Abstract
The immune response in humans is usually assessed using immunogenicity assays to provide biomarkers as correlates of protection (CoP). Flow cytometry is the assay of choice to measure intracellular cytokine staining (ICS) of cell-mediated immune (CMI) biomarkers. For CMI analysis, the integrated mean fluorescence intensity (iMFI) was introduced as a metric to represent the total functional CMI response as a CoP. iMFI is computed by multiplying the relative frequency (% positive) of cells expressing a particular cytokine with the mean fluorescence intensity (MFI) of that population, and correlates better with protection in challenge models than either the percentage or the MFI of the cytokine-positive population. While determination of the iMFI as a CoP can readily be accomplished in animal models that allow challenge/protection experiments, this is not feasible in humans for ethical reasons. As a first step towards extending the iMFI concept to humans, we investigated the correlation of the iMFI derived from a human innate immune response ICS assay with functional cytokine release into the culture supernatant, as innate cytokines need to be released to have a functional impact. Next we developed a quantitatively more correlative mathematical approach for calculating the functional response of cytokine producing cells by incorporating the assignment of different weights to the magnitude (frequency of cytokine-positive cells) and the quality (the MFI) of the observed innate immune response. We refer to this model as GiMFI (Generalized iMFI).
Keywords: GiMFI, correlation analysis, functional response, culture supernatant, cytokine, flow cytometry, antigen presenting cells, integrated mean fluorescent intensity
INTRODUCTION
While direct measurement of protection from infection after a defined challenge provides the most meaningful information in vaccine trials, in human studies intermediate biomarkers (e.g., antibody titers or various measurements of cell-mediated immunity (CMI)) are used as correlates or surrogates of protection (1). The CMI response is often determined by measuring cytokines within the cell or secreted in serum or in culture supernatant. Given that cytokines exert their function mostly after being secreted, both approaches potentially measure different aspects of CMI, yet are often used interchangeably. To our knowledge, a direct correlative comparison of these two approaches has not been conducted. While quantification of secreted cytokines can be conducted using ELISA or multiplex bead arrays, these methods do not identify the specific cell source of these secreted cytokines. Alternatively, flow cytometric analysis of intracellular cytokine staining (ICS) is able to identify the specific cell/s producing a given cytokine, but it does not allow their absolute quantification. ICS results are determined as either percent positive cells or as mean fluorescent intensity (MFI) of a population of cytokine producing cells, with both measurements compared to a control population of untreated or unstimulated cells. The integrated MFI (iMFI) (2) was devised to increase the quantitative informational content of data obtained from ICS by combining the relative amount of a cytokine produced per cell or population (the MFI) with the relative number of cells that make them (the percent positive cells). For example, Darrah et al. (2) showed that the iMFI for IFN-γ, IL-2, and TNF-α of mouse CD4+ cells independently correlated with protection in a challenge model better than either percentage of cells making cytokine or the MFI of the cytokine-positive population alone. To allow the iMFI to provide such meaningful assessment of biomarker of protection in humans, steps between production of a given cytokine inside a cell (detected via ICS) and clinical protection have to be verified. The first of these series of events is the secretion of cytokines from the cell into either culture supernatant or body fluids such as serum. In this study, we have quantified the degree of correlation between intracellular cytokine production and their subsequent secretion into culture supernatant as determined by multiplex load array (Luminex). We have also improved the mathematical modeling of the functional response of cytokine producing cells through the development of the GiMFI (Generalized iMFI), which assigns different weights to the magnitude (frequency of positive cells) and the quality (iMFI) of the response.
METHODS
Data & Wet Lab Description
Flow cytometry and Luminex data were obtained from the analysis of peripheral blood mononuclear cells (PBMC) from healthy human adult volunteers as previously described (3). The study was approved by the Clinical Research Ethics Board of the University of British Columbia. Briefly, PBMC from 20 adult subjects (8 males and 12 females) were purified using Ficoll-Hypaque density centrifugation. Half a million cells in 200 ul of RPMI supplemented with 10% human AB serum and 1% penicillin-streptomycin were plated on premade 96-well plates containing 10 ul of TLR ligands in various concentrations (final concentrations of the ligands were: PAM3CSK4 at 0.01, 0.1 and 1 ug/ml; R-FSL at 0.1, 1 and 10 ug/ml; poly I:C at 12.5, 25 and 50 ug/ml; LPS at 1, 10 and 100 ng/ml; 3M-002, 3M-003, 3M-013 at 0.1, 1 and 10 uM each, and; CpG-A, CpG-B and CpG-C at 6.25, 12.5 and 25 ug/ml each). The cells plated for the ICS were cultured for 6 hours in the presence of the Golgi transport inhibitor Brefeldin A (BFA) at 10 ug/ml with the exception of the wells containing the CpG ligands. The cells stimulated with the CpG ligands were cultured for 3 hours without BFA followed by an additional 3 hours in the presence of BFA. BFA addition was delayed because it hinders the response to CpG stimulation if added immediately (4). At the 6-hr mark, these cells were treated with EDTA (final concentration of 2 mM), spun down, and the pellet resuspended in 100 ul of Becton-Dickinson (BD) FACS Lysing Solution and then frozen in −80°C until antibody staining and cytometric analysis using a BD LSRII instrument (4). Identical set of plates except without BFA were setup and cultured for 18 hours, spun down, and 100 ul of the supernatants harvested and stored in −80°C prior to Luminex assay using kits from Millipore.
Data from the LSRII instrument were analyzed using FlowJo (TreeStar, Inc.) and CPAS (LabKey Solutions) (5). Luminex data from the Bio-plex 200 instrument and the Bio-plex Manager software (Bio-rad) were further analyzed in an in-house database, in Excel (Microsoft) and in Prism (GraphPad).
Correlation Analysis of iMFI and Culture Supernatant Response
In order to test the hypothesis that culture supernatant concentration of a particular cytokine correlated to its corresponding intracellular production cytokine as estimated by the iMFI, we computed Pearson correlation coefficients between iMFI values and culture supernatant responses of all samples (including all listed TLR ligands and concentrations) obtained from every single subject, and determined strength of linear correlations between these two values. The levels of correlations were interpreted as no correlation, weakly, moderately, strongly, and perfectly positive correlation when Pearson correlation coefficients were approximately 0, 0.2, 0.5, 0.8 and 1, respectively (6). We examined two different approaches to compute the levels of correlation. In the first approach, we computed iMFI values for all the cytokine-positive cells in the PBMC together, while in the second approach, the iMFI values were computed for the cytokine-positive cells from each specific antigen-presenting cell (APC) population (i.e., monocytes, conventional dendritic cells (cDC) and plasmacytoid dendritic cells (pDC)) separately. In both of these two approaches, the first step was to identify cytokine-expressing (positive) cells, and to measure their percentage and mean fluorescence intensity (MFI) values to compute iMFI by the following formula:
| 1 |
where P is the relative percentage of the cells expressing a specific cytokine.
Automated Identification of All Cytokine-Positive Cells of PBMC
As we measured the bulk amount of cytokines present in the culture supernatant by the luminex assay regardless of cell source, we performed our initial correlation analysis of the results from this assay with the iMFI values derived from all the cytokine-positive cells in the 6-hr ICS of the corresponding PBMC. We developed an automated method to identify the cytokine-expressing (i.e., positive) cells in our ICS assay by setting a threshold based on the assumption that 2% of unstimulated PBMC cells (i.e., in a control sample) are cytokine-positive. In other words, after identifying live cells using a FSC vs. SSC plot in FlowJo, we computed a threshold based on the 98% cutoff of the empirical cumulative distribution function (ECDF) of the cytokine intensity for the unstimulated sample (see supplementary materials), and used this threshold to identify positive cells of all other samples from the same subject. Identifying positive cells and knowing their percentages and MFI values, we computed iMFI value of each cytokine using equation 1.
Manual Identification of Antigen-Presenting Cell Populations and Detection of Cytokine-Positive Cells for Different APC Populations
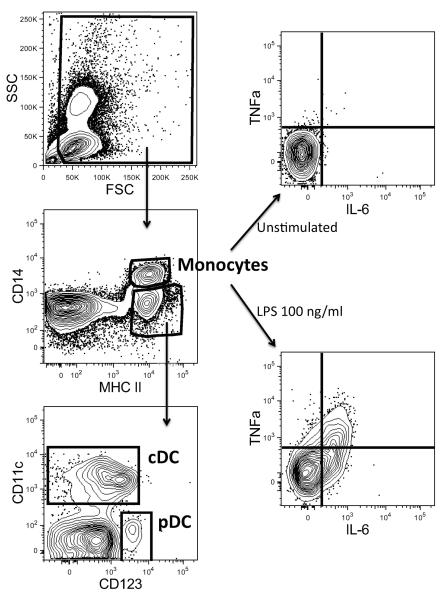
We hypothesized that Luminex assay results may correlate better with iMFI values from specific antigen-presenting cell populations instead of the iMFI values from the cytokine-positive cells in the whole PBMC, and thus computed the correlation coefficients between iMFI values of different APC populations and culture supernatant responses. Here, unlike the automated identification of the cytokine-positive cells in PBMC described above, we used FlowJo to manually identify the cDC, pDC and monocytes antigen-presenting cell populations and also the positive cells for each population. First, dead cells and cell debris were excluded by gating them out from an FSC vs. SSC plot (Figure 1). From this “live” gate, we then plotted the cells on MHC II vs. CD14. CD14high, and MHC+ cells were identified as monocytes. By plotting the CD14low/neg, MHC+ cells on CD123 vs. CD11c axes, we identified the conventional dendritic cells as the CD11c+ population while the plasmacytoid dendritic cells as CD123+. Next, the monocytes, cDC and pDC were graphed on scatter plots with the fluorescence intensity for each of the cytokines as the axes. For each subject, the quadrants inside these scatter plots which separated the cytokine-producing cells from cytokine-negative ones were manually determined based on the cells that were not stimulated with any TLR ligand.
Figure 1.
Manual identification of antigen-presenting cell populations (monocytes, cDC and pDC), and the identification of the cytokine-producing cells within cDC, pDC and monocytes. In this figure, each axis presents the intensity of the measured parameter.
Generalized iMFI (GiMFI)
The iMFI assumes the impact of MFI and P are the same when estimating a functional response of cytokine producing cells. To better model the functional response of cytokine producing cells, we relaxed this requirement, and defined our Generalized iMFI as:
| 2 |
where MFI and P are mean of fluorescent intensity and percentage of cytokine-positive cells, respectively, and α is the relative impact weight assigned to the percentage of positive cells. If 0≤α<1 then the impact weight of P in GiMFI is less significant compared to the iMFI formula. If α>1 a higher relative impact weight is assigned to P in GiMFI.GiMFI(g=a) and iMFI would have the same values for α = 1.
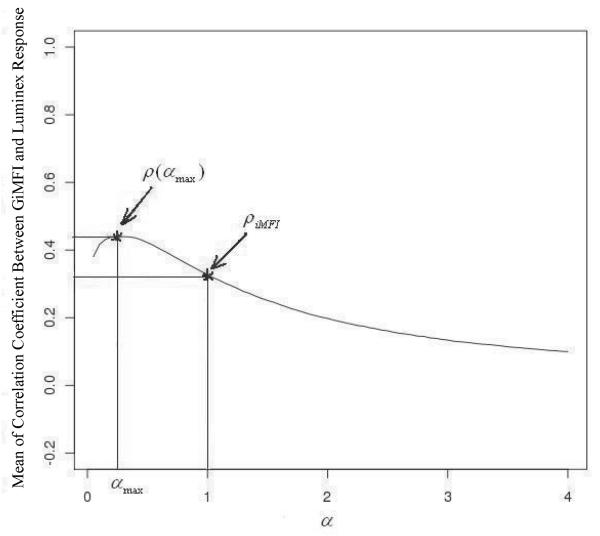
In order to find α such that GiMFI(g=a) modeled the functional response of cytokine-producing cells, we computed GiMFI(g=a) for all α in an reasonable predefined range (i.e., 0≤α≤4), and computed correlation coefficients between GiMFI(g=a) and culture supernatant cytokine response for all α in this range. Then we chose α, such that the mean of correlation coefficients (R) of all subjects was maximized. Since GiMFI(g=a) monotonically decreased after α=4, no values of α>4 were included.To keep the notation simple we will use αmax to present the value of α when the correlation coefficient between GiMFI(g=a) and culture supernatant cytokine response was at its maximum value. We also use the notation ρ(α) for correlation coefficients between culture supernatant cytokine response and GiMFI(g=a). ρiMFI is the correlation coefficient between culture supernatant cytokine response and iMFI. While ρ(α) and ρiMFI can be computed for any combination of cytokine and population, we were not interested in studying these values for those cytokines which are known not to be at least moderately produced by the population of interest. For example, we performed our correlation analysis on monocytes and cDCs for IL-12p40, IL-6 and TNF-α. Similarly, the correlation analysis for IFN-α was done only on pDCs, because cDCs and monocytes do not generate IFN-α under the culture conditions chosen (3).
RESULTS
Correlation Analysis of Whole PBMC Live Cells
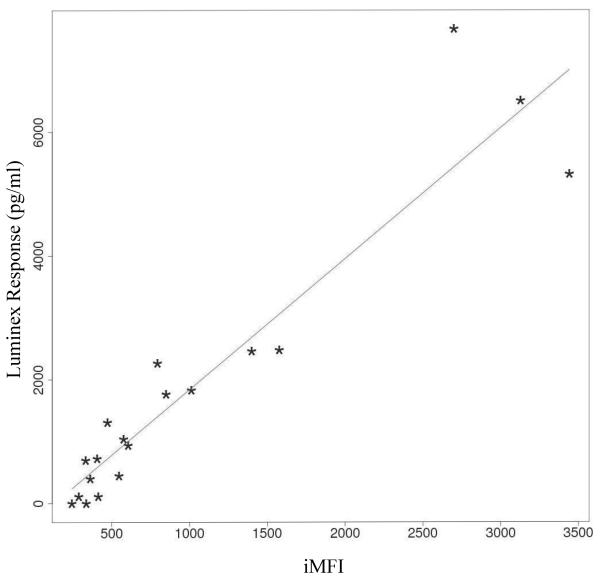
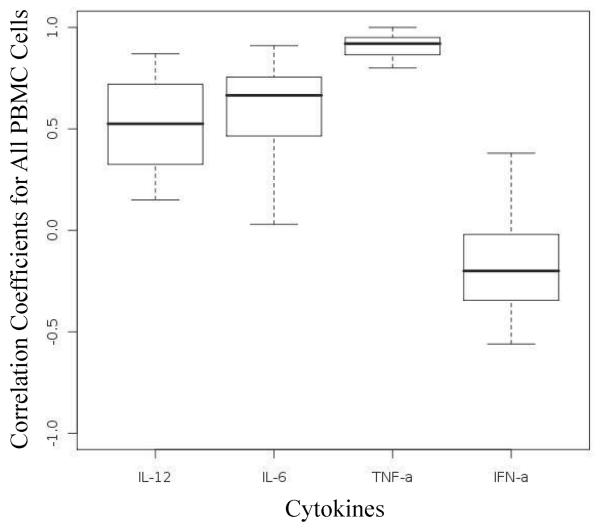
Because our Luminex assay measured the cytokine concentration in bulk without regard to cell source, we wanted to first examine if these concentrations correlate with the iMFI computed from all the cytokine-positive cells in the corresponding whole PBMC population. We computed the correlation coefficients between the iMFI values and the culture supernatant cytokine concentration for the cytokines TNF-α, IFN-α, IL-6 and IL-12p40. Figure 2 shows an example of a scatter plot of the amount of secreted IL-12p40 as measured by Luminex versus the calculated iMFI values from the intracellular cytokine staining for IL-12p40 of cells from one subject treated with different TLR ligands at different concentrations (ρiMFI =0.94). The solid line shows the best line fitted to the data based on simple regression model. Linear model parameters were estimated using the least square method for linear regression. The boxplots in Figure 3 represent the correlation coefficients computed for all four cytokines from the TLR ligand-stimulated PBMC from 20 individuals. Correlations between iMFI values and Luminex responses were strongly positive for TNF-α, while they were only moderately positive for IL-6 and IL-12p40. Almost no correlation was observed between iMFI values and Luminex responses for IFN-α.
Figure 2.
Scatter plots of the amount of secreted IL-12p40 measured by Luminex versus calculated iMFI values from the intracellular cytokine staining for IL-12p40 of cells from one subject treated with different TLR ligands at different concentrations (ρiMFI =0.94). Each (*) corresponds to a stimulations of a blood sample of the same subject. The solid line shows the best line fitted to the data based on simple regression model.
Figure 3.
Boxplots of correlation coefficients between culture supernatant cytokine response and iMFI values computed for IL-12, IL-6, TNF-α, and IFN-α) for 20 subjects. All PBMC live cells are considered together.
Correlation Analysis of Antigen-Presenting Cell Populations
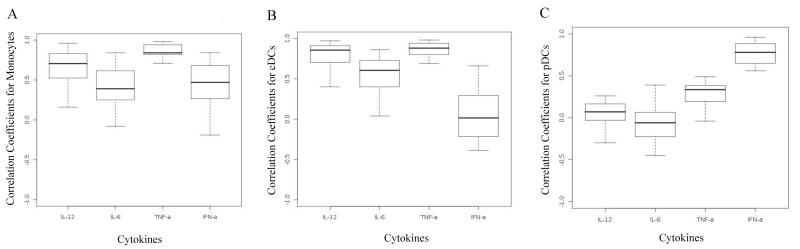
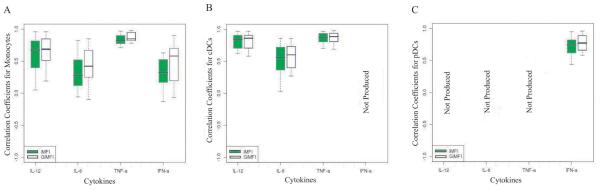
Motivated by the lack of correlation between iMFI and Luminex for IFN-α we next examined if the cytokine concentrations measured from the supernatants of our 18-hr culture would exhibit higher correlation with the computed iMFI values when these were derived from specific subpopulations in the PBMC. Figure 4 depicts boxplots of correlation coefficients between iMFI values and supernatant cytokine levels for three antigen-presenting cell populations (monocytes, cDCs, and pDCs) for 20 subjects. Figure 4C shows that for pDCs, the level of correlation between iMFI and culture supernatant cytokine response is strong for IFN-α and weak for TNF-α. No correlation was observed for IL-12p40 and IL-6. This indicated that considering only pDCs instead of whole PBMC improves the correlation between iMFI and culture supernatant cytokine response for IFN-α.
Figure 4.
Boxplots of correlation coefficients between culture supernatant cytokine response and iMFI values from the intracellular cytokine staining for four cytokines (IL-12, IL-6, TNF-α, and IFN-α) produced by (A) monocytes, (B) cDCs, and (C) pDCs.
Figure 4A shows that correlation coefficients between iMFI and culture supernatant cytokine response was strong for TNF-α, weak/moderate for IL-6 and IFN-α, and moderate for IL-12p40 in monocytes. Similarly, Figure 4B shows that for cDCs, correlation coefficients between iMFI and culture supernatant cytokine response was strong for IL-12p40 and TNF-α, while it was moderate for IL-6. No correlation was observed for IFN-α in cDCs. Table 1 summarizes the level of correlation between iMFI values and culture supernatant cytokine response. Overall, for any specific APC population, the correlation between iMFI values and culture supernatant cytokine response varies from cytokine to cytokine, though generally the more cytokine produced by the APC population, the higher the correlation. However, observing a high correlation does not always mean that the population under study was a major producer of the cytokine of interest.
Table 1.
Correlation between iMFI values and culture supernatant cytokine responses of IL-12, IL-6, TNF-α, and IFN-α produced by monocytes, cDC, and pDC based on the median of correlation coefficients.
| Cytokines | |||||
|---|---|---|---|---|---|
|
Antigen Presenting Cell Populations |
IL-12 | IL-6 | TNF-α | IFN-α | |
| Monocyte | Moderate | Weak- Moderate |
Strong | Weak-Moderate | |
| cDC | Strong | Moderate | Strong | No Correlation- Weak |
|
| pDC | No correlation | No Correlation | Weak | Strong | |
Parameter Estimation for Generalized iMFI (GiMFI) Formula
For each APC population and each cytokine, we determined the optimum α, such that the correlation coefficients between GiMFI(α) and culture supernatant cytokine response was maximized. Figure 5 illustrates our strategy for deriving αmax. Our experiments showed that for α > 4, ρ(α) (correlation coefficients between culture supernatant cytokine response and GiMFI(α)) decreases monotonically, so we did not plot ρ(α) for higher values, as we were interested only in the maximum of ρ(α). Our computation showed that range of variation of αmax was narrow for different APC populations and various cytokines. More precisely, lower quartile, mean, upper quartile and max were 0.29, 0.39, 0.47 and 0.60 respectively. Note that for α = 1, GiMFI(α) and iMFI result in the same values, that is, ρ(α = 1)= ρiMFI.
Figure 5.
Example calculation of an optimum α which maximizes the mean value of ρ(α) (correlation coefficients between culture supernatant cytokine response and GiMFI(α)). Mean of ρ(α) for IL-6 response produced by monocytes is plotted versus α in range 0 ≤ α ≤ 4.
Figure 6 presents a comparison between distributions of ρiMFI (correlation coefficients of culture supernatant response and iMFI) and ρ(αmax) (correlation coefficients of GiMFI(αmax) and culture supernatant response) for the cytokines produced moderately or highly by populations of interest (cDCs, pDCs and monocytes). This result suggests that statistically ρ(αmax) is at least as high as ρiMFI, if not higher. This was verified by applying the Wilcoxon test with the null hypothesis ρ(αmax<ρiMFI. Table 2 presents p-values computed for this test. The null hypothesis was rejected at 2% significance level for all cases other than IL-12p40 and TNF-α produced by cDCs, indicating ρ(αmax)≥ρiMFI. For these two exceptional cases, iMFI and GiMFI can be used interchangeably with no significant difference in the final results.
Fig. 6.
Distribution of ρiMFI (correlation coefficients of culture supernatant cytokine response and iMFI) plotted against distribution of ρ(αmax) (correlation coefficients of culture supernatant response and GiMFI(αmax)) for IL-12, IL-6, TNF-α, and IFN-α produced by (A) monocytes, (B) cDCs, and (C) pDCs.
Table 2.
P values obtained by applying Wilcoxon test with the null hypothesis ρ(αmax)<ρiMFI max to the correlation coefficients measured for four cytokines (IL-12, IL-6, TNF-α, and IFN-α) produced by monocytes, cDCs, and pDCs of PBMC samples from 20 healthy human adult volunteers. The exact values of αmax are given for every cytokine and cell population combination.
| Cytokines | |||||
|---|---|---|---|---|---|
|
Antigen Presenting Cell Populations |
IL-12 | IL-6 | TNF-α | IFN-α | |
| Monocyte | 0.006 (αmax = 0.3) |
0.001 (αmax = 0.25) |
0.001 (αmax = 0.45) |
0.007 (αmax = 0.1) |
|
| cDC | 0.364 (αmax = 0.60) |
0.018 (αmax = 0.45) |
0.12 (αmax = 0.55) |
Not Produced | |
| pDC | Not Produced | Not Produced | Not Produced | 0.005 (αmax = 0.45) |
|
DISCUSSION
While the measurement of cytokines in culture supernatant or serum, and the identification of intracellular cytokines by ICS represent the two most commonly employed assays of CMI, the degree to which these two assays correlate with each other has not been investigated. To quantify the degree of correlation between intracellular cytokine production and their subsequent secretion, and to optimize the mathematical modeling of this correlation (i.e., to improve the calculation of the iMFI), we analyzed the correlation of the iMFI and cytokines secreted into culture supernatants as determined by multiplex bead array (Luminex). Our experiment was designed to examine the innate immune responses in PBMC stimulated with known TLR ligands. PBMC receptors are expressed on antigen-presenting cells such as monocytes and dendritic cells that play a critical role in the process of sensing pathogens through pattern recognition (7). TLRs are strong inducers of inflammatory cytokines such as IL-6, IL-12 and TNF-α; they also induce production of type I interferon like IFN-α (8). Qualitatively, the results of our ICS-Luminex correlation testing were in line with what is currently known about the cell-type specific source of the TLR-induced cytokines we analyzed. Our analysis indicates that the iMFI and culture supernatant cytokine response to TLR stimulation correlate with each other (Figure 3), and this correlation was strong for those cytokines that are highly produced in PBMC. For example, TNF-α was produced at high levels by most monocytes, which form a significant proportion of the whole PBMC (about 10% of leukocytes in human blood for monocytes) (9) (10), thus resulting in a high level of correlation between iMFI and the concentration of TNF-α in the culture supernatant. On the other hand, for cytokines such as IFN-α, the correlation was low given that IFN-α is produced at high amounts only by pDC, which constitutes a very small fraction (around 0.2 to 0.4%) of PBMC (11) (12). Changes in the pDC cytokine production in response to TLR stimulation were hard to detect when looking at all the PBMC, as they were obscured by the lack of response by the more prevalent cell populations (e.g., monocytes and lymphocytes). This could result in critical errors in analyzing the functional response of cytokine-producing cells in PBMC, and we therefore considered each cell population separately. For each population of APC, higher correlations were obtained for those cytokines known to be produced by a given APC population (Figure 4, Table 1). For example, the level of correlation for IFN-α improved significantly when we considered pDCs alone (Figure 4C). Similarly for monocytes (the major producer of TNF-α, and a moderate producer of IL-6 and IL-12p40 (10)) the correlation was strong for TNF-α but only moderate for IL-6 and IL-12p40 (Figure 4A). As expected cDCs had a significant correlation as these cells are known to produce significant amounts of all three cytokines, IL-12p40, IL-6 and TNF-α (3) (9) (Figure 4B). However, it needs to be emphasized that although the correlation could be moderate or high for producers of cytokines, observing a moderate or high correlation does not necessarily mean that a given population under study is the producer of the cytokine of interest., that is, iMFI and culture supernatant responses could be correlated even though the level of production was low. An example would be IFN-α production by monocytes. As expected, the level of IFN-α produced by monocytes was very low, resulting in low iMFI value and a low Luminex response; yet the correlation between these two values was moderate (Figure 4A). Overall, our results confirm that a functional response as measured by intracellular cytokine detection modeled by iMFI does correlate with the level of secreted cytokine for those populations that moderately or highly generate cytokines of interest.
With the GiMFI, we improved the iMFI modeling approach by assigning different weights to the magnitude (frequency of cytokine-positive cells) and the quality (the MFI). We hypothesized that the GiMFI could provide the iMFI with a wider, more general applicability by assigning different weights to magnitude (percentage of positive cells) and quality (MFI) of the response. Our hypothesis was based on the observation that functional response of cytokine producing cells and Luminex response do correlate for populations that are major producers of cytokine-of-interest, and therefore, we tried to find a formula that maximizes this correlation. GiMFI, our proposed mathematical formula for estimating functional response of cytokine producing cells, assigns different weights to the magnitude (percentage of positive cells) and quality (MFI) of the response. In this formula, αmax is the optimum parameter that maximizes the mean correlation across different subjects. αmax was found to always be less than 1 (Table 2), suggesting that assigning a lower impact weight to the percentage of cells positive as compared to the MFI in the GiMFI formula would strengthen the correlation. The observation that αmax is always less than 1 suggests that the total cytokine production is mainly influenced by the potential of cytokine secretion per cell rather than by the quantity of cytokine-secreting cells within a given cell subset. This can be related to the fact that, after cell activation, cytokine production per cell can usually vary from several orders of magnitude over the background production, while the variation of cell frequency is more limited. In our study, we did not assign a fixed value to αmax, but adjusted it depending on the target population and cytokine-of-interest. Our results showed that the range that αmax can take is narrow, with values mostly across a limited range (lower quartile and upper quartiles were 0.29 and 0.47, respectively). Therefore, automatically assigning any constant value to αmax within this range can result in a more appropriate model, compared to iMFI where α is equal to 1. Using Wilcoxon test with null hypothesis ρ)αmax)<ρiMFI, we showed that for individual subjects, ρ(αmax) (correlation coefficients ofGiMFI(αmax) and culture supernatant response) was greater than or equal to ρiMFI (correlation coefficients of culture supernatant response and iMFI) (Table 2), confirming that GiMFI was a better model for estimating functional response of APCs compared to iMFI for most cytokines measured. However, there were two exceptions, namely IL-12p40 and TNF-α produced by cDCs. Table 2 shows that p-values for IL-12p40 and TNF-α produced by cDCs were such that the null hypothesis ρ(αmax)<ρiMFI could be neither accepted nor rejected. Figure 6B compares the distributions of ρiMFI and ρ(αmax) for these two cases, showing that ρiMFI and ρ(αmax) were equally distributed for IL-12p40 and TNF-α produced by cDCs (i.e., it should be expected that iMFI and GiMFI similarly estimated the functional response of cDCs for IL-12p40 and TNF-α produced by cDCs). For these two exceptional cases, the curvature of GiMFI(α) versus α (Figure 5) is relatively low, and therefore, the value of GiMFI(α) does not change significantly with respect α to in this range. Consequently, for these two exceptional cases, GiMFI(αmax) and iMFI = GiMFI(α = 1) get the similar values, and as a result, iMFI and GiMFI could be used interchangeably with neither having an advantage.
In summary our results show that compared to iMFI, GiMFI will as a general rule provide better estimates of the degree of correlation between intracellular cytokine detected by flow cytometry and the secretion of cytokines into culture supernatant. Therefore, since there were no cases where GiMFI provided qualitatively worse results, GiMFI is a superior approach for calculating the functional response of cytokine producing cells. We have shown here that the intracellular cytokine production of APC in response to TLR stimulation as estimated by iMFI correlated with the level of cytokines secreted into culture supernatant as measured by Luminex assay, and that this correlation was higher for those APC populations that represent the main producer of the cytokine of interest. To properly evaluate the potentially improved predictive value of the GiMFI over the iMFI as a CoP, vaccine-specific GiMFI needs to be correlated with clinical protection in human clinical trials. Our results set the stage to apply the GiMFI to adaptive immune responses in vitro, as well as measurements ex vivo in human trials. As this data accumulates, appropriate intermediate biomarkers, such as cytokine concentrations in serum or culture supernatant or intracellular cytokine determination via ICS, should allow more accurate identification of correlates of protection. It would also be interesting to know the generalizability of the αmax values for each APC subpopulation computed from our studies to other analysis that correlates iMFI with some immune response outcome.
Supplementary Material
Acknowledgements
The authors would like to thank Habil Zare and Aaron Barsky for their editorial comments.
Footnotes
This work was supported by the Michael Smith Foundation for Health Research as well asMITACS Network of Centers of Excellence, the National Institute of Allergy and Infectious Diseases, NIH (TRK; Grant number: N01 AI50023 and NIBIB Grant Number 1R01EB00840) and AllerGen NCE (TRK; Grant numbers: 07-A1A, 07-B2B). TRK is supported in part by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund, and by a CIHR Training Grant in Canadian Child Health Clinician Scientist Program, in partnership with SickKids Foundation, Child & Family Research Institute (BC), Women & Children's Health Research Institute (Alberta), Manitoba Institute of Child Health.
References
- 1.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47(3):401–9. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 2.Darrah P,A, Patel D,T, De Luca P,M, Lindsay R,W, Davey D,F, Flynn B,J, Hoff S,T, Andersen P, Reed S,G, Morris S,L, Roederer M, Seder R,A. Multifunctional TH 1 Cells Define a Correlate of Vaccine Mediated Protection Against Leishmania Major. Nat Med. 2007;13(7):843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 3.Kollmann T,R, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang X,Y, Lavoie P,M, Furlong J, Fortuno E,S, Hajjar EM, Hawkins NR, Self SG, Wilson CB. Neonatal Innate TLR-Mediated Responses Are Distinct from Those of Adults. J Immunol. 2009;183(11):7150–60. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansen K, Blimkie D, Furlong J, Hajjar A, Rein-Weston A, Crabtree J, Reikie B, Wilson C, Kollmann T. Polychromatic flow cytometric high-throughput assay to analyze the innate immune response to Toll-like receptor stimulation. J Immunol Methods. 2008;336(2):183–92. doi: 10.1016/j.jim.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shulman N, Bellew M, Snelling G, Carter D, Huang Y, Li H, Self SG, McElrath MJ, De Rosa SC. Development of an automated analysis system for data from flow cytometric intracellular cytokine staining assays from clinical vaccine trials. Cytometry A. 2008;73A(9):847–56. doi: 10.1002/cyto.a.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou K,H, Tuncali K, Silverman S,G. Correlation and Simple Linear Regression. Radiology. 2003;227(3):617–628. doi: 10.1148/radiol.2273011499. [DOI] [PubMed] [Google Scholar]
- 7.Bauer S, Muller T, Hamm S. Pattern recognition by Toll-like receptors. Adv Exp Med Biol. 2009;653:15–34. doi: 10.1007/978-1-4419-0901-5_2. [DOI] [PubMed] [Google Scholar]
- 8.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388(4):621–5. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 9.Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19(1):41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 11.Upham JW, Rate A, Rowe J, Kusel M, Sly PD, Holt PG. Dendritic Cell Immaturity during Infancy Restricts the Capacity To Express Vaccine-Specific T-Cell Memory. Infect Immun. 2006;74(2):1106–1112. doi: 10.1128/IAI.74.2.1106-1112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.