Abstract
Many forms of psychopathology and substance abuse problems are characterized by chronic ritualized forms of avoidance and escape behavior that are designed to control or modify external or internal (i.e, thoughts, emotions, bodily sensations) threats. In this functional magnetic resonance imaging investigation, we examined amygdala reactivity to threatening cues when avoidance responding consistently prevented contact with an upcoming aversive event (money loss). In addition, we examined escape responding that terminated immediate escalating money loss and approach responding that produced a future money gain. Results showed cues prompting avoidance, escape and approach behavior recruited a similar fronto-striatal-parietal network. Within the amygdala, bilateral activation was observed to threatening avoidance and escape cues, even though money loss was consistently avoided, as well as to the reward cue. The magnitude of amygdala responses within-subjects was relatively similar to avoidance, escape and approach cues, but considerable between-subject differences were found. The heightened amygdala response to avoidance and escape cues observed within a subset of subjects suggests threat related responses can be maintained even when aversive events are consistently avoided, which may account for the persistence of avoidance-coping in various clinical disorders. Further assessment of the relation between amygdala reactivity and avoidance-escape behavior may prove useful in identifying individuals with or at risk for neuropsychiatric disorders.
Keywords: approach, avoidance, escape, amydala, reward, anxiety
Introduction
"Out, damn'd spot; out I say" intoned Lady MacBeth at her imagined blood stained hands, a vision precipitated by participation in King Duncan’s murder. Despite believing “A little water clears us of this deed,” her excessive hand-washing continued, providing a measure of escape from her psychological demons. While avoidance and escape are natural adaptive behaviors, many forms of psychopathology and substance abuse problems are characterized by chronic avoidance-escape motivated by negative emotional states. For example, chronic coping in posttraumatic stress disorder often involves avoidance of uncontrollable intrusive memories (Brewin and Holmes, 2003). Features of panic disorder have been linked to avoidance of catastrophic appraisals of panic sensations (Clark, 1986). Obsessive-compulsive disorder is associated with avoidance-escape strategies designed to reduce negative cognitions or emotions, particularly in fear-contamination. Chronic avoidance-escape coping emerges in borderline personality disorder as nonsuicidal, deliberate self-harm to deal with negative thoughts or emotions. High levels of negative emotion (e.g., hostility, guilt, sadness) or anxiety may also prompt deliberate self-harm in nonclincial populations (Brown et al., 2007; Klonsky et al., 2003). Within substance abusing populations, negative affect (anxiety), social (potential loss of using friends), stress and aversive biological states associated with drug withdrawal can similarly motivate avoidance-escape in the form of drug seeking and facilitate drug relapse (Blume, 2001; Downs and Woods, 1975; Koob, 2009; Sinha, 2007). Such examples highlight how avoidance-escape commonly functions to control or modify aversive or threatening aspects of the environment or internal states (i.e, thoughts, emotions, bodily sensations), thus working as a basic emotion regulation strategy (Gross and Thompson, 2009; Hayes et al 1996).
Although avoidance-escape behavior is a recognized feature of many clinical disorders, our understanding of the neurocircuitry supporting adaptive human avoidance-escape remains poor. Consequently, progress has been limited in understanding transitions from adaptive to chronic forms of avoidance-escape and differences in avoidance neurocircuitry among clinical disorders. Neurophysiological research on adaptive forms of avoidance has implicated the amygdala in nonhuman (Poremba and Gabriel, 1997, 1999; Roozendaal et al., 1993; Werka et al., 1978) and human avoidance learning (Mobbs et al., 2009). There is also evidence linking amygdala dysfunction to mood disorders characterized by avoidance behavior (Etkin and Wager, 2007). The amygdala’s role in avoidance has been hypothesized to be limited to fear-conditioning, where a conditioned cue/threat signals the delivery of an aversive event (e.g., shock) (Cain and LeDoux, 2008; Werka et al., 1978). This view is consistent with two-factor theories of avoidance that propose threatening fear-conditioned cues motivate avoidance and removal of cues and fear-reduction serve to negatively reinforce avoidance (Miller, 1948; Mowrer, 1947; but also see Bolles, 1972, Herrnstein, 1969). Considerable evidence shows avoidance is also associated with increased reported fear and catastrophic thoughts (Eifert and Heffner, 2003) and increased skin conductance responses (Jensen et al, 2003; Mobbs et al., 2009; Rose et al., 1995; Solomon et al., 1980). Clinical research also suggests avoidance, such as the intentional suppression of undesirable or distressing emotions, can be counterproductive and can paradoxically enhance, or at least maintain, self-reported negative experiences, anxiety and physiological responses (Campbell-Sills et al., 2006; Cioffi and Holloway, 1993; Feldner et al., 2006; Spira et al., 2004). While such results suggest ties among the amygdala, avoidance and fear/anxiety, other investigations have shown that learned avoidance in nonhumans is not dependent upon the amygdala (Andrzejewski, et al., 2005; Lehmann et al., 2000; Poremba and Gabriel, 1997, 1999; Roozendaal et al., 1993), avoidance learning in humans is associated with declines in skin conductance responses to fear-conditioned cues (Lovibond et al., 2008) and avoidance cues fail to elicit amygdala activation in humans, but consistently prompts activity in the striatum (Jensen et al, 2003; Kim et al., 2006).
The relative paucity of research on the neurocircuitry supporting adaptive forms of human avoidance-escape and the mixed findings within the avoidance literature represent important gaps in our knowledge. In this functional magnetic resonance imaging investigation, our primary aim was to examine amygdala reactivity to threatening cues when avoidance responding consistently prevents contact with an upcoming aversive event, a process which models central features of many forms of psychopathology. Our secondary aim included examining reported amygdala contributions to escape from a proximal aversive event (Gold et al., 1976; Herdade et al., 2006; Mobbs et al., 2009) and to approach involving reward/reinforcement (Baxter and Murray, 2002; Hirai et al., 2009; Hommer et al., 2003; Machado and Bachevalier, 2007). The inclusion of these conditions provided a novel opportunity to compare amygdala responses to other aversive and appetitive conditions to determine if it serves a broader role as a ‘behavioral relevance’ detector (Ousdal et al., 2008; Paton et al., 2006; Sander et al., 2003; Schoenbaum et al., 2003).
Methods and Materials
Participants
Eighteen, healthy right-handed adults (9 male) participated. Subjects reported being between 18 and 50 years of age, free of medications affecting the central nervous system or the autonomic system for at least 2 weeks, and without a personal history of psychiatric disorder or substance abuse. Informed, written consent was obtained from all subjects according to the institutional guidelines established by the Johns Hopkins Human Subjects Protection Committees.
Task and Training
Prior to functional neuroimaging, subjects completed extensive training that involved learning, through trial and error, to respond appropriately to several cue-response-outcome contingencies. Training ensured stable performances during neuroimaging and eliminated possible confounds associated with regional activation reflecting acquisition of Pavlovian cue-outcome relations and operant response-outcome relations. In any task requiring decision-making, performance anxiety can also play a critical role in modulating behavior, cognition and emotion. Accordingly, pretraining also minimized the contributions of performance related anxiety to anxiety/fear that presumably would emerge during our avoidance and escape conditions.
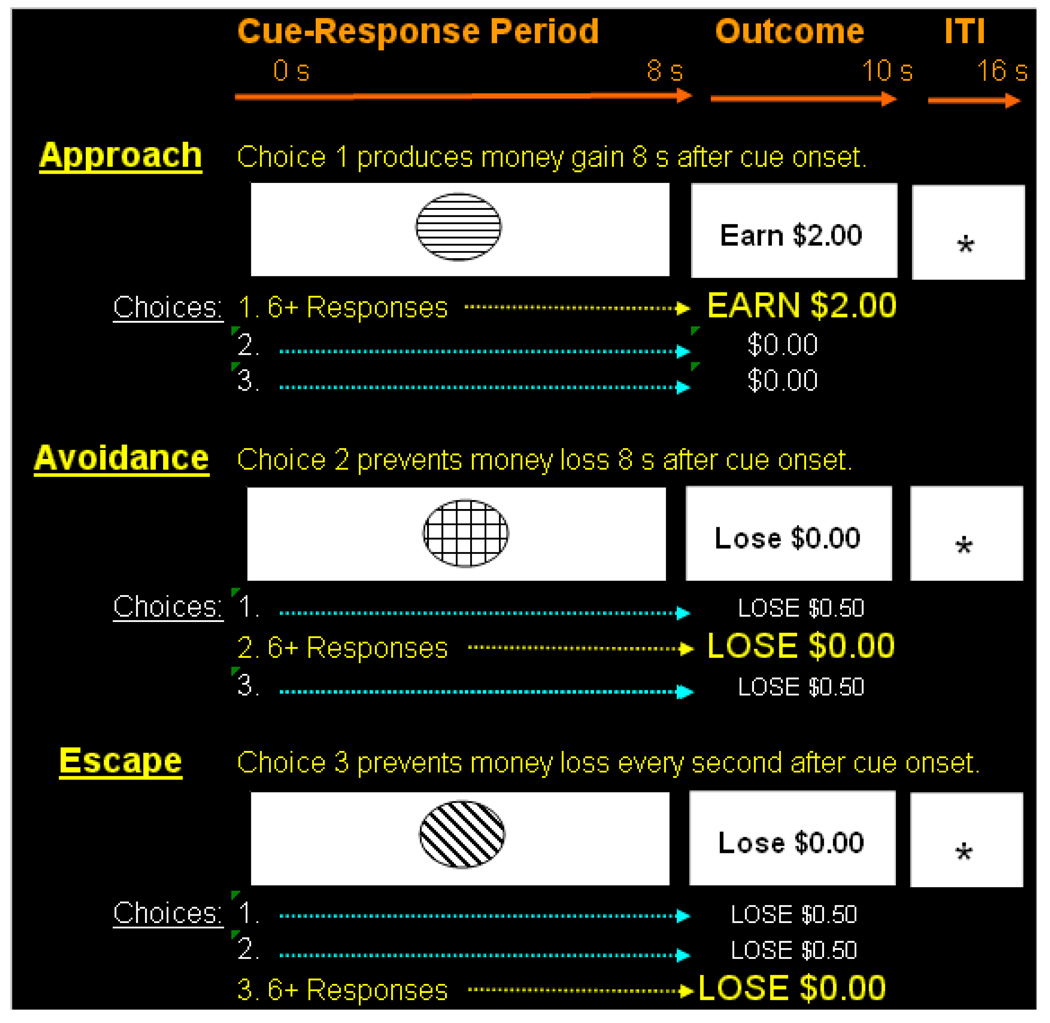
Figure 1 highlights the structure of trials and timing parameters employed during both training and neuroimaging. A trial consisted of the presentation of a visual cue for 8 s during which subjects were free to press or not press three available response buttons. Next, a 2 s outcome prompt revealed the magnitude of money gain or loss in accordance with the current contingency. A variable 4–6 s intertrial interval signaled by a fixation stimulus separated cue onsets. Three different cue-response-outcome contingencies were employed during training to establish approach, avoidance, and escape behavior. In addition, instructions highlighted that a fixation “+” stimulus (6 s in duration) would appear randomly during the task. Fixation served as the neutral baseline cue from which to identify voxels showing significant BOLD response increases in experimental conditions.
Figure 1. Approach, avoidance and escape contingencies and associated timing parameters.
The schematic shows each cue-response-outcome contingency employed and associated timing parameters. Distinct cues prompted approach responding that produced a future money gain, avoidance responding that prevented a future money loss and escape responding that terminated immediate escalating money loss. A neutral fixation cross (not shown) served as the baseline condition for imaging analyses. Pretraining using a contingency shaping procedure established stable and accurate responding during neuroimaging.
As seen in Figure 1, the approach cue was associated with a positive reinforcement contingency such that emitting greater than six presses on a target button (e.g., button #1) produced money gain ($2.00), but less than six presses or pressing other response buttons produced no monetary gain ($0.00). The avoidance cue was associated with a negative reinforcement contingency such that emitting greater than six presses on a target button (#2) cancelled a future monetary loss ($0.00), but less than six presses or pressing other response buttons resulted in loss (−$0.50). The escape cue was also associated with a negative reinforcement contingency such that presses on a target button (#3) paused escalating money loss for 1 s which began with cue onset and continued to the outcome period. Under escape, pressing other response buttons or non-responding resulted in total loss (−$0.48) and emitting less than six target responses resulted in loss proportional to the total number of responses emitted. Emitting more than 6 responses resulted in no loss ($0.00). Also included was a punishment cue where any button press produced a $0.05 loss. This cue condition was included to ensure that response-dependent money loss actually remained aversive throughout the task and loss was not overshadowed by earnings. Pretraining continued until response accuracy exceeded 90% during two consecutive blocks of 20 trials for each contingency. Response accuracy was defined as earning >90% of available reinforcers to the approach cue, avoiding >90% of losses to the avoidance cue, escaping >90% of the total programmed loss to the escape cue and not emitting any responses to the punishment cue.
Subjects were not informed about cue-response-outcome contingencies but were told that their task was to earn as much money as possible, with 100% accurate performances producing the maximum of $60.00 (the range of earnings was $58–$60). Subjects were also not informed of the minimum response requirement for each contingency. The rationale for not imposing an upper limit on the number of responses was to obtain measures of approach, avoidance and escape motivation with greater responding providing a index of greater motivation. Our rationale for using a larger amount of money for approach relative to avoidance and escape is based the suggestion that the magnitude of the response to negative stimuli is often larger than to positive stimuli which necessitated a larger reward to decrease the potential imbalance (Baumeister et al., 2001).
fMRI task and acquisition
Three ~14 minute imaging runs were completed. The imaging task employed the same trial structure and timing parameters used during training and involved presenting each cue-contingency (approach, avoidance, escape and punishment) and the baseline cue for 15 trials within an event related design. Responses were made on a hand-held button box containing three response buttons arranged vertically and labeled 1, 2, and 3. Instructions emphasized that the task was identical to the training task. Functional MRI images were collected on a 3 T Philips scanner at the F. M. Kirby Research Center for Functional Neuroimaging. T1 weighted anatomical volume images were collected for each subject using a MPRAGE sequence with a high-resolution isovoxel acquisition of 1mm3. Functional MRI data were gathered using a single shot echo planar imaging (EPI) sequence with a TR of 2 s, a TE of 30 ms, a 90 degree flip angle, 128×128 matrix size and field of view 24 cm, yielding voxels measuring 3×3 mm in plane. Approximately 43 contiguous 3 mm thick sections were obtained. The first three volumes were discarded to allow for equilibration effects. Functional images were first reconstructed from k-space to image space for further processing.
fMRI analysis
Data analysis was performed using Statistical Parametric Mapping software (SPM 2). For a subject’s imaging data to be included in the analysis, head movement was limited to less than 2 mm. Preprocessing procedures included reorientation, slice acquisition time correction, coregistration, within-subject realignment, spatial normalization to the standard Montreal Neurological Institute EPI template, resampling to 2mm3 voxel size, and spatial smoothing using a Gaussian kernel (6 mm fullwidth at half-maximum). High pass filtering was applied to the time series of EPI images to remove any low frequency drift. A canonical hemodynamic response function was used as a covariate in a general linear model and parameter estimates were generated for each voxel that corresponded to the onsets of cues and subsequent outcomes. Parameter estimates derived from the mean least squares fit of the model to the data reflect the strength of covariance between the data and the canonical response function for our events of interest. Separate contrast images were generated by contrasting activation associated with each condition cue relative to the baseline cue. Images were carried to a second-level group analysis which treated intersubject variability as a random effect. Voxel-wise comparisons were performed within a repeated measures analysis of variance to identify voxels with significant activation relative to baseline, with post-hoc paired t-tests performed to identify significant differences in activation between conditions. The statistical thresholds used for whole brain analyses were p < 0.0001, uncorrected for multiple comparisons, and an extent threshold of 50 contiguous voxels, yielding an uncorrected cluster-level threshold of p = .004. To highlight overlapping brain regions between conditions, an inclusive masking technique was used to determine the intersection (conjunction) of SPM {T} maps for the contrasts Approach>Baseline and Avoidance>Baseline, as well as Approach>Baseline and Escape >Baseline. This highlighted suprathreshold voxels for the approach cue that were also suprathreshold for avoidance and escape cues. As our a priori region of interest was the amygdala, our ROI analysis was centered on the local maxima (5 mm sphere with SVC) identified in the whole brain analysis. The location of voxels with significant activation was summarized by their local maxima separated by at least 8 mm, and by converting the maxima coordinates from MNI to Talairach coordinate space using recommended transformations (Lancaster et al, 2007). These coordinates were finally assigned neuroanatomic labels using the Talairach brain atlas (Talairach and Tournoux, 1998) and the Talairach Daemon database (Lancaster et al, 2000). Resulting statistical parametric maps were then overlaid onto a reference brain using MRIcron software (http://www.sph.sc.edu/comd/rorden/mricron/).
Results
Behavioral
Response accuracy during neuroimaging to cues prompting avoidance, escape and approach behavior exceeded 95% for all subjects. The cue signaling punishment resulted in complete response suppression, highlighting that response-dependent money loss remained aversive throughout. Mean number of responses per trial for avoidance was 18.3 (SE=1.45), escape 17.8 (SE=1.45) and approach 14.06 (SE=1.75). Results of one-sample t-tests revealed no difference between avoidance and escape responding (t(17) = 0.40, p > .05), but increased responding during avoidance relative to approach (t(17) = 2.55, p < .05) and escape relative to approach (t(17) = 2.68, p < .05) suggesting greater motivation to avoid and escape.
Brain Activation
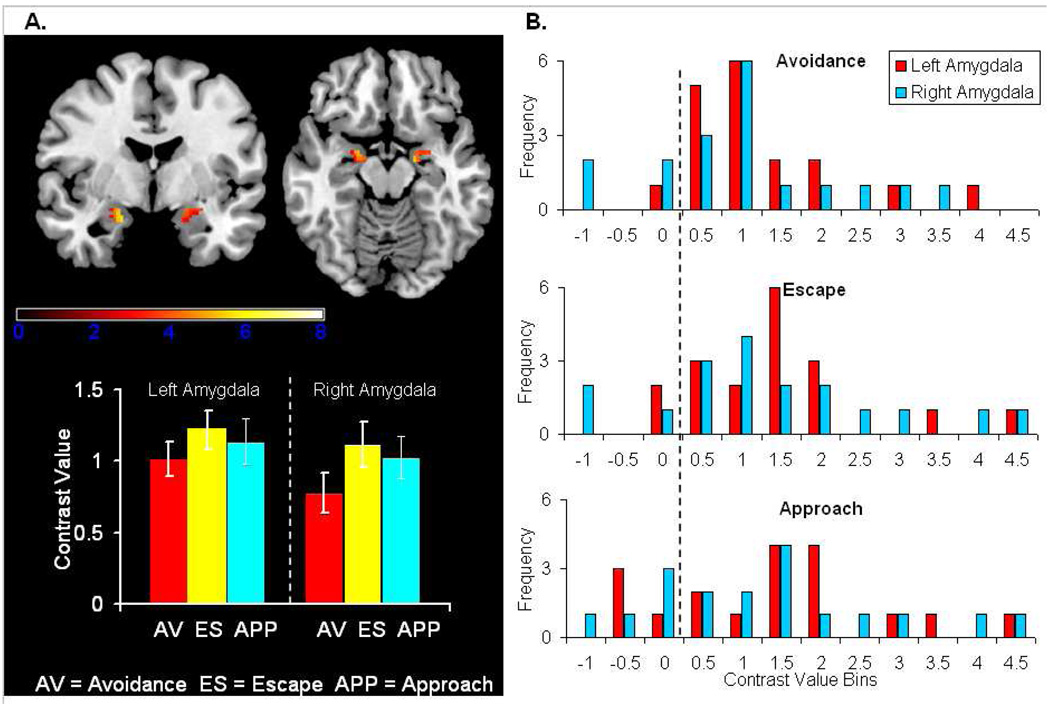
Conjunction analyses presented in Table 1 show cues prompting avoidance, escape and approach behavior recruited a similar fronto-striatal-parietal network. Bilateral activation was noted in the amygdala as well as in the striatum, inferior and middle frontal gyri, inferior and superior parietal lobules, paracentral lobule, postcentral gyrus, posterior cingulate, precuneus, superior temporal gyrus, supramarginal gryus and posterior lobes of the cerebellum. Results of our ROI analysis centering on the amygdala are presented in panel A in Figure 2 and show bilateral amygdala activation to cues prompting avoidance, escape and approach behavior. The inserted plot highlights mean contrast values---difference between parameter estimates. Post-hoc one-sample t-tests revealed no significant differences between conditions. A positive correlation between number of avoidance responses (i.e., response rate) and left amygdala activation which approached significance was also observed (r = +.47; p = .051). Frequency distributions for each amygdala by condition in panel B of Figure 2 highlight the extent of between-subject variability and reveal most subjects exhibited positive contrast values.
Table 1.
Conjunction of Approach-Avoidance and Approach-Escape.
| Approach and Avoidance |
Approach and Escape |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Cluster | t | X | Y | Z | Cluster | t | X | Y | Z | |
| Left | Middle Frontal Gyrus | 346 | 5.86 | −42 | 45 | 12 | 234 | 5.86 | −42 | 45 | 12 |
| Middle Frontal Gyrus | (117) | 4.95 | −28 | 14 | 53 | (118) | 4.95 | −28 | 14 | 53 | |
| Inferior Frontal Gyrus | (346) | 5.36 | −46 | 37 | 4 | (234) | 5.36 | −46 | 37 | 4 | |
| Inferior Frontal Gyrus | (285) | 5.23 | −57 | 11 | 27 | (281) | 5.23 | −57 | 11 | 27 | |
| Superior Frontal Gyrus | 117 | 4.99 | −36 | 20 | 49 | 118 | 4.99 | −36 | 20 | 49 | |
| Superior Temporal Gyrus | 285 | 5.39 | −57 | 6 | −5 | 281 | 5.39 | −57 | 6 | −5 | |
| Superior Temporal Gyrus | 295 | 5.53 | −55 | −25 | 0 | 298 | 5.53 | −55 | −25 | 0 | |
| Caudate Body | (13366) | 6.88 | −10 | 4 | 11 | (13508) | 6.88 | −10 | 4 | 11 | |
| Amygdala | (13366) | 5.47 | −20 | 0 | −18 | (13508) | 5.47 | −20 | −4 | −18 | |
| Postcentral Gyrus | (295) | 4.82 | −53 | −21 | 16 | (298) | 4.82 | −53 | −21 | 16 | |
| Paracentral Lobule | 51 | 4.45 | −2 | −32 | 50 | 51 | 4.45 | −2 | −32 | 50 | |
| Inferior Parietal Lobule | (13366) | 6.48 | −51 | −34 | 50 | (13508) | 6.48 | −51 | −34 | 50 | |
| Supramarginal Gyrus | (13366) | 6.73 | −40 | −43 | 37 | (13508) | 6.73 | −40 | −43 | 37 | |
| Posterior Cingulate | 134 | 5.81 | −18 | −49 | 28 | 134 | 5.81 | −18 | −49 | 28 | |
| Precuneus | (4277) | 5.56 | −14 | −66 | 40 | (4269) | 5.56 | −14 | −66 | 40 | |
| Superior Parietal Lobule | 4277 | 6.09 | −10 | −69 | 55 | 4269 | 6.09 | −10 | −69 | 55 | |
| Posterior Lobe | 13366 | 7.61 | −2 | −75 | −21 | 13508 | 7.61 | −2 | −75 | −21 | |
| Right | Middle Frontal Gyrus | 307 | 7.19 | 40 | 38 | 24 | 299 | 7.19 | 40 | 38 | 24 |
| Inferior Frontal Gyrus | ---- | ---- | ---- | ---- | ---- | (64) | 4.13 | 50 | 18 | 1 | |
| Superior Temporal Gyrus | ---- | ---- | ---- | ---- | ---- | 64 | 5.48 | 53 | 13 | −9 | |
| Anterior Cingulate | 73 | 5.07 | 12 | 6 | 35 | 80 | 5.07 | 12 | 6 | 35 | |
| Putamen | (13366) | 10.60 | 24 | −1 | 11 | (13508) | 10.60 | 24 | −1 | 11 | |
| Caudate Body | (13366) | 8.03 | 16 | −3 | 19 | (13508) | 8.03 | 16 | −3 | 19 | |
| Medial Globus Pallidus | (13366) | 6.89 | 18 | −6 | −5 | (13508) | 6.89 | 18 | −6 | −5 | |
| Amygdala | (13366) | 4.91 | 18 | −8 | −14 | (13508) | 4.91 | 18 | −8 | −14 | |
| Posterior Cingulate | (470) | 5.51 | 10 | −12 | 41 | (485) | 5.51 | 10 | −12 | 41 | |
| Paracentral Lobule | 470 | 5.78 | 2 | −13 | 45 | 485 | 5.78 | 2 | −13 | 45 | |
| Brainstem | (13366) | 6.72 | 20 | −16 | −9 | (13508) | 6.72 | 20 | −16 | −9 | |
| Middle Temporal Gyrus | 173 | 4.32 | 55 | −16 | −9 | 172 | 4.32 | 55 | −16 | −9 | |
| Postcentral Gyrus | (4277) | 6.48 | 55 | −19 | 45 | (4269) | 6.48 | 55 | −19 | 45 | |
| Precentral Gyrus | (4277) | 6.13 | 28 | −24 | 64 | (4269) | 6.13 | 28 | −24 | 64 | |
| Inferior Parietal Lobule | (4277) | 6.89 | 48 | −35 | 48 | (4269) | 6.89 | 48 | −35 | 48 | |
| Supramarginal Gyrus | (4277) | 5.16 | 48 | −45 | 35 | (4269) | 5.16 | 48 | −45 | 35 | |
| Superior Parietal Lobule | (4277) | 5.63 | 22 | −49 | 61 | (4269) | 5.63 | 22 | −49 | 61 | |
| Middle Temporal Gyrus | 167 | 7.64 | 40 | −50 | 6 | 167 | 7.64 | 40 | −50 | 6 | |
| Precuneus | (4277) | 5.97 | 4 | −52 | 54 | (4269) | 5.97 | 4 | −52 | 54 | |
| Posterior Lobe | (13366) | 7.20 | 24 | −68 | −30 | (13508) | 7.20 | 24 | −68 | −30 | |
| Cuneus | (4277) | 5.96 | 4 | −78 | 28 | (4269) | 5.96 | 4 | −78 | 28 | |
Regions in ( ) highlight secondary local maxima
Figure 2. Amygdala activation to avoidance, escape and approach cues.
Panel A highlights bilateral amygdala responses to cues that prompted avoidance, escape and approach behavior. The inserted plot highlights group mean contrast values and bars represent the standard error. Panel B presents frequency distributions of the number of subjects within a range of contrast values by condition and amygdala. Results highlight that most subjects exhibited positive contrast values and there was considerable variability between-subjects.
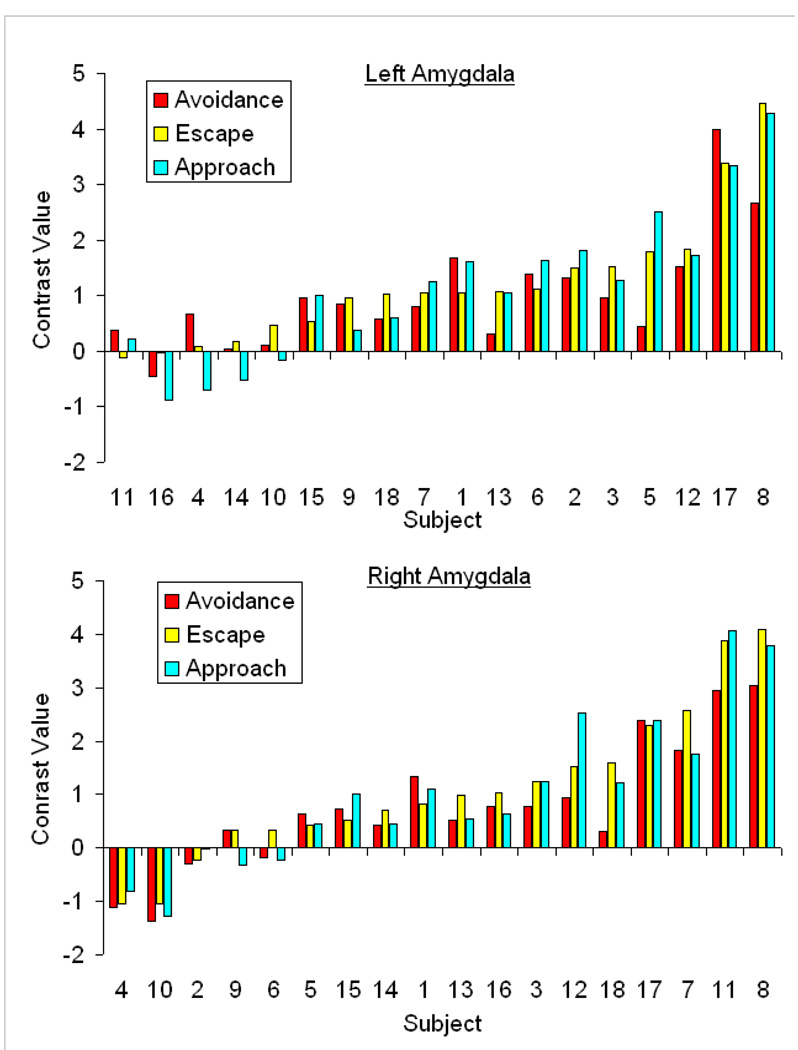
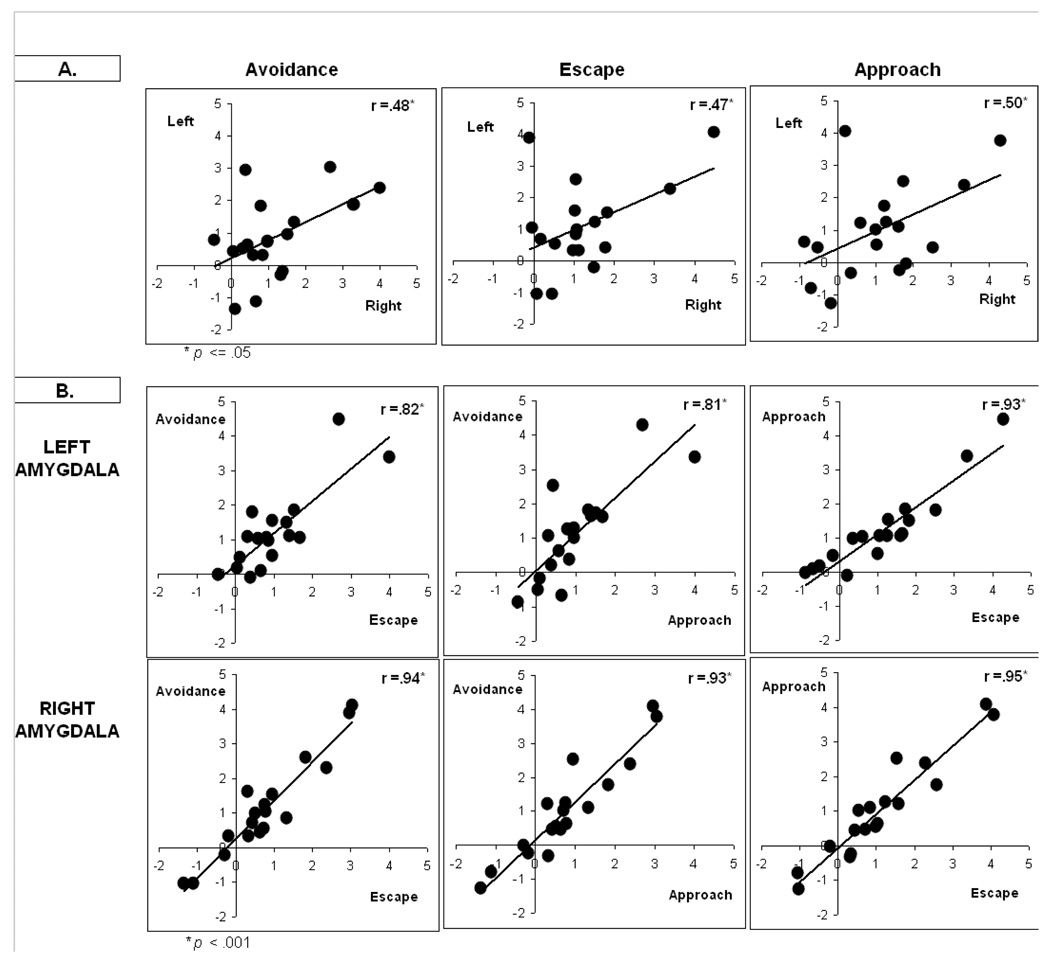
To examine the consistency of amygdala responses within-subjects, Figure 3 shows individual subject contrast values for the left amygdala (top plot) and right amygdala (bottom plot) for all conditions. Results were arbitrarily rank-ordered by escape contrast values for presentation purposes and show the magnitude of amygdala responses was relatively consistent within each subject. Results also show variability in response magnitude between-subjects, with a subset of subjects showing marked activation to avoidance, escape and approach cues. A further examination of the consistency of within-subject responses appears in Figure 4. Panel A shows moderate positive relationships between left and right amygdala activation within all conditions (r’s ranging from +.47–+.50). Panel B shows positive relationships between conditions for each amygdala (r’s ranging from +.81–+.95). Collectively, results highlight that within-subject responses were relatively similar to avoidance, escape and approach cues but between-subjects differences in response magnitude were present.
Figure 3. Distribution of individual subject contrast values.
Individual subject contrast values highlighting the magnitude of activation observed in the left amygdala (top plot) and right amygdala (bottom plot) to cues that prompted avoidance, escape and approach responding. Data were arbitrarily ordered by escape contrast values. The distributions show within-subject amygdala responses were relatively similar across avoidance, escape and approach cues but the magnitude of response varied between subjects.
Figure 4. Relationships between amygdala responses and conditions.
Panel A shows positive correlations between left and right amygdala within each condition. Panel B shows positive correlations between experimental conditions for each amygdala. Findings further highlight the consistency of within-subject amygdala responses and extent of between-subject differences.
Discussion
The primary aim of the present investigation was to examine amygdala reactivity to threatening cues even when avoidance responding consistently prevented contact with an upcoming aversive event. Our secondary aim examined reported amygdala contributions to escape from a proximal aversive event and to approach maintained by reinforcement. The inclusion of these conditions provided a novel opportunity to compare amygdala responses under a range of aversive and appetitive conditions to determine if it serves a broader role as a ‘behavioral relevance’ detector (Ousdal et al., 2008; Paton et al., 2006; Sander et al 2003; Schoenbaum et al., 2003). A number of novel observations emerged from this investigation, helping to elucidate amygdala contributions to avoidance, escape and approach and gain insight into individual differences in amygdala reactivity.
One significant finding that emerged from this investigation was observation of bilateral amygdala activation to threatening avoidance and escape cues relative to a neutral baseline cue. This occurred under conditions where aversive money loss was routinely avoided during neuroimaging. Moreover, avoidance and escape behaviors were well learned through extensive pretraining, thus activation cannot be attributed to acquisition of Pavlovian cue-outcome or operant response-outcome contingencies. Findings highlighting amygdala activation to avoidance and escape cues in humans establishes an important link between the amygdala and learned avoidance suggested by prior studies on human avoidance (Mobbs, 2009), mood disorders characterized by avoidance behavior (Etkin and Wager, 2007) and studies involving escape behavior (Gold et al., 1976; Herdade et al., 2006). Results showing amygdala involvement in supporting avoidance in adults is also consistent with results of our prior investigation on avoidance that highlighted amygdala involvement in supporting avoidance in youths (ages 9–13 years) (Schlund et al, 2010). Overall, our findings suggest the amygdala may function as one important neural mechanism within the framework of two-factor theories of avoidance which propose threatening fear-conditioned cues motivate avoidance and removal of threatening cues and fear-reduction negatively reinforce avoidance (Cain and LeDoux, 2008; Miller, 1948). At a broader level, it is also worthy to note that cues prompting avoidance, escape and approach behavior recruited a similar fronto-striatal-parietal network. Our findings implicating a role for the striatum in avoidance replicates previous findings obtained with humans (Jensen et al, 2003; Kim et al., 2006; Mobbs et al., 2009) and extends the role of the striatum to also include escape behavior.
Two additional findings of significance emerged from our within-subject analyses of amygdala responses. First, the magnitude of activation to avoidance, escape and approach cues was generally similar within-subjects. The second notable finding was the presence of considerable between-subject variability in the magnitude of amygdala activation. A clear subset of subjects showed little or no activation while another subset of subjects exhibited marked activation across conditions. The variability observed does not appear to stem from poor experimental control by programmed contingencies as behavioral performances across subjects were optimal and stable. On the one hand, the reduced level of activation observed in some subjects is consistent with evidence from nonhuman investigations that suggest learned avoidance is not dependent upon the amygdala (Andrzejewski, et al., 2005; Lehmann et al., 2000; Poremba and Gabriel, 1997, 1999; Roozendaal et al., 1993) and human neuroimaging investigations that have not observed amygdala activation (Jensen et al, 2003). Reduced activation may reflect attenuation of conditioned fear or perceived threat, which may subside once avoidance is learned because the avoidance cue predicts the absence of the aversive event (Lovibond et al., 2008;). It may also reflect greater differential control by the CS-NoUS relation or a broader reduction in the motivational salience of cues resulting from extensive experience. On the other hand, the heightened activation observed in other subjects suggests for some individuals threat related responses may be maintained even when aversive events are consistently avoided, perhaps suggesting extended control by the original CS-US relation. While the individual difference variable(s) that contribute to a sustained threat response remain unclear, our findings linking amygdala responses with avoidance are consistent with a range of clinical and basic research findings highlighting heightened behavioral and neurophysiological responses during avoidance (Eifert and Heffner, 2003; Mobbs et al., 2009; Rose et al., 1995; Solomon et al., 1980) and inefficiencies associated with avoidance in emotional regulation (Campbell-Sills et al., 2006; Cioffi and Holloway, 1993; Feldner et al., 2006: Spira et al., 2004).
The present investigation also demonstrates how human neuroimaging research on avoidance may advance by adopting instrumental avoidance paradigms developed and employed in nonhuman research. Stable and accurate avoidance and escape motivated behaviors were generated and sustained during neuroimaging using negative reinforcement processes that are hypothesized to maintain avoidance in many clinical disorders. Results contribute to other findings that demonstrate money loss can be successfully employed as a noninvasive aversive stimulus to shape and maintain avoidance and escape behaviors during neuroimaging in adults (see also Kim et al., 2006) and children (Schlund et al., 2010). The approach used illustrates one available pathway for overcoming some of the noted obstacles associated with generating convincing demonstrations of avoidance-escape in humans (Grillon et al., 2006). It also may serve as a point of departure for assessing the effects of unique human abilities and circumstances on the basic neurocircuitry supporting avoidance. For example, humans are capable of abstract and symbolic thought as well as prepositional analysis of conditioning experiences (e.g., Lovibond and Shanks 2002) that may modulate regional activation. Many forms of human avoidance-escape coping also involve experiential avoidance, in which aversive events are negative thoughts or emotions (Hayes et al., 1996). Other forms of avoidance-escape are also socially mediated, acquired not through direct experience with aversive events but rather through observational learning or instructions. Finally, there are forms of derived avoidance that are prompted by stimuli that become threatening through relational learning processes (Dymond and Roche, 2009).
Future neuroimaging research on adaptive and maladaptive forms of human avoidance faces a number of limitations and challenges. One of the most difficult questions to address because of the limited spatial resolution of fMRI relates to the function of the various amygdala nuclei. Progress in understanding the functional contributions of different amygdala nuclei in adaptive and maladaptive human avoidance may be more likely to emerge from nonhuman and human lesion studies. However, one recently developed nonhuman neurophysiological model of avoidance that may help frame future human avoidance research is the Escape-From-Fear (EFF) model. The model adopts a two-factor theoretical framework and proposes that the basolateral amygdala (BLA) aids in establishing cues as threats (i.e., fear-conditioning) and the striatum functions in modulating instrumental avoidance behavior (Cain and LeDoux, 2008). We recently reported evidence that was consistent with the model (Schlund et al., 2010) along with results implicating roles for the anterior cingulate and insula. Understanding how different neuroimaging approaches modulate basic learning processes supporting avoidance is another challenge. For example, our avoidance contingency enabled subjects to avoid money loss on every trial, which models central features of chronic avoidance-coping strategies present in many forms of psychopathology. In contrast, several prior human neuroimaging studies have used a partial avoidance contingency in which the avoidance response periodically does not prevent contact with the aversive stimulus/outcome (Jensen et al, 2003; Kim et al., 2006; Mobbs et al., 2009). The form of the avoidance contingency and subsequent beliefs about the utility of an avoidance behavior are likely to be major factors that modulate regional involvement. Similarly, the present study employed a contingency shaping procedure to establish avoidance whereas one prior study employed instructions to establish avoidance (Jensen et al., 2003) which has the potential to effectively bypassed fear conditioning processes mediated by the BLA. Future research is clearly required that examines the effects of contingency, type of aversive outcome and significant individual subject variables on regional activation.
In summary, a number of novel observations emerged from this investigation, helping to elucidate amygdala contributions to human avoidance, escape and approach behavior. At a broad level, results showed cues prompting avoidance, escape and approach behavior recruited a similar fronto-striatal-parietal network. We observed bilateral amygdala activation to threatening avoidance and escape cues, even though money loss was routinely avoided, and to a reward cue. Results highlighted that within-subject responses were relatively similar to avoidance, escape and approach cues, but between-subjects differences in response magnitude were present. The heightened amygdala response to the avoidance and escape cues observed in a subset of subjects suggests threat related responses can be maintained even when aversive events are consistently avoided, which may account for the persistence of avoidance-coping in various clinical disorders. Further assessment of the relation between amygdala reactivity and avoidance-escape behavior may prove useful in identifying individuals with or at risk for neuropsychiatric disorders.
Acknowledgements
The research and manuscript preparation was supported in part by research grant NICHD HD43178-02
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
References
- Andrzejewski ME, Spencer RC, Kelley AE. Instrumental learning, but not performance, requires dopamine D1-receptor activation in the amygdala. Neuroscience. 2005;135(2):335–345. doi: 10.1016/j.neuroscience.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev Gen Psychol. 2001;5:323–370. [Google Scholar]
- Baxter MG, Murray AE. The amygdala and reward. Nat. Rev. Neuroscience. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Holmes EA. Psychological theories of posttraumatic stress disorder. Clin Psychol Rev. 2003;23(3):339–376. doi: 10.1016/s0272-7358(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Blume AW. Negative Reinforcement and Substance Abuse: Using a Behavioral Conceptualization to Enhance Treatment. The Behav Anal Today. 2001;2001(2):86–90. [Google Scholar]
- Bolles RC. The avoidance learning problem. In: Bower GH, editor. The psychology of learning and motivation. Vol 6. New York: Academic Press; 1972. pp. 97–145. [Google Scholar]
- Brown SA, Williams K, Collins A. Past and recent deliberate self-harm: emotion and coping strategy differences. J Clin Psychol. 2007;63(9):791–803. doi: 10.1002/jclp.20380. [DOI] [PubMed] [Google Scholar]
- Cain CK, LeDoux JE. Emotional processing and motivation: In search of brain mechanisms. In: Elliot AJ, editor. Handbook of approach and avoidance motivation. New York: Psychology Press; 2008. pp. 17–34. [Google Scholar]
- Campbell-Sills L, Barlow DH, Brown TA, Hofmann SG. Effects of suppression and acceptance on emotional responses of individuals with anxiety and mood disorders. Behav Res Ther. 2006;44(9):1251–1263. doi: 10.1016/j.brat.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Cioffi D, Holloway J. Delayed costs of suppressed pain. J Pers Soc Psychol. 1993;64(2):274–282. doi: 10.1037//0022-3514.64.2.274. [DOI] [PubMed] [Google Scholar]
- Clark DA. A cognitive approach to panic. Behav Res Ther. 1986;24(4):461–470. doi: 10.1016/0005-7967(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Dymond S, Roche B. A contemporary behavior analysis of anxiety and avoidance. The Behav Anal. 2009;32:7–28. doi: 10.1007/BF03392173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs DA, Woods JH. Fixed-ratio escape and avoidance-escape from naloxone in morphine-dependent monkeys: Effects of naloxone dose and morphine pretreatment. J Exp Anal Behav. 1975;23:415–427. doi: 10.1901/jeab.1975.23-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eifert GH, Heffner M. The effects of acceptance versus control contexts on avoidance of panic-related symptoms. J Behav Ther Exper Psychiatry. 2003;34(3–4):293–312. doi: 10.1016/j.jbtep.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Zvolensky MJ, Stickle TR, Bonn-Miller MO, Leen-Feldner EW. Anxiety sensitivity-physical concerns as a moderator of the emotional consequences of emotion suppression during biological challenge: an experimental test using individual growth curve analysis. Behav Res Ther. 2006;44(2):249–272. doi: 10.1016/j.brat.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols TE. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gold PE, Rose RP, Hankins LL, Spanis C. Impaired retention of visual discriminated escape training produced by subseizure amygdala stimulation. Brain Res. 1976;118(1):73–85. doi: 10.1016/0006-8993(76)90842-8. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation; conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. New York, NY: Guilford Press; 2009. pp. 3–24. [Google Scholar]
- Grillon C, Baas JM, Cornwell B, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biol Psychiatry. 2006;60(7):752–759. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Wilson KG, Gifford EV, Follette VM, Strosahl K. Experiential avoidance and behavioral disorders: A functional dimensional approach to diagnosis and treatment. Journal of Consul Clin Psych. 1996;64:1152–1168. doi: 10.1037//0022-006x.64.6.1152. [DOI] [PubMed] [Google Scholar]
- Herdade KCP, De Andrade Strauss CV, Junior HZ, De Barros Viana M. Effects of medial amygdala inactivation on a panic-related behavior. Behav Brain Res. 2006;172(2):316–323. doi: 10.1016/j.bbr.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ. Method and theory in the study of avoidance. Psychol Rev. 1969;76(1):49–69. doi: 10.1037/h0026786. [DOI] [PubMed] [Google Scholar]
- Hirai D, Hosokawa T, Inoue M, Miyachi S, Mikami A. Context-dependent representation of reinforcement in monkey amygdala. Neuroreport. 2009;20:558–562. doi: 10.1097/WNR.0b013e3283294a2f. [DOI] [PubMed] [Google Scholar]
- Hommer DW, Knutson B, Fong GW, Bennett S, Adams CM, Varnera JL. Amygdalar recruitment during anticipation of monetary rewards: an event-related fMRI study. Ann N Y Acad Sci. 2003;985:476–478. doi: 10.1111/j.1749-6632.2003.tb07103.x. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40(6):1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O'Doherty JP. Is avoiding an aversive outcome rewarding? The neural substrates of avoidance learning in the human brain. Plos Biology. 2006;4(8):e233. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501–1508. doi: 10.1176/appi.ajp.160.8.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56 Suppl 1:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H, Treit D, Parent MB. Amygdala lesions do not impair shock-probe avoidance retention performance. Behav Neurosci. 2000;114(1):107–116. doi: 10.1037//0735-7044.114.1.107. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Saunders JC, Weidemann G, Mitchell CJ. Evidence for expectancy as a mediator of avoidance and anxiety in a laboratory model of human avoidance learning. Quarterly J Exp Psychol. 2008;61(8):1199–1216. doi: 10.1080/17470210701503229. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Shanks DR. The role of awareness in Pavlovian conditioning: empirical evidence and theoretical implications. J Exp Psychol Anim Behav Process. 2002;28(1):3–26. [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur J Neurosci. 2007;25(9):2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An Automated Method for Neuroanatomic and Cytoarchitectonic Atlas-based Interrogation of fMRI Data Sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Miller NE. Studies of fear as an acquirable drive: I. Fear as motivation and fear-reduction as reinforcement in the learning of new responses. J Exp Psychol. 1948;38(1):89–101. doi: 10.1037/h0058455. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci. 2009;29(39):12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrer OH, Lamoreaux RR. Fear as an intervening variable in avoidance conditioning. J Comp Psychol. 1946;39(1):29–50. doi: 10.1037/h0060150. [DOI] [PubMed] [Google Scholar]
- Ousdal OT, Jensen J, Server A, Hariri AR, Nakstad PH, Andreassen OA. The human amygdala is involved in general behavioral relevance detection: evidence from an event-related functional magnetic resonance imaging Go-NoGo task. Neuroscience. 2008;156(3):450–455. doi: 10.1016/j.neuroscience.2008.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Gabriel M. Amygdalar lesions block discriminative avoidance learning and cingulothalamic training-induced neuronal plasticity in rabbits. J Neurosci. 1997;17(13):5237–5244. doi: 10.1523/JNEUROSCI.17-13-05237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Gabriel M. Amygdala neurons are necessary for original acquisition but not maintenance of instrumental avoidance behavior in rabbits. J Neurosci. 1999;19:9635–9641. doi: 10.1523/JNEUROSCI.19-21-09635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Koolhaas JM, Bohus B. The central amygdala is involved in conditioning but not in retention of active and passive shock avoidance in male rats. Behav Neural Biol. 1993;59(2):143–149. doi: 10.1016/0163-1047(93)90873-g. [DOI] [PubMed] [Google Scholar]
- Rose P, McGlynn FD, Lazarte A. Control and attention influence snake phobics' arousal and fear during laboratory confrontations with a caged snake. J of Anxiety Disorders. 1995;9(4):293–302. [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14(4):303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Schlund MW, Siegle GJ, Ladouceur CD, Silk JS, Cataldo MF, Forbes EE, Dahl RE, Ryan ND. Nothing to fear? Neural systems supporting avoidance behavior in healthy youths. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.04.244. Epud ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9(5):388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Solomon S, Holmes DS, McCaul KD. Behavior control over aversive events: does control that requires effort reduce anxiety and physiological arousal? J Pers Soc Psychol. 1980;39(4):729–736. doi: 10.1037//0022-3514.39.4.729. [DOI] [PubMed] [Google Scholar]
- Spira AP, Zvolensky MJ, Eifert GH, Feldner MT. Avoidance-oriented coping as a predictor of panic-related distress: a test using biological challenge. J Anxiety Disord. 2004;18(3):309–323. doi: 10.1016/S0887-6185(02)00249-9. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: Three-dimensional proportional system: An approach to cerebral imaging. Germany: Thieme; 1998. [Google Scholar]
- Werka T, Skår J, Ursin H. Exploration and avoidance in rats with lesions in amygdala and piriform cortex. J Comp Physiol Psychol. 1978;92(4):672–681. doi: 10.1037/h0077505. [DOI] [PubMed] [Google Scholar]