Abstract
Background & Aims
NOD-like receptors are recently described cytosolic pattern recognition receptors. NOD1 and NOD2 are members of this family that recognize bacterial cell wall components, diaminopimelic acid and muramyl dipeptide, respectively. Both NOD1 and NOD2 have been associated with many inflammatory diseases, although their role in liver inflammation and infection has not been well studied.
Materials and Methods
We investigated the role of NOD receptors in mouse liver by assessing expression and activation of NOD1 and NOD2 in liver and primary isolated hepatocytes from C57BL/6 mice.
Results
Both NOD1 and NOD2 mRNA and protein were highly expressed in hepatocytes and liver. RIP2, the main signaling partner for NODs, was also expressed. Stimulation of hepatocytes with NOD1 ligand (C12-iEDAP) induced NFκB activation, activation of MAP kinases and expression of chemokines CCL5 (RANTES) and CXCL1 (KC). C12-iEDAP also synergized with interferon (IFN)γ to increase iNOS expression and production of nitric oxide. Despite activating NFκB, NOD1 ligand did not upregulate hepatocyte production of the acute phase proteins lipopolysaccharide binding protein, serum amyloid A, or soluble CD14 in cell culture supernatants, or upregulate mRNA expression of lipopolysaccharide binding protein, serum amyloid A, C-reactive protein, or serum amyloid P. NOD2 ligand (MDP) did not activate hepatocytes when given alone, but did synergize with Toll-like receptor ligands, lipopolysaccharide (LPS), and polyI:C to activate NFκB and MAPK.
Conclusions
All together these data suggest an important role for hepatocyte NOD1 in attracting leukocytes to the liver during infection and for hepatic NLRs to augment innate immune responses to pathogens.
Keywords: infection, inflammation, pattern recognition receptors, innate immunity, liver
INTRODUCTION
NOD-like receptors (NLRs) are a recently described family of pattern recognition receptors that have been associated with many inflammatory diseases in humans, highlighting their significant immunologic role [1]. NLRs are found in the cell cytosol, in contrast to membrane-associated pattern recognition receptors such as Toll-like receptors (TLRs), and contain a central nucleotide-binding and oligomerization (NOD) domain with a leucine-rich repeat domain responsible for pathogen sensing [2]. There are two major subgroups within NLRs: NODs and NACHT, leucine rich repeat and pyrin domain-containing proteins (NALP)s. NODs have amino terminal caspase activation and recruitment (CARD) domains [3], which allow them to associate with other CARD containing signaling adaptor molecules. NOD1 and NOD2 have been shown to associate with RIP2/RICK, via CARD-CARD interactions, which allow RIP2 to associate with TRAF6/TAK1 [4]. Subsequent signaling leads to activation of NFκB and upregulation of inflammatory mediators, such as interleukin (IL)-6 [4].
Specific ligands have been identified that stimulate NOD1 and NOD2. These ligands are all components of bacterial cell walls [5]. NOD1 responds to meso-diaminopimelic acid (DAP), a muropeptide found on most Gram-negative bacteria. NOD2 senses both Gram-positive and Gram-negative bacteria through peptidoglycans (PGN) and muramyl dipeptide (MDP) [5].
The liver is a sentinel organ in a unique position to monitor pathogen-associated molecules in the portal and systemic circulations. It is increasingly recognized that not only immune cells but also the parenchymal cells of the liver, including hepatocytes and liver endothelial cells, play important roles in the immune response to a wide range of liver problems, from alcoholic liver disease to acetaminophen toxicity to liver I/R injury. Hepatocytes represent the largest cell mass in the liver and we have shown these cells express TLRs. Our work [6-8] and the work of others [9,10] have shown that hepatocytes respond directly to TLR ligands and danger signals, and act together with non-parenchymal cells such as Kupffer cells (KC, resident liver macrophages) and dendritic cells (DC). The liver, and its multiple cell types, including hepatocytes, are therefore central components that initiate and regulate innate immune pathways. Little is known about the expression or function of NLRs in specific liver cell populations such as hepatocytes.
In the present study, we sought to determine whether hepatocytes express functional NOD receptors and whether NOD expression is altered in response to specific NOD or TLR ligands. We also examined the response of the liver in vivo to NOD ligand exposure. Our data show high expression of both NOD1 and NOD2 and that hepatocytes and liver respond to NOD1 ligands to activate NFκB, which results in increased CC and CXC chemokine release and increased nitric oxide (NO) production. Stimulation with NOD1 ligand did not, however, upregulate the production or release of acute phase proteins in hepatocytes. NOD2 ligand, MDP, did not by itself activate hepatocytes but did synergize with TLR ligands and lead to NFκB translocation to the nucleus.
MATERIALS AND METHODS
Reagents
Ultrapure LPS (Escherichia coli 0111:B4) from List Biological Laboratories, Inc. (Vandell Way, CA). Endotoxin-free C12-iEDAP and MDP from Invivogen (San Diego, CA). Cytokines: IFNγ (Cell Sciences Inc, Canton, MA), TNF (R&D systems, Minneapolis, MN), IL1β (Leinco Technologies, St. Louis, MO). Rabbit anti-mouse phospho-ERK, ERK, phospho-p38, p38, JNK, phospho-JNK from Cell Signaling Technologies (Beverly, MA). Anti-NOD1 and NOD2 from Imgenex (San Diego, CA). Anti-RIP2 from ProSci Inc., Poway, CA. NF-κB consensus-oligonucleotides from Promega (Madison, WI). ELISAs: RANTES, MIG, KC, MCP-1 from R&D Systems (Minneapolis, MN); LBP from Hycult Biotechnologies (Netherlands); SAA from Biosource (Camarillo, CA).
Animals
Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. C57BL/6 mice were from Charles River Laboratories (Wilmington, MA). C57BL/10 and TLR4-/- (C57BL/10ScN) mice were from Jackson Laboratories. MyD88-/- mice were on a C57BL/6 background ( a kind gift from R. Medzhitov [HHMI, New Haven]). LPS2 (TRIF-/-) mice were a kind gift from B. Beutler (Scripps Institute, CA). All mice used were specific pathogen-free, between 8-10 weeks old, and allowed rodent chow and water ad libitum. For in vivo studies mice were injected intraperitoneally with LPS (5 mg/kg), MDP (10 mg/kg), or iEDAP (5 mg/kg).
Hepatocyte isolation and cell culture
Hepatocytes were isolated from mice by an in situ collagenase (type VI; Sigma) perfusion technique, modified as described previously [11]. Hepatocyte purity exceeded 99% by flow cytometric assay, and viability was typically over 95% by trypan blue exclusion. Hepatocytes (150,000 cells/ml) were plated on gelatin-coated culture plates in Williams medium E with 10% calf serum, 15 mM HEPES, 10-6M insulin, 2 mM L-glutamine, 100 U/ml penicillin, 100 U/ml streptomycin. Hepatocytes were allowed to attach to plates overnight and prior to treatments the cell culture media was changed to serum-free media.
Analysis of chemokine, acute phase protein, and nitrite levels in cell culture supernatants
Chemokine and acute phase protein levels were detected in cell culture supernatants and plasma by ELISA according to the manufacturers’ instructions. Nitrite levels in cell supernatants were detected by Greiss reaction.
Immunoblotting and EMSA
Treated hepatocytes were washed twice in PBS. Cells were lysed, and Western blots performed as previously described [7]. Nuclei were also extracted from some cells as previously described, and NFκB was detected by EMSA as previously described [7].
Comparative PCR
Total RNA was extracted from hepatocytes or liver using RNeasy mini extraction kits from Qiagen (Valencia, CA) according to the manufacturer's protocol. cDNA was synthesized using 1μg RNA and oligo dT primers (Qiagen) and Omniscript™ reverse transcriptase (Qiagen). PCR reaction mixtures were prepared using SYBR Green PCR master mix (PE Applied Biosystems, Foster City, CA). SYBR Green two-step real-time RT-PCR was performed using forward and reverse primer pairs prevalidated and specific for NOD1, NOD2, RIP2, LBP, SAA, SAP, and CRP (Qiagen). All samples were run in triplicate. The level of gene expression for each sample was normalized to β-actin mRNA expression using the comparative Ct method.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Experimental results are analyzed for their significance by the Student's t-test. Significance was established at the 95% confidence level (p <0.05).
RESULTS
Hepatocytes highly express NOD1, NOD2, and RIP2
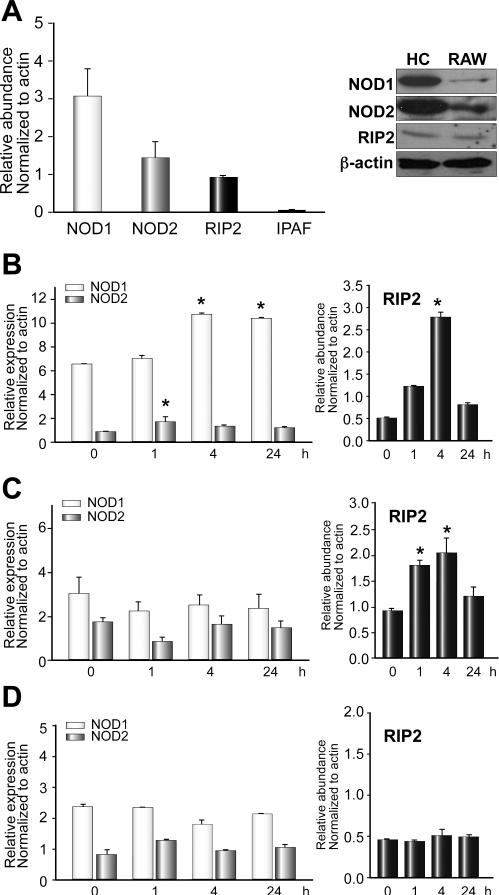
We first wanted to determine whether primary-isolated mouse hepatocytes expressed NOD1, NOD2, and RIP2 at baseline. We isolated RNA or collected whole-cell lysates from C57BL/6 mouse hepatocytes cultured overnight and performed quantitative PCR using validated specific primers for NOD1, NOD2, and RIP2 as well as Western blot analysis for NOD1, NOD2, and RIP2. Hepatocytes highly expressed both NOD1 and NOD2 mRNA and protein at baseline (Fig. 1A). Similarly RIP2 expression was also easily detected (Fig. 1A). Not all NLRs were expressed. IPAF, an NLR family member known to specifically recognize intracellular flagellin, was not expressed in hepatocytes (Fig. 1A).
Fig. 1. Expression of NOD1, NOD2, and RIP2 in primary isolated hepatocytes.
(A, left): Baseline mRNA expression of NOD1, NOD2, RIP2 and IPAF in primary isolated hepatocytes measured by quantitative PCR. Expression level normalized to actin and relative to baseline expression level in RAW264.7 macrophages (known expressers of NLRs). (A, right images): Baseline protein expression of NOD1, NOD2, and RIP2 in primary isolated hepatocytes analyzed by Western blot. Hepatocyte mRNA expression levels of NOD1, NOD2 and RIP2 at baseline (time 0), 1h, 4h, and 24h after stimulation with LPS (B), C12-iEDAP (C), MDP (D). *p <0.05 vs baseline expression. N = 3-4 for PCR experiments, and Western blot images representative of at least three separate immunoblots.
We then wanted to determine whether expression of NOD1, NOD2, or RIP2 in hepatocytes changed over time after stimulation with LPS (TLR4-ligand), C12-iEDAP (NOD1 ligand), or MDP (NOD2 ligand). NOD1 and NOD2 mRNA expression increased significantly between 1 and 4h after stimulation with 100ng/ml LPS (Fig. 1B, left). However, the functional significance of this increase is hard to appreciate as the expression of both NOD1 and NOD2 is already high. However, there was a large increase in mRNA expression of RIP2 by 4h after LPS stimulation (Fig. 1B, right), with levels nearly five times those at baseline. A similar pattern of change in expression of RIP2 mRNA was also seen after stimulation of hepatocytes with C12-iEDAP (Fig. 1C), suggesting that regulation of expression is not specific to one ligand or stimulus. NOD1 and NOD2 expression did not change, however, after stimulation for 24h with C12-iEDAP. These data may suggest that regulation of NOD1 and NOD2 signaling in hepatocytes is primarily through regulation of levels of RIP2. There was no significant increase in expression of NOD1, NOD2, or RIP2 up to 24h after stimulation of hepatocytes with MDP (Fig. 1D).
We then determined whether NOD1, NOD2, and RIP2 expression could be detected in mouse liver at baseline and at 24h after intraperitoneal injection of LPS, MDP or C12-iEDAP. No increase in expression of NOD1, NOD2, and RIP2 was measured in liver after LPS, C12-iEDAP, or MDP compared with baseline expression (data not shown). Even at 48h no significant difference in NOD1, NOD2, or RIP2 mRNA expression was determined. The discrepancy between changes of expression in hepatocytes and whole liver may reflect different patterns of NOD1, NOD2, and RIP2 expression in different liver cell types following specific stimuli.
NOD1 but not NOD2 ligand directly activates NFkB in hepatocytes
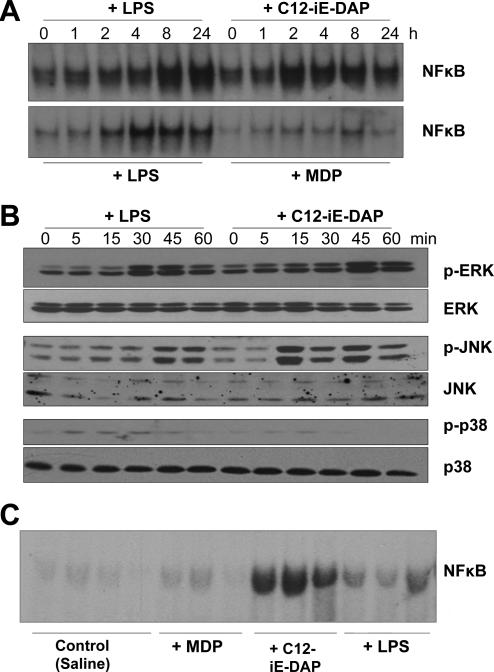
Having determined that hepatocytes express and upregulate NOD1, NOD2, and RIP2, we then wanted to determine whether hepatocytes are activated by specific ligands for NOD1 and NOD2. Activation of NOD1 and NOD2 pathways through RIP2 has been previously shown in other cell types to lead to NFκB activation [4]. Stimulation of mouse hepatocytes with C12-iEDAP activated NFκB to similar, or even stronger levels than stimulation with LPS (Fig.2A). However, MDP stimulation resulted in only a minimal increase of NFκB activation in hepatocytes (Fig.2A). These data are consistent with the literature suggesting that iE-DAP, and in particular C12-iEDAP, is able to enter cells more rapidly and with better efficiency than MDP [5] to activate intracellular NOD receptors.
Fig. 2. Increased NFκB and MAPK activation in hepatocytes after treatment with NOD1 ligand (C12-iEDAP) but not NOD2 ligand (MDP).
(A): Primary isolated mouse hepatocytes were treated for up to 60 min with LPS (100 ng/ml), C12-iEDAP (100 ng/ml) or MDP (10 μg/ml) and NFκB level detected in nuclear extracts by EMSA. (B): Whole cell lysates from primary isolated mouse hepatocytes treated for up to 60 min with LPS, C12-iEDAP, or MDP were immunoblotted for phosphorylated (active) ERK, JNK, and p38MAPK, as well as total ERK, JNK, and p38MAPK. (C): Liver was harvested from C57BL/6 mice (n = 3 or 4 per experimental group) 24h after intraperitoneal injection with saline (Control), MDP (10 mg/kg), C12-iEDAP (5 mg/kg), or LPS (5 mg/kg) and NFκB level detected in nuclear extracts by EMSA. Images representative of results obtained from at least 3 separate experiments.
Analysis of whole cell lysates from similarly treated hepatocytes showed that C12-iEDAP was also able to rapidly activate MAP kinases. ERK phosphorylation was increased by 45 min after stimulation with C12-iEDAP to similar levels as after stimulation with LPS (Fig.2B). JNK was also phosphorylated early by C12-iEDAP, and even more strongly than after LPS stimulation, although there was less phospho-p38MAPK detected compared with cells treated with LPS (Fig. 2B). MAP kinases were not activated after treatment with MDP, even after longer stimulation (data not shown).
NFκB activation in liver tissue was also assessed at 24h after intraperitoneal injection of either LPS (5 mg/kg), C12-iEDAP (5 mg/kg), or MDP (10 mg/kg). Similarly to in vitro results, C12-iEDAP strongly activated NFκB in the liver, to a greater extent than LPS at this time point (Fig. 2C). MDP, however, showed minimal activation of NFκB in the liver at 24h after stimulation (Fig. 2C). These data confirm that NOD1 is functional in vivo after stimulation with specific ligand.
NOD1 activation induces CC and CXC chemokine production in hepatocytes
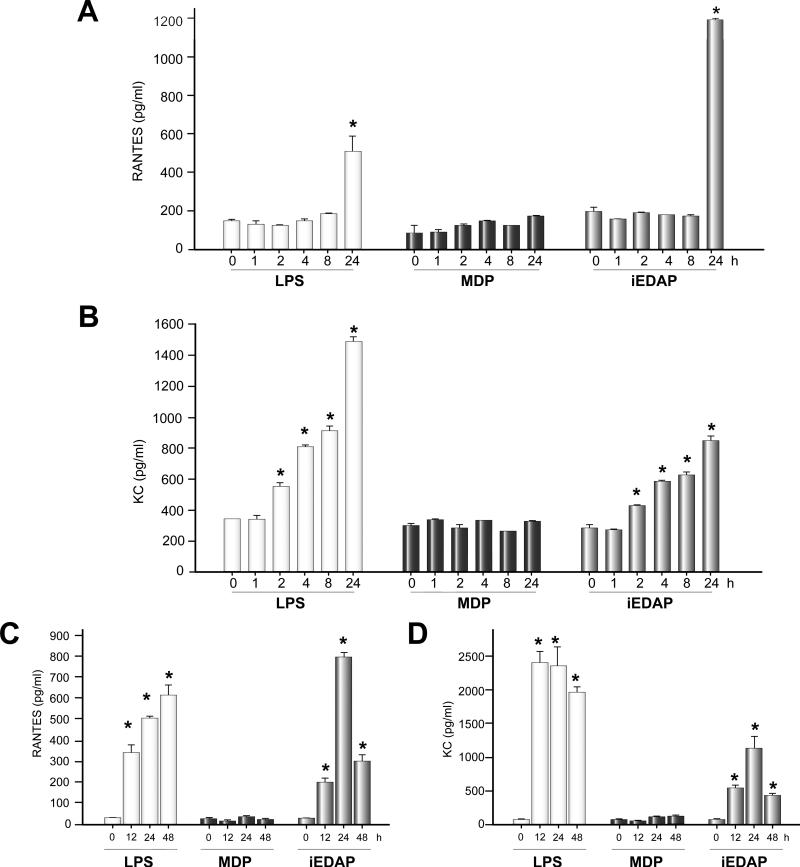
We have previously shown that hepatocytes do not produce the cytokine TNFα, IL1β or IL6 after activation of MAP kinases and NFκB by TLR ligands [7,12]. However, these cells are known to produce chemokines, NO, and acute phase proteins through the activation of NFκB [12]. We therefore determined whether chemokine expression was induced in murine hepatocytes treated for up to 24h with LPS, C12-iEDAP, or MDP.
There was no significant production of either monokine induced by IFNγ (MIG – CXCL9) or monocyte chemotactic protein (MCP)-1 (CCL2) in hepatocytes treated with LPS, C12-iEDAP or MDP (data not shown). LPS significantly induced RANTES expression in hepatocytes after 24h compared with baseline (144+/-8 pg/ml baseline vs 508+/-12 pg/ml 24h LPS, p <0.05) (Fig. 3A). C12-iEDAP also significantly increased RANTES expression in hepatocytes after 24h compared with baseline (144+/-8 pg/ml baseline vs 1198+/-10 pg/ml 24h C12-iEDAP, p <0.05), and also compared with RANTES levels at 24h after LPS (Fig. 3A). Levels of KC were significantly increased by 2h after LPS or C12-iEDAP treatment with levels increasing over the 24h time course (Fig. 3B). For KC, however, LPS was a more potent inducer, at the concentrations used, with levels significantly higher at 8h and 24h after LPS stimulation compared with 8h and 24h after C12-iEDAP stimulation (Fig. 3B). Stimulation with MDP did not increase either RANTES or KC levels in hepatocyte cell supernatants after 24h. These data suggest that NFκB activation by NOD1 and LPS induce separate and specific responses from hepatocytes. The mechanism for this specificity is not currently known but it may involve differences in activation of NFκB through RIP2 rather than MyD88.
Fig. 3. NOD1 ligand, C12-iEDAP, increases hepatocyte production of chemokines RANTES (CCL5) and KC (CXCL1).
Primary isolated hepatocytes were treated for up to 24h with LPS (100 ng/ml), MDP (10 μg/ml) or C12-iEDAP (iEDAP -100ng/ml). Cell culture supernatants were analyzed by ELISA for (A) RANTES and (B) KC expression. Plasma was harvested from C57BL/6 mice (n = 3 or 4 per experimental group) 24h after intraperitoneal injection with saline (Control), MDP (10 mg/kg), C12-iEDAP (5 mg/kg), or LPS (5 mg/kg) and analyzed by ELISA for (C) RANTES and (D) KC. *p <0.05 vs baseline chemokine level. Samples run in duplicate on ELISA, n = 3-4 for each experimental group. Graphs show mean values ± S.D.
We also wanted to confirm a role for NOD1 activation in vivo so we treated C57BL/6 mice with LPS, C12-iEDAP, or MDP as above and harvested plasma at 12, 24, and 48h. RANTES and KC levels were significantly increased in plasma by 12h after both LPS and C12-iEDAP treatment similarly to results in vitro in hepatocytes (Fig. 3C and 3D). MDP treatment did not stimulate the production of RANTES or KC even after 48h (Fig. 3C and 3D). These data suggest that hepatocytes may be a major producer of RANTES and KC after in vivo stimulation with NOD1 ligands.
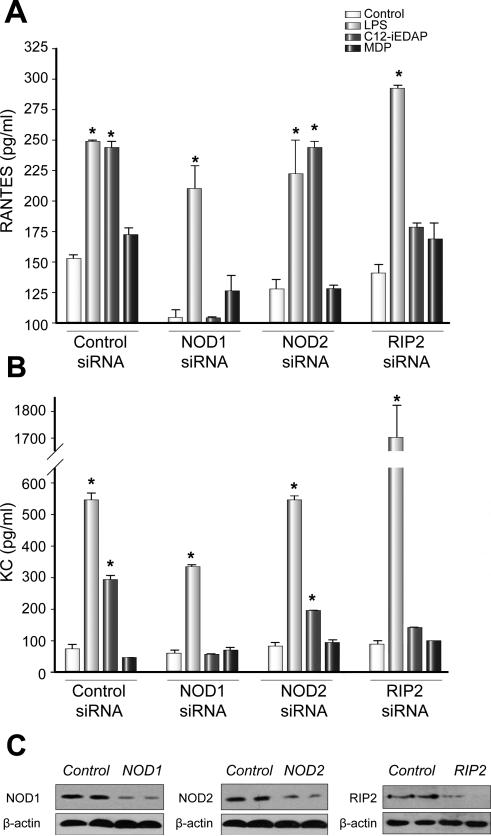
In order to confirm that increased production of RANTES and KC by C12-iEDAP in hepatocytes was dependent on NOD1 and its known signaling partner RIP2, we used siRNA to specifically knockdown either NOD1, NOD2, or RIP2. Cultured hepatocytes from C57BL/6 mice were pretreated for 24h with control (scrambled), NOD1, NOD2, or RIP2 siRNA followed by stimulation with LPS, C12-iEDAP, or MDP for a further 24h. Knockdown of each protein was confirmed by Western blot analysis (Fig. 4C). As expected, knockdown of any of NOD1, NOD2, or RIP2 did not affect hepatocyte RANTES or KC production in response to LPS. However, knockdown of NOD1 or RIP2 completely abrogated the response of hepatocytes to C12-iEDAP, with no decreased chemokine production observed when NOD2 was knocked down (Fig. 4A and 4B). MDP did not stimulate RANTES or KC production under any of the conditions tested (Fig. 4A and 4B).
Fig. 4. Increased production of RANTES and KC by NOD1-ligand (C12-iEDAP) is dependent on NOD1 and RIP2.
Cultured C57BL/6 hepatocytes were pretreated with control, NOD1, NOD2, or RIP2 siRNA for 24h before stimulation for 24h with LPS (100 ng/ml), MDP (10 μg/ml), or C12-iEDAP (iEDAP -100ng/ml). Cell culture supernatants were analyzed by ELISA for (A) RANTES and (B) KC expression. Knockdown of each protein was confirmed by Western blot (C). *p <0.05 vs baseline chemokine level. Samples run in duplicate on ELISA, n = 3-4 for each experimental group. Graphs show mean values ± S.D.
We also determined whether production of RANTES and KC was dependent on NFκB in response to NOD1 ligand (C12-iEDAP) and NOD2 ligand (MDP). We pretreated primary hepatocytes for 24h with NFκB inhibitor (Bay 11-7082) or control (DMSO) before stimulating with LPS, C12-iEDAP, or MDP as previously. As expected, both RANTES and KC production in response to stimulation with LPS, C12-iEDAP, or MDP was largely NFκB-dependent (Table 1).
TABLE 1.
RANTES and KC production by hepatocytes after 24h pretreatment with NFκB inhibitor (BAY 11-7802) or DMSO control and subsequent stimulation for 24h with LPS or NOD ligands
| Control | LPS | MDP | C12-iEDAP | |
|---|---|---|---|---|
| +DMSO | ||||
| RANTES | 74±1 | 497±24* | 97±4 | 1049±1* |
| KC | 359±22 | 3600±155* | 336±7 | 1391±32* |
| +NFκB inhibitor | ||||
| RANTES | 172±31 | 133±19 | 182±26 | 154±26 |
| KC | 4.4±1 | 23±4 | 8±7 | 11±4 |
Results expressed as mean ± S.D.
p<0.05 control vs Control(PBS), Student's t-test LPS – TLR4 ligand, MDP – NOD2 ligand, C12-iEDAP – NOD1 ligand
To further evaluate the signaling pathways involved in NFκB activation we stimulated hepatocytes isolated from WT, MyD88-/- and TRIF-/- (LPS2) mice with LPS, iEDAP, or MDP for up to 24h and measured levels of RANTES and KC in cell superntatants. As expected LPS stimulation of both RANTES and KC production was dependent mainly on MyD88, but there was also a decrease in chemokine production in TRIF-/- hepatocytes (data not shown). Neither RANTES nor KC production was diminished after stimulation with C12-iEDAP, confirming separate signaling pathways through NOD1 compared with TLR4 (data not shown).
NOD1 ligand synergizes with cytokines to induce nitric oxide in hepatocytes
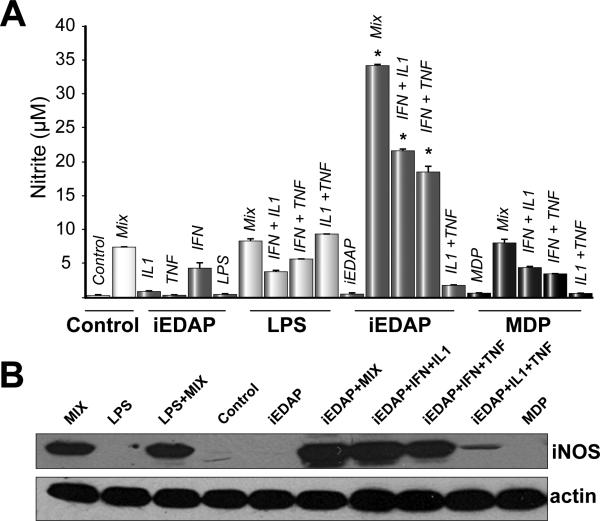
NO synthesis, via inducible NO synthase (iNOS), can be strongly upregulated in hepatocytes by cytokines, and in particular by a mix of IFNγ, IL1β and TNFα [13]. Additionally, NOD1 stimulation of mesothelial cells has been shown to cooperate with IFNγ in the production of NO [14]. We determined whether NOD1 or NOD2 ligands would induce iNOS expression and release nitrite either alone or in combination with cytokines, including IFNγ.
LPS, MDP, or C12-iEDAP alone did not significantly upregulate nitrite accumulation in supernatants (Fig. 5A) or induce iNOS expression (Fig. 5B). As expected, the cytokine mix (IFNγ, IL1β and TNFα) significantly increased nitrite levels in supernatants and induced iNOS expression in hepatocytes (Fig 5A, 5B). C12-iEDAP, but not LPS or MDP, synergized with the cytokine mix to produce significantly higher levels of nitrite in supernatants compared with cytokine mix stimulation alone (Fig.5A). Similarly, iNOS expression in hepatocytes stimulated with C12-iEDAP plus the cytokine mix was also higher than with the cytokine mix alone (Fig. 5B). Nitrite levels in supernatants were also significantly increased in cells treated with C12-iEDAP plus IFNγ, or IFNγ in combination with IL1β or TNFα (Fig.5A). This increase in nitrite was not found with stimulation with IL1β or TNFα alone or in combination with each other. Neither LPS nor MDP synergized with any of the combinations of cytokines. These data suggest an important role for NOD1 in hepatocytes to enhance the immune response to pathogens in combination with cytokines produced by other cells in the liver.
Fig. 5. NOD1 ligand, C12-iEDAP, synergizes with cytokines to increase nitrite production and iNOS expression in primary isolated mouse hepatocytes.
Hepatocytes were stimulated with LPS (100 ng/ml), MDP (10 μg/ml), C12-iEDAP (100 ng/ml) alone or with cytokine mix (Mix: IFNγ (100 U/ml) + IL1β (100 U/ml) + TNFα (500 U/ml) or with individual or double combinations of each cytokine. (A): Cell culture supernatants were collected and analyzed by Greiss reaction for nitrite levels. (B): Whole cell lysates from treated cells were immunoblotted for iNOS. Untreated cells were used as control. *p <0.05 vs nitrite level with Mix alone. Samples for Greiss reaction were run in triplicate, n = 3 for each experimental group. Data shown are mean values ± S.D. Western images are representative of iNOS levels in at least three separate experiments.
NOD1 activation does not enhance the hepatic acute phase response
The acute phase response is initiated in the liver in response to inflammatory stimuli, including bacterial pathogens. Multiple acute phase proteins are synthesized in the liver during the acute phase response and can be detected systemically, including C-reactive protein (CRP), LPS-binding protein (LBP), serum amyloid A (SAA), and serum amyloid P (SAP). Most acute phase protein expression is regulated in hepatocytes by NFκB activation, together with C/EBP in response to cytokine stimulation [15]. We therefore hypothesized that stimulation of hepatocytes with NOD1 ligand, C12-iEDAP, would increase expression of acute phase proteins through the activation of NFκB.
There were no significant increases in SAA in hepatocyte cell culture supernatants after 24h stimulation with LPS, C12-iEDAP, or MDP (Table 2). LPS, but not C12-iEDAP or MDP, significantly increased LBP production in hepatocytes after 24h (Table 2). Soluble CD14 (sCD14) is also produced by hepatocytes as part of the acute phase response. Western blots of hepatocyte supernatants showed an increased production of sCD14 after LPS stimulation, but not after treatment with C12-iEDAP or MDP (data not shown). LPS also increased hepatocyte mRNA expression of LBP, CRP, and SAP after 24h (Table 2). C12-iEDAP did not increase hepatocyte mRNA expression of any acute phase proteins measured, but there was a small increase in mRNA expression of LBP in hepatocytes treated for 24h with MDP (Table 2).
TABLE 2.
Acute Phase Protein (APP) expression in hepatocytes after 24h stimulation with NOD ligands
| APP | LPS (100ng/ml) | MDP (10μg/ml) | C12-iEDAP (100ng/ml) | |||
|---|---|---|---|---|---|---|
| Control | 24h | Control | 24h | Control | ||
| ELISA (ng/ml) | ||||||
| SAA | 0.78±0.03 | 0.84±0.03 | 0.80±0.02 | 0.82±0.06 | 0.78±0.07 | 0.84±0.10 |
| LBP | 4567±725 | 6768±1506* | 4077±672 | 3654±846 | 5053±523 | 4841±1642 |
| mRNA expression § | ||||||
| SAA | 1 | 1.52±0.08 | 1 | 1.27±0.07 | 1 | 1.17±0.05 |
| LBP | 1 | 2.11±0.48* | 1 | 1.69±0.15* | 1 | 0.92±0.10 |
| CRP | 1 | 3.28±0.51* | 1 | 1.20±0.20 | 1 | 1.01±0.11 |
| SAP | 1 | 1.68±0.14* | 1 | 1.02±0.07 | 1 | 0.95±0.10 |
Results expressed as mean ± S.D.
p<0.05 control vs 24h treatment, Student's t-test
expression relative to control level and normalized to β-actin. LPS – TLR4 ligand, MDP – NOD2 ligand, C12-iEDAP – NOD1 ligand
These results were unexpected since NOD1 ligand upregulated NFκB in hepatocytes, but did not augment the acute phase response. These data, again, suggest a level of regulation of responses through NOD1 and RIP2 that is as yet unappreciated.
MDP synergizes with TLR ligands to activate hepatocytes
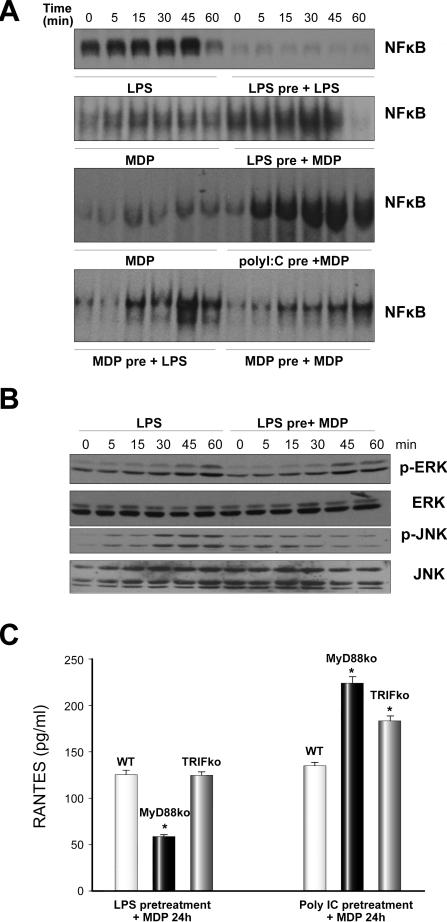
Multiple published studies have shown the ability of MDP to synergize with TLR ligands [16,17]. Our previous studies have shown that pretreatment of hepatocytes with LPS desensitizes cells to a further stimulation with LPS [8]. We confirmed these data as shown in Fig. 6A, upper image. However, hepatocytes pretreated for 24h with either LPS or polyI:C (TLR3 ligand) were able to activate NFκB in response to MDP (Fig. 6A, middle images). The pretreatment of hepatocytes with LPS followed by stimulation with MDP also increased activation of ERK and JNK (Fig. 6B). Additionally, we found that 24h-pretreatment of hepatocytes with MDP did not prevent subsequent LPS activation of NFκB in hepatocytes (Fig.6A, lower image) and unexpectedly, pretreatment of hepatocytes with MDP allowed a second MDP stimulus to activate NFκB (Fig. 6A, lower image). These data suggest that signaling for MDP and LPS is separately regulated. We also investigated the role of TLR4 signaling in LPS, MDP synergy. Primary hepatocytes from TLR4-/- (C57BL/10ScN) mice did not activate NFκB in response to LPS (as expected) or in response to MDP as shown above (data not shown). Also, LPS pretreatment did not allow later MDP signaling suggesting that LPS signaling through TLR4 is required for subsequent MDP signaling to activate NFκB (data not shown). Similarly TLR4-/- hepatocytes did not upregulate NOD1, NOD2, or RIP2 mRNA expression in hepatocytes after LPS treatment (data not shown).
Fig. 6. NFκB activation by MDP following pretreatment of hepatocytes with LPS, PolyI:C or MDP.
Primary isolated mouse hepatocytes were pretreated for 24h with LPS (100 ng/ml), PolyI:C (1 μg/ml) or MDP (10 μg/ml) followed by stimulation with MDP (10 μg/ml) for up to 60 min. (A): NFκB level was detected in nuclear extracts by EMSA. (B): ERK and JNK activation (phosphorylation) was detected by Western blot. Images shown are representative of results obtained from three separate experiments. (C). RANTES levels in supernatants of primary hepatocytes isolated from WT (C57BL/6), MyD88-/- or TRIF-/- (LPS2) mice pretreated with either LPS (100 ng/ml) or PolyI:C (1 μg/ml) for 24h followed by stimulation with MDP (10 μg/ml) for 24h. *p <0.05, Student's t-test; n = 3 per group; results representative of two repeated experiments.
It is unclear, from the above results, which TLR signaling pathway enables MDP to either enter the cell or activate its own signaling pathway as LPS and polyI:C signal through overlapping TLR-signaling pathways. We therefore pretreated hepatocytes isolated from WT, MyD88-/-, or TRIF-/- mice with either LPS or Poly IC, and then assessed RANTES production in these cells after 24h of MDP stimulation. Upregulation of RANTES production in hepatocytes by MDP after LPS pretreatment was dependent on MyD88, but not TRIF (Fig. 6C). However, upregulation of RANTES by MDP after Poly IC pretreatment was neither MyD88 nor TRIF-dependent (Fig. 6C). If anything, RANTES levels were increased in both MyD88-/- and TRIF-/- compared with WT after pretreatment with Poly IC and subsequent 24h stimulation with MDP (Fig. 6C). These results are intriguing, because they suggest that priming for MDP stimulation can occur through multiple signaling pathways in hepatocytes and also suggest that priming by Poly IC is via TRIF-dependent signaling. These data suggest that Poly IC may be activating an intracellular RNA receptor, such as a receptor from the RIG-like helicase family, rather than through TLR3 signaling. Further experiments will be needed to determine pathway interactions in hepatocytes.
DISCUSSION
In this manuscript we have examined the expression and activation of NOD1 and NOD2 in murine hepatocytes. It is clear from our data that hepatocytes highly express NOD1 and NOD2 and are activated by both NOD1 and NOD2 specific ligands. This activation likely contributes to systemic and local immune responses to pathogens. We have clearly demonstrated that NOD1 stimulation in hepatocytes induces chemokine production and synergizes with cytokines to increase NO and iNOS production. Interestingly, however, it is apparent that neither NOD1 nor NOD2 ligands stimulate the acute phase response in hepatocytes.
The liver is ideally placed to initiate and regulate immune responses to pathogens released from the gut and transported in the hepatic portal vein (e.g. after changes in gut permeability following hemorrhagic shock) or detected in the systemic blood stream. NOD1 is stimulated mainly by bacterial cell wall components from Gram-negative bacteria [18], which make up a large part of gut flora. Stimulation of chemokine expression by hepatocytes in response to NOD1 activation by gut pathogens likely, therefore, forms an important part of the mechanism involved in attracting immune cells to the liver to defend the host [19]. Similarly, chemokine production is increased in injured liver [20]. We determined that hepatocytes produce both CC and CXC chemokines in response to NOD1 stimulation. The response is somewhat specific, however, as not all CC or CXC chemokines measured were induced by C12-iEDAP.
KC (CXCL1) is a murine analog of Groα found in humans, is a chemoattractant for neutrophils and is generally produced early during immune responses to pathogens [19,21]. LPS was a more potent inducer of KC in hepatocytes than C12-iEDAP, which suggests KC production in response to NOD1 activation forms a secondary pathway of activation that may result in amplification of immune responses. RANTES (CCL5) is a lymphocyte chemoattractant [22] generally produced later in immune responses and contributes to augmentation of the adaptive immune response [22]. RANTES also contributes to hepatic wound healing and enhances hepatic fibrosis [23]. Our data suggest that hepatocyte NOD1 activation is a main pathway for RANTES production.
NOD1 and NOD2 are known to play important roles in mucosal immunity including the production of antimicrobial peptides, including defensins [24]. These data, together with our data showing NOD1 stimulation strongly activates NFκB in hepatocytes, suggested that NOD1 stimulation might also increase expression of acute phase proteins. We were surprised to find that hepatocytes did not increase expression of acute phase proteins in response to NOD1 ligand in vitro. We confirmed this lack of induction of the acute phase response in vivo in plasma of mice 24h after intraperitoneal injection of C12-iEDAP (data not shown). How hepatocytes respond in a specific manner to multiple similar infectious stimuli, each of which increases activation of NFκB, remains to be answered. It seems likely that cooperation between liver cell types will be important.
NOD2 has been associated with multiple human pathologies including inflammatory bowel disease. It was also recently described that NOD2 may be a target for regulating concanavalin A-induced liver injury [25]. From our data, as well as studies by others [5], it seems likely that MDP is not easily able to enter cells to stimulate NOD2, unless those cells are prestimulated with either a TLR ligand or another stimulus such as ATP. These findings suggest that NOD2 activation forms part of a collective immune response to pathogens and organ injury and it is interesting to speculate that hepatocyte NOD2 may play an important regulatory role in many inflammatory processes involving the liver.
Data presented in this manuscript provide important insights into the mechanism of activation of hepatocytes during infection and inflammation. Our findings indicate that hepatocytes express not only TLRs, as previously shown, but also NOD1 and NOD2 and that they respond to specific ligands for these receptors. Thus it is likely that hepatocyte NLRs are an important component of the innate immune system of the liver.
ACKNOWLEDGEMENTS
This work was supported in part by NIH grant R01-GM-05441 and by a Junior Faculty Fellowship from the Surgical Infection Society (to M.S.). Grateful thanks to Hong Liao, Carol Meiers, and Danielle Reiser for technical assistance and to Deb Williams and Kathy DiGiacomo for administrative help.
List of Abbreviations
- NOD
nucleotide oligomerization domain
- NLR
NOD-like receptor
- TLR
Toll-like receptor
- NALP
NACHT leucine rich repeat and pyrin domain containing protein
- CARD
caspase activation and recruitment domain
- RIP2
receptor-interacting protein kinase
- IL
interleukin
- DAP
diaminopimelic acid
- PGN
peptidoglycan
- MDP
muramyl dipeptide
- NO
nitric oxide
- LPS
lipopolysaccharide
- RANTES
Regulated on Activation Normal T Cell Expressed and Secreted
- MIG
Monokine induced by IFN-gamma
- KC
Keratinocyte-Derived Chemokine
- MCP1
Monocyte Chemotactic Protein 1
- SAA
serum amyloid A
- SAP
serum amyloid P
- LBP
lipopolysaccharide binding protein
- CRP
C-reactive protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006 Dec;7(12):1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 2.Kufer TA, Fritz JH, Philpott DJ. NACHT-LRR proteins (NLRs) in bacterial infection and immunity. Trends Microbiol. 2005 Aug;13(8):381–388. doi: 10.1016/j.tim.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003 Feb;4(2):95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Nunez G, et al. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. EMBO J. 2008 Jan 23;27(2):373–383. doi: 10.1038/sj.emboj.7601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J, Tattoli I, Wojtal KA, Vavricka SR, Philpott DJ, Girardin SE. pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling. J Biol Chem. 2009 Jul 1; doi: 10.1074/jbc.M109.033670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vodovotz Y, Liu S, McCloskey C, Shapiro R, Green A, Billiar TR. The hepatocyte as a microbial product-responsive cell. J Endotoxin Res. 2001;7(5):365–373. [PubMed] [Google Scholar]
- 7.Scott MJ, Billiar TR. Beta2-integrin-induced p38 MAPK activation is a key mediator in the CD14/TLR4/MD2-dependent uptake of lipopolysaccharide by hepatocytes. J Biol Chem. 2008 Oct 24;283(43):29433–29446. doi: 10.1074/jbc.M803905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott MJ, Liu S, Shapiro RA, Vodovotz Y, Billiar TR. Endotoxin uptake in mouse liver is blocked by endotoxin pretreatment through a suppressor of cytokine signaling-1-dependent mechanism. Hepatology. 2009 Feb 9; doi: 10.1002/hep.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009 Feb;119(2):305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galloway E, Shin T, Huber N, Eismann T, Kuboki S, Schuster R, et al. Activation of hepatocytes by extracellular heat shock protein 72. Am J Physiol Cell Physiol. 2008 Aug;295(2):C514–C520. doi: 10.1152/ajpcell.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Gallo DJ, Green AM, Williams DL, Gong X, Shapiro RA, et al. Role of toll-like receptors in changes in gene expression and NF-kappa B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect Immun. 2002 Jul;70(7):3433–3442. doi: 10.1128/IAI.70.7.3433-3442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HS, Loughran PA, Rao J, Billiar TR, Zuckerbraun BS. Carbon monoxide activates NF-kappaB via ROS generation and Akt pathways to protect against cell death of hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2008 Jul;295(1):G146–G152. doi: 10.1152/ajpgi.00105.2007. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Kim YG, Shaw M, Kanneganti TD, Fujimoto Y, Fukase K, et al. Nod1/RICK and TLR signaling regulate chemokine and antimicrobial innate immune responses in mesothelial cells. J Immunol. 2007 Jul 1;179(1):514–521. doi: 10.4049/jimmunol.179.1.514. [DOI] [PubMed] [Google Scholar]
- 15.Suetsugu H, Iimuro Y, Uehara T, Nishio T, Harada N, Yoshida M, et al. Nuclear factor {kappa}B inactivation in the rat liver ameliorates short term total warm ischaemia/reperfusion injury. Gut. 2005 Jun;54(6):835–842. doi: 10.1136/gut.2004.043034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritz JH, Girardin SE, Fitting C, Werts C, Mengin-Lecreulx D, Caroff M, et al. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and. Eur J Immunol. 2005 Aug;35(8):2459–2470. doi: 10.1002/eji.200526286. [DOI] [PubMed] [Google Scholar]
- 17.van Heel DA, Ghosh S, Butler M, Hunt K, Foxwell BM, Mengin-Lecreulx D, et al. Synergistic enhancement of Toll-like receptor responses by NOD1 activation. Eur J Immunol. 2005 Aug;35(8):2471–2476. doi: 10.1002/eji.200526296. [DOI] [PubMed] [Google Scholar]
- 18.Girardin SE, Travassos LH, Herve M, Blanot D, Boneca IG, Philpott DJ, et al. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003 Oct 24;278(43):41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 19.Masumoto J, Yang K, Varambally S, Hasegawa M, Tomlins SA, Qiu S, et al. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med. 2006 Jan 23;203(1):203–213. doi: 10.1084/jem.20051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurens M, Defamie V, Scozzari G, Schmid-Alliana A, Gugenheim J, Crenesse D. Hypoxia-reoxygenation-induced chemokine transcription is not prevented by preconditioning or intermittent hypoxia, in mice hepatocytes. Transpl Int. 2005 Apr;18(4):444–452. doi: 10.1111/j.1432-2277.2004.00064.x. [DOI] [PubMed] [Google Scholar]
- 21.Werts C, le BL, Liu J, Magalhaes JG, Carneiro LA, Fritz JH, et al. Nod1 and Nod2 induce CCL5/RANTES through the NF-kappaB pathway. Eur J Immunol. 2007 Sep;37(9):2499–2508. doi: 10.1002/eji.200737069. [DOI] [PubMed] [Google Scholar]
- 22.Karlmark KR, Wasmuth HE, Trautwein C, Tacke F. Chemokine-directed immune cell infiltration in acute and chronic liver disease. Expert Rev Gastroenterol Hepatol. 2008 Apr;2(2):233–242. doi: 10.1586/17474124.2.2.233. [DOI] [PubMed] [Google Scholar]
- 23.Seki E, De MS, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009 Jul;119(7):1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zilbauer M, Dorrell N, Elmi A, Lindley KJ, Schuller S, Jones HE, et al. A major role for intestinal epithelial nucleotide oligomerization domain 1 (NOD1) in eliciting host bactericidal immune responses to Campylobacter jejuni. Cell Microbiol. 2007 Oct;9(10):2404–2416. doi: 10.1111/j.1462-5822.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- 25.Body-Malapel M, Dharancy S, Berrebi D, Louvet A, Hugot JP, Philpott DJ, et al. NOD2: a potential target for regulating liver injury. Lab Invest. 2008 Mar;88(3):318–327. doi: 10.1038/labinvest.3700716. [DOI] [PubMed] [Google Scholar]