H: H2O2 treated for 10 min.
Actin depolymerization was impaired in Glrx KO MEF
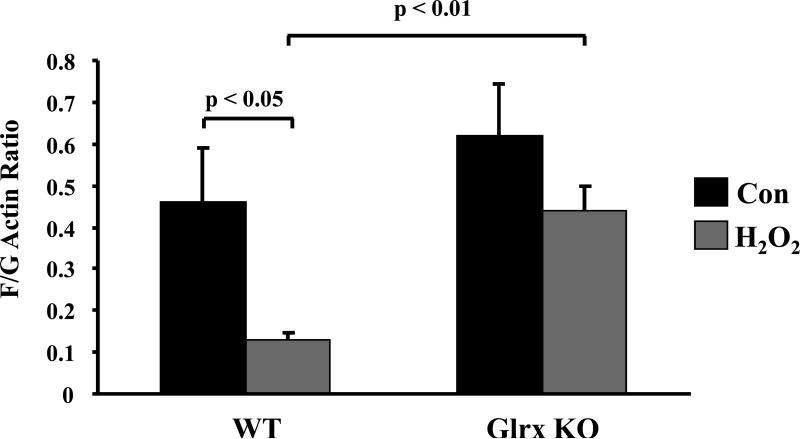
It has been demonstrated that glutathiolation of actin at cysteine374 impairs its polymerization [39]. Actin polymerization was assayed by comparing the ratio of filamentous (F)-actin to globular (G)-actin using an in vivo assay kit (Cytoskeleton, Inc). After exposure to H2O2 for 10 min, F/G was significantly decreased in WT MEF, but was decreased to a lesser extent in Glrx KO MEF (Fig 8). Similar effects were observed by immunocytochemistry with anti-actin antibody or Alexa 488-phalloidin (data not shown). This indicates that actin depolymerization or turnover is impaired in Glrx KO cells.
Figure 8. Actin polymerization (F-actin/G-actin ratio) in MEF.
MEF were exposed to H2O2 (5 mM) for 10 min and processed according to manufacturer's instruction. F/G actin ratio was decreased by H2O2 in the WT cells but not significantly in the KO cells. The result was the average of 3 assays. p < 0.05, p < 0.01.
MEF and macrophages generate less oxidant after Glrx knockdown
In order to test the hypothesis that knockdown of Glrx-1 is associated with decreased superoxide generation, DCF assay was performed in MEF stimulated with PMA, a known activator for NADPH oxidase. Tiron, a superoxide scavenger, inhibited the DCF signal in both WT and KO cells (Figure 9a). Tiron-inhibitable DCF signal was significantly less in KO vs. WT cells (WT 1.28 ± 0.17, KO 0.63 ± 0.12, p < 0.05). Cytochalasin-D, an actin polymerization inhibitor, also inhibited the DCF signal in WT cells an effect that was largely prevented in KO cells (Figure 9a), indicating actin polymerization contributes to superoxide generation in WT cells, with less contribution in KO cells. Furthermore, to test superoxide generation by NADPH oxidase, RAW 264.7 macrophages were treated with siRNA Glrx and PMA-induced oxidant generation was measured by DCF assay. After PMA stimulation, the DCF signal was approximately 50% lower in macrophages with Glrx siRNA compared to cells with control RNA (p < 0.01, Figure 9b). Lower expression of Glrx with siRNA was confirmed by Western blot (Figure 9c).
Figure 9. DCF assay in PMA stimulated cells.
a. MEF were incubated with 5 μM CMH2DCFDA in HBSS and stimulated with 1 μM PMA with or without tiron (10 mM) or cytochalasin-D (1 μM) as described in the Methods. Tiron-inhibitable fluorescent signal was significantly lower in the KO cells. Cytochalasin-D inhibited oxidants in the WT cells, but did not inhibit significantly in the KO cells. p < 0.05, p < 0.01. b. Macrophage-like RAW cells were treated with siRNA for Glrx-1 or control RNA for 2 days. PMA-induced oxidant generation was assessed by DCF assay. Oxidants generation was significantly inhibited in the cells treated with siRNA. p < 0.01. c. Western blot showed decreased expression of Glrx with siRNA.
Discussion
We showed in this study that Glrx KO mice have a diminished hypertrophic response in the heart and aorta after Ang II infusion. DHE oxidation and nitrotyrosine staining indicated that Ang II–induced oxidant production was lower in Glrx KO compared with WT aorta. Expression of iNOS was also decreased in aorta and heart in Glrx KO vs. WT mice.
Ho et al. reported that mice with targeted disruption of Glrx-1 did not demonstrate increased vulnerability to ischemia/reperfusion or hyperoxia [13]. In contrast, targeted disruption of thioredoxin in the heart was associated with increased oxidative stress and cardiac hypertrophy [42]. This suggests that the primary role of Glrx is not likely to be an indispensable antioxidant but the reduction of protein mixed disulfides (R-SSG). However, Glrx may also catalyze the reverse reaction to form R-SSG through a Glrx-SSG intermediate [9,30]. This makes the role of Glrx in vivo more obscure. In addition, other enzymes such as thioredoxins and protein disulfide isomerase can reduce mixed disulfides of GSH, although with lower efficiency [19]. Nevertheless, the Glrx KO mice demonstrated less hypertrophy and lower levels of oxidants in response to Ang II infusion.
Ang II is known to activate aortic NADPH oxidase [43]. It is well-established that NADPH oxidase-dependent superoxide production is involved in the Ang II-induced hypertrophic response [21,37,38]. Actin polymerization promotes membrane translocation and assembly of NADPH oxidase, and adequate actin fiber assembly is required for superoxide radical generation in neutrophils [23,41], eosinophils [32] and smooth muscle cells [33]. By using anti-GSH antibody and biotinylated GSH ethyl ester labeling, we show that actin is one of several proteins with higher S-glutathiolation levels in Glrx KO vs. WT fibroblasts after exposure to H2O2. Wang et al. showed that reversible S-glutathiolation of actin at Cys374 prevented G-actin from polymerization. EGF-induced deglutathiolation of G-actin led to 6-fold increase in the rate of polymerization [39,40]. Therefore, it is possible that the higher levels of glutathiolated actin in Glrx KO may prevent membrane assembly and activation of NADPH oxidase. To support this, we found a diminished DHE signal in Glrx KO aortas, indicating less superoxide radical production with Ang II infusion associated with enhanced S-glutathiolation of actin. Furthermore, PMA-stimulated oxidant production was significantly decreased in MEF from Glrx KO and in RAW 264.7 macrophages after knockdown of Glrx expression, supporting the speculation that NADPH oxidase activation is impaired in the absence of Glrx. However, in our study in MEF the F/G actin ratio was higher in Glrx KO compared with WT after H2O2 exposure, while actin-SSG was higher in Glrx KO. This is an apparent discrepancy to the previous study of Wang et al., which showed decreased polymerization with actin-SSG. However, they demonstrated that EGF caused actin deglutathiolation leading to polymerizaiton, and in a similar concept we showed that H2O2–dependent S-glutathiolation promoted actin depolymerization in WT cells. Glrx KO cells with higher S-glutathiolation decreased F/G actin ratio to a lesser extent than WT cells, indicating that actin depolymerization induced by oxidants is impaired in Glrx KO cells. We speculate that Glrx-1 maintains the redox turnover of actin (actin-SH/actin-SSG) by promoting reversible S-glutathiolation at Cys374, and the less turnover in Glrx KO cells decreases actin dynamics (polymerization/depolymerization). To support this notion cytochalasin D, an inhibitor of actin polymerization, decreased oxidant generation in WT cells but not in KO cells. Interestingly, a patient with actin mutation at Glu364 (E364K) near Cys374 demonstrated impaired superoxide generation in the neutrophils and recurrent infection [24]. Similarly actin-SSG at Cys374 may alter the binding ability to other proteins such as profilin, and modify the function of actin.
Transcription factors are other potential targets that are affected by Glrx gene deletion. S-glutathiolation of NF-κB subunit p50 at Cys62 inhibited DNA binding [27]. IKK-β activation of NF-κB activation is inhibited by S-glutathiolation of IKK-β at Cys179 and tracheal epithelial cells from Glrx KO mice demonstrated decreased NF-κB DNA binding and inflammation [28].
We found iNOS induction was suppressed in Ang II-stimulated aortic endothelium and hearts of Glrx KO mice. Since iNOS is an NF-κB-dependent gene, the lower iNOS expression levels that we observed may be attributed to inactivation of NF-κB pathway by S-glutathiolation of p50 or IKK-β. Our study in fibroblasts showed, however, that IL-1β induced IκBα phosphorylation and degradation was not significantly altered in Glrx KO cells (data not shown), suggesting that IKK-β activity may not always be affected under these condition. S-glutathiolation of c-Jun inhibits DNA binding to form the activator protein-1 (AP-1) complex [15]. Further studies are required to elucidate whether or not NF-κB, AP-1, or other transcription factors are targets of inactivation by S-glutathiolation in Glrx KO mice. Interestingly, inhibiting NF-κB activation attenuated cardiac hypertrophy [11], and c-Jun and AP-1 activation is involved in Ang II-induced hypertrophic response in cardiomyocytes [34]. Also, recently it was shown that iNOS knockout mice displayed much less cardiac hypertrophy and nitrotyrosine staining induced by aortic constriction [44], and in our unpublished data, Ang II-induced nitrotyrosine and luminol chemiluminescence were much less in iNOS KO mouse aorta, suggesting that significant amounts of peroxynitrite are derived from iNOS under Ang II infusion (Supplemental figures 2 and 3). However, medial hypertrophy was not inhibited in iNOS KO mice (unpublished, Jones and Cohen). Taken together, the Glrx KO mice displayed less iNOS, nitrotyrosine, and media hypertrophy. Both impaired NADPH oxidase activation and transcriptional inhibition of iNOS could be the mechanisms for less oxidant generation and hypertrophy in Glrx KO mice. Further studies in macrophages from Glrx KO vs. WT mice are necessary to elucidate the role of Glrx in macrophages that are important for the hypertrophic response to Ang II infusion [6].
Previously it was shown that Ang II induced smooth muscle cell hypertrophy was mediated by S-gluthathiolation and activation of the small GTPase p21ras. Overexpression of Glrx1 inactivated p21ras and blocked protein synthesis in smooth muscle cells treated with Ang II [1]. Therefore, it is surprising that Glrx KO mice did not show increase vulnerability to Ang II infusion but rather, showed less hypertrophy. The discrepancy might be attributed to a difference between cultured smooth muscle cells and intact mice, acute overexpression of Glrx1 vs. chronic in vivo deficiency, and/or the potential dual catalytic action of Glrx. Our data indicate that deletion of Glrx had protective effects following chronic infusion of Ang II, which may be explained in part through the effects of Glrx on oxidant generation through NADPH oxidase and actin polymerization.
Supplementary Material
Supplemental Data
Supplemental figure 1. iNOS expression in the mouse heart. The mouse heart was fixed with 4% formalin and embedded in paraffin. Cross-sections of the hearts (5 μm) were immunostained with anti-iNOS antibody as described in Materials and Methods. Ang II infusion increased iNOS expression in WT mice and much less increased in Glrx KO mice. Representative photos were shown at the original magnification of 400x.
Supplemental figure 2. Nitrotyrosine staining in iNOS mouse aorta. a. The aortas from Ang II infused WT and iNOS KO mice were processed for immunohistochemistry for nitrotyrosine. Representative photos show a decrease in nitrotyrosine staining in Ang II infused iNOS KO aorta compared with Ang II infused WT aorta. b. Semi-quantitative analysis was conducted by 3 observers who were blinded to the sample identity, using an arbitrary scoring range from 0 (no staining) to 3 (maximum staining). The data indicated Ang II-induced nitrotyrosine in aorta was significantly diminished by iNOS KO.
Supplemental figure 3. Luminol chemiluminescence of the mouse aorta. Isolated mouse aortas were incubated in HEPES-buffered physiological solution (HBSS) for 30 min at 37 °C, then transferred into luminometer tubes (Turner) containing 100 μM luminol in HBSS. Following a 15 min equilibration period, 10 repeated measurements were recorded in a luminometer (TD-20e, Turner), with 60 sec integration intervals and averaged. Following each determination, L-NAME (10 μM) was added to the tube, and 10 more repeated measurements were taken to determine the contribution of nitric oxide synthase to the luminol signal. After the measurement, tissues were dried in glass vials overnight at 200 °C and weighed. Data are reported as arbitrary units of emitted light per mg dry tissue weight per min. The data showed Ang II increased luminol chemiluminescence that was significantly inhibited by L-NAME or by iNOS KO. This suggests that peroxynitrite production was increased in Ang II infused aorta, which was inhibited in iNOS KO animals.
Acknowledgments
This work was supported by National Institute Health grants R01 AG027080 and P01 HL081587, and NIA R03 AG19078-01. We highly appreciate the contribution of Douglas G. Jones, Ph.D. who provided supplemental data of his iNOS KO mice study. Also, we thank to Ellen O. Weinberg, Ph.D. for reviewing our manuscript.
Reference List
- 1.Adachi T, Pimentel DR, Heibeck T, Hou XY, Lee YJ, Jiang BB, Ido Y, Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. Journal of Biological Chemistry. 2004;279:29857–29862. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 2.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nature Medicine. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay S, Starke DW, Mieyal JJ, Gronostajski RM. Thioltransferase (glutaredoxin) reactivates the DNA-binding activity of oxidation-inactivated nuclear factor I. J. Biol. Chem. 1998;273:392–397. doi: 10.1074/jbc.273.1.392. [DOI] [PubMed] [Google Scholar]
- 4.Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 5.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91phox-containing NADPH oxidase in angiotensin II-induced cardiac hypertrphy in mice. Circ. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 6.Bush E, Maeda N, Kuziel WA, Dawson TC, Wilcox JN, DeLeon H, Taylor WR. CC chemokine receptor 2 is required for macrophage infiltration and vascular hypertrophy in angiotensin II-induced hypertension. Hypertension. 2000;36:360–363. doi: 10.1161/01.hyp.36.3.360. [DOI] [PubMed] [Google Scholar]
- 7.Chrestensen CA, Starke DW, Mieyal JJ. Acute cadmium exposure inactivates thioltransferase (glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. Journal of Biological Chemistry. 2000;275:26556–26565. doi: 10.1074/jbc.M004097200. [DOI] [PubMed] [Google Scholar]
- 8.Clavreul N, Adachi T, Pimental DR, Ido Y, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. Faseb Journal. 2006;20:518–+. doi: 10.1096/fj.05-4875fje. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes AP, Holmgren A. Glutaredoxins: Glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxidants & Redox Signaling. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 10.Gladyshev VN, Liu A, Novoselov SV, Krysan K, Sun QA, Kryukov VM, Kryukov GV, Lou MF. Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2. J. Biol. Chem. 2001;276:30374–30380. doi: 10.1074/jbc.M100020200. [DOI] [PubMed] [Google Scholar]
- 11.Ha T, Li Y, Gao X, McMullen JR, Shioi T, Izumo S, Kelley JL, Zhao A, Haddad GE, Williams DL, Browder IW, Kao RL, Li C. Attenuation of cardiac hypertrophy by inhibiting both mTOR and NFkappaB activation in vivo. Free Radic. Biol. Med. 2005;39:1570–1580. doi: 10.1016/j.freeradbiomed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, Sharma RV, Engelhardt JF, Davisson RL. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics. 2006;26:180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 13.Ho YS, Xiong Y, Ho DS, Gao J, Chua BH, Pai H, Mieyal JJ. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic. Biol. Med. 2007;43:1299–1312. doi: 10.1016/j.freeradbiomed.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. European Journal of Biochemistry. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 15.Klatt P, Molina EP, de Lacoba MG, Padilla CA, Martinez-Galisteo E, Barcena JA, Lamas S. Redox regulation of c-Jun DNA binding by reversible S-glutathiolation. Faseb Journal. 1999;13:1481–1490. doi: 10.1096/fasebj.13.12.1481. [DOI] [PubMed] [Google Scholar]
- 16.Lundberg M, Fernandes AP, Kumar S, Holmgren A. Cellular and plasma levels of human glutaredoxin 1 and 2 detected by sensitive ELISA systems. Biochem. Biophys. Res. Commun. 2004;319:801–809. doi: 10.1016/j.bbrc.2004.04.199. [DOI] [PubMed] [Google Scholar]
- 17.Lundberg M, Johansson C, Chandra J, Enoksson M, Jacobsson G, Ljung J, Johansson M, Holmgren A. Cloning and expression of a novel human glutaredoxin (Grx2) with mitochondrial and nuclear isoforms. J. Biol. Chem. 2001;276:26269–26275. doi: 10.1074/jbc.M011605200. [DOI] [PubMed] [Google Scholar]
- 18.Malik G, Nagy N, Ho YS, Maulik N, Das DK. Role of glutaredoxin-1 in cardioprotection: an insight with Glrx1 transgenic and knockout animals. J. Mol. Cell Cardiol. 2008;44:261–269. doi: 10.1016/j.yjmcc.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Mannervik B, Axelsson K, Sundewall AC, Holmgren A. Relative contributions of thioltransferase-and thioredoxin-dependent systems in reduction of low-molecular-mass and protein disulphides. Biochem. J. 1983;213:519–523. doi: 10.1042/bj2130519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev. Biol. 1996;178:179–185. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 21.Matsui R, Xu S, Maitland KA, Hayes A, Leopold JA, Handy DE, Loscalzo J, Cohen RA. Glucose-6 phosphate dehydrogenase deficiency decreases the vascular response to angiotensin II. Circ. 2005;112:257–263. doi: 10.1161/CIRCULATIONAHA.104.499095. [DOI] [PubMed] [Google Scholar]
- 22.Meyer EB, Wells WW. Thioltransferase overexpression increases resistance of MCF-7 cells to adriamycin. Free Radic. Biol. Med. 1999;26:770–776. doi: 10.1016/s0891-5849(98)00247-0. [DOI] [PubMed] [Google Scholar]
- 23.Morimatsu T, Kawagoshi A, Yoshida K, Tamura M. Actin enhances the activation of human neutrophil NADPH oxidase in a cell-free system. Biochemical and Biophysical Research Communications. 1997;230:206–210. doi: 10.1006/bbrc.1996.5881. [DOI] [PubMed] [Google Scholar]
- 24.Nunoi H, Yamazaki T, Tsuchiya H, Kato S, Malech HL, Matsuda I, Kanegasaki S. A heterozygous mutation of β-actin associated with neutrophil dysfunction and recurrent infection. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8693–8698. doi: 10.1073/pnas.96.15.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okuda M, Inoue N, Azumi H, Seno T, Sumi Y, Hirata K, Kawashima S, Hayashi Y, Itoh H, Yodoi J, Yokoyama M. Expression of glutaredoxin in human coronary arteries: its potential role in antioxidant protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001;21:1483–1487. doi: 10.1161/hq0901.095550. [DOI] [PubMed] [Google Scholar]
- 26.Pimentel DR, Adachi T, Ido Y, Heibeck T, Jiang B, Lee Y, Melendez JA, Cohen RA, Colucci WS. Strain-stimulated hypertrophy in cardiac myocytes is mediated by reactive oxygen species-dependent Ras S-glutathiolation. J. Mol. Cell Cardiol. 2006;41:613–622. doi: 10.1016/j.yjmcc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Pineda-Molina E, Klatt P, Vazquez J, Marina A, de Lacoba MG, Perez-Sala D, Lamas S. Glutathionylation of the p50 subunit of NF-kappa B: a mechanism for redox-induced inhibition of DNA binding. Biochemistry. 2001;40:14134–14142. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- 28.Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EFM, Janssen-Heininger YMW. Dynamic redox control of NF-kappa B through glutaredoxin-regulated S-glutathionylation of inhibitory kappa B kinase beta. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynaert NL, Wouters EF, Janssen-Heininger YM. Modulation of glutaredoxin-1 expression in a mouse model of allergic airway disease. Am. J. Respir. Cell Mol. Biol. 2007;36:147–151. doi: 10.1165/rcmb.2006-0259RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: Role in reversible protein S-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxidants & Redox Signaling. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan DM, Wehr NB, Fergusson MM, Levine RL, Finkel T. Identification of oxidant-sensitive proteins: TNF-alpha induces protein glutathiolation. Biochemistry. 2000;39:11121–11128. doi: 10.1021/bi0007674. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki M, Kato M, Hanaka H, Izumi T, Morikawa A. Actin assembly is a crucial factor for superoxide anion generation from adherent human eosinophils. J. Allergy Clin. Immunol. 2003;112:126–133. doi: 10.1067/mai.2003.1515. [DOI] [PubMed] [Google Scholar]
- 33.Touyz RM, Yao G, Schiffrin EL. Role of the actin cytoskeleton in angiotensin II signaling in human vascular smooth muscle cells. Can. J. Physiol Pharmacol. 2005;83:91–97. doi: 10.1139/y05-006. [DOI] [PubMed] [Google Scholar]
- 34.Tu VC, Sun H, Bowden GT, Chen QM. Involvement of oxidants and AP-1 in angiotensin II-activated NFAT3 transcription factor. Am. J. Physiol Cell Physiol. 2007;292:C1248–C1255. doi: 10.1152/ajpcell.00624.2005. [DOI] [PubMed] [Google Scholar]
- 35.Urata Y, Ihara Y, Murata H, Goto S, Koji T, Yodoi J, Inoue S, Kondo T. 17Beta-estradiol protects against oxidative stress-induced cell death through the glutathione/glutaredoxin-dependent redox regulation of Akt in myocardiac H9c2 cells. J. Biol. Chem. 2006;281:13092–13102. doi: 10.1074/jbc.M601984200. [DOI] [PubMed] [Google Scholar]
- 36.Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 37.Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 38.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ. Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, Mieyal JJ, Chock PB. Reversible glutathionylation regulates actin polymerization in A431 cells. J. Biol. Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Tekle E, Oubrahim H, Mieyal JJ, Stadtman ER, Chock PB. Stable and controllable RNA interference: Investigating the physiological function of glutathionylated actin. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5103–5106. doi: 10.1073/pnas.0931345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodman RC, Ruedi JM, Jesaitis AJ, Okamura N, Quinn MT, Smith RM, Curnutte JT, Babior BM. Respiratory burst oxidase and three of four oxidase-related polypeptides are associated with the cytoskeleton of human neutrophils. J. Clin. Invest. 1991;87:1345–1351. doi: 10.1172/JCI115138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J. Clin. Invest. 2003;112:1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 44.Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ. Res. 2007;100:1089–1098. doi: 10.1161/01.RES.0000264081.78659.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data
Supplemental figure 1. iNOS expression in the mouse heart. The mouse heart was fixed with 4% formalin and embedded in paraffin. Cross-sections of the hearts (5 μm) were immunostained with anti-iNOS antibody as described in Materials and Methods. Ang II infusion increased iNOS expression in WT mice and much less increased in Glrx KO mice. Representative photos were shown at the original magnification of 400x.
Supplemental figure 2. Nitrotyrosine staining in iNOS mouse aorta. a. The aortas from Ang II infused WT and iNOS KO mice were processed for immunohistochemistry for nitrotyrosine. Representative photos show a decrease in nitrotyrosine staining in Ang II infused iNOS KO aorta compared with Ang II infused WT aorta. b. Semi-quantitative analysis was conducted by 3 observers who were blinded to the sample identity, using an arbitrary scoring range from 0 (no staining) to 3 (maximum staining). The data indicated Ang II-induced nitrotyrosine in aorta was significantly diminished by iNOS KO.
Supplemental figure 3. Luminol chemiluminescence of the mouse aorta. Isolated mouse aortas were incubated in HEPES-buffered physiological solution (HBSS) for 30 min at 37 °C, then transferred into luminometer tubes (Turner) containing 100 μM luminol in HBSS. Following a 15 min equilibration period, 10 repeated measurements were recorded in a luminometer (TD-20e, Turner), with 60 sec integration intervals and averaged. Following each determination, L-NAME (10 μM) was added to the tube, and 10 more repeated measurements were taken to determine the contribution of nitric oxide synthase to the luminol signal. After the measurement, tissues were dried in glass vials overnight at 200 °C and weighed. Data are reported as arbitrary units of emitted light per mg dry tissue weight per min. The data showed Ang II increased luminol chemiluminescence that was significantly inhibited by L-NAME or by iNOS KO. This suggests that peroxynitrite production was increased in Ang II infused aorta, which was inhibited in iNOS KO animals.