Abstract
Background & Aims
Inflammatory gene expression plays a pathological role in acute and chronic hepatic inflammation, yet, inflammation also promotes liver repair by inducing protective mechanisms to limit collateral tissue damage by priming hepatocytes for proliferation. Early growth response (Egr)-1, a transcription factor that regulates inflammatory gene expression, plays a pathological role in many animal models of acute and chronic inflammatory disease. Here we tested the hypothesis that Egr-1 is beneficial after toxic liver injury.
Methods
Acute liver injury was induced in wild-type and egr-1−/− mice by a single injection of carbon tetrachloride (CCl4). Liver injury, inflammatory, and hepatoprotective gene expression and signaling events were measured 18, 48, and 72h after CCl4 administration.
Results
Peak liver injury was greater in egr-1−/− mice compared to wild-type mice. Enhanced injury in egr-1−/− mice was associated with reduced tumor necrosis factor (TNF)α mRNA and protein expression, reduced Akt phosphorylation and nuclear localization of NFκB-p65 in nuclei of cells in the hepatic sinusoid. Expression of inducible nitric oxide synthase and cyclooxygenase-2, TNFα-regulated genes that have hepatoprotective function, was attenuated in egr-1−/− mice compared to wild-type mice. Although plasma interleukin (IL)-6 protein and hepatic accumulation of IL-6, glycoprotein 130, and IL-6 receptor α mRNA in wild-type and egr-1−/− mice were equivalent, signal transducer and activator of transcription 3 phosphorylation was attenuated in egr-1−/− mice and associated with reduced oncostatin M expression.
Conclusions
In contrast to its role in inflammation-mediated tissue injury in other models, Egr-1 expression promotes protection in the liver after CCl4 exposure.
Keywords: Early growth response-1, inflammation, hepatoprotection, carbon tetrachloride
Introduction
In response to injury, the liver is rapidly repaired to reinstate normal hepatic function and ensure survival of the affected organism; a process for which evolution has afforded a profoundly effective regenerative response. The initial response to acute liver injury, mediated by alcohol abuse, drug overdose, hepatitis virus infection or trauma, includes an increase in pro-inflammatory mediators including lipopolysaccharide (LPS), C3a, C5a, tumor necrosis factor (TNF)α, and interleukin (IL)-6. If these pro-inflammatory signals are inappropriately controlled, injury is enhanced and inflammatory disease can result. However, these signals also promote hepatoprotection. For example, inflammation-mediated induction of the cell cycle in hepatocytes by partial hepatectomy promotes protection against subsequent carbon tetrachloride (CCl4)-mediated toxic liver injury [13] while inhibition of mitosis by colchicine exacerbates thioacetamide-induced hepatotoxicity in rats [16]. Therefore, signaling pathways activated early in the time course of liver repair serve to induce hepatocyte proliferation, as well as promote hepatoprotection.
Early growth response (Egr)-1 is an immediate early gene that encodes an 80–82kDa transcription factor induced by various growth factors, LPS, hypoxia, and reactive oxygen species (ROS) in many tissues [8,21]. In addition to its requirement for luteinizing hormone β production and female fertility in mice [14], Egr-1 also contributes to the over-expression of TNFα, and many other proinflammatory genes, in animal models of acute and chronic disease where it contributes to disease pathology [21]. By contrast, Egr-1 also contributes to the expression of inflammatory genes involved in tissue repair [29]. Indeed, TNFα is a cytokine of critical importance to liver repair after partial hepatectomy or CCl4-induced liver injury; deficiency of TNFα or TNFα signaling is detrimental in these model systems [5,28]. Therefore, depending on the context in which Egr-1 is produced and whether or not it promotes appropriate (repair) or exaggerated (acute or chronic disease) expression of inflammatory mediators (i.e. TNFα), its expression and activity could be detrimental or beneficial.
The hepatic response to injury is exquisitely regulated; up and downregulation of inflammatory cytokines and growth factors occurs in a precise and step-wise progression. Misregulation in amplitude or timing has deleterious effects [5,28]. Egr-1 is an important regulator of TNFα However, Egr-1’s expression pattern and its role in CCl4-induced inflammation and inflammation-mediated hepatoprotective events is not known. Because TNFα is important for hepatoprotection and liver repair after CCl4 exposure, we hypothesized that Egr-1 expression was required for this process. We tested this hypothesis after induction of acute liver injury with a single exposure of CCl4 in wild-type and egr-1−/− mice.
Materials and Methods
Materials
CCl4 and olive oil were purchased from Sigma-Aldrich (St. Louis, MO). All primers for real-time reverse transcription PCR (real-time PCR) were synthesized by Integrated DNA Technologies (Coralville, IA, USA). Primary antibodies were purchased from the following companies: Cytochrome P450 2E1 (CYP2E1) Research Diagnostics, Inc. (Flanders, NJ, USA); Egr-1, NFκB-p65, phospho (Ser473)-Akt, and phospho (Tyr705)-STAT3, Cell Signaling Technology (Danvers, MA, USA); tErk Upstate (Temecula, CA, USA).
Animals
Female, wild-type (C57BL/6NTac) or egr-1−/− (B6.129-Egr1tm1Jmi N12) mice (8−12 weeks old) were purchased from Taconic Farms (Germantown, NY) and were originally developed by J. Milbrandt [14]. Animals were housed in standard microisolator cages and fed standard laboratory chow. All animals received humane care and all procedures were approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
CCl4 administration and sample collection
CCl4 was prediluted 1:3 in olive oil before administration. Mice received a single dose at 1 µl/g body weight of CCl4 administered by intraperitoneal injection using 100 µl Hamilton syringes fitted with 26G 5/8 inch needles. Mice were anesthetized and blood was drawn from the posterior vena cava 18, 48, and 72 hours (h) after CCl4. Plasma was separated from whole blood by centrifugation. After euthanasia, livers were removed, portions of which were fixed in 10% neutral buffered formalin, preserved in RNAlater (Ambion, Austin, TX) or snap frozen in liquid nitrogen for further analysis.
Liver histology, plasma alanine aminotransferase (ALT), and aspartate aminotransferase activities
For histological analysis, formalin-fixed tissues were paraffin-embedded, sectioned (5 µm) and stained with hematoxylin and eosin. Slides were coded prior to examination and viewed by two separate individuals. Images were captured using an Olympus microscope and digital camera. Plasma samples were assayed for ALT and AST activity using Diagnostic Chemicals Ltd. enzymatic assay kits (Oxford, CT) according to the manufacturer’s instructions.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining
Apoptotic hepatic DNA fragmentation was detected by TUNEL using the ApopTag Plus fluorescence in situ apoptosis detection kit (Millipore, Temecula, CA) and fluorescence quantified as described earlier by Pritchard et al. [22].
Cytokine analysis by real-time PCR and enzyme-linked immunosorbent assay (ELISA)
Total RNA was isolated from liver and reverse transcribed as previously described [17]. Real-time PCR amplification was performed using gene-specific primers (Table 1 in Supplemental Data) as previously described [17]. The relative amount mRNA was determined using the comparative threshold (Ct) method by normalizing target cDNA Ct values to that of 18S. Fold induction ratios were calculated relative to olive oil treated controls (basal conditions) for each genotype using the formula: 2−ΔΔCt. Statistics were performed on ΔCt values. ELISAs for plasma TNFα and IL-6 were performed as described in [22].
Liver homogenate preparation, electrophoresis, and immunoblotting
Liver homogenates were prepared and immunoblotting performed, as previously described [22].
Statistical analysis
Values reported are means +/− standard error of the mean (SEM). Due to the limited number of age-matched egr-1−/− mice, data were collected from several different experiments. The data were analyzed by general linear models procedure (SAS, Carey, NC) followed by least square means analysis of differences between groups, blocking for experiment effects when data from more than one experiment was used in any given data set. Data were log transformed to obtain a normal distribution, if necessary. In all cases, values in graphs with different alphabetical superscripts are significantly different from one another (p <0.05).
Results
Egr-1 deficiency exacerbates liver injury induced by acute CCl4 exposure
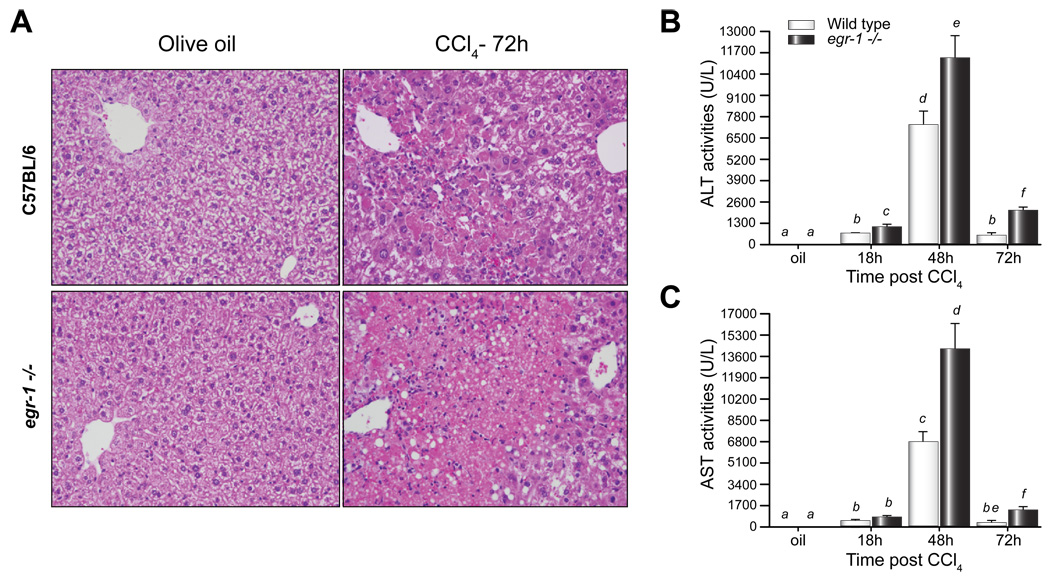
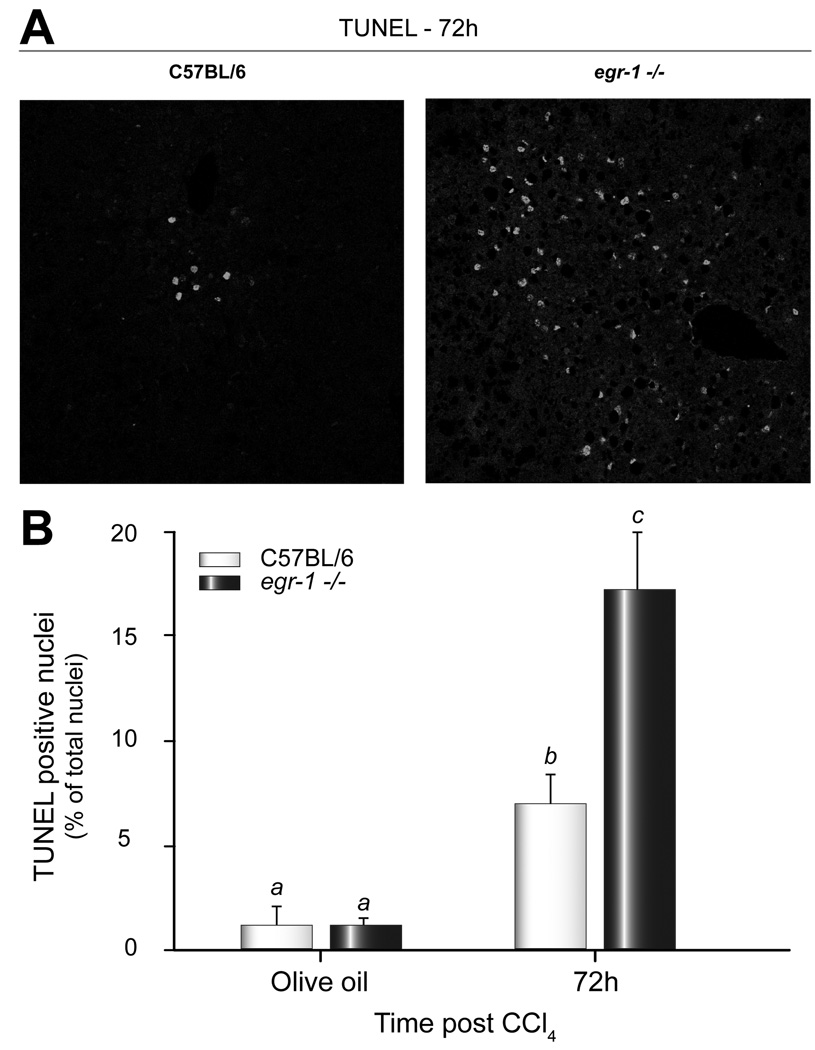
Egr-1 deficiency attenuates disease pathology in many model systems [21]. However, here we report that liver injury after a single injection of CCl4 was enhanced, not reduced, in egr-1−/− mice compared to wild-type mice. Pericentral necrosis was present in livers from both wild-type and egr-1−/− mice, but was greater in egr-1−/− mice 72h after CCl4 administration (Fig. 1A). Peak plasma ALT and AST activities, biochemical markers of liver injury, 48h after CCl4 were greater in plasma from egr-1−/− mice compared to wild-type (Fig. 1B and C). ALT and AST activities in egr-1−/− mice were also greater than wild-type 72h after CCl4 administration, but were reduced relative to peak liver injury (Fig. 1B and C). Seventy-two hours after CCl4, there was an increase in TUNEL-positive cells; this was two-fold greater in livers from egr-1−/− mice compared to wild-type mice (Fig. 2). Differences in liver injury between wild-type and egr-1−/− mice were not due to altered expression of cytochrome P450 2E1 (CYP2E1), the enzyme responsible for the bioactivation of CCl4 [33] (Supplemental Data Fig. 1).
Fig. 1. Absence of Egr-1 exacerbated CCl4-induced liver injury.
(A) Liver histology 72h after CCl4 administration, Images are representative of n = 4 – 8 mice per group. Hematoxylin and eosin, 100X magnification. Plasma isolated from whole blood was used to determine (B) ALT and (C) AST activities. Bars are means +/− SEM, n = 4–8 mice per group.
Fig. 2. Hepatocellular apoptosis was enhanced in Egr-1 deficient mice.
(A) Representative 400X images of hepatic TUNEL staining from wild-type and egr-1−/− mice 72h after CCl4. DAPI staining was used to label total hepatic nuclei (not shown). (B) TUNEL quantification. The data were calculated as percent TUNEL positive cells of total DAPI positive hepatic nuclei. Bars are means +/− SEM, n = 3–4 mice per group.
Hepatic Egr-1, TNFα, and IL-6 expression after acute CCl4 administration
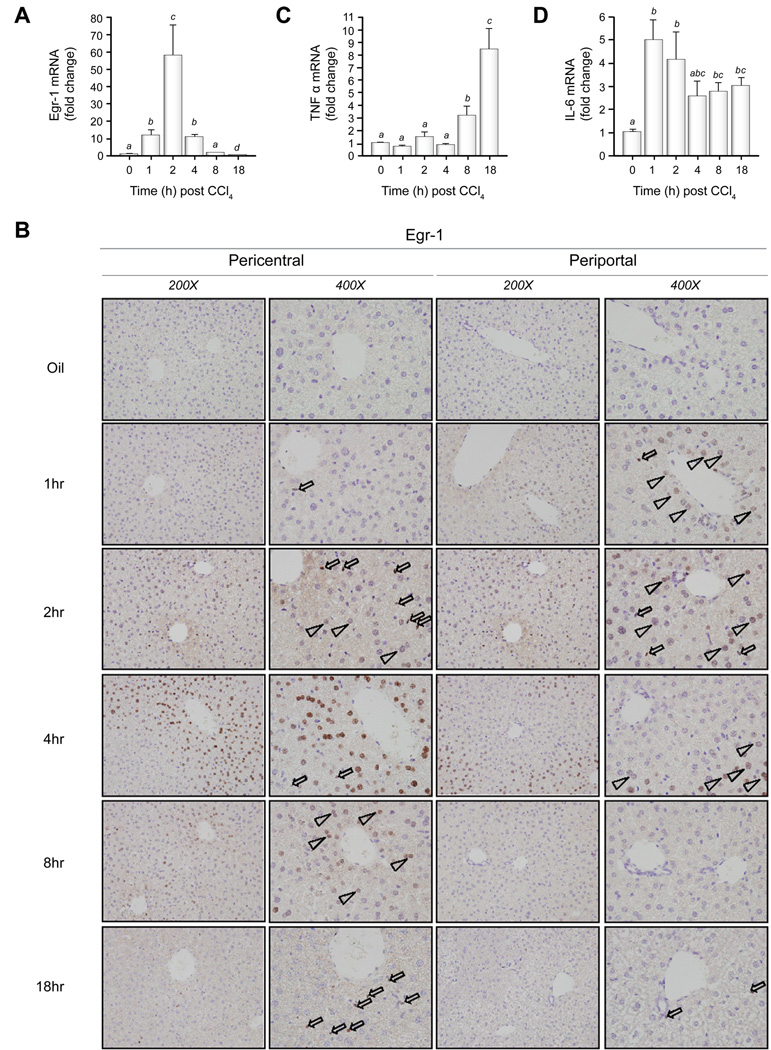
TNFα and IL-6 are required for normal hepatoprotection and liver repair after CCl4 exposure [5,28]. Egr-1 transcriptionally regulates the expression of TNFα in monocytic cells [34]. After a single exposure to CCl4, hepatic Egr-1 mRNA expression in mice was induced in wild-type mice as early as 1h, peaked at 2h and then decreased below baseline at 18h (Fig. 3A). After CCl4 admnistration, Egr-1 protein was expressed in hepatocytes and non-parenchymal cells in the hepatic sinusoid (NPC-HS), presumably Kupffer cells, the resident macrophage in the liver. In NPC-HS, nuclear Egr-1 protein was detected at 1h, peaked at 2h, was reduced 4h after CCl4 exposure, nearly absent at 8h but increased again at 18h after CCl4 (Fig. 3B). Egr-1 expression was detected in hepatocyte nuclei at 1h, peaked at 4h, declined at 8h and was absent at 18h after CCl4 exposure (Fig. 3B). Interestingly, Egr-1 protein was first found in the nuclei of hepatocytes immediately surrounding the portal vein (1h), but by 4h, staining was restricted only to hepatocyte nuclei in the pericentral area (Fig. 3B).
Fig. 3. Egr-1, TNFα, and IL-6 expression after CCl4 exposure in wild-type mice.
Real-time PCR was utilized to determine mRNA accumulation of (A) Egr-1 (C) TNFα and (D) IL-6. Bars represent means +/− SEM of n = 4 – 6 mice per experimental condition. (B) Immunohistochemistry was used to localize Egr-1 protein in liver sections. Open arrowheads indicate Egr-1-positive hepatocyte nuclei, open arrows indicate Egr-1-positive nuclei in NPC-HS. Images are representative of n = 4 mice for each experimental condition
In wild-type mice, CCl4-induced expression of TNFα mRNA was first detected 8h after CCl4 administration (Fig. 3C). TNFα was further increased to 6-fold over olive oil controls 18h after exposure (Fig. 3C). Hepatic IL-6 mRNA accumulation was detected prior to hepatic TNFα mRNA expression, 1h after CCl4 exposure in wild-type mice (Fig. 3D). IL-6 mRNA expression remained elevated over at least 18h in wild-type mice (Fig. 3D).
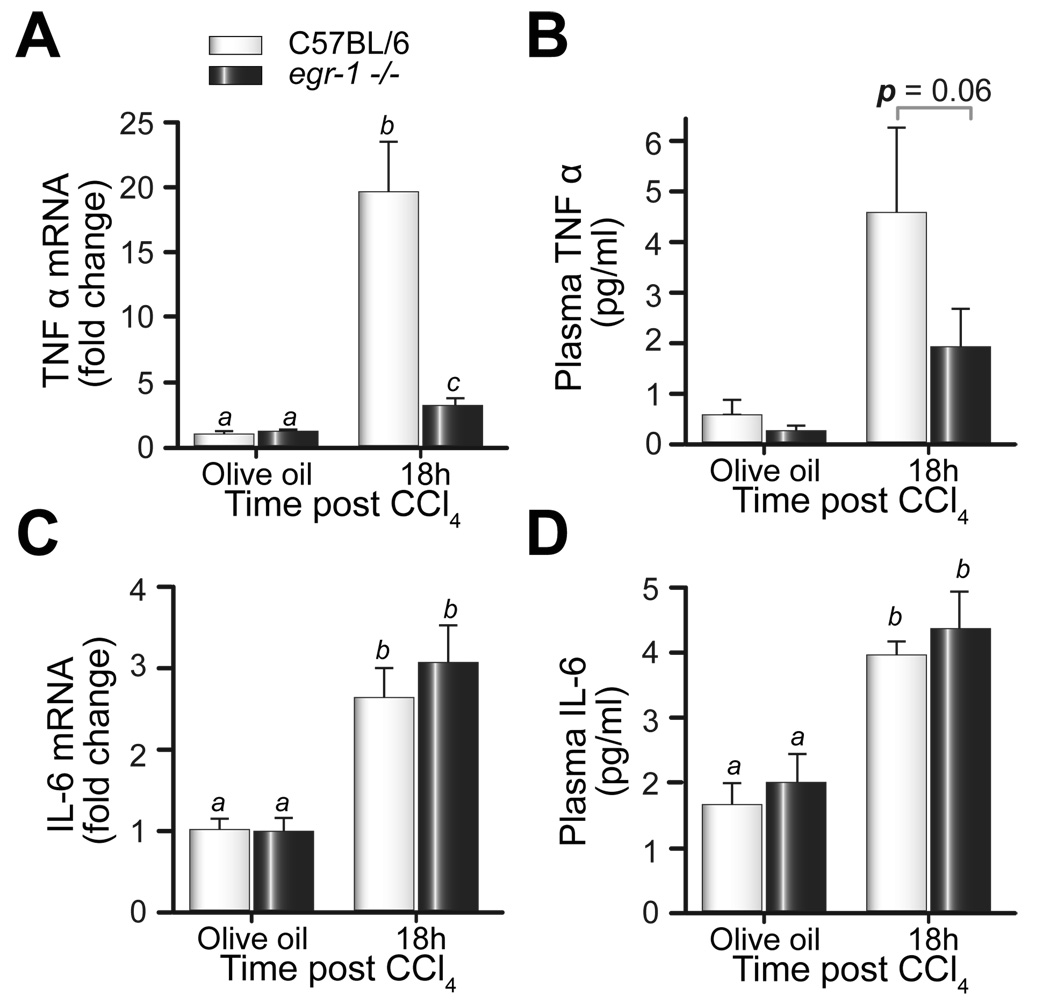
If Egr-1 is required for TNFα expression after CCl4, then TNFα expression should be reduced in egr-1−/− mice. Eighteen hours after CCl4, hepatic TNFα mRNA and protein was increased in wild-type mice and egr-1−/− mice, but this increase was less in egr-1−/− mice (Fig. 4A). In contrast, hepatic IL-6 mRNA and plasma IL-6 protein were not different between mouse strains (Fig. 4C and D). These data support the hypothesis that Egr-1 is an important regulator of TNFα, but not IL-6, expression after acute CCl4 exposure in mice.
Fig. 4. TNFα and IL-6 expression after CCl4 exposure.
Eighteen hours after CCl4 exposure, hepatic mRNA accumulation of (A) TNFα and (C) IL-6, and plasma (B) TNFα and (D) IL-6 protein were determined by real-time PCR and ELISA, respectively. Bars represent means +/− SEM, n = 5–8 mice per group.
Akt and STAT3 phosphorylation and nuclear NFκB-p65 localization were reduced in egr-1−/− mice after CCl4 exposure
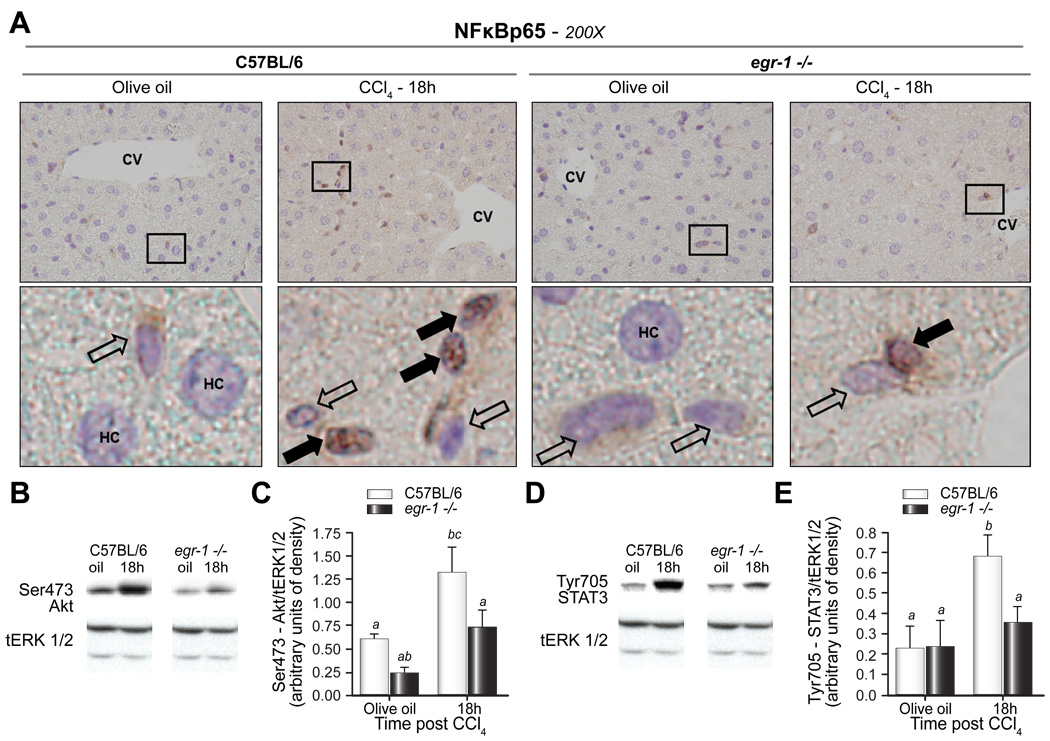
Signaling events mediated by TNFα, IL-6, and their receptor complexes are among the critical pathways required for hepatocyte priming and hepatoprotection after CCl4-induced acute injury [28]. Deficiencies in TNFα and tumor necrosis factor (TNFR)1 are associated with reduced nuclear factor κB (NFκB) activity and Akt phosphorylation while deficiencies in IL-6 and gp130 are associated with reduced signal transducer and activator of transcription (STAT)3 and Akt phosphorylation after surgical or toxin-induced liver injury [5,28]. If Egr-1 promotes hepatoprotection and cell survival during the priming phase of liver repair after CCl4-induced liver injury through a TNFα-mediated pathway, then markers of NFκB activation and phosphorylation of Akt should be attenuated in livers of egr-1−/− mice. Conversely, STAT3 phosphorylation, largely associated with IL-6 signaling [5,28], should be equivalent in wild-type and egr-1−/− mice due to their equivalent expression of IL-6. NFκB-p65 (p65) translocated to the nuclei of NPC-HS 18h after CCl4; fewer nuclei were positive in egr-1−/− mice (Fig. 5A); p65 was not found in hepatocyte nuclei of either mouse strain at this time point (Fig. 5A). Increased Akt phosphorylation was detected 18h after CCl4 in wild-type mice compared to olive oil treated controls (Fig. 5C and D). In egr-1−/− mice, phosphorylation of Akt was blunted relative to wild-type in both olive oil controls and 18h after CCl4 exposure and was not significantly increased in response to CCl4 (Fig. 5C and D). Despite equivalent IL-6 expression between the genotypes, STAT3 phosphorylation was increased only in livers from wild-type mice 18h after CCl4 administration; STAT3 phosphorylation in egr-1−/− mice remained at baseline (Fig. 5E and F). These data suggested that p65 nuclear translocation in NPC-HS as well as phosphorylation of Akt and STAT3 are required to attenuate liver injury in mice after acute CCl4 administration.
Fig. 5. Nuclear localization of NFκB-p65 in NPC-HS, and phosphorylation of Akt and STAT3 were reduced in egr-1−/− mice after CCl4 exposure.
(A) Immunohistochemistry was utilized to localize p65 in liver sections from wild-type and egr-1−/− mice. Closed arrows indicate p65-negative nuclei, while open arrows indicate p65-positive nuclei. CV = central vein; HC = hepatocyte. Outlined areas in (A) are shown enlarged below each 200X image. Images are representative of n = 4 – 8 per experimental group. Immunoblots of total hepatic protein were performed to determine expression of (B,C) phospho (Ser473)-Akt, (D,E) phospho (Tyr705)-STAT3 in livers from wild-type and egr-1−/− mice. Total (t)-Erk1/2 was used as a loading control. Representative (B) Ser473-Akt and (D) Tyr705-STAT3 immunoblots are shown. Quantification of band intensity measured by densitometry after normalization to tErk1/2 are shown in the bar graphs for (C) Ser-473 Akt and (E) Tyr705-STAT3 from n = 4–5 mice per group. Bars are means +/− SEM determined after scanning densitometry of immunoreactive bands.
iNOS and COX-2 expression were attenuated in egr-1−/− mice after CCl4 administration
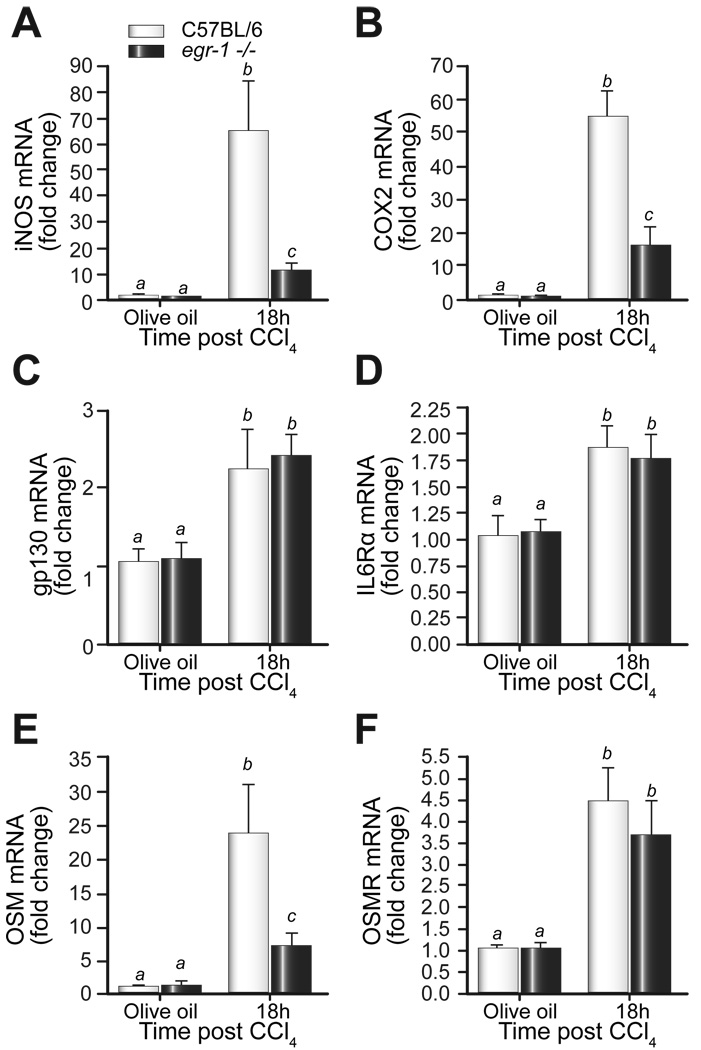
TNFα contributes to the expression of the inducible form of nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 [26,30]. In addition, both iNOS and COX-2 are induced after CCl4-administration and have well-documented, but divergent, hepatoprotective functions after toxic liver injury or partial hepatectomy [1,4,24,30]. If iNOS and COX-2 expression is dependent on TNFα, then expression of these genes should be attenuated in egr-1−/− mice. Hepatic accumulation of iNOS mRNA was induced in both strains of mice 18h after CCl4 administration, but was lower in egr-1−/− mice (Fig. 6A). Similarly, COX-2 mRNA was induced in livers from wild-type and egr-1−/− mice, but this induction was attenuated in livers from egr-1−/− mice (Fig. 6B).
Fig. 6. CCl4-induced expression of hepatoprotective molecules.
Eighteen hours after CCl4 exposure, hepatic (A) iNOS, (B) COX-2 (C) gp130 (D) IL-6Rα (E) OSM and (F) OSMR mRNA accumulation was determined using real-time PCR. Bars represent means +/− SEM, n = 5–8 mice per group.
Reduced expression of IL-6 receptor components or other IL-6 family members that, similar to IL-6, promote STAT3 activation could be responsible for reduced STAT3 phosphorylation in egr-1−/− mice, despite normal IL-6 expression. While glycoprotein (gp)130, the receptor chain shared by all IL-6 family members [11], and IL-6Rα (gp80), the IL-6-specific receptor component [11], were increased over baseline 18h after CCl4 exposure, they did not differ between wild-type and egr-1−/− mice (Fig. 6C, D). Recently, a hepatoprotective role for the IL-6 family member, oncostatin m (OSM), was described [20]. Hepatic mRNA accumulation of OSM was robustly induced in wild-type mice; however, its induction was attenuated in livers from egr-1−/− mice (Fig. 6E). The expression of the specific OSM receptor (OSMR) was induced but not different between the genotypes 18h after CCl4 exposure (Fig. 6F). Collectively, these data suggested that reduced expression of the hepatoprotective molecules iNOS, COX-2, and OSM may contribute to increased liver injury after CCl4 exposure in egr-1−/− mice.
Discussion
This is the first study to identify a critical role for Egr-1 in hepatoprotection early in the response to CCl4-induced liver injury in mice. In addition, we have identified differential expression patterns of Egr-1 in hepatocytes vs NPC-HS in response to CCl4. Reduced expression of TNFα, iNOS, COX-2, and OSM, but not IL-6, was observed in Egr-1-deficient mice. Furthermore, in the absence of Egr-1, phosphorylation of Akt and STAT3, and NFκB-p65 nuclear localization in NPC-HS were reduced. The end result of this maladaptive response to CCl4 in livers from egr-1−/− mice was enhanced peak liver injury at 48h after CCl4 compared to wild-type mice (Fig. 1). The more severe hepatic injury phenotype observed in egr-1−/− mice, concomitant with reduced expression of hepatoprotective molecules and their downstream signals, provides evidence that Egr-1 is an important regulator of hepatoprotective pathways after CCl4-induced acute liver injury.
We discovered a specific spatial and temporal pattern of Egr-1 expression in hepatocytes in response to CCl4 exposure. Nuclear Egr-1 protein occurred in a transient wave across the liver lobule. It was first observed in periportal (zone 1) hepatocytes, then zone 2 hepatocytes, then pericentral hepatocytes (zone 3); Egr-1 expression disappeared thereafter (Fig. 3). It is likely that priming events, mediated in part by a CCl4-induced increase in LPS in the portal circulation after CCl4 exposure, played a role in Egr-1 expression and nuclear localization in periportal hepatocytes. These signaling events may then provide initial Egr-1-mediated hepatoprotective signals after CCl4 exposure. However, CYP2E1-mediated CCl4 bioactivation and ROS generation, which occurs in zone 3, likely contribute to robust Egr-1 protein expression and nuclear localization in this area of the liver lobule.
Egr-1 in NPC-HS nuclei, most likely Kupffer cells, was detectable over the entire time course, suggesting that the major changes in Egr-1 mRNA accumulation in total liver reflected changes in Egr-1 expression in hepatocytes (Fig. 3). Early priming events orchestrated by gut-derived products in the portal area may provide the Kupffer cells with critical activation signals to induce synthesis of TNFα. Egr-1 directly contributes to LPS-induced TNFα expression in macrophages [34]. Here, we found that Egr-1 also contributed to TNFα expression in response to CCl4. In wild-type mice, sufficient TNFα likely induces hepatoprotective mechanisms in periportal (zone 1) hepatocytes and the more distant, zone 2 hepatocytes, limiting CCl4-induced liver injury. However, in egr-1−/− mice, TNFα is reduced and these hepatoprotective mechanisms are attenuated and instead associated with expansion of liver injury (Fig. 1 and 2).
NFκB DNA binding activity is induced after partial hepatectomy and after CCl4 exposure. Early NFκB-DNA binding activity is demonstrable by EMSA using nuclear extracts prepared from hepatocyte-enriched fractions of rat liver 30 min after partial hepatectomy [6]; this is not observed at later time points. More recent literature demonstrates that it is translocation of p65 to the nuclei of NPC-HS which is required for hepatocyte regeneration after partial hepatectomy [3]. We found that p65 nuclear localization in NPC-HS at 18h was reduced in egr-1−/− mice relative to wild-type mice; we were unable to detect p65 in hepatocyte nuclei at any time point tested [Fig. 5 and data not shown (1, 2, 4, 8, 36, 48, 72h)]. We propose that reduced TNFα protein in egr-1−/− mice 18h after CCl4 exposure is responsible for reduced p65 nuclear localization in NPC-HS, as TNFα signaling promotes p65 nuclear localization [28].
Despite the well-described roles for TNFα and signaling via TNFR1 for hepatocyte proliferation [28], the mechanisms for TNFα-mediated hepatoprotection are not well understood. One potential downstream target of TNFα is iNOS [25]. Indeed, hepatic expression of iNOS is critical for liver repair in various liver injury models [1,4,24,35]. iNOS produces nitric oxide (NO) from L-arginine; NO is associated with a shift from hepatocyte quiescence to proliferation during the priming phase of liver regeneration [4,24,35]. Induction of proliferative competence in hepatocytes provides a protective function which attenuates persistent liver injury [18]. iNOS/NO can also reduce TNFα expression [19] and protect hepatocytes from TNFα-mediated apoptotic cell death after CCl4 exposure in mice [24]. Collectively, these reports provide evidence for a critical hepatoprotective and proliferative role for iNOS/NO and suggest mechanisms by which reduced iNOS observed in this study could contribute to enhanced liver injury in egr-1−/− mice.
Another target downstream of TNFα expression is COX-2. COX-2 is the inducible COX isoform with known contributions to inflammatory processes [26]. Like iNOS, COX-2 is induced by proinflammatory cytokines including TNFα and IFNγ with roles for NFκB and C/EBPβ in this process [12,32]. Despite the potency of COX-2-mediated inflammation that spurred the wide-spread use of COX-2 inhibitors, the beneficial effects of COX-2 function cannot be overlooked. Indeed, COX-2 is implicated in hepatoprotection in lipopolysaccharide/D-galactosamine, CCl4, acetaminophen, and Concanavalin-A-induced liver injury models and after partial hepatectomy. COX-2 is required to mitigate the proinflammatory production of arachidonic acid by soluble phospholipase (sPL) A2 released from dead and dying hepatocytes after CCl4-induced toxic liver injury [2]. The hepatoprotective effect of COX-2 is due to its synthesis of prostaglandins from arachidonic acid [1,23,35]. Prostaglandins reduce TNFα expression, improve resistance to apoptotic and necrotic cell death and promote cell cycle progression [1,2,12].
Despite the eventual expression of TNFα in egr-1−/− mice, iNOS and COX2 were never induced to levels comparable to wild-type mice (Supplementary Data Fig. 3A and B). Therefore, delayed, but robust, TNFα expression in egr-1−/− mice likely contributed to increased tissue injury due to the continued absence of NO and prostaglandins to reduce apoptotic cell death as described above. Increased TUNEL-positive hepatic nuclei in egr-1−/− mice 72h after CCl4 support this hypothesis (Fig. 2).
IL-6-mediated signaling is implicated in hepatoprotection in many systems [7,27,31]. Reduced STAT3 phosphorylation was associated only with reduced OSM, an IL-6 family member [9,20]; CCl4-induced IL-6, gp130,IL-6Rα, and OSMR were not different between wild-type and egr-1−/− mice. Indeed, OSM exhibits hepatoprotective function and mediates this function, in part, through STAT3 signaling. For example, OSMR−/− mice exhibit impaired regeneration and persistent necrosis after both CCl4 and partial hepatectomy and is associated with reduced STAT3 activation [20]. In addition, OSM gene therapy in rats exposed to dimethylnitrosamine, a potent hepatotoxin, reduces hepatic necrosis, apoptosis, and liver enzyme release and enhances hepatocyte proliferation [10]. Although GC-rich sequences typical of an Egr-1-dependent promoter element exist in the OSM promoter [15], a direct role for Egr-1 in the regulation of OSM is not known. Our data linking reduced OSM expression with reduced STAT3 phosphorylation and hepatoprotection and also with increased necrosis and apoptosis in egr-1−/− mice after CCl4 are consistent with these studies but do not preclude potential for other STAT3-activating cytokines such as EGF or amiphregulin in this process [5].
Evolution has afforded the liver several apparently redundant pathways for hepatoprotection and repair after injury. This redundancy is exemplified by studies in which mice deficient in single cytokines, cytokine receptors, or signaling molecules are exposed to hepatotoxins or subjected to partial hepatectomy but recover, albeit with a delay in liver repair processes [28]. Here, we identify CCl4-induced Egr-1 expression as a very early event in response to liver injury, before TNFα expression but concomitant with IL-6 expression, which places it at a critical point to affect the regulation of several genes and/or pathways involved in hepatoprotection during the liver repair process after CCl4.
Supplementary Material
Acknowledgements
The authors would like to thank Megan R. McMullen, Emmanuelle Ogier, and Samjhana Thapaliya for technical expertise and additional support during the course of these studies.
Financial support: This work was supported by NIH grants AA015833 and AA017918 to M.T.P. and AA0138868 to L.E.N.
Abbreviations
- LPS
lipopolysaccharide
- TNFα
tumor necrosis factor α
- IL-6
interleukin 6
- CCl4
carbon tetrachloride
- Egr-1
early growth response 1
- CYP2E1
cytochrome P450 2E1
- NFκB-p65
nuclear factor κB p65 subunit
- ROS
reactive oxygen species
- h
hours
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- TUNEL
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
- Ct
threshold cycle
- ELISA
enzyme linked immunosorbent assay
- SEM
standard error of the mean
- STAT3
signal transducer and activator of transcription 3
- iNOS
inducible form of nitric oxide synthase
- COX-2
cyclooxygenase 2
- gp130
glycoprotein 130 kDa
- OSM
oncostatin M
- OSMR
oncostatin M receptor
- TNFR1
tumor necrosis factor receptor 1
- NO
nitric oxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have no financial disclosures.
References
- 1.Bhave VS, Donthamsetty S, Latendresse JR, Mehendale HM. Inhibition of cyclooxygenase-2 aggravates secretory phospholipase A2-mediated progression of acute liver injury. Toxicol Appl Pharmacol. 2008 Apr 15;228(2):239–246. doi: 10.1016/j.taap.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Bhave VS, Donthamsetty S, Latendresse JR, Muskhelishvili L, Mehendale HM. Secretory phospholipase A2 mediates progression of acute liver injury in the absence of sufficient cyclooxygenase-2. Toxicol Appl Pharmacol. 2008 Apr 15;228(2):225–238. doi: 10.1016/j.taap.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Chaisson ML, Brooling JT, Ladiges W, Tsai S, Fausto N. Hepatocyte-specific inhibition of NF-kappaB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J Clin Invest. 2002 Jul;110(2):193–202. doi: 10.1172/JCI15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diez-Fernandez C, Sanz N, Bosca L, Hortelano S, Cascales M. Involvement of nitric oxide synthesis in hepatic perturbations induced in rats by a necrogenic dose of thioacetamide. Br J Pharmacol. 1997 Jun;121(4):820–826. doi: 10.1038/sj.bjp.0701191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006 Feb;43(2) Suppl 1:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 6.FitzGerald MJ, Webber EM, Donovan JR, Fausto N. Rapid DNA binding by nuclear factor kappa B in hepatocytes at the start of liver regeneration. Cell Growth Differ. 1995 Apr;6(4):417–427. [PubMed] [Google Scholar]
- 7.Gao B. Therapeutic potential of interleukin-6 in preventing obesity-and alcohol-associated fatty liver transplant failure. Alcohol (Fayetteville, NY. 2004 Aug;34(1):59–65. doi: 10.1016/j.alcohol.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Gashler A, Sukhatme VP. Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol. 1995;50:191–224. doi: 10.1016/s0079-6603(08)60815-6. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Lechon MJ, Oncostatin M. signal transduction and biological activity. Life Sci. 1999;65(20):2019–2030. doi: 10.1016/s0024-3205(99)00296-9. [DOI] [PubMed] [Google Scholar]
- 10.Hamada T, Sato A, Hirano T, Yamamoto T, Son G, Onodera M, et al. Oncostatin M gene therapy attenuates liver damage induced by dimethylnitrosamine in rats. Am J Pathol. 2007 Sep;171(3):872–881. doi: 10.2353/ajpath.2007.060972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003 Aug 15;374(Pt 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isoda K, Koide H, Kojima M, Arita E, Ikkaku M, Higashiyama S, et al. Stimulation of hepatocyte survival and suppression of CCl4-induced liver injury by the adenovirally introduced C/EBPbeta gene. Biochem Biophys Res Commun. 2005 Apr 1;329(1):182–187. doi: 10.1016/j.bbrc.2005.01.113. [DOI] [PubMed] [Google Scholar]
- 13.Kodavanti PR, Joshi UM, Young RA, Meydrech EF, Mehendale HM. Protection of hepatotoxic and lethal effects of CCl4 by partial hepatectomy. Toxicol Pathol. 1989;17(3):494–505. doi: 10.1177/019262338901700304. [DOI] [PubMed] [Google Scholar]
- 14.Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, et al. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1) Science. 1996 Aug 30;273(5279):1219–1221. doi: 10.1126/science.273.5279.1219. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Streiff RJ, Liu J, Spence MJ, Vestal RE. Cloning and characterization of human oncostatin M promoter. Nucleic acids research. 1999 Dec 1;27(23):4649–4657. doi: 10.1093/nar/27.23.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangipudy RS, Rao PS, Mehendale HM. Effect of an antimitotic agent colchicine on thioacetamide hepatotoxicity. Environ Health Perspect. 1996 Jul;104(7):744–749. doi: 10.1289/ehp.96104744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMullen MR, Pritchard MT, Wang Q, Millward CA, Croniger CM, Nagy LE. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury in mice. Gastroenterology. 2005 Jun;128(7):2066–2076. doi: 10.1053/j.gastro.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehendale HM. Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol Pathol. 2005;33(1):41–51. doi: 10.1080/01926230590881808. [DOI] [PubMed] [Google Scholar]
- 19.Morio LA, Chiu H, Sprowles KA, Zhou P, Heck DE, Gordon MK, et al. Distinct roles of tumor necrosis factor-alpha and nitric oxide in acute liver injury induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2001 Apr 1;172(1):44–51. doi: 10.1006/taap.2000.9133. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura K, Nonaka H, Saito H, Tanaka M, Miyajima A. Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology. 2004 Mar;39(3):635–644. doi: 10.1002/hep.20086. [DOI] [PubMed] [Google Scholar]
- 21.Pritchard MT, Nagy LE. Ethanol-induced liver injury: potential roles for egr-1. Alcohol Clin Exp Res. 2005 Nov;29(11) Suppl:146S–150S. doi: 10.1097/01.alc.0000189286.81943.51. [DOI] [PubMed] [Google Scholar]
- 22.Pritchard MT, Roychowdhury S, McMullen MR, Guo L, Arteel GE, Nagy LE. Early growth response-1 contributes to galactosamine/lipopolysaccharide-induced acute liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2007 Dec;293(6):G1124–G1133. doi: 10.1152/ajpgi.00325.2007. [DOI] [PubMed] [Google Scholar]
- 23.Quiroga J, Prieto J. Liver cytoprotection by prostaglandins. Pharmacol Ther. 1993;58(1):67–91. doi: 10.1016/0163-7258(93)90067-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rai RM, Lee FY, Rosen A, Yang SQ, Lin HZ, Koteish A, et al. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc Natl Acad Sci U S A. 1998 Nov 10;95(23):13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salkowski CA, Detore G, McNally R, van Rooijen N, Vogel SN. Regulation of inducible nitric oxide synthase messenger RNA expression and nitric oxide production by lipopolysaccharide in vivo: the roles of macrophages, endogenous IFN-gamma, and TNF receptor-1-mediated signaling. J Immunol. 1997 Jan 15;158(2):905–912. [PubMed] [Google Scholar]
- 26.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 27.Streetz KL, Tacke F, Leifeld L, Wustefeld T, Graw A, Klein C, et al. Interleukin 6/gp130-dependent pathways are protective during chronic liver diseases. Hepatology. 2003 Jul;38(1):218–229. doi: 10.1053/jhep.2003.50268. [DOI] [PubMed] [Google Scholar]
- 28.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004 Oct;5(10):836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 29.Taub R, Greenbaum LE, Peng Y. Transcriptional regulatory signals define cytokine-dependent and -independent pathways in liver regeneration. Semin Liver Dis. 1999;19(2):117–127. doi: 10.1055/s-2007-1007104. [DOI] [PubMed] [Google Scholar]
- 30.Taylor BS, Alarcon LH, Billiar TR. Inducible nitric oxide synthase in the liver: regulation and function. Biochemistry (Mosc) 1998 Jul;63(7):766–781. [PubMed] [Google Scholar]
- 31.Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003 Aug;18(8):891–902. doi: 10.1046/j.1440-1746.2003.03056.x. [DOI] [PubMed] [Google Scholar]
- 32.Vila-del Sol V, Fresno M. Involvement of TNF and NF-kappa B in the transcriptional control of cyclooxygenase-2 expression by IFN-gamma in macrophages. J Immunol. 2005 Mar 1;174(5):2825–2833. doi: 10.4049/jimmunol.174.5.2825. [DOI] [PubMed] [Google Scholar]
- 33.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33(2):105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 34.Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J Biol Chem. 1997 Jul 11;272(28):17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 35.Zeini M, Hortelano S, Traves PG, Martin-Sanz P, Bosca L. Simultaneous abrogation of NOS-2 and COX-2 activities is lethal in partially hepatectomised mice. J Hepatol. 2004 Jun;40(6):926–933. doi: 10.1016/j.jhep.2004.02.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.