Abstract
Background
The prognostic meaning of an undetectable ultrasensitive prostate-specific antigen (USPSA) level after prostatectomy remains unclear.
Objective
To determine whether an undetectable USPSA level obtained after surgery is a predictor of biochemical recurrence (BCR)–free survival.
Design, setting, and participants
From the Urologic Oncology Database at the University of California San Francisco, 525 men were identified who had a USPSA measurement 1–3 mo postoperatively with at least 2 yr of follow-up. All preoperative and pathologic criteria were recorded.
Measurements
Patients were stratified based on their initial USPSA level. We defined an undetectable USPSA level at ≤0.05 ng/ml. Recurrence was defined as two consecutive prostate-specific antigen (PSA) levels ≥0.2 ng/ml or secondary treatment.
Results and limitations
We found that 456 patients (87%) had undetectable USPSA and 69 patients (13%) had detectable USPSA immediately postprostatectomy. A 5-yr recurrence-free rate of 86% was found in the undetectable USPSA group compared with 67% in the detectable USPSA group (p < 0.01). For patients with pT3 disease, men with an undetectable USPSA had a 5-yr BCR-free survival rate of 78% compared with 40% for men with a detectable USPSA (p < 0.01). A multivariable analysis confirmed that patients with an undetectable USPSA were 67% less likely to recur (hazard ratio: 0.33; 95% confidence interval: 0.20–0.55). As the detection level of PSA is lowered, the false-positive rate of BCR necessarily increases. A limitation of the study is its retrospective nature.
Conclusions
An undetectable USPSA after radical prostatectomy is a prognostic indicator of BCR-free survival at 5 yr and may aid in predicting outcome in higher risk patients.
1. Introduction
Prostate-specific antigen (PSA) was introduced as a screening tool for prostate cancer in the late 1980s, and was recognized shortly thereafter as a surrogate biomarker for residual or recurrent disease [1–4]. Assays that measure PSA to levels <0.1 ng/ml are denoted ultrasensitive PSA (USPSA) tests and are now widely available to clinicians. The utility of USPSA testing postprostatectomy has been the focus of some controversy in the urologic literature. Some authors claim that USPSA nadir values postprostatectomy are helpful in identifying cases of early biochemical relapse [5–7]. Others believe that USPSA offers minimal advantages and often causes an increase in anxiety in patients who are destined to have only clinically meaningless rises of USPSA [8].
Studies centered on the prognostic utility of an undetectable PSA postprostatectomy have not been widely reported. Our study hypothesizes that an undetectable USPSA as the initial test after radical prostatectomy (RP) will be a robust predictor of biochemical recurrence (BCR)–free survival regardless of clinical risk stratification or pathologic findings. As such, it may aid clinicians in determining who may either benefit by or forgo adjuvant therapy.
2. Patients and methods
2.1. Urologic Oncology Database
The Urologic Oncology Database (UODB) is a clinical and research resource in the Department of Urology at the University of California San Francisco (UCSF). The UODB contains diagnostic, surgical, pathologic, and clinical outcomes data on men treated for prostate cancer in the Urology Department at UCSF. Age, PSA history, biopsy findings, imaging and lab test results, surgical procedure and pathology details, BCR, secondary treatment, and mortality data are collected on patients who undergo RP or who are followed on active surveillance. Patients included in the UODB have signed consent forms that are approved and monitored by the Institutional Review Board of UCSF.
2.2. Patient population
There were 2825 patients who consented and were enrolled in the UODB database as of July 2008; of those, 2251 underwent laparoscopic or open RP. Year of surgery was restricted to 1996–2006 (n = 1674) to ensure that ultrasensitive testing was available. Men who received neoadjuvant androgen deprivation therapy (ADT) or radiation treatments (9%) were excluded to ensure consistent treatment across patients. Patients with positive lymph nodes at pathology were also excluded. All patients had ultrasensitive PSA values 1–3 mo after surgery, at least two postsurgery PSA tests to determine BCR, and at least 2 yr of follow-up.
2.3. Definition of undetectable ultrasensitive prostate-specific antigen
The definition of undetectable USPSA for this analysis was ≤0.05 ng/ml. PSA data in the UODB come from multiple laboratories that define undetectable PSA levels at either ≤0.02 ng/ml or ≤0.05 ng/ml. We used the higher cut-off when identifying undetectable PSA to minimize measurement bias.
2.4. Treatment failure
PSA recurrence was defined as two consecutive rises in PSA of ≥0.2 ng/ml at least 8 wk after surgery. This definition has been used widely and has been proposed as a national standard [9]. We also report the patient having a recurrence if the patient underwent secondary therapy (ADT or radiation therapy), regardless of whether the PSA failure definition was met, to capture patients who were deemed primary treatment failures by urologists but who did not meet PSA criteria.
2.5. Statistical analysis
Characteristics of the patients with detectable versus undetectable USPSA were compared using chi-square tests for categorical variables and analysis of variance for continuous variables. TNM staging adhered to the American Joint Committee on Cancer (AJCC) 2002 guidelines. Clinical risk groups were based on a modification of categories defined by D’Amico et al [10]. Patients were considered low risk if they had PSA ≤10 ng/ml, Gleason sum <7 with no primary or secondary Gleason of 4 or 5, and clinical stage T1–T2a. They were considered intermediate risk if they had PSA 10.1–20 ng/ml, Gleason secondary pattern 4 or 5, or clinical stage T2b–2c. High-risk patients were those who had PSA >20 ng/ml, Gleason sum >7 or Gleason primary 4 or 5, or cT3a. Life table product-limit estimates and Kaplan-Meier curves, stratified by USPSA cohort, were used to examine time to BCR >5 yr after RP. In multivariate Cox proportional hazards regression models, we examined whether USPSA cohort predicted BCR, adjusting for pretreatment age, diagnostic PSA, biopsy Gleason total, clinical stage, pathologic Gleason total, pathologic stage, and surgical margins. Significance was set at p < 0.05. All analyses were performed using SAS v.9.1 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Baseline patient characteristics
Of 1674 patients who underwent RP in 1996–2006, 1323 received no neoadjuvant or adjuvant treatments and had pN0. Of those, 525 patients had at least 2 yr of postsurgery follow-up data, including ultrasensitive PSA values measured 1–3 mo after surgery.
The median follow-up was 4.7 yr (range: 2.1–14.5 yr). There were 456 patients (87%) who had undetectable USPSA and 69 patients (13%) who had detectable USPSA immediately postprostatectomy. Most undetectable PSA values (n = 349) were ≤0.02 ng/ml. Table 1 describes the baseline characteristics of both the detectable and the undetectable cohorts.
Table 1.
Baseline characteristics of detectable and undetectable patients
| Detectable | Undetectable | p-value | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Age at diagnosis | <55 | 12 | 17 | 101 | 22 | 0.38 |
| 55–64 | 39 | 57 | 266 | 58 | ||
| ≥65 | 18 | 26 | 89 | 20 | ||
| Race | White | 59 | 87 | 391 | 91 | 0.23 |
| Other | 9 | 13 | 37 | 9 | ||
| Relationship | In relationship | 51 | 75 | 377 | 86 | 0.02 |
| No relationship | 17 | 25 | 61 | 14 | ||
| PSA (ng/ml) at diagnosis | ≤6 | 27 | 39 | 228 | 50 | 0.09 |
| >6 | 42 | 61 | 228 | 50 | ||
| cT stage | T1 | 27 | 39 | 158 | 35 | 0.47 |
| ≥T2 | 42 | 61 | 298 | 65 | ||
| Biopsy Gleason | <7 | 40 | 58 | 265 | 58 | 0.98 |
| ≥7 | 29 | 42 | 191 | 42 | ||
| Percent biopsy cores positive | <33% | 18 | 35 | 177 | 54 | 0.03 |
| 33–66% | 25 | 48 | 107 | 33 | ||
| >66% | 9 | 17 | 45 | 14 | ||
| D’Amico et al clinical risk group | Low | 27 | 39 | 164 | 36 | 0.32 |
| Intermediate | 25 | 36 | 206 | 45 | ||
| High | 17 | 25 | 86 | 19 | ||
| CAPRA clinical risk group | 0–2 | 24 | 49 | 172 | 52 | 0.38 |
| 3–5 | 19 | 39 | 136 | 41 | ||
| 6–10 | 6 | 12 | 22 | 7 | ||
| Gleason at pathology | <7 | 21 | 33 | 158 | 36 | 0.60 |
| ≥7 | 43 | 67 | 279 | 64 | ||
| Positive margins | No | 57 | 83 | 377 | 83 | 0.99 |
| Yes | 12 | 17 | 79 | 17 | ||
PSA = prostate-specific antigen; CAPRA = Cancer of the Prostate Risk Assessment.
In bivariable analysis, a higher proportion of patients with an undetectable USPSA had lower percentages of biopsy cores that were positive than those with detectable USPSA (p < 0.05). Age, race, PSA, clinical T stage, Gleason grade, and risk category were not significantly different between the two cohorts.
3.2. Biochemical recurrence–free rates between cohorts
Overall, the 5-yr failure-free recurrence rate was 86% in the undetectable USPSA group versus 67% among detectable patients (log-rank p < 0. 01, Fig. 1). Median time to recurrence was 3.5 yr (range: 0.2–12.2) in the detectable group and 4.1 yr (range: 0.4–11.4) in the undetectable group.
Fig. 1. Kaplan-Meier curves for detectable ultrasensitive prostate-specific antigen (USPSA) and undetectable USPSA to failure-free survival (log-rank p < 0.01).
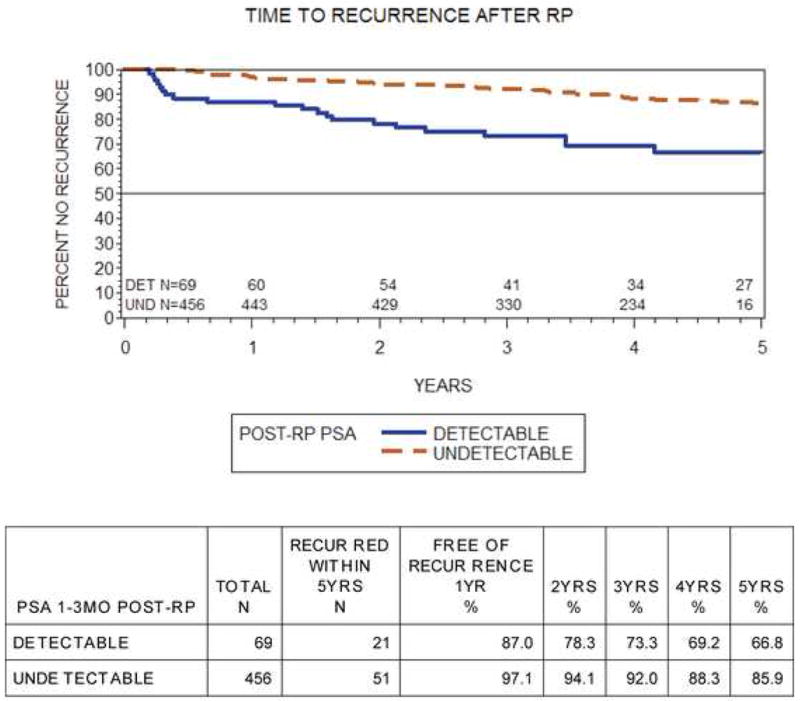
RP = radical prostatectomy; Det = detectable; Und = undetectable; PSA = prostate-specific antigen.
In the undetectable cohort, the 5-yr BCR-free rates stratified by risk category are outlined in Table 2. The 5-yr BCR-free survival rates of low-, intermediate-, and high-risk patients (based on preoperative clinical risk stratification) with undetectable USPSA were 91%, 89%, and 71%, respectively. The 5-yr recurrence-free rates for patients with low, intermediate, or high risk with detectable USPSA were 89%, 52%, and 59%.
Table 2.
Failure-free survival stratified by D’Amico et al [10] clinical risk stratification and ultrasensitive prostate-specific antigen (USPSA) level
| Free of failure*, % | ||||||||
|---|---|---|---|---|---|---|---|---|
| USPSA | Preoperative risk category | Total n | Recurred within 5 yr, n | 1 yr | 2 yr | 3 yr | 4 yr | 5 yr |
| Undetectable | Low | 164 | 10 | 98.2 | 97.6 | 96.8 | 92.6 | 90.9 |
| Intermediate | 206 | 19 | 96.6 | 94.7 | 92.4 | 90.9 | 88.7 | |
| High | 86 | 22 | 96.5 | 86.0 | 82.2 | 74.7 | 70.7 | |
| Detectable | Low | 27 | 3 | 92.6 | 88.9 | 88.9 | 88.9 | 88.9 |
| Intermediate | 25 | 11 | 92.0 | 80.0 | 67.3 | 57.7 | 51.9 | |
| High | 17 | 7 | 70.6 | 58.8 | 58.8 | 58.8 | 58.8 | |
PSA = prostate-specific antigen.
Failure defined as two consecutive PSA values ≥0.2 ng/ml or secondary treatment.
Moreover, 5-yr recurrence-free survival rates were also significantly different between detectable and undetectable postoperative PSA levels for the PSA cut-off points 0.02, 0.1, and 0.2 ng/ml. Treatment failure rates were highest for patients with postoperative PSA levels >0.2 ng/ml and lowest for patients whose postoperative levels were <0.02 ng/ml (Table 3).
Table 3.
Five-year biochemical recurrence–free survival (BFS) rates for detectable versus undetectable prostate-specific antigen (PSA) cut-off levels of 0.2, 0.1, 0.05, and 0.02 ng/ml taken 1–3 mo postprostatectomy
| PSA level* | n at risk(total) | n at risk at 5 yr | BFS at 5 yr, % | CI for BFS at 5 yr, % | Log-rank p-value | |
|---|---|---|---|---|---|---|
| ≥0.02 | Detectable | 169 | 73 | 73 | 64–81 | <0.01 |
| <0.02 | Undetectable | 356 | 113 | 88 | 81–93 | |
|
| ||||||
| ≥0.05 | Detectable | 69 | 27 | 67 | 50–79 | <0.01 |
| <0.05 | Undetectable | 456 | 160 | 86 | 80–90 | |
|
| ||||||
| ≥0.1 | Detectable | 60 | 26 | 68 | 51–80 | <0.01 |
| <0.1 | Undetectable | 465 | 161 | 85 | 79–90 | |
|
| ||||||
| ≥0.2 | Detectable | 17 | 6 | 50 | 21–74 | <0.01 |
| <0.2 | Undetectable | 508 | 175 | 85 | 79–89 | |
CI = 95% confidence interval.
Total number of men (out of 525 total) with detectable and undetectable PSA levels at each PSA cut-off level listed at baseline and at 5 yr.
3.3. Undetectable ultrasensitive prostate-specific antigen and positive surgical margins
We examined treatment failure rate stratified by surgical margins status at pathology in a subanalysis of patients with undetectable USPSA. Overall, 91 of 525 (17%) patients had positive margins at final pathology, with 79 of 456 (17%) in the undetectable group. Margin status was not associated with postoperative detection of USPSA (p = 0.99). The 5-yr BCR-free survival rates of patients with negative and positive margins were 86% and 69%, respectively (log-rank p < 0.01). For patients with positive margins, 5-yr failure-free survival was 72% for patients with an undetectable USPSA and 50% for men with a detectable USPSA (log-rank p = 0.07; Fig. 2).
Fig. 2. Kaplan-Meier curves of failure-free survival in patients with positive surgical margins stratified by ultrasensitive prostate-specific antigen level (log-rank p = 0.07).
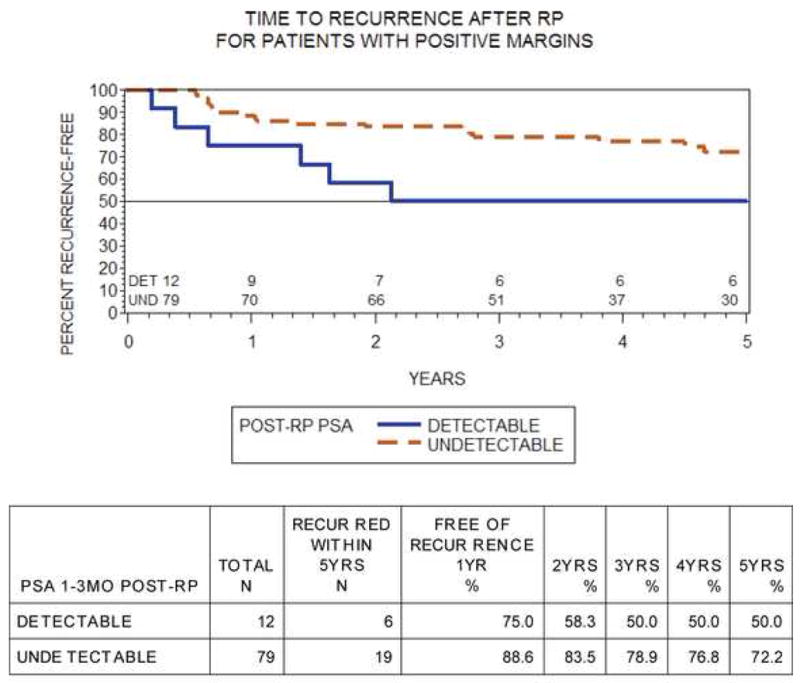
RP = radical prostatectomy; Det = detectable; Und = undetectable; PSA = prostate-specific antigen.
3.4. Ultrasensitive prostate-specific antigen and pathologic T3 disease
We examined BCR rate for men with pathologic T3 disease. In all, 151 of 525 (29%) patients had pT3 disease (pT3a: n = 102; pT3b: n = 49). Of those men, 135 of 151 (89%) had an undetectable USPSA postoperatively. The 5-yr BCR-free survival was 78% for undetectable USPSA and 40% for detectable USPSA in pT3 patients (log-rank p < 0.01; Fig. 3). Median time to biochemical relapse was 1.7 yr (range: 0.2–8.5) in the detectable group and 3.9 yr (range: 0.6–10.3) in the undetectable group.
Fig. 3. Kaplan-Meier curves of failure-free survival in patients with pT3 disease stratified by ultrasensitive prostate-specific antigen level (log-rank p ≤0.01).
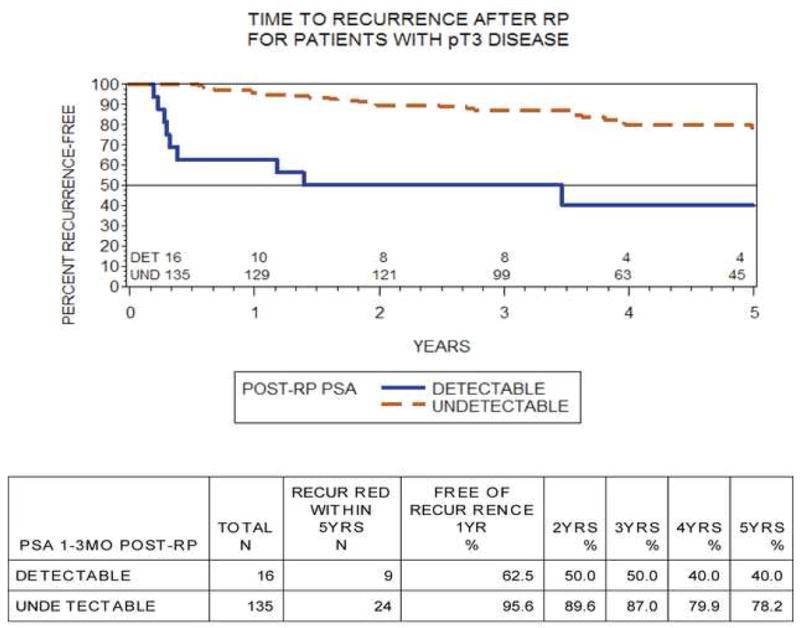
RP = radical prostatectomy; Det = detectable; Und = undetectable; PSA = prostate-specific antigen.
3.5. Multivariate analysis of time to biochemical recurrence
We performed a Cox proportional hazards regression of time to treatment failure with covariates of age at diagnosis, diagnostic PSA, biopsy Gleason score, clinical stage, postoperative USPSA at 6 wk, pathologic Gleason score, pathologic stage, and surgical margin status. Table 3 shows the significant variables established in our model. Patients with undetectable USPSA were 67% less likely to experience a recurrence (hazard ratio: 0.33; 95% confidence interval: 0.20–0.55) than those with detectable USPSA after controlling for other established risk factors. Lower PSA, pathologic Gleason total, tumor stage, and negative surgical margins were also associated with lower risk of treatment failure (Table 4).
Table 4.
Multivariate proportional hazards regression predicting treatment failure*
| p-value | Hazard ratio | 95% confidence interval | |||
|---|---|---|---|---|---|
| Age at diagnosis | Trend | 0.14 | – | – | – |
| 55–64 vs ≥65 | 0.22 | 0.73 | 0.45 | 1.21 | |
| <55 vs ≥65 | 0.05 | 0.49 | 0.24 | 1.01 | |
| PSA at diagnosis | Trend | 0.01 | – | – | – |
| 4.1–6 vs ≥10.1 | 0.001 | 0.38 | 0.21 | 0.68 | |
| 6.1–10 vs ≥10.1 | 0.03 | 0.56 | 0.32 | 0.95 | |
| <4 vs ≥10.1 | 0.20 | 0.57 | 0.24 | 1.35 | |
| Biopsy Gleason total | <7 vs ≥7 | 0.07 | 0.63 | 0.38 | 1.04 |
| T-stage at diagnosis | cT1 vs cT2/3 | 0.91 | 0.97 | 0.61 | 1.56 |
| Surgical margins | Negative vs positive | 0.001 | 0.46 | 0.29 | 0.74 |
| USPSA 4–12 wk post-RP | Undetectable vs detectable | <0.0001 | 0.33 | 0.20 | 0.55 |
| Pathologic Gleason total | <7 vs ≥7 | 0.01 | 0.39 | 0.20 | 0.79 |
| T-stage at pathology | pT2 vs pT3 | 0.001 | 0.48 | 0.30 | 0.75 |
PSA = prostate-specific antigen; USPSA = ultrasensitive prostate-specific antigen; RP = radical prostatectomy.
Failure defined as two consecutive PSA values ≥0.2 ng/ml or secondary treatment.
4. Discussion
USPSA detection following RP has been shown to provide earlier detection of BCR than standard PSA measurements [5]. However, the prognostic ability of an undetectable USPSA has not been thoroughly evaluated. This study found that an undetectable USPSA 1–3 mo postoperatively was associated with a significant treatment failure advantage compared with men who have a detectable postoperative USPSA regardless of surgical margin status or pathologic stage.
The favorable prognosis of an undetectable USPSA has been noted in other surgical series. Doherty et al noted that in their series of 200 men, only 2 men who had undetectable USPSA later had BCR [6], although the follow-up was limited to 2 yr. Similarly, Shen et al also evaluated patients with undetectable USPSA values. At New York University (NYU), men with USPSA levels <0.01 (n = 423) had a recurrence rate of 4% with a mean follow-up time of 3 yr [5]. Shen et al excluded USPSA values from outside laboratories, using only a solitary assay to assess USPSA. The current study uses the USPSA results from several laboratories throughout the country, establishing greater external validity of the current findings. Additionally, our report uses the initial postoperative USPSA rather than nadir, establishing earlier efficacy of the test.
We confirmed the prognostic ability of USPSA in a multivariate model. Not surprisingly, other well-established risk factors, pathologic stage and Gleason score, were also significantly associated with BCR.
An analysis by patient preoperative risk stratification provides evidence of the prognostic utility of an undetectable USPSA. Estimates of biochemical disease-free survival in most large, contemporary series of high-risk patients using the D’Amico et al criteria [10] (PSA >20, Gleason sum 8–10, AJCC stage T2c) are in the range of 40–60% [10,11]. Our high-risk cohort with undetectable USPSA had a 5-yr BCR-free rate of 71%. It should be noted, however, that there is wide variability among the D’Amico et al high-risk group, and this group may be substratified using a multivariable instrument such as the Cancer of the Prostate Risk Assessment (CAPRA) score [12]. While preoperative low-risk patients had similar low failure rates regardless of USPSA level (90.9% undetectable vs 88.9% detectable), intermediate-risk patients showed marked stratification by USPSA level (88.7% undetectable vs 51.9% detectable). This finding suggests that a detectable USPSA 1–3 mo after RP was predictive of BCR for higher risk patients, a fact that was confirmed by our multivariate analysis. It is important to note, however, that our number of men with a detectable USPSA (n = 69) was small, which did limit our analysis somewhat.
The effect of positive surgical margins after RP is controversial. Several groups have shown positive margins to be an independent predictor of BCR [11, 13–15], while others have not found this association [16, 17]. Our model did find a worse outcome with a positive surgical margin. It is interesting to note that the presence of a positive surgical margin did not predict a detectable USPSA. This suggests that USPSA may be able to predict micrometastatic disease at the time of surgery and the need for secondary therapy rather than acting solely as a surrogate marker for the adequacy of surgical extirpation. While immediate adjuvant therapy is a consideration for patients with adverse pathologic features [18–20], an undetectable USPSA, in light of the current data, would not favor such treatment. Additionally, as earlier salvage therapy with its inherent morbidities is discussed in the literature [21–23], the identification of patients who are most at risk of disease recurrence is crucial. With the use of USPSA, pT3 patients may be stratified into different risk categories. Men with an undetectable postoperative USPSA are at significantly decreased risk of biochemical failure compared with men with a detectable USPSA, and the benefit of adjuvant therapy in this group warrants further investigation. While adding prognostic value, a detectable postoperative USPSA does not perfectly predict ultimate BCR. Indeed, at a cut-off level of 0.05 ng/ml, 66.8% of men with detectable USPSA remained free of biochemical disease at 5 yr. This detection rate was similar to that for a PSA cut-off of 0.1 ng/ml. Nevertheless, a detectable USPSA does place the patient at higher risk for recurrence compared to those with undetectable levels. Not surprisingly, the risk of future BCR after a detectable postoperative PSA rises as the PSA cut-off increases (Table 3). Conversely, lowering the PSA cut-off will lessen the risk of recurrence with a detectable PSA level. Stated another way, a lower USPSA threshold increases the risk of detecting a clinically meaningless PSA rise. While the increased information provided by USPSA could increase patient anxiety in some patients with detectable USPSA levels, other men with undetectable USPSA levels but poor pathologic features may feel reassured. Additionally, it is important to note that as the lower limit of the serum PSA test falls, the coefficient of variation (CV) of the test itself will rise. The increased CV implies a decline in the reliability of the test at lower PSA values[em]a fact that should be considered by patients and practitioners.
Our studied end point of BCR itself remains controversial [24,25]. While some studies show few differences in overall mortality between patients with and without BCR [26], other studies show progression to metastatic disease and prostate cancer death [27,28]. Indeed, long-term studies have shown that between 18–34% of patients ultimately progress to metastatic disease, with some patients progressing in as short a time as 1 yr [27,29]. With more younger patients with few comorbidities presenting with prostate cancer than in the past, the risk of prostate cancer mortality may be increased [24,30]. In such cases, earlier information on PSA kinetics may allow for earlier intervention or secondary therapy when there may be an improved chance of altering the disease course [18]. Such suggestions warrant further investigation in prospective studies.
There are limitations to our study beyond the standard problems inherent to retrospective analysis. As a tertiary referral center, many patients are cared for by their local urologist after surgical treatment and are unavailable for follow-up at UCSF. As such, only 39.7% (525 of 1323) of patients who met our surgical inclusion criteria also had adequate 2-yr follow-up for analyses. Separate analyses comparing patients lost to follow-up with those retained for analysis revealed no differences in preoperative or pathologic variables. Thus, we assume this loss to follow-up represents uninformative censoring and should not meaningfully affect our results.
Additionally, the number of patients with a detectable USPSA is relatively small. We used the higher cut-off of ≤0.05 ng/ml when identifying undetectable PSA values reported by various laboratories. This selection bias made distinctions between detectable and undetectable patients less robust, thus favoring the null hypothesis. Despite this limitation, we saw significant differences in patient outcomes based on the initial postprostatectomy USPSA level, and the minimum of 2 yr of postsurgery follow-up data strengthened our findings.
5. Conclusions
An undetectable USPSA after RP is a useful prognostic indicator of BCR-free survival at 5 yr that may aid in postoperative risk stratification and yield an earlier assessment of postoperative PSA kinetics. While an undetectable postoperative USPSA can reassure patients with unfavorable pathology, a detectable USPSA may cause unnecessary anxiety in patients who never suffer formal BCR.
Acknowledgments
Funding/Support and role of the sponsor: Supported by National Institutes of Health/National Cancer Institute, University of California San Francisco SPORE Special Program of Research Excellence P50CA89520
Footnotes
Author contributions: Michael Eisenberg had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Carroll, Eisenberg, Davies
Acquisition of data: Eisenberg, Davies, Carroll
Analysis and interpretation of data: Eisenberg, Davies, Cooperberg, Cowan, Carroll
Drafting of the manuscript: Eisenberg, Davies, Carroll, Cowan
Critical revision of the manuscript for important intellectual content: Eisenberg, Davies, Cooperberg, Carroll, Cowan
Statistical analysis: Cowan, Eisenberg, Cooperberg, Davies
Obtaining funding: none
Administrative, technical, or material support: none
Supervision: Carroll, Other (specify): none
Take-home message
An undetectable ultrasensitive prostate-specific antigen level obtained 1–3 mo after radical prostatectomy is a useful prognostic indicator of biochemical recurrence–free survival that may aid in postoperative risk stratification.
Financial disclosures:
I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Killian CS, Emrich LJ, Vargas FP, et al. Relative reliability of five serially measured markers for prognosis of progression in prostate cancer. J Natl Cancer Inst. 1986;76:179–85. [PubMed] [Google Scholar]
- 2.Schacht MJ, Garnett JE, Grayhack JT. Biochemical markers in prostatic cancer. Urol Clin North Am. 1984;11:253–67. [PubMed] [Google Scholar]
- 3.Zajic G, Graham MD, Schacht J. Gamma-carboxyglutamate in normal and pathological human middle ear bones. Arch Otorhinolaryngol. 1984;241:51–4. doi: 10.1007/BF00457917. [DOI] [PubMed] [Google Scholar]
- 4.Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909–16. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 5.Shen S, Lepor H, Yaffee R, Taneja SS. Ultrasensitive serum prostate specific antigen nadir accurately predicts the risk of early relapse after radical prostatectomy. J Urol. 2005;173:777–80. doi: 10.1097/01.ju.0000153619.33446.60. [DOI] [PubMed] [Google Scholar]
- 6.Doherty AP, Bower M, Smith GL, et al. Undetectable ultrasensitive PSA after radical prostatectomy for prostate cancer predicts relapse-free survival. Br J Cancer. 2000;83:1432–6. doi: 10.1054/bjoc.2000.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haese A, Huland E, Graefen M, et al. Ultrasensitive detection of prostate specific antigen in the followup of 422 patients after radical prostatectomy. J Urol. 1999;161:1206–11. [PubMed] [Google Scholar]
- 8.Taylor JA, III, Koff SG, Dauser DA, McLeod DG. The relationship of ultrasensitive measurements of prostate-specific antigen levels to prostate cancer recurrence after radical prostatectomy. BJU Int. 2006;98:540–3. doi: 10.1111/j.1464-410X.2006.06294.x. [DOI] [PubMed] [Google Scholar]
- 9.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–5. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 10.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 11.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–65. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 12.Cooperberg MR, Cowan J, Broering JM, Carroll PR. High-risk prostate cancer in the United States, 1990–2007. World J Urol. 2008;26:211–8. doi: 10.1007/s00345-008-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grossfeld GD, Chang JJ, Broering JM, et al. Impact of positive surgical margins on prostate cancer recurrence and the use of secondary cancer treatment: data from the CaPSURE database. J Urol. 2000;163:1171–7. quiz 1295. [PubMed] [Google Scholar]
- 14.Palisaar RJ, Noldus J, Graefen M, et al. Influence of nerve-sparing (NS) procedure during radical prostatectomy (RP) on margin status and biochemical failure. Eur Urol. 2005;47:176–84. doi: 10.1016/j.eururo.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Yossepowitch O, Bjartell A, Eastham JA, et al. Positive surgical margins in radical prostatectomy: outlining the problem and its long-term consequences. Eur Urol. 2009;55:87–99. doi: 10.1016/j.eururo.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 16.Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999;281:1395–400. doi: 10.1001/jama.281.15.1395. [DOI] [PubMed] [Google Scholar]
- 17.Vis AN, Schroder FH, van der Kwast TH. The actual value of the surgical margin status as a predictor of disease progression in men with early prostate cancer. Eur Urol. 2006;50:258–65. doi: 10.1016/j.eururo.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 18.Thompson IM, Jr, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329–35. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 19.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572–8. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 20.Ganswindt U, Stenzl A, Bamberg M, Belka C. Adjuvant radiotherapy for patients with locally advanced prostate cancer[em]a new standard? Eur Urol. 2008;54:528–42. doi: 10.1016/j.eururo.2008.06.059. [DOI] [PubMed] [Google Scholar]
- 21.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–9. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moinpour CM, Hayden KA, Unger JM, et al. Health-related quality of life results in pathologic stage C prostate cancer from a Southwest Oncology Group trial comparing radical prostatectomy alone with radical prostatectomy plus radiation therapy. J Clin Oncol. 2008;26:112–20. doi: 10.1200/JCO.2006.10.4505. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui SA, Boorjian SA, Inman B, et al. Timing of androgen deprivation therapy and its impact on survival after radical prostatectomy: a matched cohort study. J Urol. 2008;179:1830–7. doi: 10.1016/j.juro.2008.01.022. discussion 1837. [DOI] [PubMed] [Google Scholar]
- 24.Freedland SJ, Moul JW. Prostate specific antigen recurrence after definitive therapy. J Urol. 2007;177:1985–91. doi: 10.1016/j.juro.2007.01.137. [DOI] [PubMed] [Google Scholar]
- 25.Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol. 2007;51:1175–84. doi: 10.1016/j.eururo.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Jhaveri FM, Zippe CD, Klein EA, Kupelian PA. Biochemical failure does not predict overall survival after radical prostatectomy for localized prostate cancer: 10-year results. Urology. 1999;54:884–90. doi: 10.1016/s0090-4295(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 27.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 28.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 29.Roberts SG, Blute ML, Bergstralh EJ, Slezak JM, Zincke H. PSA doubling time as a predictor of clinical progression after biochemical failure following radical prostatectomy for prostate cancer. Mayo Clin Proc. 2001;76:576–81. doi: 10.4065/76.6.576. [DOI] [PubMed] [Google Scholar]
- 30.Greene KL, Cowan JE, Cooperberg MR, et al. Who is the average patient presenting with prostate cancer? Urology. 2005;66:76–82. doi: 10.1016/j.urology.2005.06.082. [DOI] [PubMed] [Google Scholar]


