Abstract
Background & Aims
Adenosine mediates immune suppression and is generated by the ectonucleotidases CD39 (ENTPD1) and CD73 that are expressed on vascular endothelial cells and regulatory T cells (Treg). Although tumor-infiltrating immune cells include Foxp3+ Treg, it is not clear whether local adenosine generation by Treg promotes tumor growth in a CD39-dependent manner. In this study, we have examined the impact of CD39 expression by Treg on effector immune cell responses to hepatic metastases in vivo.
Methods and Results
A model of hepatic metastatic cancer was developed with portal vein infusion of luciferase-expressing melanoma B16/F10 cells and MCA38 colon cancer cells in wild type and mutant mice null for Cd39. Chimeric mice were generated by bone marrow transplantation (BMT) using Cd39 null or wild type (wt) C57BL6 donors and irradiated recipient mice. We demonstrate that hepatic growth of melanoma metastatic tumors was strongly inhibited in mice with Cd39 null vasculature or in wild type mice with circulating Cd39 null bone marrow-derived cells. We show functional CD39 expression on CD4+Foxp3+ Treg suppressed anti-tumor immunity mediated by NK cells in vitro and in vivo. Lastly, inhibition of CD39 activity by POM-1 (polyoxometalate-1), a pharmacological inhibitor of NTPDase activity, significantly inhibited tumor growth (P < .001).
Conclusions
CD39 expression on Treg inhibits NK activity and is permissive for metastatic growth. Pharmacological or targeted inhibition of CD39 enzymatic activity may find utility as an adjunct therapy for secondary hepatic malignancies.
Keywords: CD39/ENTPD1, Regulatory T cells (Treg), Cancer therapy, Liver
Introduction
Although advances in therapies for hepatic metastatic disease have occurred,1, 2 limitations of VEGF-based anti-angiogenesis strategies and other regimens remain a major clinical problem. The tumor microenvironment may regulate local cellular responses by generating purinergic mediators that dampen anti-tumor immunity.3 The effects of extracellular nucleosides and nucleotides are mediated by type 1 purinergic (P1) receptors for adenosine and P2 receptors that bind extracellular nucleotides.4 Adenosine may promote tumor growth by stimulating vascular endothelial cell proliferation, and inhibiting immune cell cytokine synthesis, trans-endothelium migration, and anti-tumor effector responses.3, 5, 6
CD39/ENTPD1 (ectonucleoside triphosphate diphosphohydrolase-1) is the dominant ectonucleotidase expressed by endothelial cells and immune regulatory T cells (Treg). CD39 drives the sequential hydrolysis of both adenosine triphosphate (ATP) and adenosine diphosphate (ADP) to adenosine monophosphate (AMP).7–10 AMP is further degraded by CD73/ecto-5′-nucleotidase, to adenosine. CD39 expression by the vasculature is an absolute requirement for tumor angiogenesis.11 We have noted that deletion of Cd39 on Treg results in heightened alloimmune responses.9 Whether CD39 expression by tumor-infiltrating immune cells facilitates tumor growth has not been explored to date.
Melanoma and colon cancers are aggressive, lethal tumors that often target the liver. In this study, we note that in our model anti-tumor activity is NK cell-dependent. CD4+Foxp3+ Treg inhibit NK cell-mediated anti-tumor functions, a pathway that is dependent on intrinsic CD39 expression. Pharmacological inhibition of CD39, using POM-1 (polyoxometalate-1),12 likewise inhibits tumor growth. These findings suggest that targeted inhibition of CD39 might find utility as an adjunct therapy for metastatic hepatic malignancy.
Materials and Methods
Animals
Six to fourteen week old male C57BL6 Cd39 null mice were used.8 Age-, sex- and strain-matched wild type mice and (γc)/Rag2−/− mice were purchased from Taconic (MA). Rag1−/− mice were from Jackson Laboratory (Bar Harbor, ME). Foxp3-GFP knock-in mice were generated as described.13
Animal Experimentation Protocols were reviewed and approved by the Institutional Animal Care and Use Committees (IACUC) of Beth Israel Deaconess Medical Center.
Antibodies and Reagents
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO). FACS studies were performed using FITC-, PE-, Cy-chrome- or APC-conjugated antibodies. The following antibodies used for FACS sorting and analysis were from eBioscience (San Diego, CA): anti-mouse CD3 (clone: eBio500A2), CD4 (GK1.5), CD8 (53–6.7), CD28 (37.51), CD39 (24DMS1), TCRβ (H57-597), NK1.1 (PK136) (BD Bioscience, San Jose, CA). FACS data were analyzed with FlowJo software (TreeStar Inc., Ashland, OR). Antibodies used for immunohistochemistry were the following: CD31, CD4, CD8, CD11b, Gr-1 (LY6C and LY6G, BD Bioscience), F4/80, Thy1.2, Foxp3, NKp46 (R&D Systems, Minneapolis, MN), and polyclonal rabbit anti-mouse CD39 antibody.14 Anti-PE MicroBeads and anti-Biotin MACSiBead Particles were from Miltenyi Biotec Inc. (Auburn, CA). NTPDase inhibitor POM-1 was obtained as described.12
Isolation of spleen and LN Treg, NK cells, and cytotoxicity assay
Lymphocytes were positively selected using MOFLO or FACSaria cell sorter (BD Bioscience, San Jose, CA) producing > 99% cell population. NK cells were sorted as NK1.1+TCRβ− cells, and Treg were sorted as CD4+GFP+ cells using Foxp3-GFP knock-in mice.13 Cytolytic activity of NK cells was tested at a number of E:T ratios against N YAC-1 cells using LIVE/DEAD Cell-Mediated Cytotoxicity Kit (Invitrogen Life Technologies, Carlsbad, CA).
Tumor Cell Lines
Luciferase-expressing B16/F10 (luc-B16/F10 on BL6) cells were developed as described.15 Syngeneic murine MCA38 colon cancer cells provided by Dr. Nicholas P. Restifo, National Cancer Institute) were maintained in RPMI 1640 medium supplemented with 10% FCS and glutamine. YAC-1 cells were purchased from ATCC (Manassas, VA).
Tumor Cell Inoculation and in vivo Bioluminescence Imaging
Luc-B16/F10 and MCA38 cells were harvested by trypsinization and resuspended with HBSS/2% FBS for injection. Luc-B16/F10 cells (1.5 × 105 cells for standard and BMT experiments, and 2 × 105 cells for adoptive transfer experiments) and MCA38 cells (1.0 × 105 cells for all experiments except 2 × 105 cells for POM-1 treatments) were infused into liver via portal vein. Tumor-bearing mice were sacrificed and examined for tumor growth at indicated time points or if any distress or suffering was observed. In vivo tumor progression was examined using the noninvasive bioimaging system IVIS (Xenogen) as described previously,15 at the Longwood Small Animal Imaging Facility. Perpendicular tumor diameters were also directly measured and tumor volume was determined by integration: Σt1+t2+….tn (t=a2 × b × 0.52; a=smaller tumor diameter, b=larger tumor diameter).6, 16
Bone Marrow Transplantation (BMT)
Six-week old male Cd39 null mice and wt mice were exposed to 10 Gy (0.28 Gy/min, 200 kV, 4 mA) γ-ray total body irradiation, using an Andrex Smart 225 (Andrex Radiation Products AS, Copenhagen, Denmark) with a 4-mm aluminum filter. The marrow from the femur and tibia of matched Cd39 null mice and wt mice were harvested and cells were purified under sterile conditions. Irradiated recipient mice received 10 × 106 bone marrow cells i.v. The success of bone marrow transplantation (BMT) was validated by FACS analysis of immune cell populations (not shown). Transplanted mice were housed in autoclaved cages for 8 wk before experimentation.17
Adoptive Transfer Experiments
Freshly sorted CD4+ T cells (1 × 106), CD8+ T cells (0.5 × 106), Treg cells (0.1 × 106), or Teff cells (0.9 × 106) from Cd39 null or wt mice, or wt NK cells (1.5 × 106), were injected into Rag1−/− mice. Wt NK cells (1 × 106), alone or with wt Treg (1 × 106), or Cd39 null Treg (1 × 106), were injected into (γc)/Rag2−/− mice.
Histology and Immunohistochemistry
Paraffin-embedded or frozen sections of tumor-bearing livers were analyzed by immunohistochemical staining as described.11, 18 Apoptosis was determined using Tunel staining (TdT) (Millipore, Temecula, CA).
Measurement of IFN-γ Production by ELISA
IFN-γ plasma concentrations was measured using Mouse IFN-γ (Femato-HS) ELISA Ready-To-Go kit from eBioscience.
Purification of Tumor- or Liver-infiltrating Mononuclear Cells
These cells were purified as described previously.19
Thin Layer Chromatography Analysis
NTPDase activity of CD39 on freshly isolated cells was analyzed using Thin Layer Chromatography (TLC), as described.9, 19
Statistical Analysis
Results in this study are expressed as means ± SEM of values (obtained from at least 4 mice per group and/or at least 3 independent in vitro experiments). All histology and immunohistochemical images are representative of at least 4 mice per group.
For statistical analyses, the two-tailed Students t-test was used. Animal time to euthanasia were analyzed using the Kaplan-Meier method, and were compared using the log-rank test (StatView 5.0 analysis software).
Significance was defined as P < .05.
Results
CD39 Expression Facilitates Tumor Growth
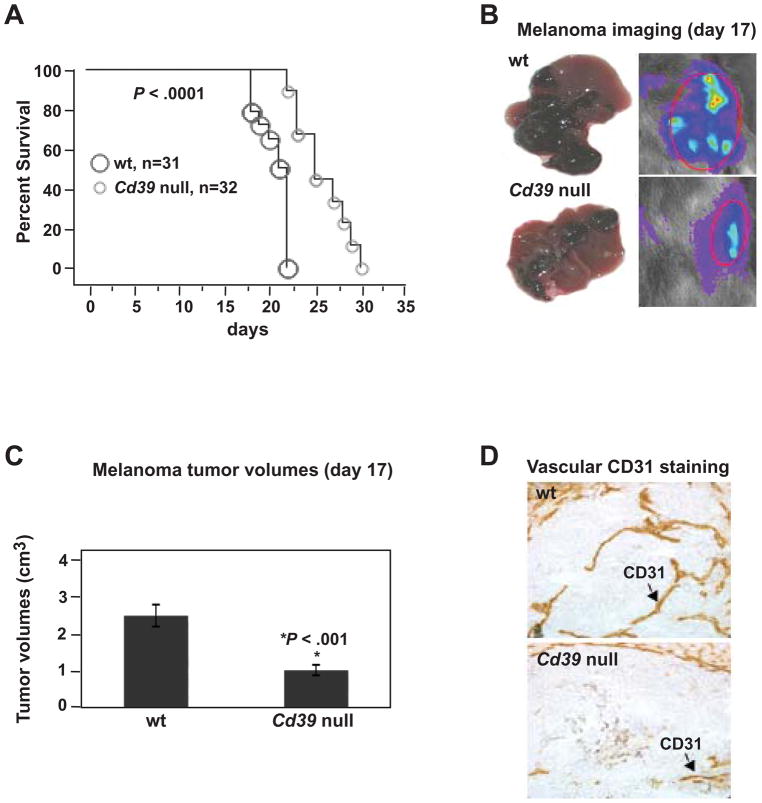
Portal vein infusion of luciferase-expressing melanoma B16/F10 (luc-B16/F10) cells and MCA38 colon cancer cells resulted in cells being retained in liver sinusoids; no tumors were found in lungs or other sites within 14 days (not shown). Melanoma-bearing Cd39 null mice had longer times to euthanasia and death than the wt mice (P < .0001, Figure 1A).
Figure 1.
Cd39 deletion inhibits metastatic melanoma growth in livers. (A) Survival and/or time to euthanasia in tumor-bearing mice post luc-B16/F10 cell infusion (1.5 × 105 cells) via portal vein (P < .0001). (B) Representative images of tumor-bearing livers on day 17 (left panel) and in vivo bioluminescent imaging of tumor metastasis (right panel). (C) Tumor volumes at day 17 (*P < .001). (D) Representative immunohistochemical staining on tissue sections obtained from (B) using anti-CD31 (a marker for endothelium) antibody (Magnification x400). Data are given as means ± SEM.
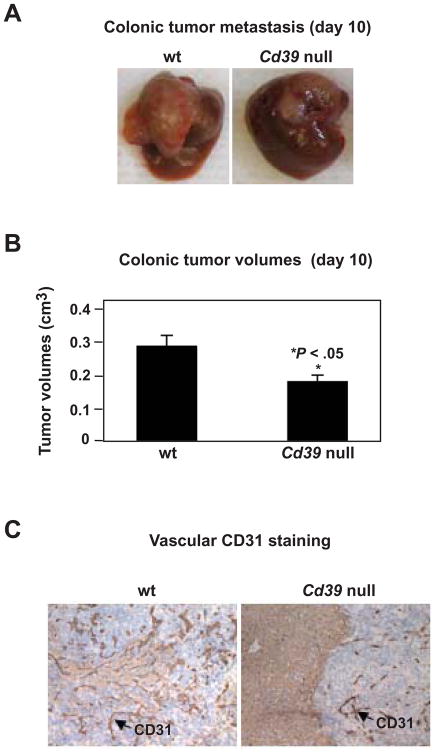
Tumor growth in liver was substantially inhibited in Cd39 null mice (melanoma data in Figure 1B–C and colonic tumor studies in Figure 2A–B), and was associated with decreased angiogenesis (Figure 1D and Figure 2C). Luc-B16/F10 cells and MCA38 cells do not express CD39 per se in vitro or in vivo at any tumor progression stages (not shown). Hence, endogenous CD39 expression is required for the development of metastatic tumors in the liver.
Figure 2.
Cd39 deletion inhibits metastatic colon tumor growth in livers. (A) Representative images of tumor-bearing livers at day 10 post portal vein infusion of 1.0 × 105 MCA38 cells. (B) Tumor volumes at day 10 (*P < .05). (C) Immunohistochemical staining on tissue sections obtained from (A) using anti-CD31 (a marker for endothelium) antibody (Magnification x400). Data are given as means ± SEM.
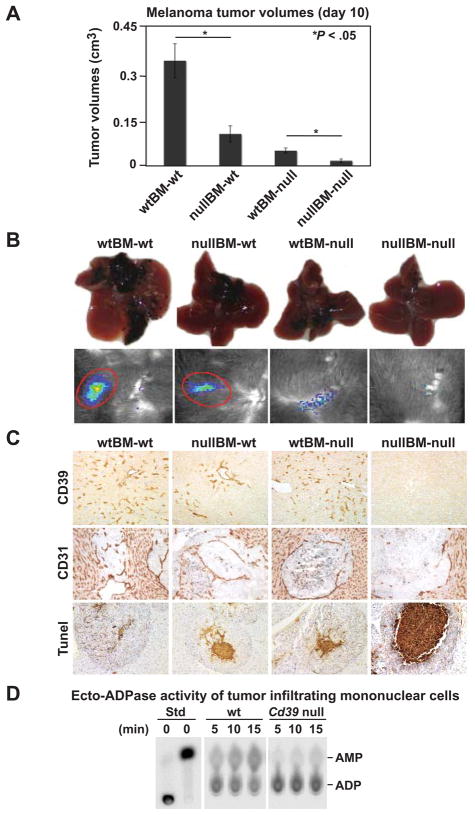
We next studies bone marrow reconstitution. Melanoma growth was significantly delayed in the livers of irradiated, reconstituted wt mice that had received Cd39 null bone marrow transplantation (BMT) (Figure 3A–B, P < .05), when compared with wt mice that received wt BMT. Here, defective angiogenesis in tumors was associated with Cd39 null vasculature. High levels of cell death were clearly demonstrated at the center of tumor nodules (Figure 3C).
Figure 3.
CD39 expression on bone marrow-derived cells facilitates tumor growth. Wild type (wt) and Cd39 null (null) chimeric mice were generated by bone marrow transplantation (BMT) (donor genotype designated first/recipients second). Eight weeks post-BMT, 1.5 × 105 luc-B16/F10 cells were infused via portal vein. (A) Tumor volumes on day 10 (n = 6–8 mice per group). (B) Representative images of tumor-bearing livers at day 10 (top) and in vivo bioluminescent imaging of tumor metastasis (bottom). (C) Representative immunohistochemical staining using anti-CD39 and anti-CD31 antibodies and Tunel staining (TdT) for apoptosis (Magnification x400 for CD39; x200 for CD31 and Tunel staining). (D) Ecto-ADPase activity in tumor-infiltrating mononuclear cells. Tumor-infiltrating mononuclear cells were isolated from tumor tissues of wt and Cd39 null tumor-bearing mice. Thin Layer Chromatography (TLC) analysis of ADP hydrolysis to AMP using 1.5 × 105 cells was analyzed. Data are given as means ± SEM. *P < .05.
Differences of hepatic melanoma growth among groups on day 14 and 21 were comparable to that seen on day 10 (Supplementary Figure 1 and not shown). Mortality was only seen in wtBM-wt mice, from day 17; and all other groups survived for at least 21 days (not shown). Taken together, these data indicate that CD39 expression by BM-derived cells is critical for tumor growth.
Characterization of Tumor-Infiltrating Cells
Melanoma tumor tissues were infiltrated by CD39+, CD4+, CD8+, Thy1.2+ cells, macrophages (F4/80+), granulocytes and leukocytes (Gr-1+), NK (NK1.1+TCRβ−) and NKT (NK1.1+TCRβ+) cells, as well as cells positive for CD11b, indicative of myeloid-derived suppressive cells (MDSCs) (Supplementary Figure 2A–B).20
A proportion of the infiltrating CD4+ cells was positive for both CD39 and Foxp3 (Supplementary Figure 2C–D), suggesting that Treg infiltrate tumors.13, 21 As Treg express CD39 (Supplementary Figure 2C–D),9 we measured ecto-ADPase activity of purified tumor-infiltrating mononuclear cells (TIMNC) by TLC assay. Cd39 null cells exhibited markedly decreased ecto-ADPase activity (Figure 3D).
Cd39 Null CD4+ T Cells Inhibit Tumor Growth
Immunity to melanoma could be mediated by a variety of immune cells e.g. NK, NKT and CD8+ T cells.15, 22–25 Treg have been shown to suppress anti-melanoma cytotoxic responses by CD8+ cytotoxic T lymphocytes (CTLs), NKT, as well as NK cells at the local tumor site.15, 22, 23, 26
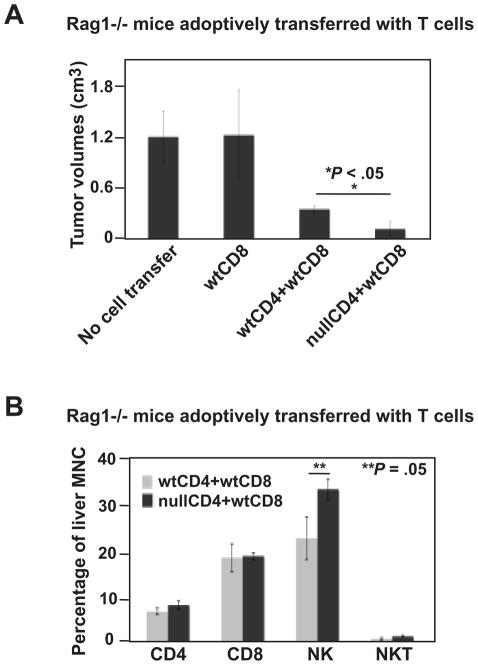
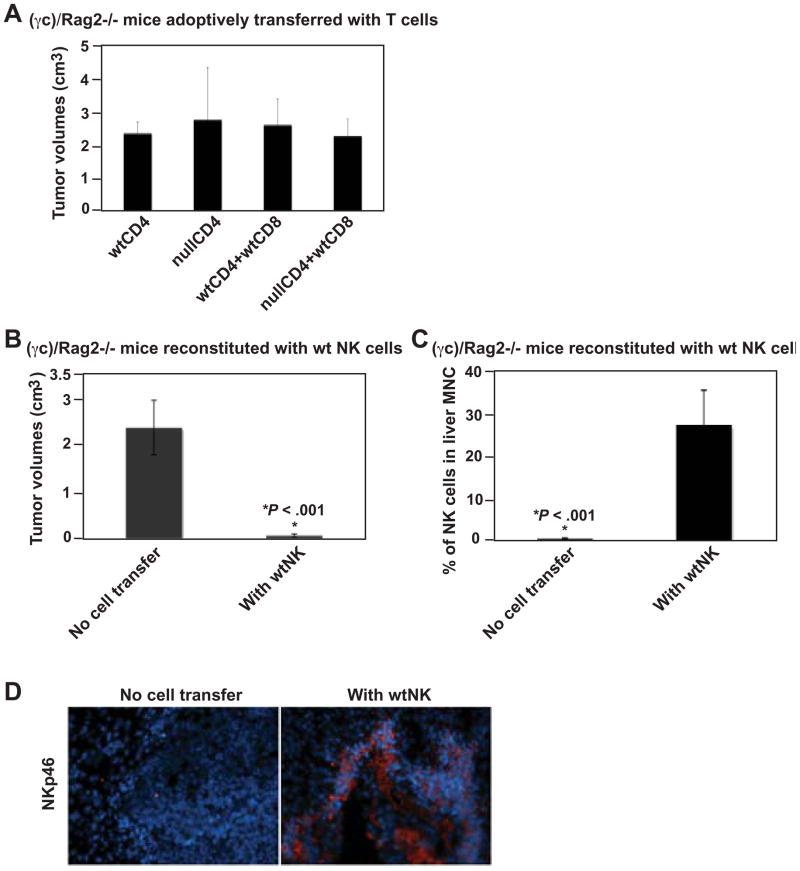
To examine first whether CD39 expression on CD4+ T cells is involved in anti-tumor immunity, wt or Cd39 null CD4+ T cells in combination with effector wt CD8+ T cells were adoptively transferred into Rag1−/− mice. CD8+ T cells alone failed to control melanoma growth unless CD4+ T cells were co-transferred (Figure 4A). Tumor growth was significantly inhibited when Rag1−/− mice were co-transferred with Cd39 null CD4+ T cells, compared to wt CD4+ T cells (P < .05, Figure 4A).
Figure 4.
Cd39 null CD4+ T cells inhibit tumor growth. (A) Tumor volumes of tumor-bearing Rag1−/− mice adoptively transferred with lymphocytes (CD4+ T cells, 1 × 106; CD8+ T cells, 0.5 × 106), followed by portal vein infusion of 2 × 105 luc-B16/F10 cells, on day 14. (B) Percent CD4+, CD8+, NK1.1+ and NKT lymphocytes of liver mononuclear cells isolated from tumor-bearing livers above. Data are given as means ± SEM. *P < .05, **P = .05.
Liver-infiltrating mononuclear cells of these mice were isolated and the percentages of CD4+, CD8+, NK and NKT cells were evaluated by FACS analysis (Figure 4B). Heightened levels of NK cells were observed in mice adoptively transferred with Cd39 null CD4+ T cells (wtCD4+wtCD8 23.25±5.24% vs nullCD4+wtCD8 34.34±8.18%, P = .05). In contrast, percentages of adoptively transferred CD4+ or CD8+ cells remained at the same level (Figure 4B).
We next transferred wt or Cd39 null CD4+ T cells into Rag1−/− mice followed by subcutaneous injection of 0.2 ml Matrigel into the flanks 24 h later. We did not observe any inhibition by CD4+ T cells on the process of endothelial angiogenesis (Supplementary Figure 3).
NK Cells Mediate Anti-tumor Immunity
We next performed similar adoptive transfer experiments using NK cell-deficient (γc)/Rag2−/− mice, instead of Rag1−/− mice. In (γc)/Rag2−/− mice, no inhibition of melanoma growth was noted even after T cell transfer (Figure 5A) suggesting that NK cells are required to inhibit melanoma growth. We next reconstituted (γc)/Rag2−/− mice with wt NK cells followed by melanoma cell inoculation. NK cell reconstitution greatly suppressed tumor growth in (γc)/Rag2−/− mice (P < .001, Figure 5B), with migration of NK cells into the liver (P < .001, Figure 5C) expressing NKp46 (Figure 5D). Anti-tumor activity of NK cells was noted to be cell number-dependent (not shown). Colonic tumor growth was also inhibited in Rag1−/− mice, as compared to (γc)/Rag2−/− mice (P < 1E-06, Supplementary Figure 4A–B), associated with migration of endogenous NK cells into the liver (P < 1E-06, Supplementary Figure 4C) and strong expression of NKp46 (Supplementary Figure 4D). These data indicate that NK cells play important roles in anti-tumor immunity.
Figure 5.
NK cells mediate anti-tumor immunity to melanoma. (A) Tumor volumes of tumor-bearing (γc)/Rag2−/− mice adoptively transferred with lymphocytes (CD4+ T cells, 1 × 106; CD8+ T cells, 0.5 × 106), followed by portal vein infusion of 2 × 105 luc-B16/F10 cells, on day 14. (B) Tumor volumes of tumor-bearing (γc)/Rag2−/− mice reconstituted with 1.5 × 106 of wild type, spleen- and lymph node (LN)-derived NK cells (sorted as NK1.1+TCRβ− using MOFLO), on day 14. (C) Percentage of NK cells among mononuclear cells isolated from tumor-bearing livers of (B). (D) Immunofluorescence staining using anti-NKp46 antibody on livers of (B): DAPI staining nuclei in blue and NKp46 in red (Magnification x200). Data are given as means ± SEM. *P < .001.
Treg Suppress NK Cell-mediated Anti-tumor Responses in a CD39-dependent Manner
NK cell-mediated anti-tumor functions may be suppressed by Treg in vitro and in vivo.22, 26 This regulation seems NKG2D-mediated and TGF-β− dependent.22, 26, 27 Extracellular purinergic mediators and related metabolites may also exert inhibitory effects on cytotoxic activity and cytokine production of activated NK cells.28, 29 Therefore, it is conceivable that CD39 expressed by Treg regulates NK cell activity.
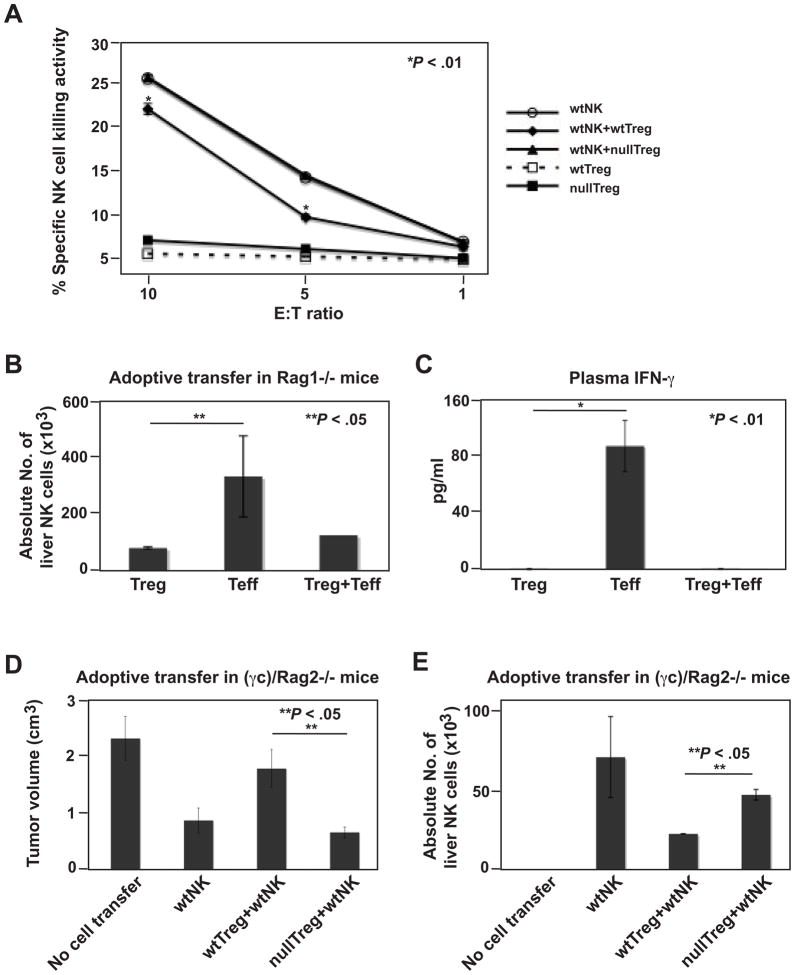
To test for this possibility, wt or Cd39 null Treg were activated with anti-CD3/CD28 and IL2 for 72 h before coculturing with wt NK cells in vitro. We demonstrated specific ability of in vitro activated Treg to suppress NK cell-mediated cytolysis of YAC-1 target cells (P < .01, wtNK vs wtNK+wtTreg; Figure 6A). Cd39 null Treg lacked inhibitory effects on NK cell-mediated cytotoxicity (Figure 6A).
Figure 6.
Treg regulate NK cell-mediated anti-tumor immunity in a CD39-dependent manner. (A) Cd39 null Treg fail to suppress NK cell cytotoxicity in vitro. NK cells were cocultured at 1.5:1 and other ratios (not shown) with activated wt or Cd39 null Treg. The sorted CD4+GFP+ Treg were stimulated with anti-CD3/CD28 bound MACSiBead Particles and IL2 (100 ng/ml) for 72 h before coculture. (*P < .01, wtNK vs wtNK+wtTreg). (B) Absolute liver NK cell numbers in tumor-bearing Rag1−/− mice adoptively transferred with sorted wt lymphocytes (Treg, 0.1 × 106; Teff, 0.9 × 106) as indicated, followed by portal vein infusion of 2 × 105 luc-B16/F10 cells, on day 14. (C) Levels of IFN-γ in plasma obtained from mice in (B). (D) Tumor volumes of tumor-bearing (γc)/Rag2−/− mice adoptively transferred with lymphocytes (Treg, 1.0 × 106; NK, 1.0 × 106), followed by portal vein infusion of 2 × 105 luc-B16/F10 cells, on day 14. Mice received PBS as controls. (E) Absolute liver NK cell numbers in tumor-bearing mice of (D). Data are given as means ± SEM. *P < .01, **P < .05.
Rag1−/− mice were then adoptively transferred with wt Treg (CD4+GFP+Foxp3+) from Foxp3-GFP knock-in mice, or with wt Teff (CD4+GFP−Foxp3−), or in combination, followed by melanoma cell inoculation. Decreased numbers of liver endogenous NK cells and lower plasma levels of IFN-γ were noted in mice transferred with Treg alone, as compared to mice transferred with Teff alone (P < .05 and P < .01, respectively) (Figure 6B–C). Effects of Treg were dominant as shown in Treg+Teff combinations (Figure 6B–C).
We reconstituted (γc)/Rag2−/− mice with wt NK cells alone, or in combination with wt or Cd39 null Treg, followed by melanoma cell inoculation. As expected, wt NK cells inhibited tumor growth. NK cells lost anti-tumor efficacy if co-transferred with wt Treg. NK cell activity was preserved when Cd39 null Treg were co-transferred, with tumor growth abrogated (P < .05) (Figure 6D). Anti-tumor activity correlated with intrahepatic enrichment of administered NK cells (Figure 6E). Hence, Treg directly suppress NK cell-controlled tumor expansion in a manner dependent on Treg-specific CD39.
Pharmacological Inhibition of CD39 Activity Impedes Tumor Growth
A high proportion of tumor-infiltrating CD4+ T cells are Foxp3+ Treg. Deleting these suppressors or mitigating suppressive activity could boost anti-tumor immunity. Pharmacological inhibition of CD39 enzymatic activity might be an anti-cancer treatment. Polyoxometalates (POMs) are anionic complexes that inhibit NTPDase activity12 and have been proposed as chemotherapeutic agents against many human cancers, e.g. colon cancer, lung cancer and breast cancer.30, 31
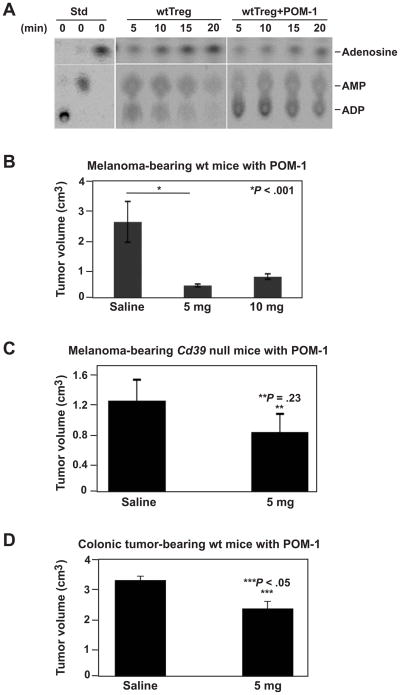
We observed that POM-1 inhibited ecto-NTPDase activity of Treg in vitro (Figure 7A). POM-1 (5 or 10 mg/kg/day) was then injected (i.p.) into wt mice for 10 days after melanoma cell challenge. Tumor growth on day 14 decreased in POM-1-treated wt mice, in contrast to saline-treated wt mice (P < .001, Figure 7B). There was no detectable liver and renal toxicity at suppressive doses (Supplementary Figure 5). In parallel, POM-1 (5 mg/kg/day) did not impact melanoma tumor growth in Cd39 null mice indicating inhibitory effects of POM-1 are mediated via CD39 (P = .23, Figure 7C). Furthermore, POM-1 (5 mg/kg/day) was shown to suppress hepatic metastatic colonic tumor growth in wt mice (P < .05, Figure 7D).
Figure 7.
Pharmacological inhibition of CD39 activity suppresses tumor growth. (A) Ecto-NTPDase activity of POM-1-treated wt Treg in vitro. Wt Treg (CD39+CD4+Foxp3+, 3 × 105) were pre-treated with 26 μm of POM-1, washed, and then subjected to TLC ADPase analysis. Treg incubated in POM-1-free medium served as control. (B) Tumor volumes of melanoma-bearing wt mice (received 2 × 105 luc-B16/F10 cells) treated with POM-1 on a daily dosage of 5 or 10 mg/kg for 10 days, on day 14. Mice received saline were used as controls. (C) Tumor volumes of melanoma-bearing Cd39 null mice (received 2 × 105 luc-B16/F10 cells) treated with POM-1 on a daily dosage of 5 mg/kg for 10 days, on day 14. Mice received saline served as controls. (D) Tumor volumes of colonic tumor-bearing wt mice (received 2 × 105 MCA38 cells) treated with POM-1 on a daily dosage of 5 mg/kg for 10 days, on day 14. Mice received saline were used as controls. Data are given as means ± SEM. *P < .001, **P = .23, ***P < .05.
Discussion
We have already shown that CD39 expression on endothelium is important for angiogenesis and is required for tumor growth in the lung.11, 18 We show now that infiltrating Treg inhibit NK cell-mediated tumor cytotoxicity in a CD39-dependent manner in the liver. Treg exhibit immunosuppressive effects on effector T cells, operational via adenosine-mediated pathways modulated by CD39 and CD73.9 Our current findings strengthen the notion that the expression of CD39 and associated changes in nucleotide/nucleoside balance are integral components of Treg cell function.
Natural killer (NK) cells are an important component of innate defenses against malignant cells. Tumor growth is accelerated in NK-deficient (γc)/Rag2−/− mice. This phenotype could be corrected by NK cell reconstitution. NK cell-mediated anti-tumor functions are susceptible to direct Treg suppression in vitro and in vivo.22, 26, 32 Suppression has been suggested to be, at least in part, NKG2D-mediated and TGF-βdependent.22, 26, 27 However, other mechanisms might also be involved.33
Adenosine interacts with four different adenosine receptor subtypes (A1, A2a, A2b, and A3 AR). A2a signaling has been shown to play a predominant role in inhibiting cytotoxicity of activated NK cells.6, 28, 34, 35 A2b receptors have low affinity and might promote tumor growth at higher concentrations of adenosine.36 Whether A2a and/or A2b are responsible for suppressing NK function in our model is currently undetermined.5, 37
NK cells express CD39, but lack CD73 expression.19, 38 Several studies have reported that purines (ATP = ADP > AMP = adenosine) inhibit cellular proliferation, cytokine production and cytotoxic activity of activated NK cells.29, 34, 39–41 Molecular mechanisms underlying purine-mediated suppression of NK cell activity are highly complex. Whether and how deletion of Cd39 impacts NK cell activity may be dependent on the types of the stimuli and the resulting balance between adenosine- and ATP/ADP-mediated signaling pathways, and is being tested further.
Solid tumors are infiltrated by Treg at a far higher frequency than seen in normal lymphoid organs. It is suggested that depleting Treg with antibodies against surface markers, e.g., CD25, would have a positive impact on the immunotherapy of cancer.42 Nevertheless, CD25 is also expressed on many types of activated immune cells and benefits of this strategy are questionable. We show here that blocking CD39 enzymatic activity with Polyoxometalate-1 (POM-1), a pharmacological inhibitor of NTPDase activity,12 could inhibit adenosine generation by Treg in vitro and abrogate tumor growth in vivo.
CD39 is also expressed on a variety of immune cells including B cells, dendritic cells (DC), macrophages, NK cells, NKT cells,9, 19, 35, 43, 44 and CD4+ T memory cells (CD4+CD44+CD62L−Foxp3−).45 Treg are also not the only tumor-infiltrating suppressor cells. Myeloid-derived suppressor cells (MDSCs) have been shown to be potent suppressors of various T-cell functions in tumor-bearing mice and in patients with cancer.20 In mice, these cells express myeloid-cell lineage differentiation antigens GR-1 and CD11b.20 Monocyte and neutrophil populations express CD3918, 46, 47 and it was demonstrated here that tumor-infiltrating immune cells contain GR-1+ and CD11b+ cells (Supplementary Figure 2).
It has been recently demonstrated that B16/F10 melanoma cells express ligands for natural cytotoxicity receptors (NCRs, NKp46 in mice) and DNAX accessory molecule-1 (DNAM-1), but not NKG2D.48 Interference with both DNAM-1 and NCRs pathways results in dramatic decrease in killing of melanoma cells.48 We have observed strong NKp46 staining on tumor-infiltrating NK cells, associated with decreased melanoma and colonic tumor growth (Figure 5 and Supplementary Figure 4).
NK cells may stimulate maturation of DC,49 and produce cytokines that impact CD4+ and CD8+ effector T cells,50 and regulate proliferation of T cells.51 Our current data clearly show that NK and Teff cells interact, particularly in the context of IFN-γ production and that NK cells could directly influence adaptive immune responses.
We have noted effects of CD39 expression on pulmonary and subcutaneous metastases of melanoma, colon and colorectal cancers (unpublished data and 11). Taken together, our studies provide an intriguing mechanism for the purinergic suppression of NK cell-mediated anti-tumor immunity, by CD39 expression on Treg. Other authors have recently shown that CD39 may be expressed on infiltrating T cells to generate adenosine in lymphoproliferative disorders,52 or on T regulatory cells associated with head and neck tumors.53, 54 Work has been also done to neutralize tumor cell expressed CD73 in a model of breast cancer with salutary effects.55 Our work strongly suggests that it is CD39 expression on T regulatory cells that modulates NK cell reactivity against tumor cells.
Detailed analyses of CD39 expression and Treg function in patients at different stages of hepatic metastatic disease may help improve our understanding of the molecular mechanisms resulting in tumor growth and metastasis. Inhibition of CD39 expression and/or activity might also find future utility as a component of anti-cancer immunotherapy.
Supplementary Material
Acknowledgments
Funding: This study has been supported by the National Institutes of Health (NHLBI PO1-HL076540 and RO1-HL094400).
We thank members of the Longwood Small Animal Imaging Facility of BIDMC for assistance with the in vivo bioluminescence imaging.
Abbreviations in this paper
- Treg
regulatory T cells
- NK cells
natural killer cells
- BMT
bone marrow transplantation
- POM-1
polyoxometalate-1
- NTPDase
nucleoside triphosphate diphosphohydrolase
- VEGF
vascular epithelial growth factor
- IFN-γ
interferon-γ
- LN
lymph node
- wt
wild type
Footnotes
Conflicts of interest: The authors disclose no conflicts.
X.S and Y.W were involved in study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and statistical analysis; W.G, K.E and E.C provided intellectual and technical support; C.E.M and T.M provided material support and critical revision of the manuscript providing important intellectual inputs; S.C.R was involved in study design, data analysis with supervision, and drafting of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogawa T, Takayama K, Takakura N, Kitano S, Ueno H. Anti-tumor angiogenesis therapy using soluble receptors: enhanced inhibition of tumor growth when soluble fibroblast growth factor receptor-1 is used with soluble vascular endothelial growth factor receptor. Cancer Gene Ther. 2002;9:633–40. doi: 10.1038/sj.cgt.7700478. [DOI] [PubMed] [Google Scholar]
- 2.Chari RS, Helton WS, Marsh RD. Chemotherapy and regional therapy of hepatic colorectal metastases: expert consensus statement by Bartlett et al. Ann Surg Oncol. 2006;13:1293–5. doi: 10.1245/s10434-006-9025-9. [DOI] [PubMed] [Google Scholar]
- 3.Hoskin DW, Reynolds T, Blay J. Adenosine as a possible inhibitor of killer T-cell activation in the microenvironment of solid tumours. Int J Cancer. 1994;59:854–5. doi: 10.1002/ijc.2910590625. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–73. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- 5.Spychala J. Tumor-promoting functions of adenosine. Pharmacol Ther. 2000;87:161–73. doi: 10.1016/s0163-7258(00)00053-x. [DOI] [PubMed] [Google Scholar]
- 6.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–7. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plesner L. Ecto-ATPases: identities and functions. Int Rev Cytol. 1995;158:141–214. doi: 10.1016/s0074-7696(08)62487-0. [DOI] [PubMed] [Google Scholar]
- 8.Enjyoji K, Sévigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–7. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 9.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–32. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 11.Jackson SW, Hoshi T, Wu Y, Sun X, Enjyoji K, Cszimadia E, Sundberg C, Robson SC. Disordered purinergic signaling inhibits pathological angiogenesis in cd39/Entpd1-null mice. Am J Pathol. 2007;171:1395–404. doi: 10.2353/ajpath.2007.070190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller CE, Iqbal J, Baqi Y, Zimmermann H, Röllich A, Stephan H. Polyoxometalates--a new class of potent ectonucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg Med Chem Lett. 2006;16:5943–7. doi: 10.1016/j.bmcl.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 14.Kaczmarek E, Koziak K, Sévigny J, Siegel JB, Anrather J, Beaudoin AR, Bach FH, Robson SC. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–22. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 15.Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. Antitumor activity of IFN-lambda in murine tumor models. J Immunol. 2006;176:7686–94. doi: 10.4049/jimmunol.176.12.7686. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Wong MK, Yi H, Watkins S, Laird AD, Wolf SF, Gorelik E. Combined therapy of local and metastatic 4T1 breast tumor in mice using SU6668, an inhibitor of angiogenic receptor tyrosine kinases, and the immunostimulator B7.2-IgG fusion protein. Cancer Res. 2002;62:5727–35. [PubMed] [Google Scholar]
- 17.Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–91. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goepfert C, Sundberg C, Sévigny J, Enjyoji K, Hoshi T, Csizmadia E, Robson S. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104:3109–15. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- 19.Beldi G, Wu Y, Banz Y, Nowak M, Miller L, Enjyoji K, Haschemi A, Yegutkin GG, Candinas D, Exley M, Robson SC. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. 2008;48:841–52. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 22.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–7. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 23.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, Fu YX. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–91. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu D, Gu P, Pan PY, Li Q, Sato AI, Chen SH. NK and CD8+ T cell-mediated eradication of poorly immunogenic B16-F10 melanoma by the combined action of IL-12 gene therapy and 4-1BB costimulation. Int J Cancer. 2004;109:499–506. doi: 10.1002/ijc.11696. [DOI] [PubMed] [Google Scholar]
- 25.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–88. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–85. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghiringhelli F, Menard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev. 2006;214:229–38. doi: 10.1111/j.1600-065X.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 28.Raskovalova T, Huang X, Sitkovsky M, Zacharia LC, Jackson EK, Gorelik E. Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells. J Immunol. 2005;175:4383–91. doi: 10.4049/jimmunol.175.7.4383. [DOI] [PubMed] [Google Scholar]
- 29.Lokshin A, Raskovalova T, Huang X, Zacharia LC, Jackson EK, Gorelik E. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res. 2006;66:7758–65. doi: 10.1158/0008-5472.CAN-06-0478. [DOI] [PubMed] [Google Scholar]
- 30.Rhule JT, Hill CL, Judd DA, Schinazi RF. Polyoxometalates in Medicine. Chem Rev. 1998;98:327–358. doi: 10.1021/cr960396q. [DOI] [PubMed] [Google Scholar]
- 31.Hasenknopf B. Polyoxometalates: introduction to a class of inorganic compounds and their biomedical applications. Front Biosci. 2005;10:275–87. doi: 10.2741/1527. [DOI] [PubMed] [Google Scholar]
- 32.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–12. [PubMed] [Google Scholar]
- 33.Ralainirina N, Poli A, Michel T, Poos L, Andres E, Hentges F, Zimmer J. Control of NK cell functions by CD4+CD25+ regulatory T cells. J Leukoc Biol. 2007;81:144–53. doi: 10.1189/jlb.0606409. [DOI] [PubMed] [Google Scholar]
- 34.Priebe T, Platsoucas CD, Nelson JA. Adenosine receptors and modulation of natural killer cell activity by purine nucleosides. Cancer Res. 1990;50:4328–31. [PubMed] [Google Scholar]
- 35.Priebe T, Platsoucas CD, Seki H, Fox FE, Nelson JA. Purine nucleoside modulation of functions of human lymphocytes. Cell Immunol. 1990;129:321–8. doi: 10.1016/0008-8749(90)90208-9. [DOI] [PubMed] [Google Scholar]
- 36.Ryzhov S, Novitskiy SV, Zaynagetdinov R, Goldstein AE, Carbone DP, Biaggioni I, Dikov MM, Feoktistov I. Host A(2B) adenosine receptors promote carcinoma growth. Neoplasia. 2008;10:987–95. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. Faseb J. 2000;14:2065–74. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 38.Christensen LD, Andersen V. Natural killer cells lack ecto-5′-nucleotidase. Nat Immun. 1992;11:1–6. [PubMed] [Google Scholar]
- 39.Miller JS, Cervenka T, Lund J, Okazaki IJ, Moss J. Purine metabolites suppress proliferation of human NK cells through a lineage-specific purine receptor. J Immunol. 1999;162:7376–82. [PubMed] [Google Scholar]
- 40.Raskovalova T, Lokshin A, Huang X, Jackson EK, Gorelik E. Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-activated NK cells: involvement of protein kinase A isozyme I (PKA I) Immunol Res. 2006;36:91–9. doi: 10.1385/IR:36:1:91. [DOI] [PubMed] [Google Scholar]
- 41.Hoskin DW, Mader JS, Furlong SJ, Conrad DM, Blay J. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (Review) Int J Oncol. 2008;32:527–35. [PubMed] [Google Scholar]
- 42.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–65. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 43.Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci. 2008;13:2588–603. doi: 10.2741/2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizumoto N, Kumamoto T, Robson SC, Sevigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–65. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Q, Yan J, Putheti P, Wu Y, Sun X, Toxavidis V, Tigges J, Kassam N, Enjyoji K, Robson SC, Strom TB, Gao W. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant. 2009;9:2303–11. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banz Y, Beldi G, Wu Y, Atkinson B, Usheva A, Robson SC. CD39 is incorporated into plasma microparticles where it maintains functional properties and impacts endothelial activation. Br J Haematol. 2008;142:627–37. doi: 10.1111/j.1365-2141.2008.07230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corriden R, Chen Y, Inoue Y, Beldi G, Robson SC, Insel PA, Junger WG. Ectonucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J Biol Chem. 2008;283:28480–6. doi: 10.1074/jbc.M800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, Capanni M, Umansky V, Paschen A, Sucker A, Pende D, Groh V, Biassoni R, Hoglund P, Kato M, Shibuya K, Schadendorf D, Anichini A, Ferrone S, Velardi A, Karre K, Shibuya A, Carbone E, Colucci F. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest. 2009;119:1251–63. doi: 10.1172/JCI36022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–24. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 50.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 51.Assarsson E, Kambayashi T, Schatzle JD, Cramer SO, von Bonin A, Jensen PE, Ljunggren HG, Chambers BJ. NK cells stimulate proliferation of T and NK cells through 2B4/CD48 interactions. J Immunol. 2004;173:174–80. doi: 10.4049/jimmunol.173.1.174. [DOI] [PubMed] [Google Scholar]
- 52.Hilchey SP, Kobie JJ, Cochran MR, Secor-Socha S, Wang JC, Hyrien O, Burack WR, Mosmann TR, Quataert SA, Bernstein SH. Human follicular lymphoma CD39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J Immunol. 2009;183:6157–66. doi: 10.4049/jimmunol.0900475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Lenzner DE, Jackson EK, Gorelik E, Lang S, Johnson JT, Whiteside TL. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin Cancer Res. 2009;15:6348–57. doi: 10.1158/1078-0432.CCR-09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 285:7176–86. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 107:1547–52. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.