Abstract
Objective:
Not only are substance-use disorders and externalizing disorders frequently comorbid, they often co-occur in families across generations. The current study examined the role of genetic and environmental influences in the relationship between paternal histories of drug dependence or alcohol dependence and offspring conduct disorder using an offspring-of-twins design.
Method:
Participants were male twins (n = 1,774) from the Vietnam Era Twin Registry, their offspring (n = 1,917), and mothers of the offspring (n = 1,202). Twins had a history of drug dependence, alcohol dependence, or neither. Based on the father's and his co-twin's drug-dependence or alcohol-dependence history and zygosity, risk groups were constructed to reflect different levels of genetic and environmental risk that were then used to predict offspring conduct disorder.
Results:
After controlling for potentially confounding variables, the offspring of men with a history of drug dependence or alcohol dependence had significantly higher rates of conduct disorder, compared with offspring of men without this history. Offspring at higher genetic risk had higher rates of conduct disorder. High-risk offspring at lower environmental risk had lower rates of conduct disorder but only in the case of paternal drug-dependence risk. Lower environmental risk did not influence rates of offspring conduct disorder when the father had an alcohol-dependence history.
Conclusions:
Genetic risk associated with both paternal drug-dependence and paternal alcohol-dependence histories predicted offspring conduct-disorder risk, but only risk associated with paternal drug-dependence history was mitigated by having a low-risk environment. These results demonstrated a significant gene-environment interaction effect.
Alcohol dependence, drug dependence, and conduct disorder frequently co-occur, and this co-morbidity appears to be the result of common etiological factors, including underlying genetic covariation (Button et al., 2007). Also, these disorders frequently co-occur within families across generations (Jacob et al., 2003). Several lines of evidence lend support to the proposition that the observed comorbidity occurs because common genetic variation underlies all three disorders: alcohol dependence, drug dependence, and conduct disorder. First, substantial genetic effects have been identified for alcohol dependence (Heath et al., 1997; Kendler, 1993; McGue, 1994), drug dependence (Kendler and Prescott, 1998; Lyons et al., 1997; Tsuang et al., 1996), and conduct disorder (Lyons et al., 1995; Slutske et al., 1998). Second, in both clinical and epidemiological samples, the rates of comorbidity between alcohol dependence, drug dependence, and conduct disorder have consistently been shown to be significantly higher than expected by chance (Hesselbrock, 1985; Holdcraft et al., 1998; Kessler et al., 1994). Third, genetic epidemiological studies have identified latent susceptibility factors that appear to account for this comorbidity, and this latent factor has been shown to be largely genetic in nature. Slutske et al. (1998) found that genetic influences accounted for more than 70% of the phenotypic association between conduct disorder and alcohol dependence and that 90% of this shared genetic risk was associated with behavioral undercontrol personality traits (Slutske et al., 2002). Krueger et al. (2002)—in a study that included alcohol dependence, drug dependence, and conduct disorder—identified a common latent externalizing factor, with additive genetic influences accounting for 81% of the variance of this latent phenotype. In an adult male twin sample, Fu et al. (2002) reported that more than half of the genetic variance on antisocial personality disorder was shared with both alcohol and marijuana dependence.
Although common genetic variation has been shown to underlie externalizing disorders, shared environmental variation has been less often demonstrated, even for substance-use disorders wherein phenotypic evidence of environmental effects has long been reported (Zucker, 1994). However, there is some accumulating evidence of shared environmental variation on conduct disorder. For example, Krueger et al. (2002), in a study of 17-year-old male and female twins, identified a significant shared environmental effect that was specific to conduct disorder; no similar specific effect emerged for substance-dependence variables. True et al. (1999), in an adult male twin sample, identified shared environmental variance that was common to conduct disorder, alcohol dependence, and marijuana dependence and that was larger for conduct disorder than for the substance-use disorders. However, in general, compared with reports of genetic effects, reports of shared environmental effects on externalizing disorders are infrequent. Thus, the study of conduct disorder may increase the likelihood of identifying shared environmental effects within a genetically informative model. In the current study, it was important to examine conduct disorder with both drug-dependence and alcohol-dependence risk within the same genetically informative study design in an effort to clarify the nature of these associations and what may be unique for one of these disorders.
The current study used an offspring-of-twins design to examine the transmission of genetic risk and family environmental risk from parents to children. This genetically informative design is also particularly sensitive to shared environmental variation that may influence offspring outcomes (Heath and Nelson, 2002; Jacob et al., 2001) and that also may methodologically broaden this literature, which is largely based on the classical twin model. Rather than relying on statistical latent variable methods to partition variance into genetic, shared environmental (including familial), and unique (nonshared) environmental sources of influence, the offspring-of-twins design permits construction of groups based on the twin parents' and his co-twin's measured characteristics that together differentiate the various levels of genetic and environmental risk. Comparisons across groups permit inferences about the contribution of genetic and environmental factors to the outcome of interest (see Jacob et al., 2003, for an example).
In our previous work, we examined genetic and environmental effects based on the alcohol-dependence status of the twin father and his co-twin and their association with offspring conduct disorder to test the proposition that the same genetic influences underlie both alcohol dependence and conduct disorder. In the current study, we extended this work to include fathers with drug dependence (with or without alcohol dependence). The goals of the current study were (a) to simultaneously, at the phenotypic level of analysis, examine offspring risk associated with paternal (and/or co-twin) history of drug dependence or alcohol dependence in contrast with offspring without this family history; (b) to confirm that common genetic variation underlies offspring conduct disorder and paternal drug dependence and alcohol dependence; and (c) to shed light on whether a low-risk environment might mitigate the effect of high genetic vulnerability on offspring conduct-disorder risk. Hypotheses included the following:
paternal history of drug dependence (with or without alcohol dependence), as well as paternal history of alcohol dependence (without drug dependence), will be associated with elevated rates of offspring conduct disorder after adjust ing for paternal and maternal antisocial personality disorder and other potential confounds—this is a test of phenotypic association;
offspring of fathers with a history of drug dependence (with or without alcohol dependence) will have higher rates of conduct disorder, compared with offspring of fathers with a history of alcohol dependence (without drug dependence) (see Dick et al., 2007);
offspring at high genetic risk (associated with mono- zygotic co-twins) will have higher rates of conduct disorder, compared with offspring at moderate genetic risk (associated with dizygotic co-twins); and
offspring at high genetic risk will have lower rates of conduct disorder when family environmental risk is lower.
Method
Subjects
The participants in this study included twin fathers who were members of the Vietnam Era Twin Registry, their co-twins, their offspring, and the biological mothers of those offspring. The Vietnam Era Twin Registry has been described in detail elsewhere (Eisen et al., 1987; Goldberg et al., 1993; Henderson et al., 1990; Tsuang et al., 1996), and only salient aspects of this registry will be described here. This national military twin registry comprises male-male twin pairs born between 1939 and 1957 in which both members served in the United States military between May 1965 and August 1975. In 1987, Registry members completed a mailed questionnaire about their military experience, general health, education, marital and family history, etc. (Eisen et al., 1989; Henderson et al., 1990). In 1992, a psychiatric interview was administered by telephone to twins who completed the questionnaire. This interview assessed a range of psychiatric disorders, including alcohol- and drug-use disorders (Tsuang et al., 1996). Information from the 1987 mailed questionnaire and the 1992 interview was used to design two offspring-of-twins studies: one that began in 2000 and focused on paternal alcohol dependence (Children of Alcoholics Study; Duncan et al., 2006; Jacob et al., 2003) and the second that began in 2003 that focused on paternal drug dependence (Twins as Parents Study; Scherrer et al., 2008). Identical offspring and maternal assessments were used in both studies, with the exception of an expanded drug-use disorder section for the offspring and their biological mothers in the second study. Thus, collected data from these studies could be combined.
Procedure: Selection of twin parents
Eligible twin pairs were drawn from the Vietnam Era Twin Registry using a selection algorithm based on the following criteria: (a) both twins completed the 1987 and 1992 surveys; (b) at least one twin reported that he had children born between 1974 and 1988; and (c) based on the Diagnostic and Statistical Manual, Third Edition, Revised (DSM-III-R; American Psychiatric Association, 1987), at least one twin met criteria for a lifetime diagnosis of alcohol dependence (selected for Study 1) or drug dependence (selected for Study 2). (A reference group was also selected in both studies from twin pairs where both members were unaffected by alcohol dependence or drug dependence.) Pairs were selected according to a high-risk sampling plan that provided oversampling of twin pairs having an alcohol-dependent or drug-dependent member. Altogether, for both Studies 1 and 2, 1,062 Vietnam Era Twin Registry pairs were selected. Even though selection was based on pairs, each twin was individually contacted for participation, and twins were not excluded if a co-twin declined to participate. Also included in the sample were the twins' biological children and the biological mothers of those children. In rare cases, a substitute mother was included if the biological mother was not involved in rearing their child between the ages of 6 to 13 years. Up to three age-eligible children (giving priority to the three youngest children) were selected. Concerted attempts were made to locate, contact, and enroll every individual selected for the study. Interviewers were blind to the twins' substance-use history. For both studies, procedures for obtaining verbal informed consent were approved by each institution's institutional review board; written consent was obtained from at least one parent for participation by minoraged offspring.
The initial data collections were conducted in 2000-2001 for the Children of Alcoholics Study and in 2002-2004 for the Twins as Parents Study. Data collection was conducted by the Institute for Survey Research at Temple University (Philadelphia, PA). Interviewers used an automated, computer-assisted telephone interview system, which provided standardization of all interview questions and probes. Response rates were similar across the studies. In Study 1, 83% (n = 1,295) of the individual twins were interviewed; while the comparable rate for Study 2 was 81% (n = 725). In Study 1, twins gave consent to contact 1,113 mothers, of whom 81% were interviewed. Consent to contact 604 mothers was given in Study 2, of whom 71% participated. Participating offspring included 85% of those for whom permission was obtained (n = 1,329) in Study 1; in comparison, consent to contact 950 offspring in Study 2 was obtained, of whom 88% (n = 839) were interviewed.
Equivalent procedures and assessments used in both studies permitted the combining of data, which resulted in 1,774 individual twins, 1,202 mothers, and 1,919 offspring. (There was some overlap during sample selection, as might be expected from comorbid phenotypes; 246 offspring were interviewed in both Study 1 and Study 2. Where two reports were obtained, data from Study 2 interviews were used. This yielded a total of 1,919 individual offspring.) Information provided from previous interviews with the twins was also combined and used for these analyses. After personal identifiers were removed by the Vietnam Era Twin Registry, data were released to the investigators.
Assessments
Interviews for the twin fathers in both Studies 1 and 2 included an updated drug-use history and a lifetime drinking history modified to include DSM, Fourth Edition (DSM-IV; American Psychiatric Association, 1994) alcohol-use disorder symptomatology. As noted previously, twin fathers' psychiatric diagnostic information was obtained from the 1992 interview. Mothers and offspring were administered adaptations of the Semi-Structured Assessment for the Genetics of Alcoholism, Version II (Bucholz et al., 1994), which provided lifetime DSM-IV diagnoses for a range of psychiatric disorders, including conduct disorder, as well as demographic and background information.
Classification of risk groups based on paternal and co-twin alcohol-dependence and drug-dependence statuses and zygosity
Based on the twin father and his co-twin's zygosity and status as drug dependent, alcohol dependent, or neither, seven mutually exclusive risk groups were constructed to reflect different levels of latent genetic and environmental risk to the offspring. The risk groups defined risks separately for offspring of drug-dependent (including comorbid drug-dependent/alcohol-dependent) fathers, compared with offspring of alcohol-dependent fathers (without drug dependence). In the drug-dependence group, the rate of comorbidity for alcohol dependence was about 70% in this sample. Given the small drug dependence-only sample size, drug dependence/alcohol dependence and drug dependence only were considered together.
The first risk group included offspring of men who had a history of drug dependence (with or without alcohol dependence). They were at high genetic (HG) risk by virtue of having a father with drug dependence and at high environmental (HE) risk by virtue of being reared by a father with drug dependence. This group was labeled HGHE-D to denote that both their genetic and environmental risks were high because of biological heritage and being reared by a father with drug dependence.
A second group of offspring was those whose fathers themselves did not have either a drug-dependence or an alcohol-dependence history but whose identical (monozygotic) co-twins did have drug-dependence history. These offspring were at the same high genetic risk as their cousins in the HGHE-D group because their father's genes were identical to those of his affected monozygotic co-twin; however, the offspring were considered to be at low environmental (LE) risk because they were reared by a father who did not have a drug-dependence or alcohol-dependence history. A label of HGLE-D was assigned to this group.
A third group comprised offspring of men without a drug-dependence or alcohol-dependence history but whose fraternal (dizygotic) co-twin had a history of drug dependence. These offspring were considered to be at moderate genetic (MG) risk because their dizygotic twin father shared, on average, only half of the genes with his dizygotic brother, but they were at low environmental risk because they were reared by a father without a drug-dependence or alcohol-dependence history. This group was labeled as MGLE-D to denote their moderate genetic and low environmental risks associated with drug dependence.
Three additional risk groups were defined that were analogous to those defined on the basis of the drug-dependent men. An HGHE-A group comprised offspring of fathers with alcohol dependence (but not drug dependence). An HGLE-A group consisted of offspring of fathers without alcohol dependence but with an identical co-twin who was affected by an alcohol-dependence history. These offspring were at high genetic risk, based on having an uncle with alcohol dependence who was the identical co-twin of their unaffected father, but at low environmental risk by virtue of being reared by a non-alcohol-dependent father. An MGLE-A group comprised offspring of fathers without alcohol dependence who had a dizygotic co-twin with alcohol dependence. Last, a seventh group was created for offspring of father–co-twin pairs who did not have either an alcohol-dependence or a drug-dependence history; these offspring were considered to be at both low genetic (LG) and low environmental risk because neither their fathers nor their father's monozygotic or dizygotic co-twins (their uncles) had drug-dependence or alcohol-dependence histories and, therefore, did not transmit either genetic or environmental vulnerability to them. Labeled as LGLE, this group served as the reference group. The structure of this seven-group basic model is presented in
Offspring conduct-disorder outcome
Offspring conduct disorder was defined as a binary variable indicating offspring endorsement of three or more DSM-IV criteria associated with a conduct-disorder diagnosis. The adolescent/young adult interview included 78 questions assessing incidence, age of onset, and age of most recent occurrence of specific behaviors corresponding with the 15 DSM-IV conduct-disorder diagnostic criteria. If three or more different conduct-disorder criteria were endorsed and if they met the age cutoffs identified in the DSM-IV, a positive status was indicated for conduct disorder.
Covariates
The analysis took into account other potentially confounding variables in the model that were selected from the literature as linked to conduct disorder. Included were paternal conduct-disorder and antisocial-personality-disorder status, based on the 1992 interview data, and maternal antisocial behaviors (diagnosis not available). Maternal alcohol dependence and maternal heavy marijuana use (>150 uses as a proxy measure for diagnosis) were also included as covariates but were not available for all mothers. Concerning psychopathology, a dichotomous variable was created to code the presence of any of the following lifetime DSM-III-R diagnoses to indicate father's internalizing disorder: panic disorder, generalized anxiety disorder, posttraumatic stress disorder, dysthymia, or depression (available from the 1992 interview). For mothers, DSM-IV lifetime major depression was derived based on their own reports of symptoms at interview. Sociodemographic covariates included father's current full-time employment, maternal and paternal highest educational attainment as having some college, whether the parents were divorced, and offspring gender and age (12-17 or 18 and older). A dummy variable was created reflecting missing maternal interview data to include those offspring whose mothers did not participate (n = 179) and to determine whether there was an effect of maternal nonparticipation on offspring conduct-disorder outcome.
Data analysis
Preliminary analyses were conducted to confirm data integrity and assumptions about family structure (e.g., 81% of offspring never lived apart from their father for >1 year). Logistic regression analyses were conducted to examine the relationships among the paternal risk groups described above and offspring conduct-disorder outcome. Tests were first conducted to determine if offspring conduct-disorder rates in the three drug-dependence risk groups differed from those of the respective alcohol-dependence risk groups, that is, whether conduct-disorder rates were different between the HGHE-D and HGHE-A groups, the HGLE-D and HGLE-A groups, and the MGLE-D and MGLE-A groups. If not different, this was taken as evidence for collapsing the drug-dependence and alcohol-dependence subgroups into one overall risk group reflecting HGHE, HGLE, or MGLE risk, respectively. Sample description analyses were conducted next for the resulting model. Covariates were then added to the model, and planned contrasts were computed. Analyses were conducted using SPSS Version 14 (SPSS Inc., Chicago, IL), SAS Version 9.1 (SAS Institute Inc., Cary, NC), and STATA Version SE11 (StataCorp LP, College Station, TX) to take the clustered nature of the data into account.
Results
Results of selection bias analyses
As described, we first conducted tests to determine whether offspring risk groups, defined by father's and co-twin's drug-dependence or alcohol-dependence diagnostic status and zygosity, could be combined. Prevalence of offspring conduct disorder for each of the seven risk groups is presented in the multifactorial model results of Table 2, along with odds ratios (ORs) comparing each risk group with the LGLE group. Individual contrast models are also presented. In the multifactorial model, results indicated that offspring conduct disorder was significantly elevated for both the HGHE-D and HGHE-A groups compared with the reference group. Offspring were 2.27 and 1.60 times as likely to have conduct disorder in the HGHE-D and the HDHE-A groups, respectively, compared to their LGLE counterparts. No other significant differences were observed. There was a suggestive finding for less elevated odds of conduct disorder among offspring in the MGLE-A group (OR = 0.48) compared with that of offspring in the LGLE group; however, this effect could only be considered a trend. Of particular interest was use of this model to determine whether drug-dependence and alcohol-dependence risk groups of the same type (e.g., both HGLE groups) could be collapsed across substance types, as described above. The OR for the contrast between HGHE-D and HGHE-A groups was found to be statistically significant (OR = 1.42, p = .03), indicating that these two groups could not be combined. These results indicated that offspring had 42% greater odds of endorsing three or more conduct-disorder symptoms when at risk as a result of their father's drug-dependence history compared with alcohol-dependence history. The comparison of ORs for the HGLE-D and HGLE-A groups and for the MGLE-D and MGLE-A groups did not yield statistically significant differences, and, as such, these groups were combined. Thus, the risk groups were reduced to five groups: HGHE-D, HGHE-A, HGLE, MGLE, and LGLE. See Table 1 (bottom half) for a summary of the final five-group model.
Table 2.
Prevalence, odds ratio, and p values for offspring conduct disorder (CD) (three or more symptoms) as an unadjusted function of paternal drug-dependence (DD) or alcohol-dependence (AD) history
| Offspring CD |
|||
| Variable | Prevalence % | OR [95% CI] | pa |
| Multifactorial model | |||
| 1. HGHE-D | |||
| Hi-G and hi-E DD/AD groupb | 21.3 | 2.27 [1.55, 3.30]*** | .000 |
| 2. HGLE-D | |||
| Hi-G and low-E DD/AD groupb | 14.7 | 1.44 [0.71, 2.92] | .32 |
| 3. MGLE-D | |||
| Mod-G and low-E DD/AD groupb | 11.1 | 1.05 [0.49, 2.23] | .91 |
| 4. HGHE-A | |||
| Hi-G and hi-E AD groupc | 16.1 | 1.60 [1.10, 2.34]* | .02 |
| 5. HGLE-A | |||
| Hi-G and low-E AD groupc | 13.5 | 1.31 [0.77, 2.23] | .32 |
| 6. MGLE-A | |||
| Mod-G and low-E AD groupc | 5.4 | 0.48 [0.22, 1.04] | .06 |
| 7. LGLE | |||
| Low-G and low-E group (ref.) | 10.7 | 1.00 (ref. group) | (ref.) |
| Overall prevalence of CD | 14.8 | ||
| Individual contrast models | |||
| Contrast: HGHE-D vs. HGHE-A | 1.42 [1.03, 1.95]* | .03 | |
| Contrast: HGLE-D vs. HGLE-A | 1.10 [0.51, 2.39] | .81 | |
| Contrast: MGLE-D vs. MGLE-A | 2.19 [0.81, 5.91] | .12 | |
Notes: OR = odds ratio; CI = confidence interval; HG = high genetic; HE = high environmental; D = drug; G = genetic; E = environmental; LE = low environmental; MG = medium genetic; A = alcohol; LG = low genetic; ref. = reference.
P values for each group in the multifactorial model are compared with Group 7, the reference group;
DD: offspring risk due to twin or cotwin history of drug dependence with or without AD;
AD: offspring risk due to twin or cotwin history of alcohol dependence (without DD).
Table 1.
Classification of offspring risk groups: Seven-group and five-group models
| Seven-group basic model | Father and cotwin's diagnostic status | G | E | n | Risk type |
| Group | |||||
| HGHE-D | DD or DD/AD | High | High | 455 | Father's DD risk |
| HGLE-D | Father unaffected, MZ cotwin DD or DD/AD | High | Low | 75 | MZ cotwin's DD risk |
| MGLE-D | Father unaffected, DZ cotwin DD or DD/AD | Moderate | Low | 81 | DZ cotwin's DD risk |
| HGHE-A | AD (without DD) | High | High | 548 | Father's AD risk |
| HGLE-A | Father unaffected, MZ cotwin AD (without DD) | High | Low | 170 | MZ cotwin's AD risk |
| MGLE-A | Father unaffected, DZ cotwin AD (without DD) | Moderate | Low | 148 | DZ cotwin's AD risk |
| LGLE | None | Low | Low | 440 | None |
| Five-group model combining Groups 2 and 5 and 3 and 6 | Dx | G | E | n | Risk type |
| Group | |||||
| HGHE-D | DD or DD/AD | High | High | 455 | Father's DD risk |
| HGHE-A | AD without DD | High | High | 548 | Father's AD risk |
| HGHE | Father unaffected, MZ cotwin in DD or AD or both | High | Low | 245 | MZ cotwin's DD or AD risk |
| MGLE | Father unaffected, DZ cotwin DD or AD or both | Moderate | Low | 229 | DZ cotwin's DD or AD risk |
| LGLE | None | Low | Low | 440 | None |
Notes: G = genetic; E = environmental; n = number of offspring; HG = high genetic; HE = high environmental; D = drug; DD = drug dependence; AD = alcohol dependence; LE = low environmental; MZ = monozygotic; MG = medium genetic; DZ = dizygotic; A = alcohol; LG = low genetic; Dx = diagnosis.
Sample characteristics
Examination of sociodemographic characteristics (see Table 3) found significant differences in the families of fathers with drug dependence or comorbid drug-dependence/ alcohol-dependence history (HGHE-D) compared with families with neither disorder. Offspring were older and had younger mothers, fathers were less frequently employed full time, family income was lower, and these parents were more likely to be divorced. These differences were not found for the alcohol-dependence risk group (HGHE-A), except for mother's younger age, and they were not evident in any other risk group. In terms of parental psychopathology, significant increases in mental disorders were exhibited in both the HGHE-D and HGHE-A groups compared with families with neither disorder, as seen in paternal history of antisocial personality disorder or conduct disorder, paternal history of internalizing disorders, maternal history of alcohol dependence, and maternal history of heavy marijuana use. HGHE-D mothers also endorsed a history of major depression, and HGHE-A mothers endorsed a history of antisocial-personality-disorder symptoms more frequently than referent mothers. For the most part, intermediate risk groups (HGLE and MGLE) did not display these characteristics.
Table 3.
Sociodemographic characteristics for four offspring risk groups compared with the reference group, percentages given for dichotomous variables, M (SD) given for continuous variables
| Family characteristics (as of date of interview) | HGHE-D Hi-G and hi-E MZ and DZ DD twins (n = 455) M (SD) or % | HGHE-A Hi-G and hi-E MZ and DZ AD twins (n = 548) M (SD) or % | HGLE Hi-G and low-E MZ twin no dx; co-twin DD or AD (n = 245) M (SD) or % | MGLE Mod-G and low-E MZ twin no dx; co-twin DD or AD (n = 229) M (SD) or % | LGLE reference Low-G and low-E MZ and DZ unaffected (n = 440) M (SD) or % | Entire sample (n= 1,917) M (SD) or % |
| Male offspring | 50.3% | 49.8% | 50.2% | 50.2% | 47.5% | 49.5% |
| Child age, yrs. | 22.1 (4.4)* | 21.1 (4.2) | 21.5 (4.3) | 21.2 (6.5) | 21.4 (4.4) | 21.4 (4.7) |
| Paternal age, yrs. | 51.6 (2.7) | 51.6 (2.9) | 51.8 (2.8) | 51.7 (2.7) | 51.6 (2.8) | 51.6 (2.8) |
| Maternal age, yrs. | 48.2 (4.4)** | 48.5 (4.4)* | 48.9 (4.7) | 48.4 (3.8) | 49.1 (4.3) | 48.6 (4.3) |
| Father educ. >high school | 61.1% | 64.8% | 63.8% | 63.2% | 66.2% | 63.9% |
| Mother educ. >high school | 60.2% | 61.5% | 64.5% | 64.5% | 63.9% | 62.6% |
| Father employed fulltime | 83.5%** | 90.0% | 87.3% | 92.6% | 90.5% | 88.5% |
| Household income group | 9.9 (6.7)** | 10.5 (6.2) | 11.8 (11.5) | 10.3 (3.2) | 11.1 (5.3) | 10.6 (6.8) |
| White race | 96.3% | 93.4% | 92.6% | 93.9% | 94.3% | 94.3% |
| Marital status: divorced* | 36.5%*** | 18.2% | 18.0% | 20.1% | 16.4% | 22.3% |
| Father ASPD or CD dx | 22.2%*** | 13.1%*** | 4.5% | 7.9%** | 2.5% | 11.1% |
| Mother ASPD symptoms | 4.8% | 12.0%** | 5.2% | 6.6% | 6.1% | 7.5% |
| Father internalizing dx | 41.8%*** | 19.5%*** | 11.4% | 10.5% | 7.3% | 19.9% |
| Mother lifetime depression dx | 16.5%*** | 4.6% | 5.3% | 9.2%* | 5.2% | 8.2% |
| Mother AD dx | 16.0%*** | 9.7%** | 5.7% | 7.4% | 4.5% | 9.2% |
| Mother marijuana >150 uses | 5.9%** | 4.4%* | 1.2% | 3.5% | 1.6% | 3.6% |
| Mother data missing | 10.8% | 9.7% | 6.9% | 10.9% | 8.0% | 9.3% |
Notes: Bold indicates statistical significance. HG = high genetic; HE = high environmental; D = drug; A = alcohol; LE = low environmental; MG = medium genetic; LG = low genetic; MZ = monozygotic; DZ = dizygotic; DD = drug dependence; AD = alcohol dependence; Dx = diagnosis; yrs. = years; educ. = education; ASPD = antisocial personality disorder. Significantp values:
p < .05;
p < .01;
p < .001.
Characterization: Covariates
As described, certain psychiatric and sociodemographic variables known to be correlated with conduct disorder were considered either theoretically necessary or were found to be substantively important in current analyses. Because parental antisocial personality is a primary confound of alcohol effects in both parents and offspring, it was theoretically necessary to model antisocial variables for both parents (i.e., paternal antisocial personality disorder/conduct disorder and maternal antisocial behaviors). The tradeoffs in doing so are discussed in Haber et al. (2005). Also theoretically necessary were maternal substance and psychiatric indicators (i.e. maternal alcohol dependence, heavy marijuana use, and depression). Paternal full-time employment and paternal education (beyond high school) were included as indicators of socio-economic status; after doing so, family-income variables and maternal education no longer influenced offspring conduct disorder and were removed. Race was not found to be associated with offspring conduct disorder after controlling for other covariates and was removed from the model. Offspring age was found to be closely associated with outcome; however, paternal and maternal ages were not associated and were removed. Marital status (divorce) was confirmed as a significant predictor. The dummy variable reflecting missing maternal data was technically necessary; using it permitted inclusion of 179 offspring whose mothers did not participate and provided an assessment of the association between maternal participation and offspring conduct disorder. Twelve covariates were retained as important to this analytic model. The final model is displayed in Table 4. As can be seen, paternal and maternal antisocial personality disorder increased the odds of offspring conduct disorder significantly (paternal OR = 1.60; maternal OR = 2.63) compared with unaffected families. Parental divorce almost doubled the odds of conduct disorder in offspring (OR = 1.91). Male gender (OR = 4.51) and older age of offspring (OR = 2.67) were also significantly associated with conduct-disorder outcome. Once key covariates were taken into account, several other variables fell below the significance threshold. Concerning the above dummy variable, results showed no statistically significant effect associated with missing maternal data (p = .81).
Table 4.
Odds ratio andp values for a binary indicator of 3 or more conduct disorder symptoms in offspring as a function of family DD/AD or AD risk status in combined groups adjusted for covariates
| Offspring conduct disorder |
|||
| Variable | OR [95% CI] | Pa | |
| Multifactorial model | |||
| HGHE-D (hi-G and hi-E DD group)b | (Hypothesis 1a) | 1.99 [1.29, 3.08] | .002 |
| HGHE-A (hi-G and hi-E AD group)c | (Hypothesis 1b) | 1.57 [1.04, 2.36] | .03 |
| HGLE (hi-G and low-E combined DD + ADd MZ group) | 1.38 [0.84, 2.26] | .21 | |
| MGLE (mod-G and low-E DD + AD combinedd DZ group) | 0.64 [0.35, 1.16] | .14 | |
| LGLE (low-G and low-E group as ref.) | 1.00 (ref. group) | — | |
| Covariatese | |||
| Paternal antisocial personality and conduct disorder | 1.60 [1.08, 2.38] | 02 | |
| Paternal internalizing psychopathologyf | 0.77 [0.54-1.09] | .14 | |
| Paternal full-time employment | 0.71 [0.48, 1.05] | .08 | |
| Paternal post-high school education | 0.80 [0.60, 1.06] | .11 | |
| Maternal antisocial personality disorder (screen) | 2.63 [1.65, 4.18] | .000 | |
| Maternal marijuana use (150 times or more) | 1.48 [0.80, 2.76] | .22 | |
| Maternal alcohol dependence | 1.01 [0.64, 1.59] | .97 | |
| Maternal major depression | 0.97 [0.60, 1.57] | .89 | |
| Maternal missing data | 1.06 [0.67, 1.66] | .81 | |
| Male offspring | 4.51 [3.32, 6.11] | .000 | |
| Offspring age 18 and older | 2.67 [1.78, 4.00] | .000 | |
| Marital divorce | 1.91 [1.41, 2.58] | .000 | |
| Individual contrast models (adjusted for covariates) | |||
| Hypothesis 2: Contrast HGHE-D vs. HGHE-A (hi-G and hi-E DD groupb vs. hi-G and hi-E AD group)c | 1.29 [0.89, 1.88] | .18 | |
| Hypothesis 3: HGLE vs. MGLE (hi-G and low-E combinedd MZ vs. hi-G and low-E combinedd DZ groups) | 2.64 [1.34, 5.21] | .005 | |
| Hypothesis 4a: Contrast HGHE-D vs. HGLE (hi-G and hi-E DD groupb vs. hi-G and low-E combined MZ groupd) | 1.63 [1.00, 2.68] | .05 | |
| Hypothesis 4b: Contrast HGHE-A vs. HGLE (hi-G and hi-E AD groupc vs. hi-G and low-E combined MZ groupd) | 1.12 [0.71, 1.78] | .62 | |
Notes: Bold indicates statistical significance. DD = drug dependence; AD = alcohol dependence; OR = odds ratio; CI = confidence interval; HG = high genetic; HE = high environmental; D = drug; G = genetic; E = environmental; A = alcohol; LE = low environmental; MZ = monozygotic; MG = medium genetic; DZ = dizygotic; LG = low genetic; ref. = reference.
P values for each group in the multifactorial model are compared with Group 7, the reference group;
DD: offspring risk due to paternal history of DD (with or without AD);
AD: offspring risk due to paternal history of AD (without DD);
DD + AD: offspring risk due to paternal DD or AD or both (combined risk group);
eliminated from model: family income, maternal education, paternal and maternal age, and race;
paternal major depression; dysthymia; and generalized anxiety, panic, and posttraumatic stress disorders.
Specific hypothesis tests
Hypothesis testing was conducted in the full multivariate model (see Table 4).
Hypothesis 1 (Phenotypic risk): To test the hypothesis of phenotypic risk, contrasts were made between the group of offspring at high genetic and high environmental risk, compared with offspring at low risk for both genetic and environmental influences. Significant differences were found for both the HGHE-D and HGHE-A groups, compared with the LGLE group. The odds of conduct disorder were almost twice as high (OR = 1.99, 95% CI [1.29, 3.08],p = .002) for offspring in the HGHE-D group, and 1.6 times as high for offspring in the HGHE-A group (OR = 1.57, 95% CI [1.04, 2.36],p = .03), compared with the reference (LGLE) group. These results confirmed Hypothesis 1 by demonstrating that having a father with a history of drug dependence or alcohol dependence is associated with an increased risk of conduct disorder in offspring, compared with those with unaffected fathers.
Hypothesis 2 (Comorbidity): Hypothesis 2 specified an increased risk of conduct disorder associated with paternal drug dependence, which is frequently comorbid with alcohol dependence, compared with paternal alcohol dependence alone. After adjusting for covariates, there were no statistically significant differences between the HGHE-D and the HGHE-A groups. Given that the paternal drug-dependence versus alcohol-dependence contrast was significant in the basic model (without covariates), key mediating covariates could be identified by removing variables systematically. Results indicated that maternal variables—specifically, maternal alcohol dependence, marijuana use, depression, and divorce—accounted for a significant part of the observed difference between paternal drug dependence and alcohol dependence on offspring conduct disorder.
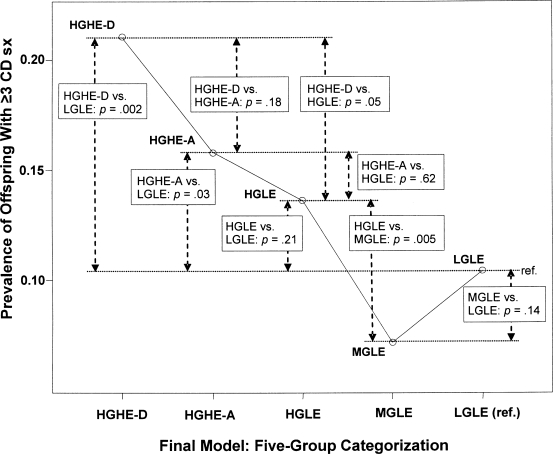
Hypothesis 3 (Genetic risk): The contrast between HGLE and MGLE groups (see Figure 1) was an examination of differences in offspring conduct-disorder risk associated with an affected co-twin of the father, but not the father himself, and associated with the greater genetic similarity of monozygotic pairs compared with dizygotic pairs. Because the latent environmental risk was low for both of these groups, this contrast differentiated genetic risk groups by examining whether there were differences in offspring conduct-disorder risk based on whether the monozygotic twin of a high-risk co-twin transmitted greater risk to offspring compared with the dizygotic twin of a high-risk co-twin. In these data, groups reflecting hypothesized high genetic risk (monozygotic co-twins) versus moderate genetic risk (dizygotic co-twins) were significantly different regarding offspring conduct-disorder scores (OR = 2.64, 95% CI [1.34, 5.21],p = .005). Evidence that risk of conduct disorder in offspring was significantly greater among those whose fathers had an affected monozygotic co-twin, compared with an affected dizygotic co-twin, demonstrated the potency of genetic influences and confirmed Hypothesis 3.
Figure 1.
Prevalence of offspring with three or more conduct-disorder symptoms: Group contrasts with adjusted probability of significant group differences. This figure shows the four key risk groups compared with the reference group and their intergroup contrasts. The pattern of significant contrasts reveals the genetic and environmental structure of observed effects. HG = high genetic risk; MG = moderate genetic risk; LG = low genetic risk, HE = high environmental risk; LE = low environmental risk; D = drug dependence history with or without alcohol dependence history; A = alcohol dependence history (no drug dependence history); CD = conduct disorder; sx = symptoms; ref. = reference.
Hypothesis 4 (Environmental risk): Contrasts associated with this hypothesis compared offspring with similarly high genetic backgrounds who differed in their family environments. A significant contrast between these groups would therefore reflect the impact of different levels of environmental risk (high vs. low). The contrast between HGHE-D and HGLE was significant (OR = 1.63, 95% CI [1.00, 2.68], p = .05). (Recall that the HGLE-D and HGLE-A groups were combined because they were not significantly different in predicting offspring conduct disorder.) Thus, low environmental risk resulted in lower odds of offspring conduct disorder, even in the presence of high genetic risk. However, the same inference did not hold when HGHE-A was contrasted with HGLE (p = .62). A similar mitigating influence of a low-risk environment was not observed when the paternal phenotype was alcohol dependence. Hypothesis 4 was confirmed with respect to the drug-dependence risk group but was not supported for alcohol-dependence risk.
Discussion
In the current study, family history risks associated with paternal drug dependence and alcohol dependence were examined simultaneously as predictors of offspring conduct disorder in an offspring-of-twins research design. Similar to other studies of comorbidity (e.g., Dick et al., 2007), 70% of fathers with drug dependence in this sample also met criteria for alcohol dependence. Examination of predictive associations between paternal drug dependence (with or without alcohol dependence) and paternal alcohol dependence (without drug dependence) and the outcome of offspring conduct disorder supported several conclusions. First, transmission of risk was evident across generations from parents to children and across diagnoses from paternal drug-dependence history and paternal alcohol-dependence history to offspring conduct disorder (confirming Hypothesis 1). Second, the drug-dependence association with conduct disorder was significantly stronger than the alcohol-dependence association with conduct disorder in a basic model without covariates but was not significantly different in a multivariate model after covarying (partialing out) key influences, notably maternal variables and divorce (partially confirming Hypothesis 2). Third, genetic influences contributed significantly and nondifferentially to the association between paternal drug dependence and/or alcohol dependence and offspring conduct disorder (confirming Hypothesis 3). Fourth, significant environmental influences were evident in the association between paternal drug-dependence risk and offspring conduct disorder but not in the association between paternal alcohol-dependence risk and offspring conduct disorder (partially confirming Hypothesis 4), findings suggestive of a significant gene-environment interaction effect for drug-dependence risk that was not observed for alcohol-dependence risk.
Hypothesis 1 was a test of phenotypic association. Those at high genetic and high environmental risk were compared with those having low genetic and low environmental risk. Even after accounting for covariation, offspring at risk because of paternal drug-dependence history and paternal alcohol-dependence history exhibited elevated rates of conduct disorder, compared with low-risk offspring. There was significant variation in each of these different phenotypes to demonstrate their separate impact on offspring outcomes and to confirm that one disorder did not account for the other. Results also demonstrated the transmission of risk across generations and diagnoses for both disorders, that is, from each paternal substance-dependence type to offspring conduct disorder. These results were consistent with findings from many published comorbidity studies and family studies within the alcohol and drug literatures, especially those focusing on family alcoholism, child development, and children of alcoholics (Loukas et al., 2001; Lynskey et al., 1994; Slutske et al., 2002; Zucker, 1994).
Hypothesis 2 specifically contrasted offspring at drug-dependence risk with offspring at alcohol-dependence risk, a test of differences in severity. In a univariate model (unadjusted), the association between paternal drug dependence and offspring conduct disorder was significantly stronger than the association between paternal alcohol dependence and offspring conduct disorder; but, in a multivariate model (adjusted for covariates), this drug dependence-alcohol dependence difference was not significant. By removing covariates systematically, it was found that maternal alcohol dependence, heavy marijuana use, depression, and divorce accounted for the drug dependence-alcohol dependence difference in severity even when maternal antisocial personality symptoms remained in the model. Therefore, variation arising from paternal history of drug dependence and from correlated maternal and familial effects both contributed importantly to the prediction of offspring conduct disorder. That is, maternal factors mediated an important part of the increase in severity seen in drug-dependence risk, compared with alcohol-dependence risk. This suggests that maternal and familial factors must be modeled to adequately characterize the impact of paternal drug dependence on offspring. Also, these results strengthen support for a small but growing literature suggesting that comorbid drug dependence/alcohol dependence is a more severe (and more heritable) form of substance dependence compared with alcohol dependence alone (Dick et al., 2007).
Hypothesis 3 addressed the role of genetic effects in the association between paternal history of drug-dependence and alcohol-dependence risk and offspring conduct disorder. Differences in zygosity were the basis for detection of genetic effects in this model, as is always the case in twin research designs. However, this design was unique in that the measured outcome was obtained from the offspring rather than from the twins themselves. Thus, monozygotic-dizygotic differences were transmitted from parents to offspring before measurement, and some attenuation during transmission would be expected. Nonetheless, monozygotic-dizygotic differences remained sufficiently strong, thus demonstrating that genetic variation accounted for a substantial part of the association between paternal histories of drug dependence and/or alcohol dependence and offspring conduct disorder. These findings from an offspring-of-twins study added multimethod validation to the growing number of classic twin studies that have identified a substantially genetic common latent factor, especially those examining externalizing disorders (Button et al., 2007; Fuller et al., 2003; Kendler et al., 1992; Krueger et al., 1994) and the molecular extensions of these studies (Dick et al., 2007).
Concerning the role of the environment, Hypothesis 4 addressed the potential mitigating influence of a low-risk environment on the expression of high genetic risk. In this comparison, all offspring were at high genetic risk by virtue of their father's or their uncle's (his monozygotic co-twin's) drug-dependence or alcohol-dependence history, but environmental risk varied depending on whether the offspring family environment included a father with a history of drug dependence or alcohol dependence or, alternatively, a father without such history (although his co-twin did have this history). Concerning drug-dependence risk, offspring at high genetic risk offspring who were also at high environmental risk as a result of having fathers with drug-dependence history had higher rates of conduct disorder. However, when environmental risk was low (because fathers did not have a history of drug dependence, even though father's monozygotic co-twin did), offspring had significantly lower rates of conduct disorder. These results demonstrated that, in the case of high genetic risk associated with drug-dependence family history, the absence of environmental risk mitigated the expression of genetic risk. In contrast, and contrary to expectations, analyses of alcohol-dependence risk did not produce a similar finding. That is, among offspring at high genetic risk, the presence or absence of environmental risk did not differentiate offspring conduct-disorder outcomes. By definition, when the effects of genetic risk vary according to the level of an environmental factor, a gene-environment interaction is evident. Therefore, it can be concluded that, in the case of drug-dependence risk, a gene-environment interaction was demonstrated. This finding is noteworthy because gene-environment interactions are rarely reported in the literature.
The greater influence of environmental factors on drug-dependence risk (but not alcohol dependence) can be at least partially explained by the sociodemographic and psychopathological characteristics of drug-dependence families as seen in Tables 3 and 4. For instance, father's antisocial-personality-disorder/conduct-disorder diagnoses were twice as frequent in drug-dependence families, compared with alcohol dependence, and were nine times greater than in low-risk families; and both paternal and maternal antisocial personality were significantly associated with offspring conduct disorder even after accounting for all other covariates. In addition, the families of fathers with drug-dependence history had (a) about double the divorce rate of any other group, which approximately doubled the odds for offspring conduct disorder; (b) about twice the rate of paternal internalizing disorders of that in alcohol-dependence families, which was six times greater than in low-risk families; (c) about twice the rate of maternal alcohol-dependence diagnoses, which was four times greater than in low-risk families; and (d) lower rates of employment and lower household income, compared with all other groups. As can be seen, when the father had a history of drug dependence, the family environment included significant disadvantages that have been shown to be associated with increased offspring adversity (Jacob et al., 2000). These environmental disadvantages help explain the gene-environment interaction observed in drug-dependence risk families.
Limitations
It should be noted that this study did not include direct measurement of the environment of the offspring. Instead, environmental risk was handled as a latent environmental variable, in that environmental risk was inferred from a father's history and his role as father within the offspring's family environment (Jacob et al., 2000). For drug-dependence family risk, this latent environmental construct was sufficiently robust as to differentiate offspring conduct-disorder outcomes, but conclusions would be stronger if the environment were directly measured. These concerns will be addressed in subsequent studies. Also, the offspring-of-twins design depends on certain assumptions that were not tested in this study. The reader is referred to other studies from our group (and others) that have specifically tested key assumptions. For instance, Duncan et al. (2006) empirically examined assumptions about high-risk offspring exposure to paternal drinking; and Koenig et al. (2010) examined assumptions about low-risk offspring exposure to alcoholic uncles (father's co-twins). These studies support the validity of the offspring-of-twins design assumptions.
Acknowledgments
The authors are grateful for the continued cooperation and participation of the members of the Vietnam Era Twin Registry and their families. Without their contribution, this research would not have been possible.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants AA11667, AA11822, and DA14363; by NIAAA center grant AA11998; and by a merit review grant from the Department of Veterans Affairs Medical Research Service, Washington, DC, awarded to Theodore Jacob. The U.S. Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including the Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; and Institute for Survey Research, Temple University (Philadelphia, PA).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: Author; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Button TMM, Rhee SH, Hewitt JK, Young SE, Corley RP, Stallings MC. The role of conduct disorder in explaining the comorbidity between alcohol and illicit drug dependence in adolescence. Drug and Alcohol Dependence. 2007;87:46–53. doi: 10.1016/j.drugalcdep.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Wang JC, Hinrichs A, Bertelsen S, Bucholz KK, Bierut LJ. Alcohol dependence with comorbid drug dependence: Genetic and phenotypic associations suggest a more severe form of the disorder with stronger genetic contribution to risk. Addiction. 2007;102:1131–1139. doi: 10.1111/j.1360-0443.2007.01871.x. [DOI] [PubMed] [Google Scholar]
- Duncan AE, Sartor CE, Scherrer JF, Grant JD, Heath AC, Nelson EC, Bucholz KK. The association between cannabis abuse and dependence and childhood physical and sexual abuse: Evidence from an offspring of twins design. Addiction. 2008;103:990–997. doi: 10.1111/j.1360-0443.2008.02210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AE, Scherrer J, Fu Q, Bucholz KK, Heath AC, True WR, Jacob T. Exposure to paternal alcoholism does not predict development of alcohol-use disorders in offspring: Evidence from an offspring-of-twins study. Journal of Studies in Alcohol. 2006;67:649–656. doi: 10.15288/jsa.2006.67.649. [DOI] [PubMed] [Google Scholar]
- Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in Vietnam Era Twin Registry: An approach using questionnaires. Clinical Genetics. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: Method of construction. Acta Geneticae Medicae et Gemellologiae. 1987;36:61–67. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, Eisen SA. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: Contribution of antisocial personality disorder in men. Archives of General Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- Fuller BE, Chermack ST, Cruise KA, Kirsch E, Fitzgerald HE, Zucker RA. Predictors of aggression across three generations among sons of alcoholics: Relationships involving grandparental and parental alcoholism, child aggression, marital aggression and parenting practices. Journal of Studies on Alcohol. 2003;64:472–483. doi: 10.15288/jsa.2003.64.472. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Henderson WG, Eisen SA, True W, Ramakrishnan V, Lyons MJ, Tsuang MT. A strategy for assembling samples of adult twin pairs in the United States. Statistics in Medicine. 1993;12:1693–1702. doi: 10.1002/sim.4780121805. [DOI] [PubMed] [Google Scholar]
- Haber JR, Jacob T, Heath A. Parental alcoholism and offspring conduct disorder: Evidence for the 'common genes' hypothesis. Twin Research. 2005;8:120–131. doi: 10.1375/1832427053738782. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heath AC, Nelson EC. Effects of the interaction between genotype and environment: Research into the genetic epidemiology of alcohol dependence. Alcohol Research and Health. 2002;26:193–201. [PMC free article] [PubMed] [Google Scholar]
- Henderson WG, Eisen S, Goldberg J, True W, Barnes JE, Vitek ME. The Vietnam Era Twin Registry: A resource for medical research. Public Health Report. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock VM. Drinking style of parents of alcoholic and control probands. Alcohol. 1985;2:525–528. doi: 10.1016/0741-8329(85)90128-4. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono W, McGue M. Antisocial personality disorder and depression in relation to alcoholism: A community-based sample. Journal of Studies on Alcohol. 1998;59:222–226. doi: 10.15288/jsa.1998.59.222. [DOI] [PubMed] [Google Scholar]
- Jacob T, Haber JR, Leonard K, Rushe R. Home interactions of high and low antisocial male alcoholics and their families. Journal of Studies on Alcohol. 2000;61:72–80. doi: 10.15288/jsa.2000.61.72. [DOI] [PubMed] [Google Scholar]
- Jacob T, Sher KJ, Bucholz KK, True WR, Sirevaag EJ, Rohrbaugh J, Heath AC. An integrative approach for studying the etiology of alcoholism and other addictions. Twin Research. 2001;4:103–118. doi: 10.1375/1369052012218. [DOI] [PubMed] [Google Scholar]
- Jacob T, Waterman B, Heath A, True W, Bucholz KK, Haber R, Fu Q. Genetic and environmental effects on offspring alcoholism: New insights using an offspring-of-twins design. Archives of General Psychiatry. 2003;60:1265–1272. doi: 10.1001/archpsyc.60.12.1265. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness: Current status and future directions. Archives of General Psychiatry. 1993;50:905–915. doi: 10.1001/archpsyc.1993.01820230075007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. Journal of the American Medical Association. 1992;268:1877–1882. [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. American Journal of Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Koenig LB, Jacob T, Haber JR, Xian H. Testing the equal environments assumption in the children of twins design. Behavior Genetics. 2010;40:533–541. doi: 10.1007/s10519-010-9345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R, Hicks B, Patrick C, Carlson S, Iacono W, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Schmutte P, Caspi A, Moffitt T, Campbell K, Silva P. Personality traits are linked to crime among men and women: Evidence from a birth cohort. Journal of Abnormal Psychology. 1994;103:328–338. doi: 10.1037//0021-843x.103.2.328. [DOI] [PubMed] [Google Scholar]
- Loukas A, Fitzgerald HE, Zucker RA, von Eye A. Parental alcoholism and co-occurring antisocial behavior: Prospective relationships to externalizing behavior problems in their young sons. Journal of Abnormal Child Psychology. 2001;29:91–106. doi: 10.1023/a:1005281011838. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Fergusson DM, Horwood L. The effect of parental alcohol problems on rates of adolescent psychiatric disorders. Addiction. 1994;89:1277–1286. doi: 10.1111/j.1360-0443.1994.tb03306.x. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Toomey R, Meyer JM, Green AI, Eisen SA, Goldberg J, Tsuang MT. How do genes influence marijuana use? The role of subjective effects. Addiction. 1997;92:409–417. [PubMed] [Google Scholar]
- Lyons MJ, True WR, Eisen SA, Goldberg J, Meyer JM, Faraone SV, Tsuang MT. Differential heritability of adult and juvenile antisocial traits. Archives of General Psychiatry. 1995;52:906–915. doi: 10.1001/archpsyc.1995.03950230020005. [DOI] [PubMed] [Google Scholar]
- McGue M. Genes, environment and the etiology of alcoholism. In: Zucker R, Boyd G, Howard J, editors. The development of alcohol problems: Exploring the biopsychosocial matrix of risk (NIAAA Research Monograph No. 26, NIH Publication No. 94–3495. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 1994. pp. 1–40. [Google Scholar]
- Scherrer JF, Grant JD, Duncan AE, Pan H, Waterman B, Jacob T, Bucholz KK. Measured environmental contributions to cannabis abuse/dependence in an offspring of twins design. Addictive Behaviors. 2008;33:1255–1266. doi: 10.1016/j.addbeh.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Cronk NJ, Sher KJ, Madden PAF, Bucholz KK, Heath AC. Genes, environment and individual differences in alcohol expectancies among female adolescents and young adults. Psychology of Addictive Behaviors. 2002;16:308–317. [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PAF, Bucholz KK, Dunne MP, Martin NG. Common genetic risk factors for conduct disorder and alcohol dependence. Journal of Abnormal Psychology. 1998;107:363–374. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- True WR, Heath AC, Scherrer JF, Xian H, Lin N, Eisen SA, Tsuang MT. Interrelationship of genetic and environmental influences on conduct disorder and alcohol and marijuana dependence symptoms. American Journal of Medical Genetics-Part B: Neuropsychiatric Genetics. 1999;88:391–397. doi: 10.1002/(sici)1096-8628(19990820)88:4<391::aid-ajmg17>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: A study of 3,372 twin pairs. American Journal of Medical Genetics-Part B: Neuropsychiatric Genetics. 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Volk HE, Scherrer JF, Bucholz KK, Todorov A, Heath AC, Jacob T, True WR. Evidence for specificity of transmission of alcohol and nicotine dependence in an offspring of twins design. Drug and Alcohol Dependence. 2007;87:225–232. doi: 10.1016/j.drugalcdep.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Zucker RA. Pathways to alcohol problems and alcoholism: A developmental account of the evidence for multiple alcoholisms and for contextual contributions to risk. In: Zucker R, Boyd G, Howard J, editors. The development of alcohol problems: Exploring the biopsychosocial matrix of risk (NIAAA Research Monograph No. 26, NIH Publication No. 94-3495. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 1994. pp. 255–289. [Google Scholar]