Abstract
Human cathelicidin LL-37, a host defense peptide derived from leukocytes and epithelial cells, plays a crucial role in innate and adaptive immunity. Not only does it eliminate pathogenic microbes directly, LL-37 also modulates host immune responses. Emerging evidence from tumor biology studies indicates that LL-37 plays a prominent and complex role in carcinogenesis. While overexpression of LL-37 has been implicated in the development or progression of many human malignancies, including breast, ovarian and lung cancers, LL-37 suppresses tumorigenesis in gastric cancer. These data are beginning to unveil the intricate and contradictory functions of LL-37. The reasons for the tissue-specific function of LL-37 in carcinogenesis remain to be elucidated. Here, we review the relationship between LL-37, its fragments and cancer progression as well as discuss the potential therapeutic implications of targeting this peptide.
Mini-Review
Cathelicidins, a family of host defense peptides discovered in mammals, birds, fish, and reptiles, provide the first-line defense against infection by serving as “natural antibiotics”.[1][2] The only cathelicidin in humans is expressed as a 18-kDa preproprotein (hCAP-18) that consists of an N-terminal signal sequence, a well conserved cathelin-like domain, and a C-terminal antimicrobial peptide domain. Proteolytic cleavage of hCAP-18 is required for the release of the bioactive peptide, LL-37. [1][2] The peptide is expressed in leukocytes and on surfaces in contact with the outside environment like skin and gastrointestinal epithelium.[3][4][5][6][7] LL-37 plays an important role in the maintenance of natural immunity. For instance, LL-37 exhibits potent antimicrobial activities against a broad spectrum of pathogens and chemotactic activities toward different types of leukocytes, including neutrophils, monocytes, T-cells, eosinophils and mast cells.[8][9][10][11][12][13] More noteworthy is that LL-37 often reaches high levels at sites of acute infection, in which LL-37 also regulates inflammatory response and promotes tissue repair.[14][15][16][17][18][19][20][21] In contrast to it traditional roles, recent evidence suggests that LL-37 is involved in carcinogenesis. Several laboratories have shown that LL-37 expression is dysregulated in ovarian, breast, lung and gastric cancers and, depending on context, this peptide may promote or suppress tumorigenesis.[22][23][24][25] In this review, the structural characteristics of LL-37 as well as its biological effects on various cancers and the signaling pathways involved will be discussed. Based on recent data, we also describe possible use of LL-37 fragments to control cancers.
Structural studies of LL-37 by NMR spectroscopy
The protective role of human cathelicidin LL-37 against infection is well established. Antimicrobial assays have revealed that LL-37 shows a wide spectrum of activities against bacteria, fungi, and viruses.[3][4][5][6][7] LL-37 is a 37-residue amphipathic peptide with a net charge of +6 (Table 1). It is proposed that this positively charged cathelicidin targets negatively charged bacterial membranes.[26][27] Similarly, in cancer cells, the anionic phosphorylserine is exposed. [28] We therefore hypothesize that LL-37 may also bind to and have an effect on cancer cells. To identify the bioactive region of human LL-37, we have synthesized several fragments. While the N-terminal fragment LL-12 corresponding to residues 1–12 (Table 1) is inactive against bacteria or cancer cells, the C-terminal fragment IG-27 (Table 1) corresponding to residues 13–37 is active. In this regard, IG-27 inhibits the proliferation of drug-resistant KBv and drug-sensitive KB epidermoid carcinoma cells.[29] Moreover, we have succeeded in identifying the active region within IG-27, in which both GF-17 (Gly + residues 17–32) and FK-13 (residues 17–29) (Table 1) have retained antibacterial and antitumor effects.[29] Other laboratories have reported the anticancer activity of additional antimicrobial peptides.[30] According to the antimicrobial peptide database (http://aps.unmc.edu/AP/main.php), 101 such peptides are known to have anticancer effects.[31]
Table 1.
Amino Acid Sequences of LL-37 and Its Fragments
| Peptide | Sequence1 | AA2 | Net Charge |
|---|---|---|---|
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | 37 | +6 |
| LL-12 | LLGDFFRKSKEK | 12 | +2 |
| IG-27 | IGKEFKRIVQRIKDFLRNLVPRTES | 27 | +4 |
| GF-17 | GFKRIVQRIKDFLRNLV-NH2 | 17 | +5 |
| FK-13 | FKRIVQRIKDFLR-NH2 | 13 | +5 |
| LL-25 | LLGDFFRKSKEKIGKEFKRIVQRIK | 25 | +6 |
NH2 indicates C-terminal amidation.
AA stands for the number of amino acid residues in the peptide. AA and net charge were calculated using the Prediction Interface of the APD [31].
To elucidate the mechanism of action, we have determined the 3D structures of human LL-37 fragments by 2D nuclear magnetic resonance (NMR) spectroscopy.[29] For solution NMR studies, detergent micelles are commonly utilized to mimic bacterial membranes.[32] In complex with sodium dodecylsulfate, a short helix (residues 3–7) is found in LL-12 with the rest of the residues poorly defined (Fig. 1A). Because LL-12 is inactive against bacteria or cancer cells, we conclude that the short helix at the N-terminal fragment of LL-37 is insufficient to confer antimicrobial or antitumor activity to the peptide. In contrast, a longer amphipathic helix (residues 17–30) has been defined in IG-27 (Fig. 1B). Because this helical region corresponds well to the short active peptide FK-13, we propose that the long helix in the C-terminal fragment of LL-37 is responsible for its antimicrobial and anticancer activity. In micelles, FK-13 is found to be entirely helical.[29]
Figure 1.
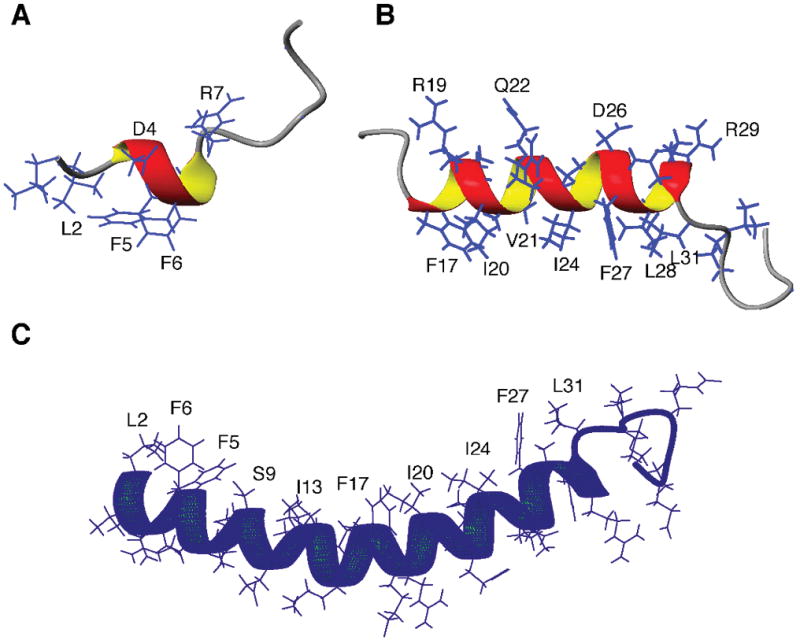
Structures of LL-37 and its fragments. (A) LL-12 corresponding to residues 1–12 of LL-37 is inactive to bacteria or cancer cells. (B) IG-27 corresponding to residues 13–37 of LL-37 is bioactive, which contains a long amphipathic helix structure for residues 17–30. (C) High-quality structure of LL-37 as determined by 3D NMR spectroscopy.
The two-dimensional NMR spectroscopy, however, does not provide sufficient spectral resolution for structural determination of intact LL-37 in complex with sodium dodecylsulfate.[29] It is necessary to obtain isotope-labeled LL-37 using bacterial expression systems. As LL-37 is toxic to the expression host Escherichia coli, the peptide has been expressed as a fusion protein. Recombinant LL-37 is released from the fusion protein by chemical cleavage as enzyme cleavage has turned out to be problematic due to protein aggregation.[33] The high-quality LL-37 structure, determined by 3D NMR spectroscopy, is displayed in Figure 1C. It consists of a long amphipathic helix spanning residues 2–31 with the C-terminal residues 32–37 unstructured. Heteronuclear nuclear overhauser effect (NOE) measurements have verified that the C-terminal residues of LL-37 are indeed mobile.[34] Another noticeable feature is that the structure is curved with a train of hydrophobic side chains (L2, F5, F6, I13, F17, I20, V21, I24, F27, L28, and L31) on the concave surface. Such a cationic structure is perfect to associate with anionic micelles. Using dioctanoyl phosphatidylglycerol (D8PG) as a novel bacterial membrane-mimetic model, we have found that LL-37 adopts a similar structure in this anionic lipid micelle.[34] Furthermore, F5, F6, F17, and F27 show intermolecular NOE cross-peaks with D8PG, verifying the location of the peptide on lipid micelles. In addition, Arg-PG cross-peaks have been detected, providing evidence for direct interactions between cationic antimicrobial peptides and anionic biological membranes.[35] Indeed, our more recent work indicates that electrostatic interactions of cationic LL-37 fragments with anionic lipids can induce lipid domain formation in lipid bilayers.[36] We propose that similar electrostatic interactions may also play a role when LL-37 fragments interact with cancer cells.
Ovarian cancer -Involvement of FPR2 and mesenchymal stem cells
Expression of hCAP-18/LL-37 is upregulated in various ovarian tumor subtypes, including serous adenocarcinomas, mucinous adenocarcinomas and granulosa cell tumors when compared with normal ovarian tissues.[22] Tumor and stromal cells (i.e. leukocytes, fibroblasts and endothelial cells) both express hCAP-18/LL-37 in these tumors, whereas expression is limited to mainly CD45+ leukocytes in normal tissue. Importantly, normal ovarian surface epithelial cells do not produce the peptide suggesting that acquired mutations in these cells lead to hCAP-18/LL-37 upregulation. In agreement with its inflammatory and pro-angiogenic roles, hCAP-18/LL-37 expression in tumors also correlates with increased hematopoietic cell infiltration and microvessel density.[22][37][38] However, further studies are warranted to determine whether hCAP-18/LL-37 expression is predictive of poor prognosis or worsened survival of patients with ovarian tumors.
Several studies now indicate that LL-37 is a positive regulator of cancer progression in the ovary. We have found that ovarian cancer cell lines treated with LL-37 in vitro are stimulated to proliferate at a similar rate as other mitogens, such as epidermal growth factor (EGF).[22][39] Likewise, administration of LL-37 neutralizing antibodies to tumor-bearing mice results in a profound reduction in tumor volume and markedly decreases the proliferative index of ovarian xenograft tumors.[40] The stimulatory effect of LL-37 on cell proliferation is found to be independent of G-protein coupled receptors (GPCR). Aside from cell proliferation, LL-37 enhances the metastatic capacity of ovarian cancer cells presumably through upregulation of tissue remodeling enzymes, including matrix metalloproteinase (MMP)-2.[22][39] The pro-invasive effect of LL-37 is mediated by the GPCR, formyl peptide receptor 2 (FPR2; formerly known as formyl peptide receptor like-1/FPRL-1), as pharmacologic inhibition or knockdown of the receptor attenuates invasion and inhibits the ability of LL-37 to increase MMP-2 gene expression in ovarian cancer cells. These data, together with analysis of activated signaling pathways, transcription factors, and modulated expression of genes at both the mRNA and protein levels, implicate involvement of two receptors through which LL-37 affects ovarian cancer cells.[39] This will be an important consideration when designing anti-cancer therapeutics.
In addition to its autocrine actions on tumor cells, LL-37 has been shown to promote tumor progression through its influence on a particular progenitor cell population known as mesenchymal stromal/stem cells (MSCs).[40] MSCs comprise a group of heterogeneous, non-hematopoietic multipotent cells that can be isolated from several adult tissues and expanded ex vivo. MSCs preferentially home to damaged tissue where they release immunomodulatory and pro-angiogenic factors, which facilitate tissue repair and vascularization.[41][42] These cells maintain a perivascular location in normal tissue and are now thought to be analogous to pericytes (or subpopulations of pericytes).[43][44][45][46][47] However, in xenograft tumor models, exogenously delivered MSCs are rarely found surrounding blood vessels and mainly contribute to the fibroblast or myofibroblast population.[40][48][49] We found that LL-37 is directly involved in recruitment of MSCs to tumors through a GPCR – possibly FPR2. Furthermore, LL-37 enhances the release of immunodulatory and pro-angiogenic molecules, such as interleukin (IL)-6, IL-10, CCL5, MMP-2 and vascular endothelial growth factor (VEGF), from MSCs suggesting that LL-37 augments the pro-tumorigenic nature of these cells. As such, neutralization of ovarian tumor-derived LL-37 may abrogate tumor progression by preventing its association with both tumor cells and stromal cells.[40]
Breast Cancers -ErbB2 Signaling & Metastasis
The normal mammary gland produces and secretes LL-37, which may contribute to the anti-infectious properties of breast milk.[50] It has been shown that the expression of LL-37/hCAP-18 is highly upregulated in human breast cancer tissues, when compared with normal breast tissues, as demonstrated by in situ hybridization, immunohistochemistry and Western blot. Upregulation of LL-37 is also more prominent in high-grade tumors.[23] Clinical sample analysis has revealed that hCAP-18 expression is associated with lymph node metastases in oestrogen receptor-positive tumours. Interestingly, the mRNA expression of hCAP-18 also positively correlates with that of ErbB2.[51] ErbB2 belongs to the family of ErbB receptors whose other members include ErbB1/EGF receptor (EGFR), ErbB3/HER3 and ErbB4/HER4. Upon binding of ligands, the ErbB receptors form homodimers or heterodimers with other ErbB family members, after which the dimerized receptors are internalized and autophosphorylated on their tyrosine residues and transduce oncogenic signals.[52] It is noteworthy that ErbB2 has a functionless ligand-binding domain and its activation relies on dimerization with other activated ErbB family members.[52] To this end, LL-37 has been shown to amplify the activation of mitogen-activated protein kinase (MAPK) signaling induced by heregulin (an ErbB3/ErbB4 ligand) through upregulation of ErbB2, which leads to enhanced migration and anchorage-independent growth in breast cancer cells. Transgenic overexpression of hCAP-18 in breast cancer cells also enhances MAPK signaling and promotes the development of metastases in SCID mice, further substantiating the pro-metastatic effect of LL-37.[51]
Lung cancer - EGFR transactivation
It has been shown that LL-37 stimulates proliferation and anchorage-independent growth of cultured lung cancer cells as well as promotes tumorigenicity and formation of larger tumors in a lung cancer xenograft model.[24] Immunohistochemical staining has revealed that the peptide is expressed mostly in adenocarcinoma and squamous cell carcinoma. The mitogenic effect of LL-37 on lung cancer cells is accompanied by the phosphorylation of EGFR and the subsequent activation of Ras/MAPK cascade.[24] EGFR signaling plays a crucial role in lung carcinogenesis by direct contribution to cell proliferation, resistance to apoptosis, and promotion of angiogenesis, invasion and metastasis.[53] The mechanism by which LL-37 activates EGFR in lung cancer cells remains obscure but it has been reported that LL-37 transactivates EGFR in normal lung epithelial cells and other cell types through a process known as ectodomain shedding. Most of the EGFR ligands, such as transforming growth factor α and heparin-binding EGF, are expressed as transmembrane precursors in which protease-mediated cleavage is required for releasing the soluble form. These cleaved ligands then freely diffuse to bind to and activate EGFR.[54] In this respect, LL-37 has been shown to transactivate EGFR to induce IL-8 secretion in airway epithelial cells through MMP-mediated, ectodomain shedding of pro-EGFR-ligands.[19] EGFR transactivation by LL-37 has also been reported in keratinocytes.[55] These findings suggest that EGFR transactivation is an important mechanism mediating the oncogenic effect of LL-37 in certain tissues. Nevertheless, whether upregulation of ErbB2 is involved in amplifying the effect of EGFR transactivation in lung cancer cells has not yet been investigated.
Gastric cancer -Proteasome inhibition & BMP signaling
The expression of LL-37 is dysregulated during Helicobacter pylori-associated gastric carcinogenesis.[7] In normal stomach, the expression of LL-37 is restricted to differentiated surface epithelial cells, chief cells and parietal cells, and is present in the gastric secretion. In response to H. pylori infection, the expression of LL-37 is induced along the whole gastric gland, which may help the host to fight against the infection initially. However, during progression from atrophic gastritis to adenocarcinoma, the expression of LL-37 is reduced. It has been observed that LL-37 is absent or expressed at very low levels in gastric hyperplastic polyps, tubular adenomas, and adenocarcinomas.[7] Our recent study suggests that LL-37 may function as a putative tumor-suppressing peptide in gastric carcinogenesis.[25] We found that exogenous LL-37 inhibits proliferation and induces G0/G1-phase cell cycle arrest in gastric cancer cells. Furthermore, depletion of endogenous LL-37 by RNA interference stimulates gastric cancer cell DNA synthesis, suggesting that the antiproliferative effect of LL-37 occurs at physiological concentrations. The direct anticancer activity of LL-37 in vivo has also been confirmed using a gastric cancer xenograft model in nude mice. Furthermore, the antiproliferative effect of LL-37 cannot be blocked by pertussis toxin, which inactivates Gαi and Gαo G proteins, excluding the involvement of FPR2.[25] These findings suggest that, unlike in other cancers, LL-37 negatively regulates gastric cancer growth.
Bone morphogenetic protein (BMP) signaling is a crucial tumor-suppressing pathway in gastric carcinogenesis. For instance, BMP2, a ligand of BMP receptor, inhibits gastric cancer cell proliferation and is epigenetically silenced in gastric cancer tissues.[56][57] Conditional knockout of BMP receptor I also promotes gastric cancer formation.[58] BMP signaling is initiated by the binding of BMP ligands to BMP receptors which, upon activation, recruit and phosphorylate the downstream Smad 1/5/8. The phosphorylated Smads then form heterodimers with Smad4 and translocate into nucleus to act as transcription factors, inducing genes that mediate biological effects of BMPs.[59] Interestingly, we found that LL-37 increases BMP4 expression and Smad 1/5 phosphorylation and subsequently induces the mRNA and protein expression of p21Waf1. Activation of BMP signaling as well as the inhibition of cell proliferation induced by LL-37 can be partially blocked by RNA interference targeting BMP receptor II. Moreover, LL-37 downregulates cyclin E2 expression in a BMP-independent manner.[25] It is known that both p21Waf1 and cyclin E2 regulate cell cycle progression through the late G1-phase.[60] It is therefore speculated that the concurrent upregulation of p21Waf1 and downregulation of cyclin E2 contributes to the antitumor effect of LL-37 in gastric cancer. This hypothesis is further supported by the positive correlation between hCAP18 and p21Waf1 mRNA expression in primary gastric cancer tissue samples.[25] In relation to BMP signaling, we and other investigators have previously reported that inhibition of proteasome, a multimeric protein complex with proteolytic activity, results in upregulation of BMP ligands and induction of Smad1/5/8 phosphorylation.[61][62][63] To this end, we found that LL-37 substantially inhibits the chymotrypsin-like and caspase-like activity of 20S proteasome. Moreover, the proteasome inhibitor MG-132 induces the BMP/p21 cascade to inhibit cell proliferation, producing effects similar to those of LL-37 in gastric cancer cells. These data suggest that the antimitogenic effect of LL-37 is mediated through proteasome inhibition-induced activation of BMP signaling (Fig. 2).[25]
Figure 2.
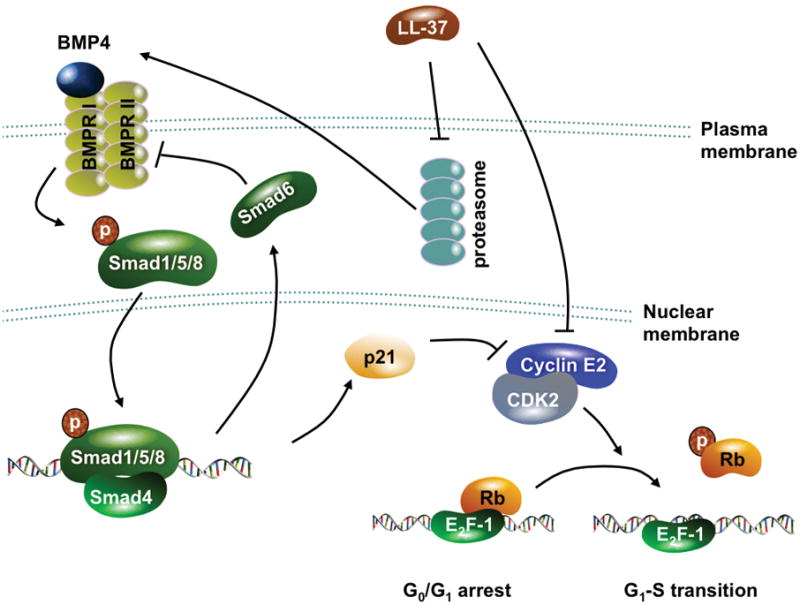
Proposed antitumor mechanism of LL-37 in gastric cancer. LL-37 inhibits proteasomal activity via an unknown mechanism, which leads to upregulation of BMP4 and subsequent activation of BMP signaling. LL-37 also downregulates cyclin E2. These changes result in cell cycle arrest at the late G1-phase.
LL-37 as immunotherapeutics
Aside from the antitumor activity in gastric cancer, LL-37 has been used as an immunostimulant or adjuvant to promote elimination of cancer cells by the host immune system. In immunotherapy, CpG oligodeoxynucleotides are employed to enhance the tumor-suppressing activity of the host immune cells through stimulation of Toll-like receptor (TLR)-9. LL-37 can enhance the sensing of CpG oligodeoxynucleotides by B lymphocytes, plasmacytoid dendritic cells and natural killer (NK) cells.[64][65] Chuang et al. have also reported that co-administration of CpG oligodeoxynucleotides and LL-37 produces synergistic antitumor effects against ovarian cancer in mice. Moreover, CpG oligodeoxynucleotides and LL-37 enhance the proliferation and activation of peritoneal NK cells, whose depletion by antibody reverses the antitumor effects.[65] In line with this finding, a recent study by Büchau et al. has shown that cathelicidin is absolutely required for the tumor-suppressing activity of NK cells.[66]
Concluding remarks & future perspectives
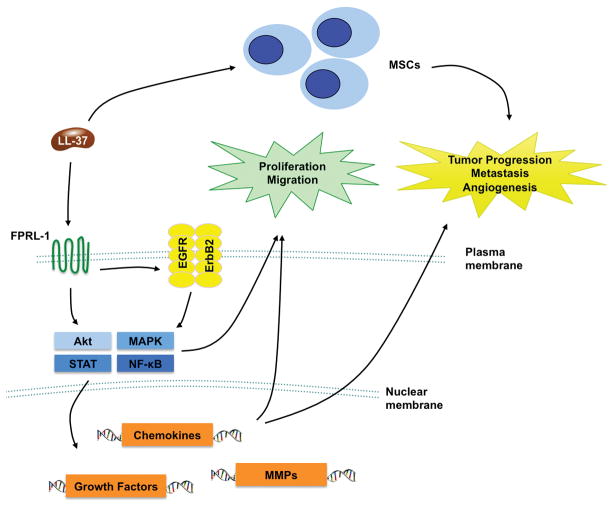
The host defense peptide LL-37 has emerged as a novel modulator of tumor growth and metastasis in carcinogenesis of various types of cancers. In ovarian cancer, LL-37 promotes cell proliferation and invasiveness via FPR2-independent and -dependent pathways, respectively. In breast and lung cancers, LL-37 promotes cell proliferation and metastasis mainly through ErbB-mediated pathways including sensitizing of ErbB signaling by upregulation of ErbB2 or transactivation of EGFR (Fig. 3). Concordant with its putative oncogenic functions, LL-37 expression is upregulated in these tumors.[22][23][24][39][51] However, in gastric cancer where LL-37 expression is downregulated, this peptide inhibits cell proliferation through a novel mechanism involving proteasome inhibition and activation of BMP signaling.[7][25] LL-37 also exhibits antitumor effects on epidermoid carcinoma cells, suggesting that the effect of LL-37 on cancer phenotypes depends on the tissue origin of the tumor.[29] The tumor microenvironment may also take part in determining the effect of LL-37 as this peptide has been shown to recruit MSCs to tumors and enhance their expression of pro-inflammatory and pro-angiogenic cytokines.[40] The complex biological actions of LL-37 in both healthy and diseased tissues and its dual role as a tumor-suppressor/oncogene render systemic administration or targeting of LL-37 an undesirable approach. Tissue-specific modulation or directed-drug delivery may thus provide an alternative to circumvent unwanted effects in other tissues, and maintain the efficacy of LL-37-directed therapeutics at targeted diseased organs. While LL-37 is demonstrated to promote cancer metastasis, another fragment LL-25 (Table 1) abrogates its effects.[51] We have shown that the C-terminal fragments of LL-37 (Table 1) also have tumor inhibitory effects.[29] These findings suggest that the fragments of LL-37 have the potential to be developed into anticancer agents. Future elucidation of the structure of LL-37 and its fragments in complex with FPR2 or other receptors will provide a basis for structure-based anticancer drug design. As an alternative strategy, LL-37 has been shown to enhance the antitumor effects induced by CpG oligodeoxynucleotides through stimulating the tumor-suppressing activity of NK cells. [64][65][66] With a further understanding of the complex biology of LL-37 in cancer, novel therapeutic strategies will emerge.
Figure 3.
Proposed mechanism by which LL-37 promotes cancer growth. LL-37, through activation of FPR2 and transactivation of EGFR/ErbB2, stimulates multiple oncogenic pathways to stimulate cell proliferation and migration. In addition, LL-37 recruits MSCs to tumors to promote angiogenesis and metastasis.
Acknowledgments
This work was supported by a research grant from National Basic Research Program of China (973 Program, 2010CB529305) awarded to D. Fan, K. Wu, J.J.Y. Sung and J. Yu and NIH 1P20RR20152-01, DOD OC073102 Concept Awardawarded to A.M. Betancourt.
Footnotes
Conflict of Interests
The authors declared no conflict of interests.
References
- 1.Elsbach P. What is the real role of antimicrobial polypeptides that can mediate several other inflammatory responses? J Clin Investig. 2003;111:1643–45. doi: 10.1172/JCI18761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaiou M, Gallo RL. Cathelicidins, essential gene-encoded mammalian antibiotics. J Mol Med. 2002;80:549–61. doi: 10.1007/s00109-002-0350-6. [DOI] [PubMed] [Google Scholar]
- 3.Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238:325–32. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 4.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jörnvall H, Wigzell H, Gudmundsson GH. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–93. [PubMed] [Google Scholar]
- 5.Frohm M, Agerberth B, Ahangari G, Stâhle-Bäckdahl M, Lidén S, Wigzell H, Gudmundsson GH. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 6.Schauber J, Svanholm C, Termén S, Iffland K, Menzel T, Scheppach W, Melcher R, Agerberth B, Lührs H, Gudmundsson GH. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52:735–41. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, Ohtake T, Obonyo M, Gallo RL, Eckmann L, Kagnoff MF. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology. 2003;125:1613–25. doi: 10.1053/j.gastro.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol. 2004;172:1763–7. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 9.López-García B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005;125:108–15. doi: 10.1111/j.0022-202X.2005.23713.x. [DOI] [PubMed] [Google Scholar]
- 10.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A. 1998;95:9541–6. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, uses formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tjabringa GS, Ninaber DK, Drijfhout JW, Rabe KF, Hiemstra PS. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int Arch Allergy Immunol. 2006;140:103–12. doi: 10.1159/000092305. [DOI] [PubMed] [Google Scholar]
- 13.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, Nagaoka I. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20–6. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivas-Santiago B, Hernandez-Pando R, Carranza C, Juarez E, Contreras JL, Aguilar-Leon D, Torres M, Sada E. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun. 2008;76:935–41. doi: 10.1128/IAI.01218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chromek M, Slamová Z, Bergman P, Kovács L, Podracká L, Ehrén I, Hökfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–41. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 16.Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, Ohtake T, Obonyo M, Gallo RL, Eckmann L, Kagnoff MF. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology. 2003;125:1613–25. doi: 10.1053/j.gastro.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 17.Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, Roche FM, Mu R, Doho GH, Pistolic J, Powers JP, Bryan J, et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol. 2006;176:2455–64. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 18.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. 2004;172:4987–94. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 19.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, Rabe KF, Hiemstra PS. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 20.Heilborn JD, Nilsson MF, Kratz G, Weber G, Sørensen O, Borregaard N, Ståhle-Bäckdahl M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–89. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 21.Shaykhiev R, Beisswenger C, Kändler K, Senske J, Püchner A, Damm T, Behr J, Bals R. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol. 2005;289:L842–8. doi: 10.1152/ajplung.00286.2004. [DOI] [PubMed] [Google Scholar]
- 22.Coffelt SB, Waterman RS, Florez L, Höner zu Bentrup K, Zwezdaryk KJ, Tomchuck SL, LaMarca HL, Danka ES, Morris CA, Scandurro AB. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int J Cancer. 2008;122:1030–9. doi: 10.1002/ijc.23186. [DOI] [PubMed] [Google Scholar]
- 23.Heilborn JD, Nilsson MF, Jimenez CI, Sandstedt B, Borregaard N, Tham E, Sørensen OE, Weber G, Ståhle M. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer. 2005;114:713–9. doi: 10.1002/ijc.20795. [DOI] [PubMed] [Google Scholar]
- 24.von Haussen J, Koczulla R, Shaykhiev R, Herr C, Pinkenburg O, Reimer D, Wiewrodt R, Biesterfeld S, Aigner A, Czubayko F, Bals R. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59:12–23. doi: 10.1016/j.lungcan.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Wu WK, Sung JJ, To KF, Yu L, Li HT, Li ZJ, Chu KM, Yu J, Cho CH. The host defense peptide LL-37 activates the tumor-suppressing bone morphogenetic protein signaling via inhibition of proteasome in gastric cancer cells. J Cell Physiol. 2010;223:178–86. doi: 10.1002/jcp.22026. [DOI] [PubMed] [Google Scholar]
- 26.Johansson J, Gudmundsson GH, Rottenberg ME, Berndt KD, Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998;273:3718–24. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 27.Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J. 1999;341:501–13. [PMC free article] [PubMed] [Google Scholar]
- 28.Schröder-Borm H, Bakalova R, Andrä J. The NK-lysin derived peptide NK-2 preferentially kills cancer cells with increased surface levels of negatively charged phosphatidylserine. FEBS Lett. 2005;579:6128–34. doi: 10.1016/j.febslet.2005.09.084. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Li Y, Han H, Miller DW, Wang G. Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J Am Chem Soc. 2006;128:5776–85. doi: 10.1021/ja0584875. [DOI] [PubMed] [Google Scholar]
- 30.Mader JS, Hoskin DW. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin Investig Drugs. 2008;15:933–46. doi: 10.1517/13543784.15.8.933. [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Li X, Wang Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009;37 (Database issue):D933–7. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G. NMR of membrane-associated peptides and proteins. Curr Protein Pept Sci. 2008;9:50–69. doi: 10.2174/138920308783565714. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Li X, Wang G. Cloning, expression, isotope labeling, and purification of human antimicrobial peptide LL-37 in Escherichia coli for NMR studies. Protein Expr Purif. 2006;47:498–505. doi: 10.1016/j.pep.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Wang G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J Biol Chem. 2008;283:32637–43. doi: 10.1074/jbc.M805533200. [DOI] [PubMed] [Google Scholar]
- 35.Wang G. Determination of solution structure and lipid micelle location of an engineered membrane peptide by using one NMR experiment and one sample. Biochim Biophys Acta. 2007;1768:3271–81. doi: 10.1016/j.bbamem.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Epand RF, Wang G, Berno B, Epand RM. Lipid segregation explains selective toxicity of a series of fragments derived from the human cathelicidin LL-37. Antimicrob Agents Chemother. 2009;53:3705–14. doi: 10.1128/AAC.00321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koczulla R, von Degenfeld G, Kupatt C, Krötz F, Zahler S, Gloe T, Issbrücker K, Unterberger P, Zaiou M, Lebherz C, Karl A, Raake P, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coffelt SB, Tomchuck SL, Zwezdaryk KJ, Danka ES, Scandurro AB. Leucine leucine-37 uses formyl peptide receptor-like 1 to activate signal transduction pathways, stimulate oncogenic gene expression, and enhance the invasiveness of ovarian cancer cells. Mol Cancer Res. 2009;7:907–15. doi: 10.1158/1541-7786.MCR-08-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coffelt SB, Marini FC, Watson K, Zwezdaryk KJ, Dembinski JL, LaMarca HL, Tomchuck SL, Honer zu Bentrup K, Danka ES, Henkle SL, Scandurro AB. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc Natl Acad Sci U S A. 2009;106:3806–11. doi: 10.1073/pnas.0900244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 42.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–24. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brighton CT, Lorich DG, Kupcha R, Reilly TM, Jones AR, Woodbury RA., 2nd The pericyte as a possible osteoblast progenitor cell. Clin Orthop Relat Res. 1992:287–99. [PubMed] [Google Scholar]
- 44.Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–8. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crisan M, Deasy B, Gavina M, Zheng B, Huard J, Lazzari L, Peault B. Purification and long-term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: myoendothelial cells and pericytes. Methods Cell Biol. 2008;86:295–309. doi: 10.1016/S0091-679X(08)00013-7. [DOI] [PubMed] [Google Scholar]
- 46.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Paquet-Fifield S, Schlüter H, Li A, Aitken T, Gangatirkar P, Blashki D, Koelmeyer R, Pouliot N, Palatsides M, Ellis S, Brouard N, Zannettino A, et al. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J Clin Invest. 2009;119:2795–806. doi: 10.1172/JCI38535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–8. [PubMed] [Google Scholar]
- 49.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 50.Murakami M, Dorschner RA, Stern LJ, Lin KH, Gallo RL. Expression and secretion of cathelicidin antimicrobial peptides in murine mammary glands and human milk. Pediatr Res. 2005;57:10–5. doi: 10.1203/01.PDR.0000148068.32201.50. [DOI] [PubMed] [Google Scholar]
- 51.Weber G, Chamorro CI, Granath F, Liljegren A, Zreika S, Saidak Z, Sandstedt B, Rotstein S, Mentaverri R, Sánchez F, Pivarcsi A, Ståhle M. Human antimicrobial protein hCAP18/LL-37 promotes a metastatic phenotype in breast cancer. Breast Cancer Res. 2009;11:R6. doi: 10.1186/bcr2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol. 2006;16:649–656. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010;277:301–8. doi: 10.1111/j.1742-4658.2009.07448.x. [DOI] [PubMed] [Google Scholar]
- 54.Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. J Cell Physiol. 2009;218:460–6. doi: 10.1002/jcp.21635. [DOI] [PubMed] [Google Scholar]
- 55.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, Yahata Y, Dai X, Tohyama M, Nagai H, Yang L, Higashiyama S, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–8. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 56.Wen XZ, Miyake S, Akiyama Y, Yuasa Y. BMP-2 modulates the proliferation and differentiation of normal and cancerous gastric cells. Biochem Biophys Res Commun. 2004;316:100–6. doi: 10.1016/j.bbrc.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 57.Wen XZ, Akiyama Y, Baylin SB, Yuasa Y. Frequent epigenetic silencing of the bone morphogenetic protein 2 gene through methylation in gastric carcinomas. Oncogene. 2006;25:2666–73. doi: 10.1038/sj.onc.1209297. [DOI] [PubMed] [Google Scholar]
- 58.Bleuming SA, He XC, Kodach LL, Hardwick JC, Koopman FA, Ten Kate FJ, van Deventer SJ, Hommes DW, Peppelenbosch MP, Offerhaus GJ, Li L, van den Brink GR. Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Res. 2007;67:8149–55. doi: 10.1158/0008-5472.CAN-06-4659. [DOI] [PubMed] [Google Scholar]
- 59.Varga AC, Wrana JL. The disparate role of BMP in stem cell biology. Oncogene. 2005;24:5713–21. doi: 10.1038/sj.onc.1208919. [DOI] [PubMed] [Google Scholar]
- 60.Bartek J, Lukas J. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 2001;490:117–22. doi: 10.1016/s0014-5793(01)02114-7. [DOI] [PubMed] [Google Scholar]
- 61.Wu WK, Sung JJ, Yu L, Cho CH. Proteasome inhibitor MG-132 lowers gastric adenocarcinoma TMK1 cell proliferation via bone morphogenetic protein signaling. Biochem Biophys Res Commun. 2008;371:209–14. doi: 10.1016/j.bbrc.2008.04.059. [DOI] [PubMed] [Google Scholar]
- 62.Wu WK, Sung JJ, Wu YC, Li ZJ, Yu L, Cho CH. Bone morphogenetic protein signalling is required for the anti-mitogenic effect of the proteasome inhibitor MG-132 on colon cancer cells. Br J Pharmacol. 2008;154:632–8. doi: 10.1038/bjp.2008.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garrett IR, Chen D, Gutierrez G, Zhao M, Escobedo A, Rossini G, Harris SE, Gallwitz W, Kim KB, Hu S, Crews CM, Mundy GR. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003;111:1771–82. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chung CM, Monie A, Wu A, Mao CP, Hung CF. Treatment with LL-37 peptide enhances the antitumor effects induced by CpG oligodeoxynucleotides against ovarian cancer. Hum Gene Ther. 2009;20:303–13. doi: 10.1089/hum.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hurtado P, Peh CA. LL-37 promotes rapid sensing of CpG oligodeoxynucleotides by B lymphocytes and plasmacytoid dendritic cells. J Immunol. 2010;184:1425–35. doi: 10.4049/jimmunol.0902305. [DOI] [PubMed] [Google Scholar]
- 66.Büchau AS, Morizane S, Trowbridge J, Schauber J, Kotol P, Bui JD, Gallo RL. The host defense peptide cathelicidin is required for NK cell-mediated suppression of tumor growth. J Immunol. 2010 Jan 1;184(1):369–78. doi: 10.4049/jimmunol.0902110. [DOI] [PMC free article] [PubMed] [Google Scholar]