Abstract
This study examined the use of polyvinylphosphonic acid (PVPA), as a potential matrix metalloproteinase (MMP) inhibitor and how brief cross-linking of demineralized dentin matrix that did not affected its mechanical properties enhanced the anti-MMP activity of PVPA. The anti-MMP potential of five PVPA concentrations (100–3,000 μg/mL) was initially screened using a rhMMP-9 colorimetic assay. Demineralized dentin beams were treated with the same five concentrations of PVPA to collagen and then aged for 30 days in a calcium- and zinc-containing medium. The changes in modulus of elasticity, loss of dry mass and dissolution of collagen peptides via three-point bending, precision weighing and hydroxyproline assay, respectively were measured. All tested PVPA concentrations were highly effective (p<0.05) in inhibiting MMP-9. Aging in the incubation medium did not significantly alter the modulus of elasticity of the five PVPA-treatment groups. Conversely, aged dentin beams from the control group exhibited a significant decline in their modulus of elasticity (p<0.05) over time. Mass loss from dentin beams and the corresponding increase in hydroxyproline of from the medium in the five PVPA treatment groups were significantly lower than the control (p<0.05). PVPA is a potent inhibitor of endogenous MMP activities in demineralized dentin. It may be used as an alternative to chlorhexidine for preventing collagen degradation within hybrid layers to extend the longevity of resin-dentin bonds.
Keywords: acid-etch, dentin, inhibitor, hydroxyproline, matrix metalloproteinase, modulus of elasticity, polyvinylphosphonic acid
1. Introduction
The National Institute of Dental and Craniofacial Research 2009–2013 Strategic Plan on Biotechnology/Regenerative Medicine/Biomaterials (RFA-DE-10-004) indicated that tooth-colored resin restorations have an average replacement time of 5.7 years due to secondary caries precipitated by failure of resin-dentin bonds [1]. Composite-dentin bonds are challenged by the harsh environment of the oral cavity. Human in vivo data indicate that dentin bonding is not as durable [2–4] as when the dentin hybridization concept was first proposed in the 1980s [5]. Replacement dentistry costs about 5 billion dollars annually in the US alone. Additional tooth structure must also be sacrificed during replacement of deteriorated fillings. Thus, there is a compelling need to pursue methods to extend the longevity of resin-based restorations.
Dentin bonding with the use of current bonding technologies requires demineralization of 0.5–8 μm of the intertubular dentin matrix for infiltration of adhesive resin monomers to achieve micromechanical retention of resin composites. The acid-etching step in the application of etch-and-rinse adhesives and the use of self-etch adhesives expose and activate endogenous dentin matrix metalloproteinases (MMPs) [6–8]. These enzymes are zinc and calcium-dependent hydrolases that add water across specific peptide linkages in collagen peptides [9] and result in the progressive loss of collagen fibrils from the hybrid layers [2–4,10,11]. Chlorhexidine prevents proteolytic degradation by commercial and cell-bound bacterial proteases [12]. More recent studies have shown that the use of chlorhexidine as an inhibitor of MMP-2, -8 and -9 [13] could prevent the degradation of hybrid layers [2–4,14,15]. As chlorhexidine binds electrostatically to different substrates [16,17], it may eventually desorb from a denuded collagen matrix. Ongoing research is currently conducted on identifying quaternary ammonium methacrylate resin monomers with anti-MMP properties and other anti-MMP agents that can be chemically cross-linked to dentin collagen as a means of creating hybrid layers with sustained anti-MMP potential.
Similar to chlorhexidine, PVPA also binds electrostatically to dentin collagen, but may be trapped in collagen matrices by chemically cross-linking the collagen via the use of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) within 1–5 min to minimize its desorption from dentin collagen by ionic competition [18].
It is possible that some of the denuded collagen fibrils at the base of the hybrid layer may be degraded by exposed acid-activated, dentin matrix-bound MMP-2, -8 and -9 [19–20] over time. Since preliminary data (Pashley et al., unpublished results) indicated that PVPA possesses anti-collagenolytic activity against bacterial collagenase, we hypothesized that PVPA may inhibit both soluble MMPs and the endogenous MMP activity in demineralized dentin. The objective of the present paper was to examine the potential of PVPA as an inhibitor of endogenous MMP activity in demineralized dentin. The null hypotheses tested were that PVPA does not inhibit soluble MMPs, and PVPA has no effect on the endogenous MMP activity of demineralized dentin matrices.
2. Materials and methods
2.1 Human MMP-9-based anti-MMP screening
This assay employed purified human recombinant MMP-9 (Cat#72009) and the Sensolyte Generic MMP colorimetric assay kit (Cat#72095) from AnaSpec, Inc. (San Jose, CA, USA) for screening anti-MMP activity of compounds of interest. Although MMP-9 has been traditionally classified as a gelatinase, a recent study indicated that similar to MMP-2, MMP-9 is able to cleave native soluble, monomeric forms of type I collagen at both 37°C and 25°C along the three-quarter/one-quarter locus of the collagen molecule [21]. The assay involves incubating a constant concentration of rhMMP-9 with a proprietary chromogenic substrate. The latter is a thiopeptolide that is cleaved by the MMPs and collagenases to release a sulfhydryl group. The sulfhydryl group reacts with 5,5’-dithiobis(2-nitrobenzoic acid) to produce the colored reaction product 2-nitro-5-thiobenzoic acid which can be detected at 412 nm.
Polyvinylphosphonic acid (Sigma-Aldrich) was used without further purification and dissolved in deionized water to achieve the designated concentrations (100, 200, 500, 1,000 and 3,000 μg/mL).
The thiopeptolide substrate solution was diluted to 0.2 mM with the supplied assay buffer in a 1:50 volume ratio. The 92-kDa rhMMP-9 was activated with 10 μg/mL of trypsin at 37ºC for 2 h immediately before the experiment to generate its 68-kDa active form. The trypsin was then inactivated with trypsin inhibitor. The assay was performed in a 96-well plate using five replicate wells for each experimental and control variable. Each well in the experimental groups (i.e. the 5 PVPA concentrations) contained 2 μL of rhMMP-9 (19.6 ng/well), 10 μL of the potential MMP inhibitor and 50 μL of the thiopeptolide substrate solution (additional buffer added to achieve a total of 100 μL for each well). As this resulted in a 10-fold dilution of the potential anti-MMP agent, the five PVPA solutions were prepared to 10 times their designated concentrations.
The control groups consisted of: 1) a positive control containing rhMMP-9 only without the potential anti-MMP agent, 2) an inhibitor control containing rhMMP-9 and 10 μL of 20 μM GM6001, a known MMP inhibitor supplied in the assay kit, 3) a test compound control containing assay buffer and the potential anti-MMP agent, and 4) a substrate control containing assay buffer. Additional assay buffer was added to bring the total volume of all control wells to 50μL prior to adding 50 μL of the thiopeptolide substrate solution to obtain the 100 μL volume.
The reagents were mixed completely by shaking the plate gently for 30 sec and then read kinetically every 10 min for 60 min and absorbance was measured at 412 nm using a 96-well plate reader (Synergy HT, BioTek Instruments, Inc., Winooski, VT, USA).
Background absorbance was determined using the mean absorbance readings from the “substrate control” wells and subtracted from the readings of other wells containing the thiopeptolide substrate. The potencies of MMP-9 inhibition by the proprietary MMP inhibitor GM6001 (“inhibitor control”), the five PVPA concentrations and 0.3 M EDC were expressed as percentages of the adjusted absorbance of the “positive control”, which was taken to be 100% inhibition. The data were statistically analyzed to examine the effect of PVPA concentrations and the EDC cross-linking agent on MMP-9 inhibition. As the normality and homoscedasticity assumptions of the data appeared to be valid, % inhibition in the six groups was analyzed using one-way ANOVA on ranks and Tukey multiple comparison tests at α = 0.05.
2.2 Preparation of demineralized dentin beams
Thirty extracted human third molars were obtained with patient (18–22 yr old) informed consent under a protocol approved by the Human Assurance Committee of the Medical College of Georgia. Ninety percent of the teeth had completely formed roots. None of the teeth were carious. Half were impacted and half were erupted. The teeth were stored at 4ºC in 0.9% NaCl supplemented with 0.02% sodium azide to prevent bacterial growth and used within three months after extraction. For each tooth, the enamel and superficial dentin were removed by horizontal sectioning at 1 mm below the deepest central fissure using a diamond-coated copper disk (Isomet saw, Buehler Ltd., Lake Bluff, IL, USA) under water cooling. A 1 mm thick dentin disk consisting of mid-coronal dentin was prepared from each tooth. Two 5 x 2 x 1 mm dentin beams were sectioned from each of the middle of disk under water cooling to obtain sixty beams.
Prior to demineralization, a dimple was made at the end of one of the two 5 x 2 mm surfaces to allow for repeated measurements to be performed on the same surface. The beams were submerged in 10% phosphoric acid for 18 h at 25 °C to completely demineralize the dentin. Absence of residual minerals was confirmed using digital radiography. The initial modulus of elasticity of each demineralized dentin beam was determined using three-point flexure (see below). The beams were distributed to six groups (five experimental groups and one control; N = 10) so that the mean initial elastic modulus of each group was similar in all groups.
2.3 PVPA trapping by cross-linking and incubation of dentin beams
Five solutions were freshly prepared by dissolving 200, 400, 1,000, 2,000 and 6,000 μg/mL of PVPA in deionized water. Prior to cross-linking, a 0.6 M solution of EDC, a cross-linking agent, was added in a 1:1 volume ratio to the respective PVPA solution to yield a final PVPA concentration of 100, 200, 500, 1,000 and 3,000 μg/mL of PVPA and 0.3 M EDC in the respective solution. The demineralized dentin beams from the five experimental groups were immersed in the respective EDC-containing solutions for 5 min at ambient temperature. Each beam was removed, rinsed with deionized water for 30 sec and placed in individually labeled propylene tubes with threaded caps containing 1 mL of an incubation medium. The latter consisted of 5 mM HEPES, 2.5 mM CaCl2, 0.05 mM ZnCl2, 0.3 mM NaN3 adjusted to pH 7.4 with 1 N HCl. The control group consisted of demineralized dentin beams that were immersed in 0.3 M EDC only for 5 min; those beams were rinsed with deionized water for 30 sec and aged similarly in the incubation medium. The tubes were placed in a shaking water bath and agitated at 37°C and 60 cycles/min for 30 days.
2.4 Indirect evaluation of endogenous MMP activity in demineralized dentin
2.4.1. Loss of modulus of elasticity
Each demineralized dentin beam was placed on a miniature three-point flexure device with a 2.5 mm separation between supports. Using a 1N load cell (Transducer Techniques, Temecula, CA, USA) mounted on a Vitrodyne universal testing machine (Model V1000, John Chatillon & Sons, Greensboro, NC, USA). The beams were loaded to a 10% strain at a displacement rate of 0.5 mm/min while immersed in deionized water. After maximum displacement, the load was returned immediately to 0% stress within 15 s without further holding to prevent creep of the demineralized collagen matrix [22–23]. The rationale for using a 10% strain was based on a pilot study showing that such a strain value did not create permanent plastic deformation of the demineralized dentin beam (i.e. the load-displacement curve remained linear throughout the flexing procedure) [24]. After initial testing, each beam was returned to its designated polypropylene tube containing the original incubation medium. After 30 days of incubation, the beams were rinsed free of salts for 10 min with running deionized water, and retested with three-point flexure by loading them to 10% strain.
Stress-strain curves were prepared from the load-displacement data. The modulus of elasticity (E; in MPa) of each specimen was calculated as the slope of the linear stress-strain curve using the formula: E = mL3/4bh3, where “m” is the slope of the linear load-displacement curve (N/mm), “L” is the span length (2.5 mm), “b” is the width of the test specimen (2.0 ± 0.1 mm) and “h” is the beam thickness (1.0 ± 0.05 mm). As this equation is based on beams whose length is many times longer than their thickness, the absolute values of our apparent moduli of elasticity are only approximate. However, they can detect differences in the stiffness among control versus experimental groups.
The data were analyzed using a two-factor repeated measures analysis of variance to examine the effects of PVPA concentrations (i.e. control - no PVPA, 100, 200, 500, 1,000 and 3,000 μg/mL PVPA) and the repeated factor storage time (i.e. baseline vs 30 days) and the interaction of these two factors on the modulus of elasticity. As the normality and homoscedasticity assumptions of the data appeared to be violated, the data were expressed as least square means and the common standard error of the least square means prior to the analysis. Least square means are the expected values of group or subgroup means for a balanced design involving the group variables with all covariates at their common mean values. Pair-wise multiple comparisons were performed using the Tukey test. Statistical significance was set at α = 0.05.
2.4.2. Loss of dry mass over time
After the baseline modulus of elasticity measurements, the beams were transferred to individually labeled polypropylene tubes and placed in a sealed plastic box containing anhydrous calcium sulfate (Drierite, W.A. Hammond Drierite Company, Xenio, OH, USA). With the vial cap off, each beam was desiccated to a constant weight within 8 h. The initial dry mass was measured to the nearest 0.001 mg using an analytical balance (XP6 Microbalance, Mettler Toledo, Hightstown, NJ, USA). After dry mass measurement, each dried and shrunken dentin beam was placed in its corresponding polypropylene tube containing the original aqueous incubation medium. This completely rehydrated the dried beam, reversibly recovered its original dimensional volume [25–26] and restored the beam from a shrunken state to its original unstrained relaxed state. After 30 days of incubation, determination of the dry mass of each beam was repeated under the same conditions following evaluation of its modulus of elasticity. As the normality and homoscedasticity assumptions of the data were violated, the loss of dry mass over time in the six groups was evaluated using Kruskal-Wallis one-way ANOVA on ranks and Dunn’s multiple comparison tests at α = 0.05.
2.4.3. Analysis of incubation media for dissolved collagen peptides
When dentin matrix-bound MMPs were incubated in calcium- and zinc-containing incubation medium [9], the activated forms of the enzymes slowly digested the demineralized collagen matrix, with the solubilized peptide fragments accumulating in the aging medium over the 30-day period [27]. At the end of incubation period, 400 μL of the medium was collected from each vial and transferred to an individually labeled ampule. The medium was diluted with an equal volume of 12 N HCl to give a final concentration of 6 N HCl. Ampules were sealed using an ampule sealer (Ampulmatic, Biosciences Inc, PA, USA). The sealed ampules were hydrolyzed at 120 °C in an oil bath for 18 h. After hydrolysis, the ampules were opened and placed in a large glass desiccator containing anhydrous calcium sulfate and trays of sodium hydroxide pellets to trap the HCl vapor that was released as the hydrolyzate evaporated to dryness in a mild vacuum. After drying, the hydroxyproline content of the hydrolyzate was analyzed using a spectrophotometer (Model UV-A180, Shimadzu, Tokyo, Japan) at 558 nm [28]. The amount of hydroxyproline (μg/mL) was determined from a pre-established calibration curve derived from a linear regression equation of the absorbance of hydroxyproline against known concentrations of hydroxyproline in those standards. The resulting amount of hydroxyproline was used to estimate the percent of solubilized collagen fragments in the aged medium. This procedure was based on the assumption that 90% of the dry mass of the demineralized dentin beams consisted of type I collagen [29] and that the dentin collagen contains 9.6 mass% of hydroxyproline [30]. For each specimen, the dissolved collagen from the demineralized dentin beam was expressed as μg of hydroxyproline/mg of the dry mass of the baseline demineralized dentin. As the normality and homoscedasticity assumptions of the data were violated, the amounts of dissolved collagen from the demineralized dentin beams in the six groups were evaluated using Kruskal-Wallis one-way ANOVA on ranks and Dunn’s multiple comparison tests at α = 0.05.
3. Results
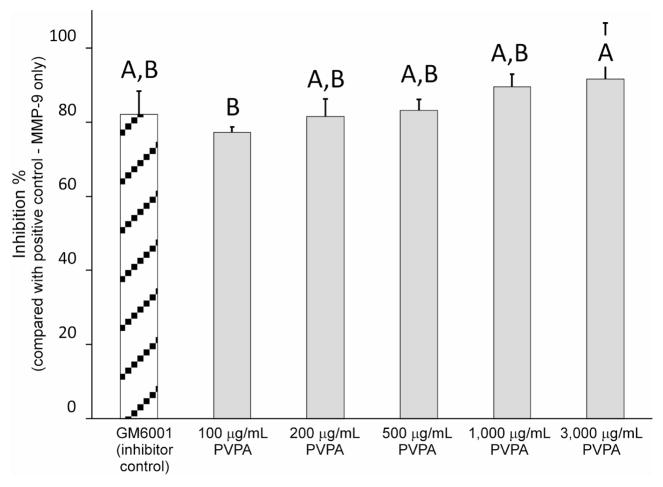
Results of the MMP-9-based anti-MMP screening assay are represented in Figure 1. The relative percentage of rhMMP-9 inhibition by the proprietary GM6001 inhibitor control was 82.1±6.3%. The relative percentages of rhMMP-9 inhibition by PVPA were 77.2±1.4%, 81.5±4.7%, 83.1±3.1%, 89.5±3.4%, and 91.6±15.1% for 100, 200, 500, 1,000 and 3,000 μg/mL PVPA, respectively. The difference among the six groups were statistically significant (p = 0.041). Inhibition of MMP-9 by 100 μg/mL PVPA was significantly lower than 3,000 μg/mL PVPA (p < 0.05) but was not significantly different from the inhibitor control and the other three PVPA groups. There was no statistically significant difference in the anti-MMP potential of 200, 500, 1,000 and 3,000 μg/mL PVPA (p > 0.05).
Fig. 1.
A bar chart comparing the percentage inhibition of rhMMP-9 by the control (GM 6001) and the potential MMP inhibitors. Values are means and standard deviations. Groups with the same letters on top of the bars are not statistically different (p > 0.05).
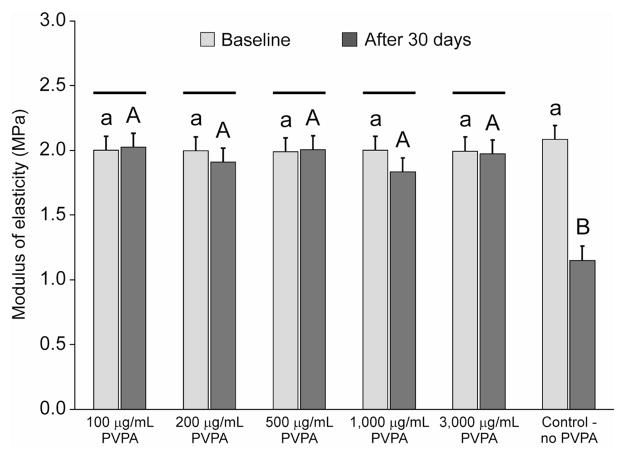
The initial elastic moduli (E) and the changes in elastic moduli of the demineralized dentin beams after 30 days of incubation are shown in Figure 2. The initial modulus of elasticity of the demineralized dentin beams were low and were in the range of 2.01 ± 0.54 MPa (data from the six groups pooled together). Two-way repeated measures ANOVA revealed that the effect of the factor “PVPA concentrations” had no significant effect on the modulus of elasticity of those beams (p = 0.390). That is, cross-linking PVPA to demineralize dentin with EDC for 5 min did not result in a further increase in its modulus of elasticity. Conversely, the repeated factor “storage time” had a highly significant effect on the modulus of elasticity (p = 0.003). Moreover, the effect of different levels of “PVPA concentrations” was dependent on the level of “storage time”, as there was a highly significant interaction between those two factors (p < 0.001). Multiple comparison tests indicated that there was no difference in the modulus of elasticity of the pre-incubated beams (p > 0.05). After incubation in the respective medium, beams from the control group exhibited decline in their modulus of elasticity that was significantly different from all the five PVPA treatment groups (p < 0.05). There were no differences among the different PVPA-containing groups at the 30-day period (p > 0.05). When the initial and 30-day moduli of elasticity were compared for each of the six experimental and control groups, a significant decline in the modulus of elasticity was only observed in the control group (p < 0.05).
Fig. 2.
Baseline and 30-day modulus of elasticity of completely demineralized dentin beams cross-linked with different PVPA concentrations (100 - 3,000 μg/mL) and incubated in a calcium- and zinc-containing aging medium for 30 days. Specimens without PVPA cross-linking immersed in same aging medium were used as the control. Data are presented as least square mean with a common standard error of the least square mean (0.109 MPa). For the baseline period, groups with the same lower case letter are not statistically significant (p > 0.05). For the 30-day period, groups with the same upper case letter are not statistically significant (p > 0.05). For each PVPA concentration as well as the control, time-period columns connected by a solid black bar are not statistically significant from one another (p > 0.05).
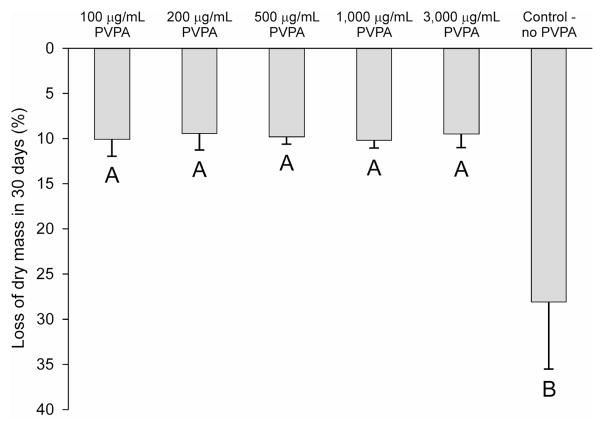
Loss of dry mass from the demineralized dentin beams over time was depicted in Figure 3. The control group showed a large decrease in dry mass (28.1%) while the decreases in dry mass in the experimental PVPA cross-linked groups were in the range of 9–10%. A highly significant difference was identified among the groups (p < 0.001). Multiple comparison tests further indicated that loss of dry mass from the control group was significantly higher than each of the experimental group (p < 0.05). The losses of dry mass from the experimental groups were not significantly different from one another (p>0.05).
Fig. 3.
Loss of dry mass from completely demineralized dentin beams cross-linked with different PVPA concentrations (100 - 3,000 μg/mL) and the control after incubation in a calcium- and zinc-containing aging medium for 30 days. The loss of dry mass from each beam was calculated as a percentage of the dry mass of that beam at baseline. Groups with the same upper case letter are not statistically significant (p > 0.05).
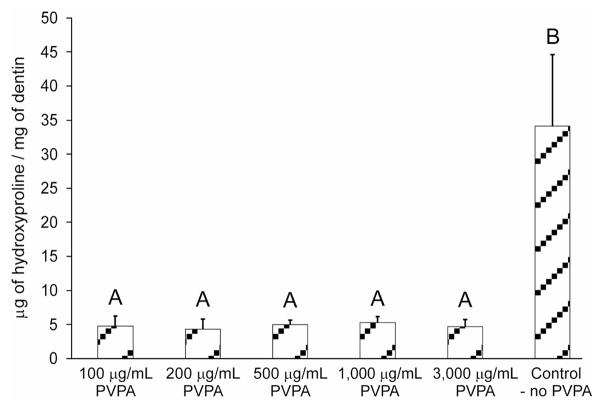
A similar trend was observed when the incubation medium was hydrolyzed to release all amino acids from the dissolved collagen peptides, as revealed by the hydroxyproline assay (Figure 4). The control group released 34 μg of hydroxyproline/mg dry mass of the collagen matrix. All groups that were cross-linked with PVPA released between 4.3–5.3 μg of hydroxyproline/mg dry mass of the collagen matrix. The results were statistically significant (p = 0.003). Dunn’s multiple comparison tests further identified that the hydroxyproline content in the control group was significantly higher than all PVPA-treatment experimental groups (p < 0.05), which were not significantly different from one another.
Fig. 4.
Hydroxyproline content derived from the aging medium of the PVPA cross-linked demineralized dentin beam groups and the control after a 30-day incubation period. For each specimen, the dissolved collagen from the demineralized dentin beam was expressed as μg of hydroxyproline/mg of the dry mass of the baseline demineralized dentin. Groups with the same upper case letter are not statistically significant (p > 0.05).
4. Discussion
As the anti-MMP potentials of the five different PVPA concentrations were either similar to or higher than the GM6001 inhibitor control, we have to reject the first null hypothesis that PVPA does not inhibit soluble MMP-9. The results of this part of the study indicated that PVPA is a relatively potent inhibitor of MMP-9 even in the lowest concentration (i.e. 200 μg/mL). Polyvinylphosphonic acid is similar to the class of bisphosphonate drugs in that the –C-O-P ester group in phosphoric acid are replaced by the –C-P phosphonate group. Matrix metalloproteinases are calcium- and zinc-dependent endopeptidases [9]. Bisphosphonate drugs are effective MMP inhibitors, the actions of which were thought to be mediated via the chelation of calcium [31] and zinc cations [31]. Unlike bisphosphonates which contains two phosphonate groups covalently linked to a central carbon atom, PVPA is a long chain polymer containing multiple phosphonate groups, each separated by a methylene group in the molecule backbone. Polyvinylphosphonic acid is a very effective chelator of divalent cations such as Zn2+, particularly at pH values higher than 3, at which its first ionization was completed [32]. Although its binding with Ca2+ was not investigated in that study, its binding capacities with other divalent ions were in the range of 60–113 mg metal per gram of polymer. The polychelatogenic property of PVPA probably accounts for its potent anti-MMP property.
The rationale for using EDC as a cross-linking agent to increase the uptake of PVPA [18] is based on the molecular sieving characteristics of insoluble type I collagen matrices. The packing density of collagen microfibrils is such that the matrices limit the size of molecules that can penetrate into its internal water compartment [33]. Molecules smaller than 40 kDa are completely excluded, while those smaller than 6 kDa freely diffuse to all water compartments of collagen fibrils [33]. The average molecular size of PVPA reported by the manufacturer is listed as 24 kDa but is really a mixture of PVPA polymers that include some that are smaller and larger than 24 kDa [34]. The smaller polymers can enter the water compartments of collagen matrices. By then cross-linking collagen with EDC, a zero-length cross-linking agent, we can increase the sieving properties of collagen and trap PVPA with the matrix. The evidence supporting this hypothesis is published in Gu et al. [18]. In that study, desorption of PVPA from the collagen matrix by 0.13 M and 0.5 M NaCl was reduced when the matrices were cross-linked with EDC, when compared to electrostatic binding of PVPA to the collagen matrix. For the latter, PVPA was completely desorbed from the collagen matrix after 24 h. Although cross-linking of collagen with EDC for 1 min only was not as effective to cross-linking of PVPA for 5 min, the extent of desorption PVPA from cross-linked collagen following a 5-min application of EDC was similar to a 4–24 h application of the cross-linking agent [18]. Conventionally, carbodiimides are used to cross-link collagen molecules by activating the carboxylic acid groups of glutamic or aspartic acid residues to react with amine groups of another chain, forming amide bonds [35]. As a member of the zero-length class of cross-linkers, EDC does not remain in the chemical bond but is released as a substituted urea molecule [36]. However, cross-linking of collagen matrix by EDC to cause increases in its stiffness requires 4–6 h and the presence of N-hydroxysuccinimide to be effective [35–38]. Moreover, the thiopeptolide employed in the MMP assay has the structure Ac-Pro-Leu-Gly-SCH[CH2CH(CH3)2]CO-Leu-Gly-OC2H5 [39], which does not contain free carboxylic acid groups to enable intramolecular cross-links to be developed.
The 5 min cross-linking technique was not intended to improve the mechanical properties of the collagen matrix via improved cross-linking of the collagen fibrils, a process that requires 4–6 h. The hypothesis that this particular use of the EDC cross-linking agent did not result in appreciable cross-linking of the collagen matrix was further confirmed by the similar modulus of elasticity measured from demineralized collagen beams in the five PVPA experimental groups before they were treated with the PVPA solutions for 5 min, and after they were aged in the incubation buffer for 30 days. Improvements in the mechanical properties and resistance to collagenase degradation of collagen-based biomaterials have been reported with prolonged EDC treatment in the presence of N-hydroxysuccinimide [37,40]. Treatment of demineralized dentin beams with alternative cross-linking agents such as glutaraldehyde and grape seed extract for 4 h resulted in the increase in their modulus of elasticity from 4.8–6.2 MPa to 34.9–242.5 MPa [41]. In the present study, the modulus of elasticity of demineralized dentin beams immersed in 0.3 M EDC only (control) dropped significantly after aging in the calcium- and zinc-containing incubation medium for 30 days. The decrease in dry mass of the aged control beams and the hydroxyproline content of the aged control supernatant were also significantly higher than the corresponding values in the five experimental PVPA groups. Thus, it is unlikely that the very short period of EDC-mediated cross-linking of PVPA to demineralized dentin could have resulted in appreciable cross-linking more than the peripheral collagen matrix. Conversely, the modulus of elasticity of the demineralized collagen beams in the five PVPA experimental groups neither increase nor decrease after aging in the incubation medium. On one hand, this indicated that EDC cross-linked demineralize dentin did not stiffen the collagen matrix further. One the other hand, the combined results of the stable modulus of elasticity and the significantly lower loss of dry mass and dissolution of the collagen matrix in the five experimental PVPA groups suggested that those results were attributed to the effect of PVPA on inhibiting the endogenous MMP activity of demineralized dentin matrices. Thus, the second null hypothesis that PVPA has no effect on the endogenous MMP activity of demineralized dentin matrices has to be rejected.
Even if there were indeed minor increases in collagen cross-linking in the presence of EDC, scission of the collagen molecules at their three-quarter/one-quarter locations could have only resulted in segregated but interlocked cross-linked fragments that were incapable of conferring additional resistance to bending moments [38]. The ability of the PVPA in preventing the degradation of collagen fibrils is a far more important attribute than increasing the cross-links in demineralized dentin collagen, which, already is highly cross-linked [42]. The durability of resin-dentin bonds requires stable collagen fibrils within the hybrid layer.
It is interesting that the lower anti-MMP-9 inhibition efficacy seen in the 100 μg/ml PVPA group was not reflected in the indirect assays of functional MMP activities of the demineralized dentin beams. This leads us to speculate that EDC per se may have some inherent anti-MMP potential. Cross-linking agents can inhibit MMPs by altering their tertiary protein structure such as inactivating or masking some of the functional domains that are critical for their enzymatic activities. Although the application of cross-linking agents such as glutaraldehyde (24 h) and diphenylphosphorylazide (24 h) completely abolished endogenous MMP-9 and MMP-2 activities in bovine and porcine pericardium, treatment with EDC in the presence of N-hydroxysuccinimide (4 h) was less effective in inhibiting endogenous MMP-9 and MMP-2 activities [43]. Thus, it would be interesting to examine the potential of EDC in inhibiting dentin matrix-bound MMPs in a future study design that involves the use of different molar concentrations of EDC. If indeed a short period of EDC application (i.e. less than 5 min) to a demineralized dentin matrix can inhibit matrix-bound MMPs by cross-linking their protein structures and inactivating their catalytic sites, this may represent a completely new concept and a supplementary strategy to the currently suggested use of MMP inhibitors, which can only inhibit certain types of MMPs. Apart from endogenous MMPs, other host proteases such as cysteine cathepsins are present in sound and carious dentin [44]. These host proteases also contribute to the degradation of demineralized dentin but may not be effectively inhibited by currently employed MMP inhibitors.
Young teeth are known to contain higher MMP-2 activities [45] than are found in older teeth. This was recently confirmed by Tersariol et al. [44], who also reported that the cysteine cathepsin activity in young teeth was higher than in older teeth. The use of young teeth in the current study is justified because most of the replacement resin-composite restorations are done in young patients. The higher protease activity in young teeth makes them more at risk for degradation following dentin bonding procedures and during caries progression.
5. Conclusions
Within the limits of the present study, it may be concluded that PVPA is a potent inhibitor of endogenous MMP activities in demineralized dentin collagen matrices. The use of PVPA should extend the longevity of resin-dentin bonds, in the manner that chlorhexidine has been used [2–4,6,14,15] prior to bonding to prevent the degradation of the water-rich, resin-sparse collagen network at the base of the hybrid layer. As it is possible to trap PVPA in dentin [18], the use of PVPA as a potential MMP inhibitor in dentin bonding may be more advantageous than chlorhexidine in that the latter could only be bound electrostatically to the collagen matrix. Preliminary studies indicated that the microtensile bond strengths of One-Step (Bisco, Inc., Schaumburg, IL, USA) and Adper Single Bond Plus (3M ESPE, St. Paul, MN, USA) to acid-etched dentin were not affected by trapping PVPA in the matrix using EDC-mediated collagen cross-linking prior to the application of the adhesives (Takahashi et al., unpublished results). Further work should be performed to examine whether EDS-induced cross-linking of dentin collagen can result in the preservation of the long-term integrity of the hybrid layer, in the manner previously performed with the use of chlorhexidine.
Acknowledgments
This study was supported in part by, Grants R01 DE015306-06 (PI. David H. Pashley), R21 DE019213-01 (PI. Franklin R. Tay) from the National Institute of Dental and Craniofacial Research and by Grant # 8126472 (PI. Arzu Tezvergil-Mutluay) from Academy of Finland. The authors are grateful to Dr. Lisha Gu for preparing the polyvinylphosphonic acid solutions and Mrs. Michelle Barnes for her secretarial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NIDCR Strategic Plan 2009–2013. http://grants.nih.gov/grants/guide/rfa-files/RFA-DE-10-004.html#PartII. [PubMed]
- 2.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 3.Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, et al. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86:529–33. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 4.Brackett MG, Tay FR, Brackett WW, Dib A, Dipp FA, Mai S, et al. In vivo chlorhexidine stabilization of hybrid layers of an acetone-based dentin adhesive. Oper Dent. 2009;34:379–83. doi: 10.2341/08-103. [DOI] [PubMed] [Google Scholar]
- 5.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16:265–73. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 6.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 7.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, et al. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27:4470–6. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, et al. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci. 2006;114:160–6. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 9.Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006;85:22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto M, Tay FR, Ohno H, Sano H, Kaga M, Yiu C, et al. SEM and TEM analysis of water degradation of human dentinal collagen. J Biomed Mater Res B Appl Biomater. 2003;66:287–98. doi: 10.1002/jbm.b.10560. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong SR, Vargas MA, Chung I, Pashley DH, Campbell JA, Laffoon JE, et al. Resin-dentin interfacial ultrastructure and microtensile dentin bond strength after five-year water storage. Oper Dent. 2004;29:705–12. [PubMed] [Google Scholar]
- 12.Grenier D. Reduction of proteolytic degradation by chlorhexidine. J Dent Res. 1993;72:630–3. doi: 10.1177/00220345930720031301. [DOI] [PubMed] [Google Scholar]
- 13.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin Diagn Lab Immunol. 1999;6:437–9. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrilho MR, Carvalho RM, de Goes MF, di Hipólito V, Geraldeli S, Tay FR, et al. Chlorhexidine preserves dentin bond in vitro. J Dent Res. 2007;86:90–4. doi: 10.1177/154405910708600115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, et al. Chlorhexidine stabilizes the adhesive interface: A 2-year in vitro study. Dent Mater. 2009 doi: 10.1016/j.dental.2009.11.153. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slee AM, Tanzer JM. Studies on the relative binding affinities of chlorhexidine analogs to cation exchange surfaces. J Periodontal Res. 1979;14:213–9. doi: 10.1111/j.1600-0765.1979.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 17.Blackburn RS, Harvey A, Kettle LL, Manian AP, Payne JD, Russell SJ. Sorption of chlorhexidine on cellulose: mechanism of binding and molecular recognition. J Phys Chem B. 2007;111:8775–84. doi: 10.1021/jp070856r. [DOI] [PubMed] [Google Scholar]
- 18.Gu LS, Kim Y-K, Liu Y, Takahashi K, Arun S, Wimmer CE, Osorio R, Ling J-Q, Looney SW, Pashley DH, Tay FR. Immobilization of a phosphonated analog of matrix phosphoproteins within cross-linked collagen as a templating mechanism in biomimetic mineralization. Acta Biomaterials. 2010 doi: 10.1016/j.actbio.2010.07.036. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52:121–7. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Mazzoni A, Pashley DH, Tay FR, Gobbi P, Orsini G, Ruggeri A, Jr, et al. Immunohistochemical identification of MMP-2 and MMP-9 in human dentin: correlative FEI-SEM/TEM analysis. J Biomed Mater Res A. 2009;88:697–703. doi: 10.1002/jbm.a.31920. [DOI] [PubMed] [Google Scholar]
- 21.Bigg HF, Rowan AD, Barker MD, Cawston TE. Activity of matrix metalloproteinase-9 against native collagen types I and III. FEBS J. 2007;274:1246–55. doi: 10.1111/j.1742-4658.2007.05669.x. [DOI] [PubMed] [Google Scholar]
- 22.Balooch M, Wu-Magidi IC, Balazs A, Lundkvist AS, Marshall SJ, Marshall GW, et al. Viscoelastic properties of demineralized human dentin measured in water with atomic force microscope (AFM)-based indentation. J Biomed Mater Res. 1998;40:539–44. doi: 10.1002/(sici)1097-4636(19980615)40:4<539::aid-jbm4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Pashley DH, Agee KA, Wataha JC, Rueggeberg F, Ceballos L, Itou K, et al. Viscoelastic properties of demineralized dentin matrix. Dent Mater. 2003;19:700–6. doi: 10.1016/s0109-5641(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 24.Tay FR, Carvalho RM, Yiu CKY, King NM, Zhang Y, Agee K, Bouillauet S, Pashley DH. Mechanical disruption of dentin collagen fibrils during resin-dentin bond testing. J Adhes Dent. 2000;2:175–92. [PubMed] [Google Scholar]
- 25.Pashley DH, Agee KA, Nakajima M, Tay FR, Carvalho RM, Terada RS, et al. Solvent-induced dimensional changes in EDTA-demineralized dentin matrix. J Biomed Mater Res. 2001;56:273–81. doi: 10.1002/1097-4636(200108)56:2<273::aid-jbm1095>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 26.Wood JD, Wang R, Weiner S, Pashley DH. Mapping of tooth deformation caused by moisture change using moiré interferometry. Dent Mater. 2003;19:159–66. doi: 10.1016/s0109-5641(02)00025-8. [DOI] [PubMed] [Google Scholar]
- 27.Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, et al. Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. J Biomed Mater Res B Appl Biomater. 2009;90:373–80. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;15(112):70–5. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 29.Lindhe A. Noncollagenous proteins and proteoglycans in dentinogenesis. In: Lindhe A, editor. Dentin and Dentinogenesis. Chapter 9. II. CRC Press; 1984. p. 56. [Google Scholar]
- 30.Butler WT. Dentin collagen, chemical structure and role in mineralization. Chapter 8. In: Linde A, editor. Dentin and Dentinogenesis. II. CRC Press; Boca Raton: 2000. p. 40. [Google Scholar]
- 31.Heikkilä P, Teronen O, Moilanen M, Konttinen YT, Hanemaaijer R, Laitinen M, et al. Bisphosphonates inhibit stromelysin-1 (MMP-3), matrix metalloelastase (MMP-12), collagenase- 3 (MMP-13) and enamelysin (MMP-20), but not urokinase-type plasminogen activator, and diminish invasion and migration of human malignant and endothelial cell lines. Anticancer Drugs. 2002;13:245–54. doi: 10.1097/00001813-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000;60:2949–54. [PubMed] [Google Scholar]
- 33.Rivas BL, Pereira E, Gallegos P, Homper D, Geckeler KE. Metal ion binding capacity of the water-soluble poly(vinyl phosphonic acid) for mono-, di-, and trivalent cations. J Appl Polym Sci. 2004;92:2917–22. [Google Scholar]
- 34.Toroian D, Lim JE, Price PA. The size exclusion characteristics of type I collagen: implications for the role of noncollagenous bone constituents in mineralization. J Biol Chem. 2007;282:22437–22447. doi: 10.1074/jbc.M700591200. [DOI] [PubMed] [Google Scholar]
- 35.Stranberg C, Rosenauer C, Wegner G. Poly(vinyl phosphonic acid): Hydrodynamic properties and SEC-calibration in aqueous solution. Macromol Rapid Comm. 2010;31:374–9. doi: 10.1002/marc.200900605. [DOI] [PubMed] [Google Scholar]
- 36.Olde Damink LHH, Dijkstra PJ, Van Wachem PB, Van Luyn MJA, Nieuwenhuis P, Feijen J. In vitro degradation of dermal sheep collagen cross-linked using water-soluble carbodiimide. Biomaterials. 1996;17:679–84. doi: 10.1016/0142-9612(96)86737-8. [DOI] [PubMed] [Google Scholar]
- 37.Kunkel GR, Mehrabian M, Martinson HG. Contact-site crosslinking agents. Mol Cell Biochem. 1981;34:3–13. doi: 10.1007/BF02354846. [DOI] [PubMed] [Google Scholar]
- 38.Powell HM, Boyce ST. EDC cross-linking improves skin substitute strength and stability. Biomaterials. 2006;27:5821–7. doi: 10.1016/j.biomaterials.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, van der Werf KO, Fitié CF, Bennink ML, Dijkstra PJ, Feijen J. Mechanical properties of native and cross-linked type I collagen fibrils. Biophys J. 2008;94:2204–11. doi: 10.1529/biophysj.107.111013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weingarten H, Martin R, Feder J. Synthetic substrates of vertebrate collagenase. Biochemistry. 1985;24:6730–4. doi: 10.1021/bi00344a064. [DOI] [PubMed] [Google Scholar]
- 41.Caruso AB, Dunn MG. Changes in mechanical properties and cellularity during long-term culture of collagen fiber ACL reconstruction scaffolds. J Biomed Mater Res A. 2005;73:388–97. doi: 10.1002/jbm.a.30233. [DOI] [PubMed] [Google Scholar]
- 42.Bedran-Russo AK, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J Biomed Mater Res B Appl Biomater. 2008;86B:330–4. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 43.Kuboki Y, Mechanic GL. Comparative molecular distribution of cross-link in bone and dentin collagen. Structure-function relationships. Calcif Tissue Int. 1982;34:306–8. doi: 10.1007/BF02411256. [DOI] [PubMed] [Google Scholar]
- 44.Calero P, Jorge-Herrero E, Turnay J, Olmo N, López de Silanes I, Lizarbe MA, et al. Gelatinases in soft tissue biomaterials. Analysis of different crosslinking agents. Biomaterials. 2002;23:3473–8. doi: 10.1016/s0142-9612(02)00054-6. [DOI] [PubMed] [Google Scholar]
- 45.Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, Carrilho MR, Pashley DH, Tay FR, Salo T, Tjäderhane L. Cysteine cathepsins in human dentin-pulp complex. J Endo. 2010;36:475–481. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 46.Martin-de las Heras S, Valenzuela A, Overall CM. Gelatinase A in human dentin has a new biochemical marker for age estimation. J Forensic Sci. 2000;45:807–11. [PubMed] [Google Scholar]