Abstract
Preclinical models have consistently demonstrated the importance of the mesocorticolimbic (MCL) brain reward system in drug dependence, with critical molecular and cellular neuroadaptations identified within these structures following chronic cocaine administration. Cocaine dependent individuals manifest alterations in reward functioning that may relate to changes induced by cocaine or to pre-existing differences related to vulnerability to addiction. The circuit level manifestations of these drug-induced plastic changes and predispositions to drug dependence are poorly understood in preclinical models and virtually unknown in human drug dependence. Using whole-brain resting-state fMRI connectivity analysis with seed voxels placed within individual nodes of the MCL system, we report network-specific functional connectivity strength decreases in cocaine users within distinct circuits of the system, including between ventral tegmental area (VTA) and a region encompassing thalamus/lentiform nucleus/nucleus accumbens, between amygdala and medial prefrontal cortex (mPFC), and between hippocampus and dorsal mPFC. Further, regression analysis on regions showing significant functional connectivity decrease in chronic cocaine users revealed that the circuit strength between VTA and thalamus/lentiform nucleus/nucleus accumbens was negatively correlated with years of cocaine use. This is the first evidence of circuit-related changes in human cocaine dependence and is consistent with the range of cognitive and behavioral disruptions seen in cocaine dependence. As potential circuit level biomarkers of cocaine dependence, these circuit alterations may be usefully applied in treatment development and monitoring treatment outcome.
Introduction
Drug addiction is a serious public health problem that manifests as a compulsive drive to take the drug without regard to severe adverse consequences (Volkow and Li, 2005). Neuroimaging studies in drug dependent individuals have revealed significant alterations in brain structure (Franklin et al., 2002; Matochik et al., 2003), neurotransmitters (Goldstein and Volkow, 2002), metabolism (Volkow et al., 1993), functional activity (Kalivas and Volkow, 2005; Kaufman et al., 2003), and biochemistry (Li et al., 1999; Yang et al., 2009), notably in regions generally considered to be part of the brain s mesocorticolimbic (MCL) reward circuitry. These MCL components include the ventral tegmental area (VTA) and nucleus accumbens (NAcc), involved in responding to rewarding stimuli; the amygdala and hippocampus, involved in memory functions, especially related to learning cue and context associations; mediodorsal thalamus, an intermediary node linking midbrain and prefrontal cortex, and a key component of thalamo-cortico-basal ganglia circuits implicated in aberrant habit learning disorders; and prefrontal/orbitofrontal cortex (PFC/OFC) and anterior cingulate cortex (ACC), involved in emotional regulation, cognition and executive function, especially inhibitory control processes (Everitt and Robbins, 2005).
Cellular and molecular studies in animal models of drug dependence show enduring glutamatergic neuroadaptations in the PFC of rats following chronic cocaine administration, and such alterations appear to play a critical role in drug addiction and subsequent relapse to drug-seeking behavior (Baker et al., 2003). Electrophysiological recordings in rats trained to self-administer cocaine demonstrate significant decreases in the baseline, tonic firing of neurons in the NAcc (Hollander and Carelli, 2007; Peoples and Cavanaugh, 2003). While these and other studies have shown changes within individual MCL components following chronic drug administration, little is known about the consequences of these regional alterations on dynamic, functional interactions among and between MCL components and their projections in human chronic cocaine users. Noninvasive neuroimaging offers an important tool to investigate potential circuit level alterations in this disease.
Intrinsic, dynamic interactions between brain regions can be investigated using synchronized spontaneous fluctuations in the resting-state blood-oxygenation-level-dependent (BOLD) functional magnetic resonance imaging (fMRI) signal (Biswal et al., 1995). Brain functional connectivity maps in the absence of external stimulation have been observed, in awake humans and anesthetized monkeys and rodents, in specific brain circuits, including sensorimotor, visual, auditory, and the “default mode” system (Fox et al., 2005; Greicius et al., 2003; Lowe et al., 1998; Lu et al., 2007; Vincent et al., 2007; Xiong et al., 1999). Altered resting-state functional connectivity (rsFC) has been previously demonstrated in several brain disorders, including Alzheimer s disease (Li et al., 2002), schizophrenia (Liang et al., 2006), major depression (Greicius et al., 2007), epilepsy (Waites et al., 2006), and attention deficit hyperactivity disorder (Cao et al., 2009). Addiction related rsFC changes have recently been reported. Hong et al (2009) reported rsFC between dorsal ACC and striatal regions that correlated negatively with severity of nicotine addiction. Ma et al (2010) reported rsFC from MCL seeds in methadone maintained opiate addicts that indicated stronger connectivity in circuits involved in identifying salient stimuli and weaker connectivity in circuits involved in executive functioning.
While rsFC by definition does not involve task related activation, numerous studies have indicated a relationship between rsFC strength and activation during tasks involving that circuit (Hampson et al., 2006a; Hampson et al., 2006b; Kelly et al., 2008; Seeley et al., 2007). Based upon such a relationship, prominent theories of drug addiction suggest several testable rsFC hypotheses.
Koob and Le Moal propose that repeated drug use results in a shift in hedonic set point such that natural rewards are no longer sufficiently rewarding to motivate behavior such that hyper-physiological drug-induced rewards are sought to provide sufficient stimulation to motivate behavior (Koob and Le Moal, 1997). Based on this theory, reduced rsFC from salience identifying and processing regions including VTA, NAcc, rACC, amygdala and hippocampus might be expected. However, Robinson and Berridge suggest that responses to drug-related stimuli become uniquely sensitized, implying that circuits involved in responding to cues, i.e., those involving VTA, NAcc, amygdala and rACC would show increased rsFC (Robinson and Berridge, 1993). More recent theories emphasizing over-learned habit formation (Di Chiara, 1999; Everitt and Robbins, 2005) would predict increases in circuits involving thalamo-cortico-basal ganglia loops. In addition, hypo-functioning in cognitive control regions, as posited by Jentsch and Taylor (1999) and Goldstein and Volkow (2002) would predict decreased rsFC between prefrontal control regions such as ACC, dorsolateral PFC and medial PFC and areas involved in responding to drug cues such as VTA, NAcc, amygdala and rACC.
Based on the above, we hypothesized specific rsFC circuits related to the MCL system would be altered in chronic cocaine dependence and, as these changes are thought to relate to repeated exposure to cocaine and cocaine cues and to underlie addictive behaviors, we hypothesized that one or more of these circuits will be related to duration of use and/or intensity of use.
Materials and methods
Subjects
Thirty-nine active cocaine users (CU) and thirty-nine healthy controls (HC) were recruited under a protocol approved by the Institutional Review Board of the National Institute on Drug Abuse Intramural Research Program. Signed informed consents were obtained from all participants prior to study enrollment. Potential subjects were assessed with a comprehensive history and physical examination, general laboratory panel, a computerized SCID with clinical interview follow-up, and a guided interview assessing substance use. Subjects were excluded if they had any major illness, history of neurological or psychiatric disorders other than current dependence on cocaine or nicotine (i.e. meeting full criteria within the past month). In an attempt to control for the potential influence of other drugs and alcohol in the CU group, the HC group was allowed to have some current or prior substance use, but no current or past DSM-IV dependence (except nicotine). On the day of scanning, all subjects were assessed for recent alcohol use with breathalyzer and for recent drug use with urine drug screen for amphetamine, barbiturates, benzodiazepines, cocaine, methadone, PCP, tricyclic antidepressants and THC. Participants with a positive breathalyzer report were rescheduled or discharged from the study. Participants who smoked were allowed to smoke ad lib prior to scanning.
Data Acquisition
Functional MRI data were collected on a 3-T Siemens Allegra MR scanner (Siemens, Erlangen, Germany) equipped with a quadrature volume head coil. Head movement was minimized by using a polyurethane foam helmet individually made for each participant. During the 6-min resting scan, participants were instructed to keep their eyes closed and not to think of anything in particular. Thirty-nine 4-mm thick AC-PC parallel slices without interslice gap were prescribed to cover the whole brain. The resting data were acquired using a single-shot gradient-echo echo-planar imaging (EPI) sequence with repetition time (TR) of 2 s, echo time (TE) of 27 ms, flip angle (FA) of 77°, field of view (FOV) of 220×220 mm, and an in-plane resolution of 3.44×3.44 mm. For registration purpose, high resolution anatomical images were acquired using a 3-D magnetization prepared rapid gradient echo (MPRAGE) T1-weighted sequence with TR of 2.5 s, TE of 4.38 ms, FA of 7°, and a voxel size of 1×1×1 mm.
Data Processing
Data processing and analyses were conducted in AFNI (Cox, 1996), SPM5 (Friston et al., 1995) and MATLAB (The MathWorks, Inc., Natick, MA). Preprocessing included slice-timing correction, motion correction, quadratic detrending, and low-pass temporal filtering with a cutoff frequency of 0.1 Hz to retain the low frequency fluctuation components that contribute to functional connectivity (Biswal et al., 1995; Lowe et al., 1998). To facilitate group analysis, resting data were spatially normalized to the standard Talairach space with a resampled resolution of 3×3×3 mm3. Spatial smoothing with a 6 mm Gaussian kernel was performed to increase spatial signal to noise ratio. To further align the resting-state functional data across subjects, an unbiased group-wise nonlinear registration method was used to deform each spatially smoothed image to an implicit group reference image based on a small deformation elastic model (Geng et al., 2009).
Five seed regions were defined by placing bilateral 3-mm spherical regions of interest (ROIs) in the NAcc, amygdala, hippocampus, MD thalamus, and rACC (BA24); a sixth ROI seed encompassed total VTA. (See Table 2 for center coordinates of all seed regions.) The spherical centers were positioned based on the coordinates provided by the Talairach Daemon database (Lancaster et al., 2000) in AFNI (Cox, 1996) where available or literature values (Talairach et al., 1993). Reference time courses from each of the six seed regions were generated by averaging the time courses of all voxels within the ROIs. Subsequently, a cross-correlation coefficient (cc) map for each seed region was obtained by correlating each voxel s time course with the corresponding reference time course. In an attempt to examine the specificity and selectivity of any changes seen in the above six hypothesized MCL regions, seeds were also placed in the primary motor, auditory, and visual cortices, regions not generally associated with the behavioral manifestations of cocaine dependence.
Table 2.
Center coordinates of the seed regions (± indicates seed regions were defined bilaterally.)
| Seed Region | Center Coordinates (Talairch: mm) | |||
|---|---|---|---|---|
| x | y | z | ||
| MCL seeds | Ventral Tegmental Area (VTA) | 0 | −16 | −7 |
| Nucleus Accumbens (NAcc) | ±12 | 8 | −8 | |
| Amygdala | ±23 | −5 | −15 | |
| Hippocampus | ±30 | −24 | −9 | |
| Mediodorsal Thalamus | ±6 | −16 | 8 | |
| Rostral Anterior Cingulate Cortex (BA 24) | ±4 | 36 | 8 | |
| Sensory- motor seeds | Primary Motor Cortex | ±44 | −8 | 38 |
| Primary Auditory Cortex | ±44 | −34 | 14 | |
| Primary Visual Cortex | ±10 | 88 | 5 | |
Global fluctuations, originating presumably from such systemic effects as respiration and cardiac-induced pulsations, were accounted for individually by extracting the first three principal components from the white matter voxel time courses ensemble and the first three principal components from the time courses ensemble of the cerebrospinal fluid (CSF) voxels (Behzadi et al., 2007). The white matter and CSF mask were generated by segmenting the high resolution structural images in SPM5 and down-sampling the obtained white matter and CSF masks to the same resolution as the functional data. In addition to these physiological regressors, time courses of the six motion parameters also served as uninteresting covariates. All regressors were applied before low-pass temporal filtering.
Data Analysis
Before proceeding to group analysis, the CC maps were transformed by Fisher s Z-transformation ( ) to approach a normal distribution. Two-sample t-tests were performed on z-value maps to obtain group functional connectivity maps and to assess group differences between CU and HC subjects for each seed region. A threshold of t(38) > 3.8 with a cluster size of 38 voxels (pcorrected < 0.001 based on Monte Carlo simulations (Cox, 1996)) was used to generate functional connectivity maps for each group; only clusters within grey matter were retained. The grey matter mask was generated in a similar way as the white matter and CSF masks using segmentation of high-resolution structural images and then down-sampling. Considering that partial volume effects (for white and grey matter) are usually large in subcortical regions and image segmentation results are usually less accurate in these regions, the grey matter mask was applied only to the superior part of the brain (z > 6 mm). Functional connectivity differences between the CU and HC group were assessed for each seed using the general linear model with nicotine dependency as a fixed factor. To improve the sensitivity of detection while still controlling for the false positive rate, a threshold of pcorrected < 0.05, t(76) > 2.4 combined with a cluster threshold of 43 – 81 voxels (VTA: 43 voxels; NAcc: 65 voxels; amygdala: 81 voxels; hippocampus: 69 voxels; MD thalamus: 61 voxels; rAcc: 72 voxels), was used to obtain the group difference map, with the restriction that significant clusters must belong to significant regions in one or both groups connectivity maps. The different cluster size thresholds reflect different number of comparisons in the connectivity maps from different seed ROIs.
Connectivity strength and cocaine use variables
To investigate the relationship between rsFC strength and cocaine use, regression analysis against the number of years of cocaine use and current cocaine use with nicotine dependency as a fixed factor were performed using SPSS16.0 in the regions showing group difference (see Table 3) to determine the correlation between the connectivity strength (represented by the average z values) and the cocaine use and dependence measures.
Table 3.
Regions showing significant difference between cocaine users and matched control subjects (CU vs HC).
| Seed Regions | Regions of Difference (Brodmann Area) | Hemisphere | Peak (Talairach) | Peak t | Cluster Size (voxels ) | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| MCL Seeds | VTA | Thalamus/Lentiform nucleus/NAcc | Left | −26 | −17 | 3 | −4.01 | 186 |
| Thalamus | Right | 8 | −20 | 9 | −5.05 | 45 | ||
| Amygdala | Medial prefrontal cortex/Rostral ACC (BA10/9/24) | Bilateral | 2 | 56 | 6 | −4.12 | 316 | |
| Hippocampus | Medial prefrontal cortex/Superior frontal cortex (BA8/6/9) | Bilateral | 5 | 44 | 39 | −3.76 | 85 | |
| Rostral ACC (BA32/10) | Left | −8 | 44 | 3 | −3.80 | 70 | ||
| MD Thalamus | Lentiform nucleus/Putamen | Right | 26 | −8 | −4 | −3.30 | 83 | |
| Lentiform nucleus/Putamen | Left | −29 | −20 | 3 | −3.68 | 62 | ||
| rACC (BA24) | Transverse temporal gyrus/Insula (BA41/13) | Right | 47 | −26 | 12 | −3.56 | 171 | |
| Parahippocampal gyrus/Amygdala/Hippocampus (BA35) | Left | −23 | −20 | −10 | −3.54 | 146 | ||
| Parahippocampal gyrus/Amygdala/Hippocampus | Right | 29 | −17 | −13 | −3.57 | 75 | ||
| NAcc | No difference | |||||||
| Sensory- motor Seeds | Primary Visual (BA17) | Fusiform gyrus/Lingual gyrus (BA18) | Right | 17 | −83 | −22 | 3.89 | 100 |
| Fusiform gyrus/Lingual gyrus (BA18/17) | Left | −17 | −92 | −19 | 3.35 | 94 | ||
| Primary Motor | No difference | |||||||
| Primary Auditory | No difference | |||||||
Effect of Recency of Use
To assess the impact of recency of cocaine use on rsFC strength, the CU group was divided into those who presented on the scan day with a cocaine-positive urine and those with a cocaine-negative urine. A two-sample t-test assessed differences in the combined connectivity map of each seed between the cocaine positive group and the cocaine negative group. A threshold of t(35) > 2.5 combined with a cluster threshold of 43 – 81 voxels, same as the cluster sizes used in generating group difference maps, was used to investigate the effect of recency of use.
Results
Participants in CU group and HC group were matched on gender, age, race, WAIS vocabulary score, and education. There was no significant difference in the number of nicotine dependents in each group (15 dependents in the CU group vs. 9 dependents in the HC group, χ2 = 1.50, p = 0.22). The demographic characteristics of participants and drug use information for both groups are listed in Table 1. A clinical interview based on DSM-IV criteria revealed current cocaine usage of $200±129 (range $38–$560) per week, 13.8±6.3 (range 1–25) years of cocaine use and 4.3±2.0 (range 0–7) DSM-IV dependence criteria met. Thirty-four of thirty-nine CU met DSM-IV criteria for current cocaine dependence. All users reported a frequency of use between daily and weekly, except one CU who reported monthly 3–4 day binges. On the day of scanning, the urine screens of fifteen CUs were negative for all the drugs tested. There were twenty-one individuals in CU group showing positive urine results for cocaine, one of which was also positive for THC. For the remaining three CUs, one CU had urine positive for amphetamine and THC, one CU was positive for THC only, and one CU participant s urine screen results were missing.
Table 1.
Demographic characteristics and substance dependence information for cocaine users and healthy controls
| Cocaine Users (n = 39) | Healthy Controls (n = 39) | |||
|---|---|---|---|---|
| Age (years)1 | 38 ± 6.2 (27–47) | 40 ± 5.1 (25–49) | ||
| Education (years)1 | 13.2 ± 1.7 (10–16) | 12.9 ± 1.3 (10–18) | ||
| WAIS vocabulary score1 | 58 ± 8.2 (45–76) | 58 ± 7.2 (44–73) | ||
| Gender (male/female)1 | 23/16 | 29/10 | ||
| Ethnicity1 | ||||
| African American | 34 | 28 | ||
| Asian | — | 1 | ||
| Caucasian | 3 | 9 | ||
| Hispanic | — | 1 | ||
| mixed | 2 | — | ||
| Drug | Dependence/Abuse/Recreational Use2 | Dependence/Abuse/Recreational Use | ||
| Current | Past | Current | Past | |
| Cocaine | 34/4/1 | 22/10/7 | 0/0/0 | 0/0/0 |
| Alcohol | 0/2/31 | 3/9/23 | 0/1/19 | 0/2/24 |
| Amphatamine | 0 | 1/0/1 | 0 | 0 |
| Barbiturate | 0 | 0/0/1 | 0 | 0 |
| Downer | 0 | 0/1/0 | 0 | 0 |
| Heroin | 0/0/1 | 1/2/4 | 0 | 0 |
| Hallucinogen | 0 | 0/1/1 | 0 | 0 |
| Minor tranquilizer | 0 | 0/0/1 | 0 | 0 |
| Nicotine | 15/2/14 | 12/1/18 | 9/0/5 | 9/0/5 |
| PCP | 0 | 0/1/2 | 0 | 0 |
| Qualude | 0 | 1/0/0 | 0 | 0 |
| THC | 0/5/15 | 4/9/19 | 0/0/0 | 0/0/4 |
Mean ± SD (range) are shown.
There was no significant difference between the groups in age (t(76) = −1.54, p = 0.13), education years (t(76) = 1.05, p = 0.30), WAIS vocabulary score (t(76) = −0.25, p = 0.80), gender (χ2 = 1.44, p = 0.23), or ethnicity (χ2 = 7.58, p = 0.11)
Dependence and abuse classifications were based on DSM-IV criteria. Recreational use included people who had at least 5 lifetime usage of that substance.
Connectivity maps
Although reduced in extent in the CU group, the rsFC maps in the CU and HC subject groups were similar for each of the six MCL system seed regions, and are generally consistent with known anatomical connections as defined from post mortem human and non-human primates (Parent A., 1996). See Fig. 1 for representative connectivity maps from the six MCL seeds. All MCL seed ROIs are functionally connected to other components within the limbic system and its closely related structures. In contrast, seed regions within the primary motor, visual and auditory cortices yielded no limbic system connected components (See Fig. S1 in the supplementary materials).
Figure 1.
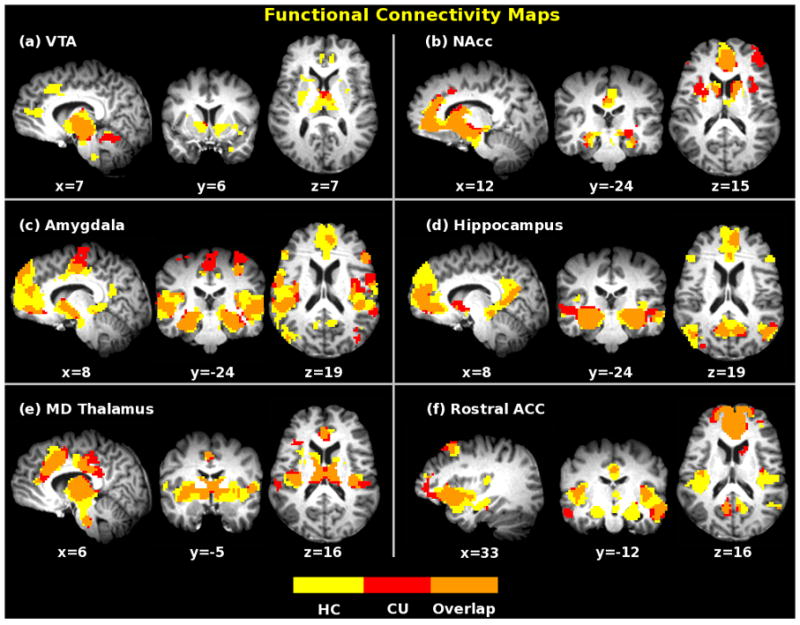
Functional connectivity maps of six MCL seeds for healthy controls (yellow) and cocaine users (red) under resting state (pcorrected < 0.001, with t(38) > 3.8 and a cluster size of 38 voxels). Maps were overlaid together for display purpose. Orange color indicates overlapped areas for both groups.
Tables S1 and S2 in the supplementary materials list all the regions functionally connected to the six seed regions in HC and CU subjects, respectively, and are summarized below. The VTA shows significantly correlated fluctuations with basal ganglia, NAcc, thalamus, parahippocampal area, rACC/mPFC, and medial frontal gyrus. The NAcc seed map includes striatum, mPFC, ACC, PCC, and parahippocampal area. The amygdala shows connectivity with parahippocampal area, mPFC/ACC, striatum, insula, NAcc, temporal gyrus, pre- and postcentral gyri. The hippocampus map encompasses presumed uncinate fasciculus and/or fornical connections with the gyrus rectus and medial frontal cortex as well as projections of non-fornical fibers to the entorhinal cortex and PCC/retrosplenial area. MD thalamus shows connectivity with dorsal and rostral ACC, medial frontal gyrus, insula, and basal ganglia. Finally, the rACC seed demonstrates connections with OFC, amygdala, striatum, NAcc, PCC and insula.
Functional connectivity differences between HC and CU subjects
When compared with matched HC participants, CU individuals show prominent rsFC strength decreases from five of six MCL seed regions; no differences were seen with the NAcc seed (see Fig. 2 for each of the five seed difference maps). The amygdala and the hippocampus seeds show decreased synchrony with unique portions of mPFC. Reciprocal decreased connectivity is seen between rostral ACC and amygdala and between rostral ACC and hippocampus. VTA and MD thalamus seeds both show decreased rsFC with lentiform nucleus/putamen. Decreased rsFC strength was also observed between the VTA seed and bilateral thalamus and right NAcc. Rostral ACC shows decreased strength of connectivity with posterior insula and portions of temporal gyrus in addition to amygdala. Table 3 lists all the regions showing significant rsFC differences in CU compared to HC subjects and Fig. 3 schematically summarizes these group differences. No significant differences between CU and HC subjects were seen when seeds were placed in the primary motor and primary auditory cortices. However, when seeds were placed in the primary visual cortex, significant rsFC increases are seen in bilateral fusiform and lingual gyri. (See Fig. S1 in the supplementary materials.)
Figure 2.
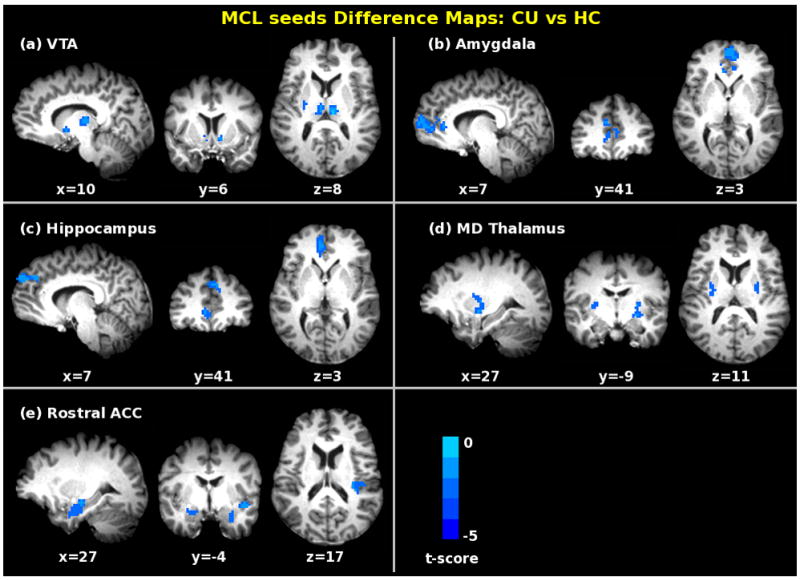
Significant differences in functional connectivity in cocaine addicts as compared to matched control subjects (pcorrected < 0.05 with t(76) > 2.4 and a cluster size of 43 – 81 voxels), when seed regions were located in the (a) VTA; (b) amygdala; (c) hippocampus; (d) MD thalamus; and (e) rostral ACC (BA24). For further details, see Table 3.
Figure 3.
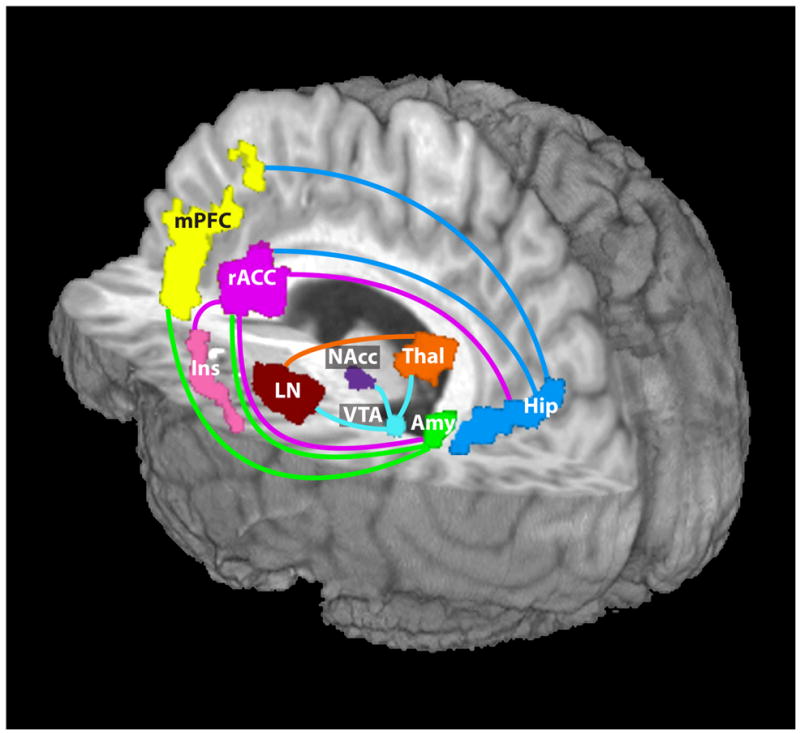
Schematic representation of regions showing decreased functional connectivity (indicated by the colored lines) in cocaine users compared with matched healthy controls. The lines match the color of the seed regions where the decreased connectivity was associated. Amygdala and rACC, as well as the hippocampus and rACC, each showed a reduction when the other was the seed region. VTA showed reduced connectivity to much of the thalamus, including the MD thalamus seed region. VTA: ventral tegmental area; NAcc: nucleus accumbens; Amy: amygdala; Hip: hippocampus; Thal: thalamus; rACC: rostral anterior cingulate cortex; mPFC: medial prefrontal cortex; Ins: insula; LN: lentiform nucleus.
Connectivity strength and years of cocaine use
Regression analysis in the regions showing group connectivity differences reveals a significant negative correlation between the rsFC strength and years of cocaine use in two circuits: seed VTA – right thalamus and seed VTA – left thalamus/left leniform nucleus/right NAcc (pcorrected < 0.05) (Fig. 4). To test for anatomical specificity, the VTA-connected clusters showing significant negative correlations were manually divided into subregions of thalamus and lentiform nucleus based on standard anatomical delineation. The same regression analysis was performed and the resultant connectivity strength regressions between the VTA seed and individual sub-regions all show similar negative correlation with years of cocaine use as that seen in the total clusters. No significant relationship between current cocaine use and functional connectivity strength was seen.
Figure 4.
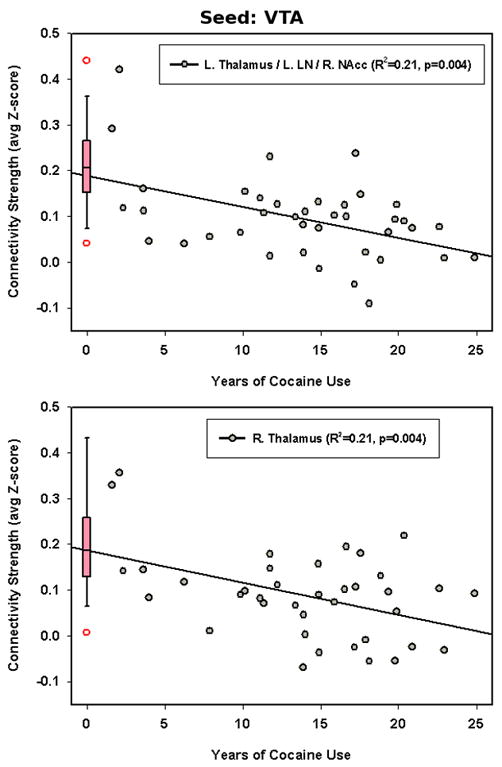
Multiple regression analysis (pcorrected < 0.05) shows negative correlation between rsFC strength (indicated by average z value) and the number of years of cocaine use in the VTA – thalamus/leniform nucleus/NAcc circuit (R = −0.47, puncorrected = 0.004). The box plot on the left summarizes the connectivity strength from the HC group in the corresponding ROI, with the open symbols representing the outliers in the HC group.
Effect of Recency of Use
The cocaine-positive urine group consisted of twenty CUs with urine positive for cocaine only and one CU with urine positive for cocaine and THC. The cocaine-negative urine group included fifteen CUs negative for all drugs tested and one CU with urine positive for THC only to balance one positive THC in the cocaine-positive urine group. There were no significant differences between the two groups on age, gender, race, IQ, and years of cocaine use, however the cocaine-positive urine group had an average of one year less education (12.5±1.2 vs. 13.5±1.2, p=.02). There was no significant difference between the two groups in rsFC strength for any MCL seed except amygdala, where those with positive urine tests show decreased rsFC with left posterior insula (pcorrected < 0.05). In addition, increased rsFC strength was observed in the cocaine-positive urine group between the primary motor cortex seed and left anterior insula. (See Fig. S2 in the supplementary materials.) These regions did not overlap with the regions of difference between CU and HC.
Discussion
In this study, we identified six functional networks related to the MCL system (VTA, NAcc, MD thalamus, amygdala, hippocampus and rACC) based on synchronized resting-state BOLD signal fluctuation. These functional networks involve multiple cortical and subcortical regions that are known to engage in reward processes, learning, memory and emotional regulation and are generally consistent with the known anatomical connections between these regions. When compared with matched healthy controls, cocaine users demonstrate similar network connection but with almost universally reduced connectivity strength for every MCL region studied except the NAcc. In contrast, while the primary motor, auditory and visual cortex seeds show connectivity with expected cortical and thalamic regions, there was no significant difference between the CU and HC group maps when the seeds were in primary motor or auditory cortex; the visual cortex seed did show increased connectivity to bilateral fusiform and lingual gyri in the CU group.
Although rsFC, by definition, does not directly speak either to brain activation during task performance or its correlation with task behavior, rsFC strength has been demonstrated to predict behavioral task performance requiring the use of that circuit (Hampson et al., 2006a; Hampson et al., 2006b; Kelly et al., 2008; Seeley et al., 2007). These observations suggest that synchronous fluctuations during the resting state are related to the ability of particular circuits to perform appropriately when challenged to perform a task. Indeed, Smith et al recently reported that resting networks identified using independent component analysis (a data driven analysis method) in 36 subjects demonstrated virtually identical networks as revealed in a meta-analysis consisting of nearly 30,000 subjects performing cognitive tasks categorized under one or more of 66 behavioral domain classifications, suggesting that functional networks utilized by the brain to perform virtually all cognitive processing are communicating even in the absence of formal task engagement (Smith et al., 2009). Thus, the current results suggest circuit alterations throughout the MCL reward system that might underlie behavioral dysfunctions seen in cocaine dependent individuals.
In particular, the amygdala seed showed decreased rsFC in the CU group with mPFC, a connection known to be important for reversal learning (Chachich and Powell, 1998). This construct has been shown to be deficient in cocaine addicts (Ersche et al., 2008) and is thought to relate to user s difficulty in learning that cocaine cues may no longer signal reward after the development of problematic use. The hippocampus demonstrated decreased rsFC with mPFC, a region known to be involved in the processing of self-relevant information and emotional memories (Buckner et al., 2008). Cocaine users demonstrate a notable lack of ability to recall negative consequences of past use and integrate such memories into decision-making processes (Washton, 1986). Such difficulty in recall could also reflect difficulty in learning from negative outcomes. The reduced rsFC strength of the VTA – NAcc circuit could be reflective of an impaired Temporal Difference Error (TDE) dopamine signaling system, which has been shown to necessary for learning new stimulus-reward associations (Schultz, 2002)
The MD thalamus seed yielded decreased cocaine group rsFC with extensive striatal regions. These circuits are thought to be important for focusing and maintaining desired behaviors while suppressing unwanted behaviors (Haber and McFarland, 2001). Cocaine dependent individuals are known have difficulties with response inhibition (Kaufman et al., 2003; Verdejo-Garcia et al., 2007), which likely contributes to their propensity to relapse in the presence of cocaine-related cues. Finally, seeds in the rACC demonstrated decreased connectivity in CU subjects with the insula and amygdala, areas important for emotional functioning (Stein et al., 2007). Emotional difficulties are characteristic of cocaine withdrawal (American Psychiatric Association., 1994) and are highly comorbid with cocaine use disorders, although the current study excluded those who met diagnostic criteria for mood or anxiety disorders.
The lack of differences from the NAcc seed was unexpected. However, cocaine dependence has been hypothesized to relate to aberrant habit formation. If true, then altered connectivity, most likely increased, might be expected in dorsal, rather than ventral striatum/NAcc, as a shift in striatal circuits from ventral to dorsal has been demonstrated as drug dependence progresses (Porrino et al., 2007).
Ma et al. conducted a study similar to ours in heroin dependent individuals, most of whom were maintained on methadone (Ma et al., 2010). They reported increased functional connectivity from NAcc and amygdala to various frontal regions and decreased functional connectivity between frontal regions involved in executive function. An important difference between the two studies, aside from the obvious difference in drug use, is that their participants were mostly under the acute effects of a drug, i.e., methadone. As pointed out in their discussion, methadone can enhance responses to drug cues (Curran et al., 1999; Langleben et al., 2008) along with profound effects on mu opiate receptor circuits (Gray et al., 2006). The difference between our results may, therefore, relate to the acute drug effects present in their study. Further examination of cocaine users under the influence of acute cocaine and heroin users not in methadone treatment will be needed to resolve these discrepant results.
While none of our participants were acutely intoxicated, twenty-one had positive urine tests for cocaine, indicating use within the last 3–4 days. Recent use was associated with decreased connectivity between the amygdala seed and left posterior insula and with increased connectivity between the primary motor cortex seed and left anterior insula. However, these recency affected regions did not overlap with those regions showing decreased cocaine group rsFC. While the insula is important for processing information on internal bodily sensations that are associated with cocaine use and has been implicated in craving, the specific significance of connectivity from amygdala and motor cortex to insula awaits further investigation.
Our results are in general agreement with findings of reduced gray matter density in medial frontal, rACC and insula in cocaine users (Franklin et al., 2002) and with reduced white matter integrity in inferior frontal regions, also in cocaine dependent individuals (Lim et al., 2008). Gray matter density reductions could result in reduced functional connectivity, depending on the cell populations responsible for the density difference. To the extent that functional connectivity reflect anatomical connections (Krienen and Buckner, 2009), reduced white matter integrity would likely result in reduced functional connectivity.
From a theoretical standpoint, our results are most consistent with the hypothesized reduction in hedonic set point (Koob and Le Moal, 1997). If rsFC strength is related to the ability to engage a circuit when required to perform a task, reduced connectivity throughout the MCL system would suggest a greater than normal stimulation requirement in order to engage reward (and other cognitive) pathways, thus leading to a shift from interest in and the ability of natural rewards to activate the system to the need for supra-physiological, drug-induced rewards to engage MCL circuits. This hypothesis could be directly tested in users in the absence and presence of acute cocaine administration.
Recent evidence strongly points to functional connectivity as reflective of and constrained by underlying neuroanatomical pathways (Krienen and Buckner, 2009). In the present study, the VTA seed connectivity pattern demonstrated the expected extensive relationship with the striatum and prefrontal regions. Somewhat less expected was the strong connectivity seen between the VTA and the thalamus in both HC and CU groups, with large portions of thalamus significantly different between groups. While the VTA is known to project to numerous thalamic nuclei including the MD nucleus, the function of these projections has received little attention in the literature (Oades and Halliday, 1987), despite evidence that MD thalamus lesions reduce cocaine self-administration in rats (Weissenborn et al., 1998). The thalamus has recently been reported to be hypoactive in cocaine users performing a visual attention task, a result hypothesized to be related to attention and perception difficulties in addicts (Tomasi et al., 2007). In light of these findings, it would seem that thalamic functions in cocaine dependent individuals warrant further investigation.
An important issue in interpreting results of a cross-sectional study such as ours is whether differences between groups are a consequence of chronic drug use or alternatively, reflect pre-existing differences that predispose some individuals to addiction. The preclinical literature provides extensive support for the notion that chronic cocaine use produces long-lasting alterations in MCL circuits and indeed, provided the inspiration for our choice of seed regions (Koob and Le Moal, 2001). However, considerable data also point to genetic influences in cocaine dependence (Kreek et al., 2005; Saxon et al., 2005), which necessarily predate the effects of cocaine. As a first step in identifying circuits that have been changed by chronic cocaine use, we correlated connectivity strength with both years of cocaine use and current cocaine usage. A significant negative correlation with years of cocaine use was found between the VTA seed and bilateral thalamus/left lentiform nucleus/right NAcc. Critically, those individuals with fewer years of use showed connectivity strength in the range of healthy controls while those with more years of use showed weaker connectivity strength. Since reduced connectivity is thought to indicate more difficulty volitionally engaging a circuit when needed, this observation appears consistent with the clinical observation that cocaine dependent individuals seek out the supraphysiological stimulation of cocaine over natural rewards (American Psychiatric Association., 1994). Preclinical studies also support the stronger reward activating capacity of cocaine; when given a choice, rats will self-administer cocaine over natural rewards (Grigson and Twining, 2002). Further, only cocaine self administration, but not passive cocaine or food, can induce a persistent VTA synaptic enhancement that is resistant to behavioral extinction, which may represent a fundamental phenomenon driving pathological drug-seeking behavior (Chen et al., 2008). Although the number of years of cocaine use has not been shown to be a predictor of treatment outcome (Poling et al., 2007), these data may still speak to important neuroadaptations that characterize the clinical condition. This above conjecture requires further confirmation, perhaps using reward task activation and choice paradigms in cocaine users,
While most human imaging studies report altered limbic and frontal regions in cocaine users, a possible role for primary sensory and motor region involvement in cocaine dependence has been previously suggested. Kosten et al (Kosten et al., 2006) report that relapse to cocaine use is associated with increased activation to cocaine cues in sensory association and motor cortical areas, along with posterior cingulate cortex. Tomasi et al (Tomasi et al., 2007) showed that cocaine users had increased occipital activation in a sustained visuospatial attention task. While we saw no differences in connectivity strength between the HC and CU groups when seed regions were placed in motor or auditory cortex, we did find increased connectivity between a visual cortex seed with bilateral fusiform and lingual gyri. The significance of these observations is not clear, as extensive behavioral characterization of our subjects requiring visual processing was not performed. Nevertheless, this result, taken together with those cited above, support the importance of understanding plastic changes in brain function between cocaine users and healthy control individuals outside traditional reward related circuits. Finally, the null results with seeds in the primary motor and auditory cortex along with the enhanced visual seed connectivity in the CU group support the selectivity and specificity of the MCL-limited connectivity reductions observed herein.
Several limitations of this study warrant discussion. It should be emphasized that circuit directionality between nodes cannot be determined from rsFC analysis and is thus not implied in these data. The question of directionality of influence in affected circuits will need to be investigated with other techniques. It also should be noted that the presence of direct anatomical connections should not be concluded from functional connectivity analysis, although as noted, evidence strongly supports this assumption. However, as cocaine affects all three major monoamine systems (dopamine, norepinephrine and serotonin) which are thought to, at least in part, have a modulatory neuronal processing function, our observed differences in functional connectivity may relate to altered common input from any one or more of these systems.
Another limitation is that participants were not directly debriefed on what they were thinking about during the resting scan. While no one in the CU group showed overt signs of craving, it is possible that common thought patterns among the addicts could have contributed to the differences seen in this study. Finally, while there was no significant difference in number of dependent smokers in the CU and HC groups, there were more casual smokers among the CU group, reflecting the relatively common occurrence that many cocaine users smoke only when using cocaine. Therefore, it is difficult to completely disentangle effects related to nicotine from those related to cocaine. Insofar as drugs of abuse are thought to utilize a final common pathway involving MCL structures, it might be that our results reflect differences common to both nicotine and cocaine. The lack of differences from MCL seeds when comparing dependent smokers to the rest of the cohort supports the conclusion that our results are largely due to properties unique to the cocaine users. Further studies are needed to address fully this issue.
To the best of our knowledge, this study reports the first circuit level abnormalities in human cocaine users and demonstrates widespread reductions in the connectivity of multiple MCL system components, implying possible difficulty in appropriately activating reward, learning and emotional circuitry in cocaine dependent individuals. It would be of great interest to further investigate how plastic these apparent neural adaptations are and their potential candidacy as biomarkers that can be used to assess treatment matching and outcome prediction. Prospective studies are recommended to explore the relationship between connectivity within MCL pathways and specific behavioral features of addiction.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cao X, Cao Q, Long X, Sun L, Sui M, Zhu C, Zuo X, Zang Y, Wang Y. Abnormal resting-state functional connectivity patterns of the putamen in medication-naive children with attention deficit hyperactivity disorder. Brain Res. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Chachich M, Powell DA. Both medial prefrontal and amygdala central nucleus lesions abolish heart rate classical conditioning, but only prefrontal lesions impair reversal of eyeblink differential conditioning. Neurosci Lett. 1998;257:151–154. doi: 10.1016/s0304-3940(98)00832-5. [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Curran HV, Bolton J, Wanigaratne S, Smyth C. Additional methadone increases craving for heroin: a double-blind, placebo-controlled study of chronic opiate users receiving methadone substitution treatment. Addiction. 1999;94:665–674. doi: 10.1046/j.1360-0443.1999.9456654.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van E, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith RS, Frackowiak SJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Geng X, Christensen GE, Gu H, Ross TJ, Yang Y. Implicit reference-based group-wise image registration and its application to structural and functional MRI. Neuroimage. 2009;47:1341–1351. doi: 10.1016/j.neuroimage.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray AC, Coupar IM, White PJ. Comparison of opioid receptor distributions in the rat central nervous system. Life Sci. 2006;79:674–685. doi: 10.1016/j.lfs.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116:321–333. [PubMed] [Google Scholar]
- Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist. 2001;7:315–324. doi: 10.1177/107385840100700408. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. Journal of Neuroscience. 2006a;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. Neuroimage. 2006b;31:513–519. doi: 10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol Rev. 2005;57:1–26. doi: 10.1124/pr.57.1.1. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O’Brien CP, Childress AR. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165:390–394. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- Li SJ, Li Z, Wu G, Zhang MJ, Franczak M, Antuono PG. Alzheimer Disease: evaluation of a functional MR imaging index as a marker. Radiology. 2002;225:253–259. doi: 10.1148/radiol.2251011301. [DOI] [PubMed] [Google Scholar]
- Li SJ, Wang Y, Pankiewicz J, Stein EA. Neurochemical adaptation to cocaine abuse: reduction of N-acetyl aspartate in thalamus of human cocaine abusers. Biol Psychiatry. 1999;45:1481–1487. doi: 10.1016/s0006-3223(98)00230-3. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci USA. 2007;104:18265–18269. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR. Addiction related alteration in resting-state brain connectivity. NeuroImage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Parent A. Carpenter’s Human Neuroanatomy. 9. Williams & Wilkins; Baltimore: 1996. [Google Scholar]
- Peoples LL, Cavanaugh D. Differential changes in signal and background firing of accumbal neurons during cocaine self-administration. J Neurophysiol. 2003;90:993–1010. doi: 10.1152/jn.00849.2002. [DOI] [PubMed] [Google Scholar]
- Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Saxon AJ, Oreskovich MR, Brkanac Z. Genetic determinants of addiction to opioids and cocaine. Harv Rev Psychiatry. 2005;13:218–232. doi: 10.1080/10673220500243364. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, Missir O. Referentially oriented cerebral MRI anatomy an atlas of sterotaxic anatomical correlations for gray and white matter. G. Thieme Verlag; Stuttgart: 1993. [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, ia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van E, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Li TK. The neuroscience of addiction. Nat Neurosci. 2005;8:1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006;59:335–343. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- Washton AM. Structured outpatient treatment of cocaine abuse. Adv Alcohol Subst Abuse. 1986;6:143–157. doi: 10.1300/J251v06n02_10. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Whitelaw RB, Robbins TW, Everitt BJ. Excitotoxic lesions of the mediodorsal thalamic nucleus attenuate intravenous cocaine self-administration. Psychopharmacology (Berl) 1998;140:225–232. doi: 10.1007/s002130050761. [DOI] [PubMed] [Google Scholar]
- Xiong J, Parsons LM, Gao JH, Fox PT. Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum Brain Mapp. 1999;8:151–156. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<151::AID-HBM13>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Salmeron BJ, Ross TJ, Xi ZX, Stein EA, Yang Y. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users - A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res. 2009;174:171–176. doi: 10.1016/j.pscychresns.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


