Abstract
Aims
Mounting evidence suggests that individuals smoke, in part, to regulate affective experience (e.g., tension reduction, mood enhancement). Implicit in such motives is the expectancy or belief that smoking will decrease negative affect and increase positive affect. The contribution of cognitively-driven expectancies to the initiation and continuation of smoking during adolescence remains largely uninvestigated. The current study examined the influence of negative affect relief expectancies (NAREs) for smoking on smoking behavior and nicotine dependence using longitudinal data from a study on the emotional and social contexts of youth smoking.
Methods
Participants were 568 adolescents with smoking experience (mean age 15.67, 56.7% female). Three separate mixed regression models were estimated to determine the relative contribution of NAREs to smoking behavior and nicotine dependence measured at 4 time points over 2 years.
Results
NAREs for smoking influenced all smoking outcomes at baseline and predicted increases in smoking behavior and nicotine dependence over time, even after controlling for anxious and depressive symptoms and baseline nicotine dependence.
Conclusions
Outcome expectancies for affect management emerged as an important risk factor for smoking escalation and the development of nicotine dependence during adolescence. The present findings highlight the potential importance of cognitively-driven expectancies as a risk factor for smoking escalation during this critical developmental period.
Keywords: Nicotine Dependence, Negative Affect, Smoking, Adolescents, Expectancies
1. Introduction
Although smoking among adolescents has decreased since peaking in 1997 (Johnston et al., 2009), the rate of decline has decelerated (Johnston et al., 2005), and many adolescents smoke despite well documented negative health consequences. This observed trend warrants concern because even minimal experience with smoking during adolescence significantly increases the risk for smoking in adulthood (Chassin et al., 1990). Given the costly health risks associated with tobacco use, further examination of the underlying mechanisms thought to influence smoking behavior in this developmental stage is sorely needed.
Among the many proposed explanatory factors that render smoking reinforcing, affect management has long-been cited by smokers as a primary motive for smoking (e.g., Ikard et al., 1969; Gilbert and Wesler, 1989; Kassel et al., 2003). Indeed, individuals are motivated to engage in cigarette smoking in large part to reduce negative affect (c.f., Kassel et al., 2003). Slight variations of this view of smoking motivation have been articulated within the widely accepted stress-coping (Wills and Shiffman, 1985) and self-medication (Khantzian, 1997) models of substance use, and more recently in the affective processing model of negative reinforcement (Baker et al., 2004b). Implicit in these smoking motives is the expectancy that use of a substance (e.g., nicotine) will facilitate achievement of a particular outcome (e.g., negative affect reduction). Accordingly, outcome expectancies for substance use are the learned beliefs stored in long-term memory about the various consequences of using a substance (e.g., (Heinz et al., 2009; Urbán and Demetrovics, 2010). Moreover, it is important to note that a drug’s effect on reducing negative affect is not necessarily the same phenomenon as its potential to increase positive affect (see Kassel et al., 2007).
Despite mixed evidence as to whether smoking reliably reduces negative affect (see Kassel et al., 2003; Baker et al., 2004a), it is compelling that smokers appear to hold dearly to this expectancy (Wetter et al., 1994; Copeland et al., 1995; Brandon et al., 1996; Brandon et al., 1999; Urbán and Demetrovics, 2010). Outcome expectancies for numerous substances have emerged as unique and powerful predictors of substance use behavior. Furthermore, this cognitive construct has been consistently implicated across different phases and levels of substance use. For instance, expectancies for smoking to help one cope with negative affective states has been identified as an important factor in the maintenance of smoking behavior (Brandon, 1994) as well as smoking relapse (e.g., Hall et al., 1993; Shiffman et al., 1997). They have also been associated with level of nicotine dependence, cessation success, and severity of withdrawal symptoms in adult smokers (Wetter et al., 1994; Copeland et al., 1995).
Several studies of adult smokers have attempted to demonstrate a causal relationship between expectancies and a variety of smoking-related outcomes. Brandon and colleagues (1996) examined the role of expectancies on situation-specific motivation to smoke following a negative affect induction and found that negative reinforcement expectancies predicted ad-lib cigarette consumption in nicotine deprived participants. Further, these expectancies marginally moderated the relationship between negative affect and urge to smoke, suggesting that stronger expectancies for smoking to relieve negative affect significantly increased urge to smoke. Using the balanced placebo design, Juliano and Brandon (2002) found that non-nicotine deprived individuals who expected smoking to relieve negative affect (following an anxious mood induction) experienced improved mood, even when they smoked a placebo cigarette. These findings highlight the need to disentangle the complex mechanisms (i.e., stress-reduction vs. withdrawal alleviation) by which smokers develop and maintain expectancies for smoking to relieve negative affect (Baker et al., 2004a), and subsequently, how these expectancies influence smoking behavior and emotional response.
1.1 Adolescent smoking and outcome expectancies
Burgeoning evidence points to a robust link between negative affect (as indexed by depression and anxiety) and smoking. The large magnitude of this relationship among adolescents is reflected in comorbidity rates of affective disorders and smoking (Kaplan et al., 1984; Reynolds and Rob, 1988; Covey and Tam, 1990), and in the potent risk conferred by smoking for affective disorders. For example, early depression and anxiety in adolescence have been prospectively linked to smoking initiation, experimentation, maintenance, relapse, and nicotine dependence (e.g., Patton et al., 1996; Sonntag et al., 2000). Further, a longitudinal study by Wills and colleagues (2002) on the temporal association of negative affect and smoking in adolescents, showed that despite being dynamically related (Orlando et al., 2001), stress and negative affect (e.g., depression, anxiety) were also etiological risk factors for smoking – not just consequences of smoking (e.g., Parrott, 1999).
Desire to reduce negative affect has also been observed in adolescent smokers and has been shown to demonstrate convergent validity with relevant smoking measures (e.g., Wahl et al., 2005; Hine et al., 2007). Findings from a youth smoking cessation study by Stevens and colleagues (2005) indicated that smoking to reduce negative affect significantly predicted future smoking intentions and lower self-efficacy to quit smoking. Correspondingly, several studies have established the predictive validity of adolescents’ smoking expectancies for subsequent smoking initiation and cessation (Chassin et al., 1981; Chassin et al., 1984; Rose et al., 1996).
Kassel and colleagues (2007) conducted the first laboratory study examining the acute effects of nicotine on emotion in adolescents. Smoking a cigarette resulted in a reduction of negative affect regardless of the nicotine content of the cigarette smoked (i.e., high-yield vs. denicotinized). Importantly, this effect was moderated by affect-related expectancies such that participants who smoked a high-yield cigarette and held strong expectancies for smoking to alleviate negative affect experienced the greatest reductions in negative affect.
Few longitudinal studies have examined possible relationships among smoking expectancies, negative affect, and smoking behavior in adolescents and young adults. Wahl and colleagues (2005) found that expectancies about the ability of cigarettes to reduce negative affect were associated with different developmental trajectories of smoking behavior in a sample of 273 8th and 10th grade early experimenters. Relative to adolescents who experimented with smoking but did not escalate, adolescents’ who reported increased smoking (over a period of eighteen months) held significantly stronger baseline expectancies for smoking to alleviate negative affect. Adolescents’ expectancies for smoking to facilitate negative affect control have also been found to mediate the effects of both baseline current smoking and peer smoking on smoking behavior three months later (Hine et al., 2002). Cohen and colleagues (2002) reported that at least part of the relationship between negative affect and change in smoking behavior over time was explained by negative reinforcement smoking expectancies. Furthermore, a recent cross-sectional analysis of college freshman and sophomores revealed that negative affect relief expectancies for smoking fully mediated the positive relationship between depressive symptoms and level of smoking behavior (Schleicher et al., 2009). Taken together, these studies suggest that expectancies for affect regulation are related to smoking behavior in a variety of important and meaningful ways.
To date, the influence of affectively laden outcome expectancies on the etiological pathway to nicotine dependence and related smoking behaviors has yielded intriguing preliminary findings. However, the extent to which expectancies for smoking to relieve negative affect predict changes in nicotine dependence and smoking behaviors over time among adolescents has not been determined. The current study examined whether expectancies for smoking to relieve negative affect predicted changes in smoking behaviors (i.e., number of days smoked in the past month, number of cigarettes smoked per day in the past month) and nicotine dependence over the course of 24 months. In addition, gender differences were examined because females traditionally report higher levels of negative affect, specifically depression and anxiety, than males. Anxious and depressive symptoms were also hypothesized to positively predict smoking outcomes. Accordingly, the current study employed multilevel linear and non-linear models to address these research questions. Based upon influential theories of addiction emphasizing the role of negative reinforcement (e.g., Baker et al., 2004b), we hypothesized that stronger expectancies for smoking to relieve negative affect would predict increases in smoking behavior and nicotine dependence over the course of two years.
2. Methods
2.1 Overview of Design
Data for this study come from the baseline, 6-month, 15-month, and 24-month assessment waves of a longitudinal study of the social and emotional contexts of adolescent smoking patterns funded by the National Cancer Institute (total n of 1,263).
2.2 Participants
Participants were recruited from sixteen Chicago area high-schools. The sample was derived in a multi-stage process. All 9th and 10th graders at the schools (N = 12,970) completed a brief screening survey of smoking behavior to determine eligibility for the study. Students were eligible to participate if they fell into one of four levels of smoking experience: 1) never smokers; 2) former experimenters (smoked ≥ 1 cigarette in the past, have not smoked in the last 90 days, and have smoked < 100 cigarettes in their lifetime); 3) current experimenters (smoked in the past 90 days, but smoked < 100 cigarettes in lifetime); and 4) regular smokers (smoked in the past 30 days and have smoked ≥ 100 cigarettes in their lifetime). As the objective of the current study was to assess the role of adolescents’ expectancies on smoking experience, only data from participants who endorsed having smoked at least once in the past thirty days (at baseline) were analyzed (N = 568). Data were collected from 568 (at baseline), 519 (at 6-months), 502 (at 15-months), and 505 (at 24-months) participants at each time point, respectively.
2.3 Procedures
The “Health Habits” portion of the longitudinal study used self-report, paper-and-pencil questionnaires that were mailed to the participants a few weeks prior to each data collection wave. Participants were instructed to complete the questionnaires and bring them to an appointment set up by the field team. Participants received a payment of $20 for each completed questionnaire that was returned at each time point.
2.4 Measures
Demographic information was collected using paper-and-pencil self-report questionnaires and included gender, grade, age, and race/ethnicity. The following measures were collected at each time point.
Smoking Expectancies
Negative affective relief expectancies (NAREs) for smoking were measured with one subscale from the Smoking Expectancies Scale (), based on Brandon and Baker’s (1991) Smoking Consequences Questionnaire. Participants were asked to indicate their agreement with a series of 10 questions regarding smoking (i.e., “Smoking helps calm me down when I feel nervous; When I’m upset with someone, a cigarette helps me cope.”). Responses were made on a 4-point scale where 1 = disagree, 2 = disagree a little, 3 = agree a little, and 4 = agree and averaged [within-waves] to create the overall scale score. Coefficient alpha for the overall scale was high (coefficient alpha = .91).
Modified Fagerstrom Tolerance Questionnaire (mFTQ)
Nicotine dependence was measured using the seven-item version of the Fagerstrom Tolerance Questionnaire modified for use with adolescent smokers (Prokhorov et al., 1996; Prokhorov et al., 1998; Prokhorov et al., 2000). A total score was created by averaging all items (coefficient alpha = .66).
Current Smoking Behavior – Smoking in past month
The number of days smoked in the past month was assessed with the item, “Now think about the past 30 days. On how many days did you smoke or try cigarettes?” Response options ranged from “0 days” to “all 30 days” and numbers of days were separated by 9 categories. The number of cigarettes smoked on the days that participants endorsed smoking was assessed with the item, “Think about the past 30 days. On the days you smoked cigarettes, about how many cigarettes did you smoke each day?” Response options ranged from “I did not smoke during the past 30 days” to “more than 20 cigarettes per day,” and amounts were separated into 11 different categories. In order to avoid non-proportionality owing to sparsity in higher categories, categories 10 (one pack a day) and 11 (more than 20 cigarettes per day) were collapsed into one category for analyses.
Mood & Anxiety Symptom Questionnaire (MASQ)
Adolescent anxiety and depressive symptoms were assessed with 12 items from the 90-item MASQ (Watson and Clark, 1991; Watson et al., 1995). The shortened version of the scale used in the current study contains items from each of the three MASQ subscales – general distress symptoms, anxious arousal, anhedonic depression symptoms. Adolescents rated the extent to which they had experienced each symptom in the past week according to a 5-point Likert-type scale, ranging from 1 (not at all) to 5 (extremely). Item scores were averaged to yield an overall negative affect score, with strong internal reliability (coefficient alpha = .81).
2.5 Data Analyses
The current study employed the HLM6 program (Raudenbush, 2004) to estimate a series of multilevel linear and non-linear ordinal regression models to test whether nicotine dependence and smoking behaviors over time could be explained as a function of negative affect relief expectancies, symptoms of anxiety and depression, and gender. Briefly, the non-linear models adhere to the “threshold concept” (Bock, 1975) which implies that an unobserved continuous latent variable underlies the observed ordinal responses. The latent variable is therefore characterized by increasing thresholds (or cutpoints) that function to separate and assign values of the latent variable into the observed ordinal responses (Hedeker, & Mermelstein, 2000). With these thresholds in place, HLM6 provides estimates of cumulative probabilities which are the expected log odds that if given X, the value of a latent variable (Y) falls within a specified range (i.e., is less than or equal to a specified value – the threshold). Of note, these models are characterized by the proportional odds assumption whereby the predictor, X, is believed to affect the odds ratio for every category of Y in the same way. Accordingly, expected differences in X do not depend on a particular category of the dependent variable Y (see Raudenbush, & Bryk, 2002). Lastly, random effects for the intercept and linear time slopes were explored in all models to determine whether their inclusion provided a better fit of the data.
Selected time-varying covariates (i.e., Negative Affect Relief Expectancies, Anxiety-Depression subscale of the MASQ) were partitioned to test the assumption of homogeneity of between- and within-subjects’ effects. Briefly, this assumption implies that the mean (Xi) of the time-varying covariate X for all observations j of person i (i.e., between-subjects time constant variable) is roughly the same as person i’s deviation around their mean (Xij - Xi; within-subjects time-varying variable). Decomposition of the these effects allows one to determine the degree to which an individuals’ deviation around their mean of predictor variable X is associated with change in the outcome variable Y (e.g., Hedeker et al., 2006). Meaningful within-subject fluctuations were anticipated for expectancies because the sample employed was at high-risk for smoking escalation; individual variation in anxiety and depression was examined as mood variability is common in adolescence. The assumption of homogeneity of the between- and within-subjects effects for covariates was rejected in the current study because the decomposed effects provided a better fit of the data (AIC - Akaike’s Information Criterion; Akaike, 1981), produced a notable difference in regression coefficient sizes, and/or accounted for more variance explained in the dependent variable (compared to the non-partitioned variable).
Restricted Maximum Likelihood (REML) with robust standard errors was employed to estimate the initial fully specified model and the final model for the mFTQ, so as to yield unbiased estimates of the variance and covariance parameters. Full Maximum Likelihood (ML) was used to estimate the ordinal multi-level models. ML estimation with robust or “Huber-corrected” standard errors for regression coefficients from all models was used in the model fitting and trimming process. Model trimming for all outcome variables was conducted such that nonsignificant (p > .05) covariates were removed individually until a parsimonious model was obtained. The amount of variance in nicotine dependence [mFTQ] scores explained by Negative Affect Relief Expectancies (after controlling for MASQ scores representing anxious and depressive symptomology) was derived by calculating the difference in level 1 residual variance values with and without NAREs included in the model. The amount of variance in smoking behavior outcomes attributed to NAREs was not calculated because methods for deriving a pseudo-R2 for ordinal regression models are not available in HLM6 and are more generally, not well developed.
2.6 Level Descriptions
The level 1 model was constructed to explain variance in outcome variables within subjects over the four time points. The regression coefficients in the level 1 equation (i.e., fixed effects) included the intercept, the linear time trend, and the quadratic time trend which is the level of the dependent variable at baseline, the average linear time slope of the dependent variable, and the average quadratic time slope of the dependent variable, respectively. Time-varying covariates and the residuals (i.e., observed versus predicted values of Y for subject i), were also included on level 1. The level 2 model was constructed to explain variance between subjects. In addition, time-invariant moderators were entered on the intercept and fixed cross-level interactions were entered on the linear time and quadratic time slopes (continuous moderators were grand-mean centered). Briefly, variables entered on the intercept function as [predicted] moderators of baseline levels of the dependent variable whereas variables entered on the linear and quadratic time slopes test for interactive effects of time and the proposed variable in relation to level of the dependent variable. Lastly, random effects were entered to determine the presence of reliable within-subject random variation on the intercept and linear time slopes. For each outcome variable, model 1 examined the relationships between smoking outcomes and time (e.g., baseline, linear and quadratic time slopes) and model 2 examined the contribution of specified covariates in explaining the proposed relationships.
3. Results
3.1 Descriptive Statistics
Of the 568 participants in the current study, 56.7% were female, approximately half (49.3%) were 9th graders at baseline, and participants’ mean age was 15.67 years (SD = .62) at baseline. The majority of participants were Caucasian (59.5%), 20.1% were Hispanic, 12% were African American, 2.1% were Asian/Pacific Islander, 0.4% were American Indian and 6.0% were Other/Unknown. Descriptive statistics for smoking behaviors, nicotine dependence, and other proposed covariates for the entire sample at each time point are provided in Table 1. The results of the initial (i.e., model 1) and final (i.e., model 2) models for each dependent variable are presented in Tables 2 and 3, respectively. Of note, the mFTQ was analyzed using a continuous normal model (REML) whereas smoking behaviors were analyzed using an ordinal logistic mixed model. As such, the results generated from the ordinal models are described in terms of logarithmic odds.
Table 1.
Descriptive Statistics for Predictor Variables, Covariates, and Smoking Outcomes
| Baseline | 6 months | 15 months | 24 Months | |
|---|---|---|---|---|
| N = 568 | N = 519 | N = 502 | N = 505 | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
|
Anxious Depressive Symptomology (MASQ) |
17.19(8.29) | 16.44(8.31) | 15.02(7.83) | 14.32(7.21) |
| Negative Affect Relief Expectancies | 1.35(.96) | 1.31(1.02) | 1.36(1.06) | 1.22(1.03) |
|
Modified Fagerstrom Tobacco Questionnaire |
1.88(1.27) | 1.98(1.43) | 2.15(1.53) | 2.21(1.59) |
| N, % | N, % | N, % | N, % | |
| Number of Days Smoked in Past 30 Days | ||||
| 0 days | 0 by design | 155, 30.1% | 174, 34.9% | 178, 35.2% |
| 1day | 144, 25.4% | 51, 9.9% | 44, 8.8% | 26, 5.1% |
| 2–3 days | 131, 23.1% | 47, 9.1% | 36, 7.2% | 35, 6.9% |
| 3–5 days | 50, 8.8% | 39, 7.6% | 30, 6.0% | 25, 5.0% |
| 6–7 days | 52, 9.2% | 24, 4.7% | 14, 2.8% | 18, 3.6% |
| 8–10 days | 46, 8.1% | 41, 8.0% | 25, 5.0% | 23, 4.6% |
| 11–20 days | 48, 8.5% | 50, 9.7% | 30, 6.0% | 36, 7.1% |
| 21–29 days | 48, 8.5% | 50, 9.7% | 50, 10.0% | 59, 11.7% |
| 30 or more days | 49, 8.6% | 58, 11.3% | 96, 19.2% | 99, 19.6% |
| Number of Cigs Smoked per Day on Days Smoked, in Past 30 Days | ||||
| 0 cigs | 17, 3.0% | 154, 29.9% | 177, 35.3% | 180, 36% |
| <1 cig | 189, 33.3% | 65, 12.5% | 57, 11.4% | 44, 8.8% |
| 1 cig | 128, 22.5% | 78, 15.0% | 60, 12.0% | 52, 10.4% |
| 2 cigs | 102, 18% | 66, 12.7% | 33, 6.6% | 43, 8.6% |
| 3 cigs | 47, 8.3% | 56, 10.8% | 43, 8.6% | 52, 10.4% |
| 4 cigs | 29, 5.1% | 25, 4.8% | 27, 5.4% | 37, 7.4% |
| 5 cigs | 26, 4.6% | 22, 4.2% | 32, 6.4% | 15, 3.0% |
| 6–10 cigs | 23, 4.0% | 36, 6.9% | 45, 9.0% | 49, 9.8% |
| 11–19 cigs | 4, .7% | 10, 1.9% | 14, 2.8% | 20, 4.0% |
| 20 cigs | 2, .4% | 4, .8% | 10, 2.0% | 6, 1.2% |
| 20 plus cigs | 1, .1% | 3, .5% | 4, .5% | 2, 0.4% |
Table 2.
Initial Model Regression Coefficients for Fixed and Random Effects with Robust Standard Errors
| Outcome Variable | Effects | Fixed Effects | Random Effects |
|---|---|---|---|
| Coefficient(SE) | Variance Component(SD) | ||
| Nicotine Dependence |
Intercept | 1.88(.06)** | 1.18(1.08)** |
| Linear Time Slope | 0.28(.10)* | 0.26(0.51)** | |
| Quadratic Time Slope | −0.05(.05) | ||
| ICC+ | 60.17% | ||
| Num. Cigs Per Day | Intercept | −6.41(.33)** | 2.12(1.46)** |
| on Days Smoked | Linear Time Slope | −.47(.23)* | 1.32(1.15)** |
| Quadratic | .17(.11) | ||
| ICC++ | 49.61% | ||
| Num. Days Smoked |
Intercept | −3.02(.14)** | 2.18(1.48)** |
| Linear Time Slope | −.94(.23)** | 1.49(1.22)** | |
| Quadratic Time Slope | 0.44(.11)** | ||
| ICC++ | 52.49% |
Note.
p< .05
p< .001.
ICC = Intraclass Correlation
= ρ = (τ00 / σ2 + τ00)
= ρ = (σ2/ σ2+ π2/3)
Table 3.
Final Model Regression Weights for Fixed and Random Effects with Robust Standard Errors
| Model 1 | Fixed Effect | Term | Coefficient (SE) | t-ratio | |
|---|---|---|---|---|---|
| MFTQ | Intercept | βoj | 1.602(.086)** | 18.734 | |
| Nicotine | Mean Expectancy | γ01 | .687(.058)** | 11.935 | |
| Dependence | Male | γ02 | .272(.092)* | 2.968 | |
| Linear Time Slope | β1ij | .290 (.110)* | 2.651 | ||
| Mean Expectancy | γ11 | .162(.037)* | 4.355 | ||
| Male | γ12 | .112(.061)*** | 1.842 | ||
| Quadratic Time Slope | β2ij | −.088(.055) | −1.613 | ||
| MASQ | β3ij | .008(.003)* | 2.632 | ||
| Expectancy Variability | β4ij | .303(.036)** | 8.453 | ||
| Random Effect | Term | Variance Component (SD) | |||
| Intercept | U0i | .793(.890)** | |||
| Linear Time Slope | U1i | .187(.433)** | |||
| Level 1 Error Variance | Rij | .590(.768) | |||
| Model 2 | Effect | Term | Coefficient (SE) | t-ratio | |
|---|---|---|---|---|---|
| Num. of Cigarettes Smoked | Intercept | βoj | −8.901(.383)** | −23.237 | |
| per day (Past 30 days) | Mean Expectancy | γ01 | .510(.088)** | 5.780 | |
| Male | γ02 | .133(.143) | .926 | ||
| Linear Time Slope | β1ij | −1.183(.233)** | −5.068 | ||
| Mean Expectancy | γ11 | 1.776(.271)** | 6.553 | ||
| Male | γ12 | .358(.127)* | 2.819 | ||
| Quadratic Time Slope | β2ij | .409 (.117)* | 3.492 | ||
| Mean Expectancy | γ21 | −.670 (.133)** | −5.040 | ||
| MFTQ | β3ij | .928(.053)** | 17.614 | ||
| MASQ | β4ij | −.003(.006) | −.536 | ||
| Expectancy Variability | β5ij | .573(.075)** | 7.611 | ||
| Random Effect | Term | Variance Component (SD) | |||
| Intercept | U0i | .336(.580)* | |||
| Linear Time Slope | U1i | .441(.664)** | |||
| Model 3 | Effect | Term | Coefficient (SE) | t-ratio | |
|---|---|---|---|---|---|
| Number of Days Smoked | Intercept | βoj | −5.105(.244)** | −20.878 | |
| in the Past 30 Days | Mean Expectancy | γ01 | .769(.093)** | 8.230 | |
| Male | γ02 | −.107(.142) | −.752 | ||
| Linear Time Slope | β1ij | −1.749(.241)** | −7.270 | ||
| Mean Expectancy | γ11 | 1.505(.285)** | 5.276 | ||
| Male | γ12 | .284(.133)* | 2.140 | ||
| Quadratic Time Slope | β2ij | .757(.121)** | 6.234 | ||
| Mean Expectancy | γ21 | −.516 (.143)* | −3.614 | ||
| MFTQ | β3ij | 1.028(.063)** | 16.240 | ||
| MASQ | β4ij | −.018(.007)* | −2.682 | ||
| Expectancy Variability | β5ij | .726(.077)** | 9.465 | ||
| Random Effect | Term | Variance Component (SD) | |||
| Intercept | U0i | .411 (.641)* | |||
| Linear Time Slope | U1i | .645(.803)** | |||
Note.
p< .05
p< .001
p<.06.
i = Person, j = Time. MFTQ = Modified Fagerstrom Tobacco Questionnaire; MASQ = Mood and Anxiety Symptom Questionnaire
3.2 Nicotine Dependence as Measured by the mFTQ
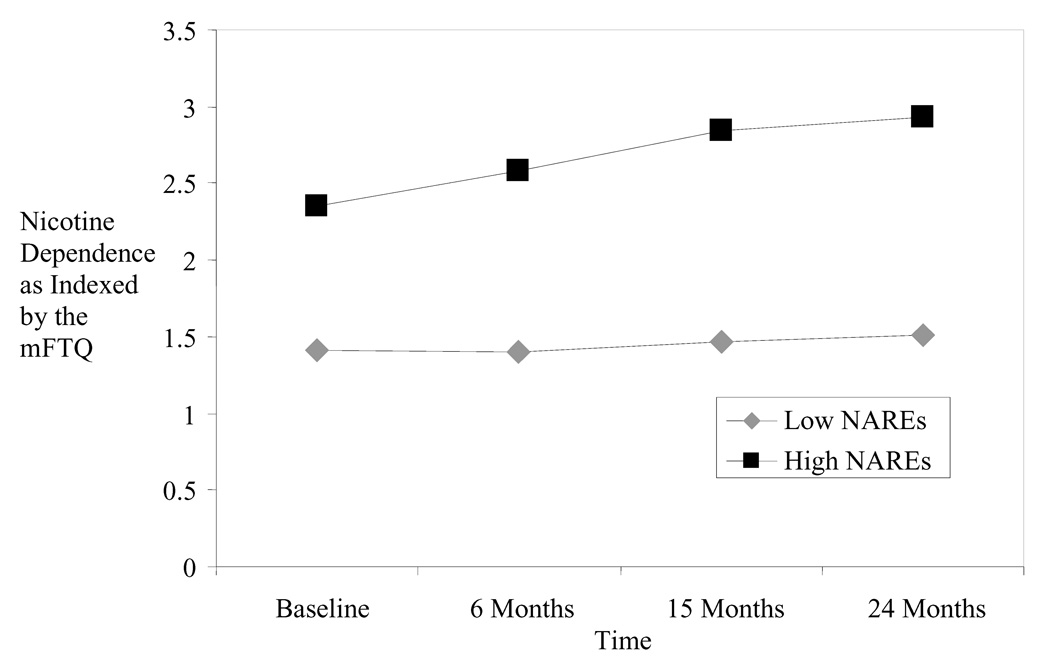
At baseline participants with higher mean levels of negative affect relief expectancies (NAREs) for smoking had higher mFTQ scores (γ02 = .69, p < .001) and males had significantly higher mFTQ scores (2.05) compared to females (1.75) (γ01 = .27, p < .01); though it should be noted that these mean mFTQ scores are indicative of low levels of dependence. The sample as a whole demonstrated increases in nicotine dependence over the course of the study and a significant random effect of time emerged, indicating that there was individual heterogeneity in mFTQ scores over time (σ2U1i = .79(.89), p < .001). In addition, there was a significant linear time slope by mean NARE cross level interaction (γ11 = .16 (.04), p < .001) such that participants with higher mean NARE levels demonstrated more positive mFTQ slopes (i.e., demonstrated a faster increase in nicotine dependence) whereas this was not the case for participants with lower mean NARE levels. A significant quadratic time slope was not detected. Variance in mFTQ scores was also accounted for by anxious and depressive symptomology (β3 = .01, p < .05; 1.50% of the variance) and by within subject NARE variation (β4 = .30, p < .001). After controlling for growth curve models (i.e., linear and quadratic time slopes) and MASQ scores, within subject variation in NAREs explained 1.06% of the variance in mFTQ scores; nondecomposed NARE scores explained 1.70% of the variance. Figure 1 illustrates the relationship between mFTQ scores and time as a function of high and low mean NAREs (derived from a median split).
Figure 1.
Nicotine dependence, as measured by the MFTQ, plotted over time as a function of high and low mean level of negative affect relief expectancies derived from a median split.
3.3 Number of Cigarettes smoked per day in the past 30 days
At baseline, the expected log odds of smoking zero cigarettes per day in the past thirty days [versus all other amounts] for an adolescent with an average level of mean NAREs and a random effect of zero, was −8.90 (i.e., low probability of group membership). Correspondingly, the expected log-odds for an adolescent smoking zero cigarettes or less than one cigarette per day versus all other amounts was slightly higher (−7.46; −.8.90 + 1.44[THOLD 2]). Participants’ mean NAREs moderated the expected log odds of smoking zero cigarettes per day versus all other amounts of cigarettes at baseline (γ01 =.51, p < .001). That is, the expected log odds of smoking zero cigarettes per day versus all other amounts decreased as mean level of NAREs increased. The negative coefficient for the linear time slope (β1= −1.18(.23); p < .001) indicated that over time, the expected log-odds of reporting zero cigarettes per day in the past 30 days versus all other amounts was decreased by a factor of 1.18. A significant mean NARE by linear time slope fixed cross level interaction (γ11 = 1.78, p < .001) indicated that over time, the expected log odds of smoking zero cigarettes per day (versus all other amounts) only decreased for participants with higher mean NAREs. Participant gender also interacted with time such that males reported smoking more cigarettes per day than females over time (γ12 = .36, p < .01). A significant quadratic time slope (β4= .41(.12); p < .01) was found suggesting that the expected log odds for smoking zero cigarettes per day versus all other amounts decreased towards the end of the assessment period. However, as evidenced by a significant mean NARE by quadratic slope cross level interaction (γ41 = −.67, p < .001), the effect of NAREs on number of cigarettes smoked per day became less pronounced.
The expected log odds for number of cigarettes smoked per day was also accounted for by nicotine dependence (β3= .93, p < .001) and within-subject NARE variation (β5= .57, p < .001), though not by anxious and depressive symptomology (i.e., MASQ scores). For example, at baseline, a unit increase in NARE decreased the odds of smoking zero cigarettes per day relative to all other amounts of cigarettes by a factor of 1.33 (exp(.57 *.499914)= 1.33), holding all other variables constant at their means. In the same fashion, a unit increase in nicotine dependence decreased the odds of endorsing smoking zero cigarettes per day relative to all other amounts of cigarettes by a factor of 1.59 (exp(.93 *.499914)) at baseline.
3.4 Number of days smoked in the past thirty days
At baseline, the expected log-odds of smoking zero days in the past month relative to all other amounts of days for an adolescent with an average level of mean NAREs and a random effect of zero, was −5.12. Correspondingly, the expected log-odds for an adolescent smoking zero days or 1 day versus all other amounts of days at baseline was −3.60 (−5.12+ 1.52). At baseline, participants’ mean NAREs moderated the expected logs odds of smoking zero days versus all other amounts of days (γ01 =.769, p < .001). Thus, holding all other variables constant, the expected log odds of smoking zero days versus all other amounts decreased as mean level of NAREs increased. The significant negative linear time slope indicated that the expected log odds of having smoked zero days versus all other amounts of days was −1.75 (β1= −1.75(.24); p < .001), holding all other variables constant. A significant mean NARE by linear time slope cross level interaction (γ11 = 1.51, p < .001) indicated that over time, the expected log odds of smoking zero days versus all other amounts only decreased for participants with higher mean NAREs. Furthermore, participant gender interacted with time to predict number of days smoked such that males had a higher likelihood of endorsing categories representing more days smoked than females (γ12 = .284, p < .05). A significant and positive quadratic time slope (β5= .76(.12); p < .001) indicated that the expected log odds for smoking zero days versus all other amounts of days increased towards the end of the assessment period.
Of note, a significant mean NARE by quadratic slope cross-level interaction (γ51 = −.52, p < .01) indicated that the effect of NAREs on number of days smoked became less pronounced towards the end of the assessment period. Variance in the expected log odds of having smoked zero days versus all other number of days was partially explained by participant level of variation in NAREs (β5 =.73, p < .001). For instance, at baseline, holding all other variables constant at their means, for every one unit increase in NARE, the expected log odds of smoking zero days versus smoking all other amounts of days, decreased by a factor of 1.78 (exp(.79 *.73)). The variance in number of days smoked was also accounted for by nicotine dependence (i.e., mFTQ scores; β3= 1.03, p < .001). At baseline, a unit increase in nicotine dependence decreased the expected log odds of smoking zero cigarettes per day relative to all other amounts of cigarettes by a factor of 2.26 (exp(1.03 *.79)). Contrary to what was predicted, anxious and depressive symptomology [as indexed by the MASQ] were inversely related to the number of days smoked such that higher MASQ scores were related to fewer number of days smoked (β4= −.02, p < .01).
Note that no NARE by MASQ interaction was found among any of the smoking outcomes. Further, individual’s mean MASQ scores, averaged over time, did not moderate the level of smoking outcomes at baseline, nor did it interact with the linear and quadratic time slopes. Table 2 presents the initial model regression coefficients (covariates not included) for fixed and random effects for all models while Table 3 provides regression coefficients for fixed and random effects in the final models.
4. Discussion
Although a host of explanatory mechanisms underlying adolescent smoking are well characterized in the literature, the role of cognitively-driven risk factors in smoking escalation and progression to nicotine dependence is not fully understood. As such, the intersection of affect and cognition has drawn increased attention as an area rich with plausible etiological risk factors for smoking initiation and escalation. However, no prior studies have explicitly examined the link between negative affect relief expectancies (NAREs) for smoking and smoking outcomes using longitudinal data with within-subject approach. Accordingly, the objective of the current study was to assess the influence of cognitively-driven expectancies for smoking to ameliorate negative affect on nicotine dependence and smoking behaviors over time, in a sample of adolescents with smoking experience. In addition, we sought to determine whether gender, individuals’ levels of anxious and depressive symptomology over time (as indexed by the MASQ), and individual’s NAREs over time moderated baseline smoking outcomes and interacted with linear and quadratic time slopes.
Our analyses indicated that within-subject variability in NARE (the degree to which a single observation deviates from an individual’s overall mean) predicted increases in nicotine dependence and smoking behavior over time, even after controlling for anxious and depressive symptoms and nicotine dependence (for smoking behaviors). Individual mean NAREs positively moderated all smoking outcomes at baseline such that higher NAREs were associated with higher levels of nicotine dependence and smoking behavior at the beginning of the assessment period. Additionally, at baseline, gender moderated level of nicotine dependence (males had significantly higher mFTQ scores than females). Individual’s mean NAREs also interacted with linear time slopes such that only stronger expectancies were associated with increases in smoking outcomes over time. As predicted, nicotine dependence scores explained variance in smoking behaviors over time and anxious and depressive symptoms, as measured by the MASQ, were associated with increases in nicotine dependence. By contrast, MASQ scores were associated with decreases in the number of days smoked in the past 30 days. MASQ scores were not related to the number of cigarettes smoked per day in the past 30 days. The inconsistency of these relationships in considered below.
Although the contribution of higher mean NAREs to smoking behavior was initially pronounced, quadratic time slope by mean NARE interactions revealed that only high mean NAREs were associated with decreased smoking behavior toward the end of the assessment period. Upon closer examination of the data, we observed that participants with lower mean NAREs had far steeper negative linear time slopes (i.e., less smoking behavior over time) than participants with high mean NAREs. However, toward the end of assessment, the slope became significantly less steep for participants with low mean NAREs but not for those with high mean NAREs. Taken together, this pattern may suggest that adolescents with stronger expectancies are initially more vulnerable to smoking escalation, though as expectancies become stronger with increased smoking experience, their effect becomes less pronounced, or stabilizes. Alternatively, there may be a ceiling effect for adolescents at the higher end of smoking such that the ability of NARE’s to predict smoking behavior is constrained because both variables are range limited at this point in the assessment period. Further research is needed to characterize the patterns by which smoking expectancies and smoking experience are reciprocally determined in adolescence.
Several explanations have been proffered for the well-documented relationship between negative affect and smoking. For instance, Choi and colleagues (1997) proposed that adolescents who experience difficultly with negative affect regulation are more sensitive to the effects of nicotine and are thus more likely to employ smoking as a primary coping mechanism. This line of reasoning introduces an important caveat to the current study in that we did not differentiate among sources of negative affect over time and whether they elicited similar expectancies for smoking. Perhaps negative affect was primarily engendered by stress early in the assessment period, though toward the end of the study, as smoking became a primary coping mechanism, the source of negative affect shifted to nicotine withdrawal. In their review of motivational influences on smoking, Baker and colleagues (2004a) echoed this concern and concluded that additional research is required to disentangle the mechanisms by which smokers develop and maintain expectancies for smoking to relieve negative affect. We can only speculate that adolescents may initially smoke in part to reduce stress-related negative affect, though over time, with increased smoking experience, withdrawal-related negative affect comes to sustain smoking behaviors (see also Audrain-McGovern et al., 2009).
Several other limitations should be acknowledged. First, deviation in NAREs (the extent to which participant’s specific observations deviated from their overall mean at each time point) predicted a significant, though relatively small, amount of variance in nicotine dependence scores (variance explained could not be calculated for ordinal models – though addition of NAREs significantly improved model fit). As such, other variables (e.g., established smoking risk factors) are needed to explain additional variance in the smoking outcomes assessed. Second, results from the current study are derived from a sample with smoking experience and therefore may not generalize to questions addressing smoking initiation or first experiences.
Lastly, assessing anxiety and depression in the context of a single measure (the MASQ) may have masked the independent contributions of each construct to smoking outcomes in this study. Specifically, there is some evidence to suggest that anxiety symptoms may yield a prophylactic effect on smoking initiation (e.g., Kassel et al., 2003). Future studies should assess anxiety and depression separately to determine the independent influence of each construct on smoking outcomes. It may also be the case that anxiety and depression, at sub-clinical levels in particular, may be better characterized as more proximal antecedents of situational smoking, which our global measures did not capture. Nevertheless, our findings indicated that anxious and depressive symptomology, as assessed by the MASQ, were related to some smoking outcomes. Further research is needed to determine whether treatment of subthreshold levels of emotional distress proves effective.
In sum, the current study is the first to establish a predictive relationship between negative affect relief expectancies and increases in nicotine dependence and smoking behavior over time in adolescents. To the extent that expectancies are modifiable, interventionists should aim to challenge and cognitively restructure these potent mechanisms to improve smoking-related outcomes. Additionally, better coping mechanisms for addressing negative affect should be disseminated among youth who attribute their smoking or failed cessation attempts to this particular consequence. Future studies should continue to identify the underlying mechanisms and processes that influence and subserve youth vulnerability to nicotine dependence, especially as they relate to affect regulation.
Contributor Information
Adrienne J. Heinz, Department of Psychology, 1007 W. Harrison St. Behavioral Sciences Building, Room 1009, Chicago, IL 60607
Jon D. Kassel, Department of Psychology, 1007 W. Harrison St. Behavioral Sciences Building, Room 1009 Chicago, IL 60607, , (O) 312-413-9162, (F) 312-413-4122, jkassel@uic.edu
Michael Berbaum, Institute for Health Research and Policy, University of Illinois at Chicago, 1747 West Roosevelt Road, Room 558, Chicago, IL 60608
Robin Mermelstein, Institute for Health Research and Policy, University of Illinois at Chicago, 1747 West Roosevelt Road, Room 558, Chicago, IL 60608
References
- Akaike H. Likelihood of a model and information criteria. Journal of Econometrics. 1981;16:3–14. [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Kassel JD. Adolescent smoking and depression: evidence for self-medication and peer smoking mediation. Addiction. 2009;104:1743–1756. doi: 10.1111/j.1360-0443.2009.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annual Review Of Psychology. 2004a;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004b;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bock RD. Multivariate statistical methods in behavioural research. New York: McGraw Hill; 1975. [Google Scholar]
- Brandon TH. Negative Affect As Motivation To Smoke. Curr Dir Psychol. 1994;3:33–37. [Google Scholar]
- Brandon TH, Baker TB. The Smoking Consequences Questionnaire: The subjective expected utility of smoking in college students. Psychological Assessment. 1991;3:484–491. [Google Scholar]
- Brandon TH, Juliano LM, Copeland AL. Expectancies for tobacco smoking. In: Kirsh I, editor. How Expectancies Shape Experience American Psychological Association. Washington, DC: 1999. pp. 263–299. [Google Scholar]
- Brandon TH, Wetter DW, Baker TB. Affect, expectancies, urges, and smoking: Do they conform to models of drug motivation and relapse? Exp Clin Psychopharmacol. 1996;4:29–36. [Google Scholar]
- Chassin L, Corty E, Presson CC, Olshavsky RW, Bensenberg M, Sherman SJ. Predicting Adolescents Intentions To Smoke Cigarettes. Journal Of Health And Social Behavior. 1981;22:445–455. [PubMed] [Google Scholar]
- Chassin L, Presson CC, Sherman SJ, Corty E, Olshavsky RW. Predicting the onset of cigarette smoking in adolescents: A longitudinal study. Journal of Applied Social Psychology. 1984;14:224–243. [Google Scholar]
- Chassin L, Presson CC, Sherman SJ, Edwards DA. The Natural-History Of Cigarette-Smoking - Predicting Young-Adult Smoking Outcomes From Adolescent Smoking Patterns. Health Psychol. 1990;9:701–716. doi: 10.1037//0278-6133.9.6.701. [DOI] [PubMed] [Google Scholar]
- Choi WS, Patten CA, Gillin JC, Kaplan RM, Pierce JP. Cigarette smoking predicts development of depressive symptoms among US adolescents. Annals Of Behavioral Medicine. 1997;19:42–50. doi: 10.1007/BF02883426. [DOI] [PubMed] [Google Scholar]
- Cohen LM, McCarthy DM, Brown SA, Myers MG. Negative affect combines with smoking outcome expectancies to predict smoking behavior over time. Psychol Addict Behav. 2002;16:91–97. doi: 10.1037//0893-164x.16.2.91. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Brandon TH, Quinn EP. The smoking consequences questionnaire adult: Measurement of smoking outcome expectancies of experienced smokers. Psychological Assessment. 1995;7:484–494. [Google Scholar]
- Covey LS, Tam D. Depressive Mood, The Single-Parent Home, And Adolescent Cigarette-Smoking. American Journal Of Public Health. 1990;80:1330–1333. doi: 10.2105/ajph.80.11.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DG, Wesler R. In: Emotion anxiety and smoking. Ney T, Gale A, editors. Chichester, England: Smoking and Human Behavior Wiley; 1989. pp. 171–196. [Google Scholar]
- Hall SM, Munoz RF, Reus VI, Sees KL. Nicotine, Negative Affect, And Depression. J Consult Clin Psychol. 1993;61:761–767. doi: 10.1037//0022-006x.61.5.761. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Berbaum M, Mermelstein R. Location scale models for multi-level ordinal data: between and within subjects variance modeling. Journal of Probability and Statistical Science. 2006 [Google Scholar]
- Hedeker D, Mermelstein R. Making the most out of data analysis and interpretation: Analysis of longitudinal substance use outcomes using ordinal random-effects regression models. Addiction. 2000;95:S381–S394. doi: 10.1080/09652140020004296. [DOI] [PubMed] [Google Scholar]
- Heinz AJ, Kassel JD, Smith EV. Caffeine expectancy: Instrument development in the Rasch measurement framework. Psychology of Addictive Behaviors. 2009;23:500–511. doi: 10.1037/a0016654. [DOI] [PubMed] [Google Scholar]
- Hine DW, Honan CA, Marks ADG, Brettschneider K. Development and validation of the smoking expectancy scale for adolescents. Psychol Assess. 2007;19:347–355. doi: 10.1037/1040-3590.19.3.347. [DOI] [PubMed] [Google Scholar]
- Hine DW, McKenzie-Richer A, Lewko J, Tilleczek K, Perreault L. A comparison of the mediational properties of four adolescent smoking expectancy measures. Psychol Addict Behav. 2002;16:187–195. [PubMed] [Google Scholar]
- Ikard FF, Green D, Horn D. A scale to differentiate between types of smoking as related to management of affect. International Journal Of The Addictions. 1969;4:649–659. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Decline in Teen Smoking Appears to be Nearing its End. 2005 Retrieved May 11th, 2008 from http://www.monitoringthefuture.org/pressreleases/05cigpr.pdf.
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Smoking continues gradual decline among U.S. teens, smokeless tobacco threatens a comeback. Ann Arbor, MI: University of Michigan News Service; 2009. Dec 14, Retrieved March 26, 2010 from http://www.monitoringthefuture.org. [Google Scholar]
- Juliano LM, Brandon TH. Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. Journal Of Abnormal Psychology. 2002;111:88–97. doi: 10.1037//0021-843x.111.1.88. [DOI] [PubMed] [Google Scholar]
- Kaplan SL, Landa B, Weinhold C, Shenker IR. Adverse Health Behaviors And Depressive Symptomatology In Adolescents. Journal Of The American Academy Of Child And Adolescent Psychiatry. 1984;23:595–601. doi: 10.1016/s0002-7138(09)60353-8. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Evatt DP, Greenstein JE, Wardle MC, Yates MC, Veilleux JC. The acute effects of nicotine on positive and negative affect in adolescent smokers. J Abnorm Psychol. 2007;116:543–553. doi: 10.1037/0021-843X.116.3.543. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review Of Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Orlando M, Ellickson PL, Jinnett K. The temporal relationship between emotional distress and cigarette smoking during adolescence and young adulthood. Journal Of Consulting And Clinical Psychology. 2001;69:959–970. doi: 10.1037//0022-006x.69.6.959. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Does cigarette smoking cause stress? American Psychologist. 1999;54:817–820. doi: 10.1037//0003-066x.54.10.817. [DOI] [PubMed] [Google Scholar]
- Patton GC, Hibbert M, Rosier MJ, Carlin JB, Caust J, Bowes G. Is smoking associated with depression and anxiety in teenagers? American Journal Of Public Health. 1996;86:225–230. doi: 10.2105/ajph.86.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhorov AV, De Moor C, Pallonen UE, Hudmon KS, Koehly L, Hu SH. Validation of the modified Fagerstrom Tolerance Questionnaire with salivary cotinine among adolescents. Addict Behav. 2000;25:429–433. doi: 10.1016/s0306-4603(98)00132-4. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, Koehly LM, Pallonen UE, Hudmon KS. Adolescent nicotine dependence measured by the modified Fagerstrom tolerance questionnaire at two time points. J Child Adolesc Subst Abus. 1998;7:35–47. [Google Scholar]
- Prokhorov AV, Pallonen UE, Fava JL, Ding L, Niaura R. Measuring nicotine dependence among high-risk adolescent smokers. Addict Behav. 1996;21:117–127. doi: 10.1016/0306-4603(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Raudenbush S. HLM 6. Lincolnwood, IL: Scientific Software International Inc; 2004. [Google Scholar]
- Raudenbush S, Bryk AS. Hierarchical linear models: Application and data analysis methods. Second ed. Vol. 1. Thousand Oaks, California: Sage Publications; 2002. [Google Scholar]
- Reynolds I, Rob MI. The Role Of Family Difficulties In Adolescent Depression, Drug-Taking And Other Problem Behaviors. Medical Journal Of Australia. 1988;149:250. doi: 10.5694/j.1326-5377.1988.tb120597.x. [DOI] [PubMed] [Google Scholar]
- Rose JS, Chassin L, Presson CC, Sherman SJ. Prospective predictors of quit attempts and smoking cessation in young adults. Health Psychology. 1996;15:261–268. doi: 10.1037//0278-6133.15.4.261. [DOI] [PubMed] [Google Scholar]
- Schleicher HE, Harris KJ, Catley D, Nazir N. The Role of Depression and Negative Affect Regulation Expectancies in Tobacco Smoking Among College Students. J Am Coll Health. 2009;57:507–512. doi: 10.3200/JACH.57.5.507-512. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Richards T, Kassel JD. Individual differences in the context of smoking lapse episodes. Addict Behav. 1997;22:797–811. doi: 10.1016/s0306-4603(97)00063-4. [DOI] [PubMed] [Google Scholar]
- Sonntag H, Wittchen HU, Hofler M, Kessler RC, Stein MB. Are social fears and DSM-IV social anxiety disorder associated with smoking and nicotine dependence in adolescents and young adults? European Psychiatry. 2000;15:67–74. doi: 10.1016/s0924-9338(00)00209-1. [DOI] [PubMed] [Google Scholar]
- Stevens SL, Colwell B, Smith DW, Robinson J, McMillan C. An exploration of self-reported negative affect by adolescents as a reason for smoking: Implications for tobacco prevention and intervention programs. Prev Med. 2005;41:589–596. doi: 10.1016/j.ypmed.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Urbán R, Demetrovics Z. Smoking outcome expectancies: A multiple indicator and multiple cause (MIMIC) model. Addict Behav. 2010 doi: 10.1016/j.addbeh.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Wahl SK, Turner LR, Mermelstein RJ, Flay BR. Adolescents' smoking expectancies: Psychometric properties and prediction of behavior change. Nicotine Tob Res. 2005;7:613–623. doi: 10.1080/14622200500185579. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. Mood and Anxiety Symptom Questionnaire. 1991 [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss M, McCormick RA. Testing a tripartite model I: Discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Smith SS, Kenford SL, Jorenby DE, Fiore MC, Hurt RD, Offord KP, Baker TB. Smoking Outcome Expectancies - Factor Structure, Predictive-Validity, And Discriminant Validity. J Abnorm Psychol. 1994;103:801–811. doi: 10.1037//0021-843x.103.4.801. [DOI] [PubMed] [Google Scholar]
- Wills TA, Sandy JM, Yaeger AM. Stress and smoking in adolescence: A test of directional hypotheses. Health Psychology. 2002;21:122–130. [PubMed] [Google Scholar]
- Wills TA, Shiffman S. In: Coping and substance use: A conceptual framework. Shiffman S, Wills TA, editors. New York: Coping and Substance use Academic Press; 1985. pp. 3–24. [Google Scholar]