Abstract
Preclinical findings suggest that the inhibition of NMDA glutamatergic neurotransmission may have beneficial effects in the treatment of cocaine dependence. We hypothesized that memantine, a low potency, uncompetitive NMDA receptor antagonist, would be safe and effective in the treatment of cocaine dependence, particularly in preventing relapse to cocaine use in abstinent individuals.
Cocaine dependent patients (N =112) were enrolled. The trial began with a 2-week placebo lead-in period during which patients received high-value voucher contingency management to induce abstinence. Participants were then randomized to receive either memantine 20 mg bid (N=39) or placebo (N=42) for 12-weeks in combination with individual relapse-prevention therapy. The randomization was stratified by abstinence status during the lead-in period. The primary outcome was the weekly proportion of days of cocaine use.
There were no significant differences in cocaine use outcome between the groups treated with memantine versus placebo. Thus, the efficacy of memantine 40 mg/d for the treatment of cocaine dependence was not supported. Urine-confirmed abstinence during the lead-in period was achieved by 44% of participants, and was a strong predictor of subsequent cocaine abstinence during the trial. This suggests that this clinical trial design, an intensive behavioral intervention during a lead-in period, resolves cocaine dependent patients into two subgroups, one that rapidly achieves sustained abstinence and may not need a medication, and another that displays persistent cocaine use and would most likely benefit from a medication to help induce abstinence. Targeting the latter subgroup may advance medication development efforts.
Keywords: Cocaine dependence, Pharmacotherapy trials, NMDA receptors, Placebo lead-in, High value contingency reinforcement
1. Introduction
Cocaine use remains a serious public health problem worldwide, particularly in North and South America and in Western and Central Europe (UNODC, 2009). Most individuals seeking treatment for cocaine use disorders receive only psychosocial treatments, but treatment dropout and relapse rates are substantial (Crits-Christoph et al., 1999). Thus, effective pharmacological strategies are needed. However, despite a concerted effort over the past two decades, the great majority of pharmacotherapy trials for cocaine dependence have been negative, and no widely effective and acceptable medication is yet available (Karila et al., 2008; Vocci and Ling, 2005).
To date, most of the pharmacological strategies for cocaine dependence have directly targeted dopamine (DA) and other mono-aminergic systems. An alternative strategy, which may alter the chronic, relapsing course of the disease, is to target the enduring changes in brain function that are produced by exposure to cocaine (Kalivas and Volkow, 2005). One hypothesis holds that cocaine addiction is due to cocaine-induced neuroadaptations in the glutamatergic corticolimbic circuitry (Kalivas and O’Brien, 2008) which increases the reactivity to cocaine-associated cues related to relapse (Everitt and Wolf, 2002). Glutamate-dependent mechanisms have been shown to mediate neuroadaptive changes and behavioral effects resulting from chronic exposure to drugs of abuse, including tolerance, sensitization, physiological dependence, and reinforcement (Gass and Olive, 2008) and are also involved in drug-conditioned behavioral responses that are essential to the maintenance of cocaine-seeking behavior (Cornish et al., 1999; Hayes et al., 2003; Hotsenpiller et al., 2001; Vorel et al., 2001). Thus, modulating glutamatergic neurotransmission could be effective in reversing glutamate-dependent mechanisms that contribute to the maintenance of cocaine dependence.
We therefore conducted a placebo-controlled trial of memantine for treatment of cocaine dependence. Memantine is a glutamatergic receptor antagonist that has been in widespread clinical use for the treatment of Parkinson’s Disease, spasticity, and dementia. As an uncompetitive NMDA receptor antagonist, memantine blocks transmission when this receptor is in the active (open) state, such as in conditions of excess glutamate concentration (Parsons et al., 2008). Compared to other NMDA receptor antagonists such as ketamine or phencyclidine, memantine is a low affinity antagonist with good tolerability at clinically relevant doses (20 to 40 mg/day). Memantine has produced mixed results in animal laboratory models of cocaine self-administration (Blokhina et al., 2005; Hyytia et al., 1999; Newman and Beardsley, 2006). More consistent positive effects have been observed in animal models of relapse where memantine decreased the conditioned reinforcing effects of cocaine-associated stimuli (Bespalov et al., 2000b; Kotlinska and Biala, 2000; Maldonado et al., 2007; Newman and Beardsley, 2006). In human laboratory studies, memantine increased selected subjective and cardiovascular effects of cocaine but had no effect on cocaine use (Collins et al., 2006, 2007; Collins et al., 1998).
Traditionally, clinical trials of medications for psychostimulant dependence have enrolled all participants with the diagnosis, with exclusions mainly for safety reasons. This approach likely selects for a heterogeneous sample. Some clinical trials have suggested that medications have greater efficacy in more homogeneous subsets of patients, such as those differing in the baseline level of use (Bisaga et al., 2006; Elkashef et al., 2008; Grabowski et al., 2001; Shoptaw et al., 2008) or the existence of comorbid conditions (Anderson et al., 2009). We have consistently observed that a substantial subset (30–40%) of cocaine dependent patients initiating clinical trial participation achieve abstinence rapidly, in the first 1–2 weeks, perhaps in response to initiating psychosocial treatment and to the social reinforcers present in the therapeutic milieu of a clinic (Bisaga et al., 2006).
We hypothesize that a rapid response, versus a non-response early in treatment may reflect a fundamental biological difference between patients in the functioning of the brain reward system and the ability to respond to alternative reinforcers, possibly resulting in differential response to medications. We therefore designed this clinical trial with a two-week, single-blind placebo lead-in period, during which participants received intensive, high-value voucher incentives contingent on abstinence. The purpose of this intensive contingency reinforcement behavioral strategy was to separate the sample into subgroups that do, versus those who do not, achieve initial abstinence in response to behavioral treatment. We hypothesized that the effect of memantine in cocaine dependence would be relapse prevention, and explored whether the effect of medication was stronger among those patients who achieved initial abstinence. We also explored the alternative hypothesis that efficacy of memantine was greater among those individuals who did not initially respond to behavioral treatment.
2. Methods
2.1. Participants
Individuals who applied for treatment at Columbia University’s Substance Treatment and Research Service (STARS) outpatient clinic in New York City, USA, were recruited for this study. Clinical screening was conducted by trained masters and doctoral level clinical psychologists, and included the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (SCID Axis I/P version; (First et al., 1995) and a clinical interview assessing substance abuse severity. Medical assessment included history, laboratory tests, electrocardiogram (ECG), a physical examination, and a psychiatric evaluation. Included were men and women 18–60 years old, who met DSM-IV criteria for current cocaine dependence and used cocaine at least 4 days in the previous month, and a urine sample positive for cocaine metabolites. Individuals with major affective or psychotic disorder, or attention-deficit hyperactivity disorder (ADHD), were excluded. Other exclusion criteria included: 1) physical dependence on opiates, sedative-hypnotics or alcohol, or if the principal drug of dependence was not cocaine; 2) ongoing treatment with psychotropic agents or other substance use treatment; 3) unstable physical disorders which might make participation hazardous; 4) pregnancy or lactation.
2.2. Study Procedures
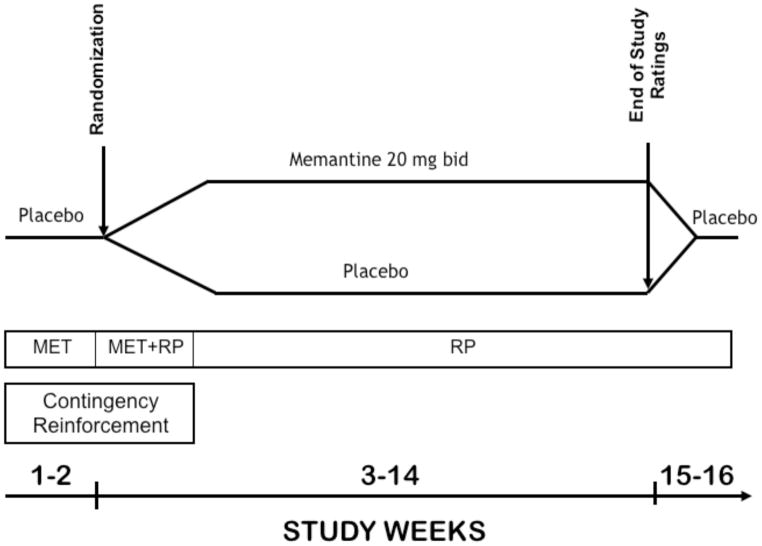
Following study consent, patients entered a 2-week, single-blind placebo lead-in period. To be eligible for randomization, patients were required to attend at least 2 of 4 scheduled therapy sessions and to submit at least 4 of 6 scheduled urine samples during the two weeks of the lead-in period. This requirement was designed to obtain a sufficient number of urine samples to correctly classify patients’ level of cocaine use and to reduce the number of non-compliant patients prior to randomization. The lead-in was followed by a 12-week double-blind trial, in which patients were randomized to receive placebo or memantine. After completing the 12-week double-blind trial, medication was gradually discontinued during a 2-week, single-blind lead-out phase (Figure 1). Patients who did not come for a visit to submit a urine sample for 14 consecutive days during the 12-week medication trial were classified as study drop-outs.
Figure 1.
Diagram of study design. MET: Motivational Enhancement Therapy, RP: Relapse Prevention-Cognitive Behavioral Treatment.
Memantine 10 mg tablets were purchased and encapsulated with 25 mg of riboflavin (added as a urine marker to assess compliance; see below) by the research pharmacy of the New York State Psychiatric Institute. A matching capsule containing folic acid tablets and riboflavin was used as a placebo. Throughout the 16 weeks of the study, participants received 2 medication capsules twice daily. Memantine dose was gradually increased to a target dose of 20 mg twice per day over a 12-day period. After the collection of the end of study ratings during week 12, medication was gradually discontinued over a period of 7 days, with a subsequent 7 days of placebo lead-out. We selected the target dose of memantine 40 mg/day to ensure that brain levels would be sufficient to achieve antagonist effects at the NMDA receptor (see Danysz et al. 1997; Wenk et al. 1995). The target dose is lower than 60 mg/day, a maximum dose safely administered in human laboratory studies (Bisaga et al., 2001; Collins et al., 2007), however this dose is higher than doses used in patients with Alzheimer’s disease (up to 20 mg/d).
Participants were required to attend the clinic three times per week. During each visit, patients gave an observed urine specimen and completed self-reported measures of drug use, craving, and mood. At each visit, a research nurse obtained vital signs, inquired about side-effects, collected unused medication, and dispensed a new supply of medication. All urine specimens were sent to the laboratory for semi-quantitative assessment of cocaine metabolite, with results available the following day. Each urine sample submitted during the trial was observed under UV light for riboflavin fluorescence. Any absence of fluorescence, indicating medication non-adherence, was noted and brought to the attention of the treatment team, and discussed with the patient. Blood was collected for serum medication levels and βHCG in females at weeks 4, 8, and 12. Participants were reimbursed $10 per week during the treatment period and $25 for follow-up visits, for their time taken to complete the research instruments.
2.3. Lead-In Contingency Reinforcement Behavioral Strategy
Starting at the first meeting with the study therapist, all participants were introduced to the voucher incentive schedule and the Contingency Management (CM) therapy. The goal of this therapy was to promote abstinence from cocaine at the study outset in participants who had a capacity to respond to alternative, non-drug reinforcers. Patients had the opportunity to earn high-value vouchers if they decreased or refrained from cocaine use, which was assessed using the concentration of cocaine metabolite (benzoylecgonine: BE) in the urine specimen submitted during the visit. During the first 3 weeks of the trial, a shaping schedule of reinforcement was used such that patients received vouchers for decreases in cocaine use (Preston et al., 2001). Specifically, participants were asked to come thrice weekly, at 48 to 72 hour intervals, and vouchers were given when concentration of BE in the urine specimen was not higher than 50 % of the concentration detected in the specimen collected during the previous visit (Preston et al., 1997). The monetary value of the voucher increased with each consecutive voucher delivered, and was reset to the initial value in the case that a visit was missed or the BE concentration was too high to earn a voucher. The combined value of vouchers that could be earned for the first week was $75, $120 for the second week, and $165 for the third week of consecutively reinforced urine specimens. During the fourth week of the trial, patients received vouchers, valued at $50, only for complete abstinence (urine specimens negative for BE). During each visit participants submitted a urine sample and they were told if the voucher was earned at the subsequent visit, when results of the urine toxicology test become available. Participants could opt to receive a specific voucher during that visit or wait and accumulate value of the vouchers for larger purchases. The maximum value of voucher that was available for earning during the trial was $510 per participant. No vouchers were available after the first four weeks of the trial, when the study medication memantine was expected to have reached a steady state level.
2.4. Behavioral Therapy Platform During the Trial
The 16-week psychosocial intervention included Motivational Enhancement Therapy (MET), and Cognitive Behavioral Treatment - Relapse Prevention (CBT-RP). The combined intervention was designed to address the following problems related to the treatment of cocaine dependence: 1) the continued cocaine use, partly related to ambivalence for abstinence, 2) high attrition, and 3) poor clinical outcome associated with poor medication and treatment compliance. All participants received MET at their first two sessions, where they were introduced to the voucher incentive schedule (see below) and participated in MET to enhance motivation to change (Miller et al., 1992). Starting with the third therapy session, the CBT-RP component was introduced to help the patient identify, garner and practice the most effective strategies to maintain cocaine abstinence and prevent relapse (Carroll, 1998). All therapy sessions were conducted by trained clinical psychologists. Sessions were in an individual format and delivered on a weekly basis. All therapists participated in weekly group supervision to assure adherence to the intervention procedures and prevent therapeutic drift. At study consent, and every two weeks thereafter, patients met with a research psychiatrist, who monitored patients’ progress in treatment, including the review of medication safety and adherence.
2.5. Stratification
Following the completion of the two-week long placebo lead-in, participants were stratified on two parameters and randomized by a research pharmacist to receive memantine or placebo. The first parameter was the level of cocaine use during the two weeks of lead-in (abstinent versus non-abstinent). The abstinent group included patients who provided four or more urine specimens that showed no new episodes of cocaine use. Abstinence at baseline has been found to be a strong predictor of cocaine use during treatment (Alterman et al., 1997; Bisaga et al., 2005b; Kampman et al., 2002) and was an important component of our relapse-prevention hypothesis that memantine would be more effective in patients who had achieved abstinence at the time of medication initiation. The second parameter was the principal route of cocaine administration (smoking versus intranasal), which has also been found to be predictive of treatment outcome (Alterman et al., 1997; Bisaga et al., 2005; Ehrman et al., 2001; Preston et al., 1998).
2.6. Outcome measures
The primary outcome measure was the proportion of days with cocaine use per week. This was determined by combining results of thrice weekly urine toxicology and participants’ self-report of cocaine use days. The urine samples were tested for cocaine metabolite (benzoylecgonine; BE) concentration, and occasions of new drug use were determined using Preston’s rules (Preston et al., 1997). The corroborated self-report and toxicology data were used to compute the number of cocaine use days based on the modified version of the rules proposed by Winhusen (2007). Specifically, if participants reported having used cocaine since their previous visit, the number of days with cocaine use as reported would be used to compute the outcome, regardless of urine toxicology result. However, if the participants reported not having used cocaine on any of the days since their last visit, yet their urine result indicated positive use, then one day of positive use would be considered for that assessment period. If the self-report was negative or missing and urine concentration of BE was more than 50% lower compared to a previous sample, collected no more than two days earlier, than the positive urine result was considered a carry-over from a previous use episode. If urine toxicology and self-report information were both missing, then that particular data point was considered missing and excluded from the analysis. The primary outcome was computed as the number of positive use days over the number of days assessed per week.
The secondary outcome measures were: 1) a binary indicator of sustained abstinence, defined as three consecutive weeks of no cocaine use, obtained by self-report and verified using negative urine toxicology results, at any point of the trial; 2) proportion of days per week with craving for cocaine, based on self-reported number of days in which participants experienced cocaine craving; 3) retention in treatment, defined as the number of study weeks completed beyond the 2-week lead-in (range 1 to 12 weeks); and 4) Clinical Global Improvement (CGI) ratings by a psychiatrist.
2.7. Data Analyses
All analyses were performed on an intent-to-treat basis and all statistical tests were two-tailed at a significance level of 0.05. Retention in treatment was compared between groups using Kaplan-Meier survival curves and the log-rank statistic. The primary outcome, weekly proportion of days with cocaine use over the course of the study, was analyzed using generalized estimating equations (GEE, as implemented by SAS’s PROC-GENMOD). The log link function was used, as the proportion of days with cocaine use was computed as a function of the number of reported days with cocaine use over the number of days assessed, and was ascertained using urine toxicology. Models with different working correlations were specified (independent, exchangeable, autoregressive), and the estimates obtained were consistent across all structures. Robust estimates are reported. We examined the effects of treatment condition, time (weeks), time by treatment interaction, and the stratification variables, level and route, in the model. Both the interaction term and the route of administration were found to be insignificant, and were later excluded from the analysis. The final model included treatment condition, time, and baseline level of use. Model fit was assessed using deviance and Pearson chi-square criteria, as well as examination of residual plots.
Additional exploratory analyses were conducted to examine the impact of alcohol use disorder (AUD) and gender on the primary outcomes, as it has been suggested that memantine may be effective in reducing alcohol drinking (Krupitsky et al., 2007) and both the gender and the presence of comorbind alcohol dependence may alter the effect of medication on cocaine use behavior in pharmacotherapy trials (Anderson et al., 2009; Pettinati et al., 2008). To explore these associations we fit separate models by adding a dichotomous AUD term (or a gender term) and a treatment by AUD term (or a treatment by gender term) as covariates into our final model.
The secondary outcome of abstinence was assessed using logistic regression, and modeled as a function of treatment condition, and baseline level of use. Craving and CGI ratings were assessed using time-course analyses. Specifically, craving was analyzed using GEE, with a log link function, since it was computed as the weekly proportion of days with craving over the number of days assessed. CGI was analyzed using a longitudinal mixed model (SAS’s PROC MIXED).
3. Results
3.1. Sample description
We screened in person 537 individuals for eligibility (See Figure 2 for the CONSORT Flow Diagram). Of those who were screened, 171 individuals refused to participate (133 failed to complete the evaluation, 25 lost interest in treatment, and 13 wanted a different treatment (no medication or inpatient) and 149 individuals were not eligible to participate (21 had low-level of cocaine use, 28 had significant psychiatric comorbidities, 32 had significant medical problems, 21 were taking other psychotropic medications, 4 had language barrier, and 43 were not eligible for other reasons). In addition, 105 individuals had comorbid Major Depressive Disorder or ADHD and entered other studies being conducted at our research clinic at that time. A total of 112 individuals consented to the study and entered the single blind, placebo lead-in phase. Thirty one participants who consented were not eligible for randomization due to non-compliance with study requirements during a 2-week long, single-blind placebo lead-in period. Following the lead-in period, thirty nine individuals were randomized to memantine arm and forty two were randomized to placebo.
Figure 2.
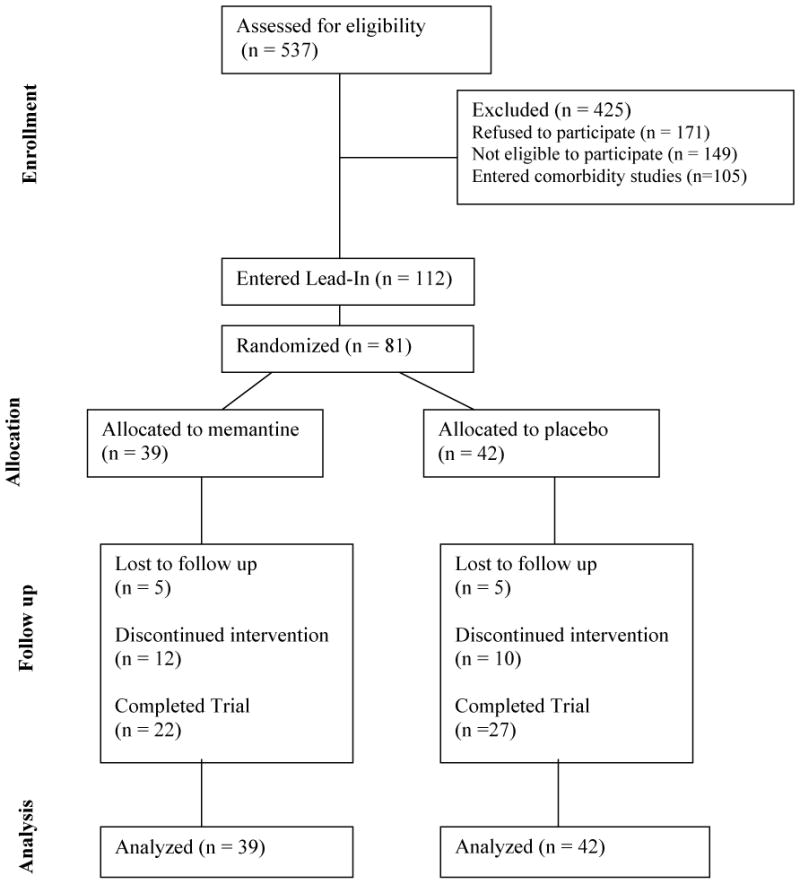
Consort diagram summarizing participant flow
Table 1 describes the demographics of the study sample by treatment group. Participants were on average 40 years of age (SD=7.9), mostly male (79.0%), either White (40.7%) or Black (37.0%), more likely to snort than smoke cocaine (61.7%), and before treatment entry used cocaine approximately 2.6 days per week (SD=2.2), with an average of $60.80 (SD=62.8) per week spent on cocaine. There were no significant differences in baseline characteristics between the treatment groups.
Table 1.
Baseline Demographic and Clinical Characteristics of Randomized Patients*
| Placebo (N=42) | Memantine (N=39) | ||
|---|---|---|---|
| Demographics | Mean (SD) | Mean (SD) | |
| Age | 40 (9) | 40 (7) | |
| n (%) | n (%) | ||
| Male | 33 (79%) | 31 (79%) | |
| Race | Black | 15 (36%) | 15 (38%) |
| Hispanic | 8 (19%) | 7 (18%) | |
| White | 18 (43%) | 15 (38%) | |
| Other | 1 (2%) | 2 (5%) | |
| Education (Post high school) | 22 (52%) | 20 (51%) | |
| Currently employed** | 25 (60%) | 22 (56%) | |
| Pattern of Cocaine Use at Randomization | |||
| Snort*** | 27 (64%) | 23 (59%) | |
| Abstinence at baseline**** | 19 (45%) | 17 (44%) | |
| CGI severity score at baseline | 4.4 (1.0) | 4.6 (1.3) | |
| Days used in last 30 days | 12.5 (10.5) | 11.6 (9.8) | |
| Amount spent per day in last 30 days ($) | 68.9 (72.8) | 61.5 (53.6) | |
| Baseline Drug and Alcohol Use | |||
| Alcohol | |||
| Days used in last 30 days | 9.7 (9.3) | 10.4 (10.0) | |
| Amount spent per day in last 30 days ($) | 3.7 (3.4) | 4.5 (7.0) | |
| Marijuana | |||
| Days used in last 30 days | 4.5 (8.7) | 7.6 (12.3) | |
| Amount spent per day in last 30 days ($) | 1.5 (3.2) | 1.9 (3.3) | |
| Heroin | |||
| Days used in last 30 days | 1.5 (5.9) | 0.1 (0.3) | |
| Amount spent per day in last 30 days ($) | 1.3 (5.2) | 0.2 (0.8) | |
Data obtained during screening for trial prior to initiation of any study procedures. Values in the table are n (%) for categorical variables, or mean (SD) for continuous variables.
Defined as full-time or part-time employment, or student
Patients used cocaine either through the route of smoking or snorting
Abstinence was defined as four or more negative urine samples collected at baseline
3.2. Change in Drug Use during lead-in period
During the first two weeks of the study, participants had an opportunity to earn vouchers in response to a reduction of cocaine use. In response to this intervention, about two fifths (44.4%) of the participants who started the study were able to achieve abstinence, defined as four or more urine specimens that showed no new episodes of cocaine use. The two groups (abstinent vs. non-abstinent) were not statistically different on baseline levels of drug and alcohol use.
3.3. Retention
Of the 81 randomized participants, 90.1% (n=73) completed at least 4 weeks of treatment, and 60.5% (n=49) completed all 12 weeks of the trial (n=22 in memantine group and n=27 in placebo group) (Figure 3). Among the 32 participants who dropped out of the trial, the majority (n=10) were removed due to non-compliance with study procedures. Of the twenty-two other participants, one participant assigned to placebo dropped out because he was no longer interested in therapy. Of participants assigned to memantine, one participant was removed due to psychiatric worsening, and another participant found no therapeutic effect with his participation in the trial. Others left the trial without specifying the reason. There were no differences between those who completed the trial and those who did not in demographic or clinical characteristics. Kaplan Meier survival analysis (Figure 3) and the log-rank test indicated that retention did not differ by treatment group and baseline abstinence (X2(3)=0.89, p=0.83). (Insert Figure 3)
Figure 3.
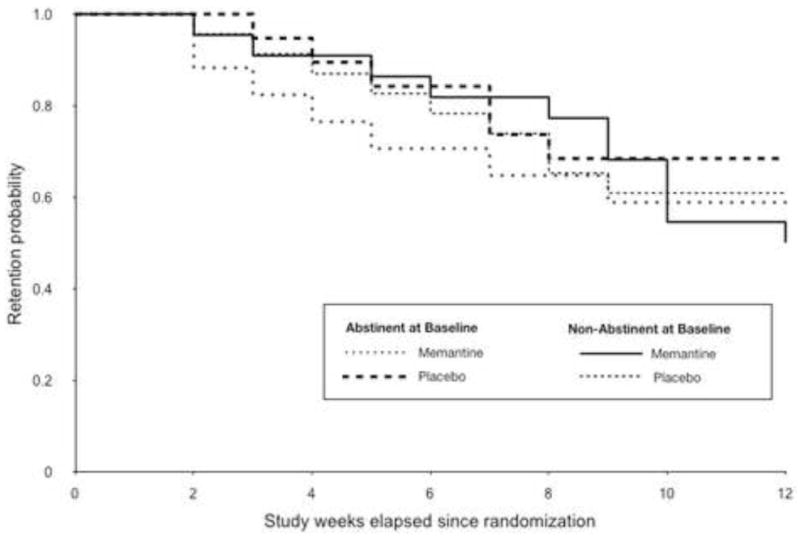
Kaplan-Meier curve of patient retention throughout the 12-week medication trial, by treatment condition and abstinence at baseline
3.4. Primary outcome (Cocaine Use)
Results from the GEE analysis indicated that treatment had no significant effect on the weekly proportion of days with positive cocaine use throughout the trial (β=0.03, SE=0.17, X2(1)=0.03, p=0.87). Nonetheless, time (β=−0.03, SE=0.01, X2(1)=5.52, p=0.02) and baseline abstinence (β=−1.20, SE=0.18, X2(1)=42.6, p<0.0001) were significant predictors of cocaine use. As shown in Figure 4, which presents the GEE model of the mean proportion of days with cocaine use per week throughout the twelve-week trial across treatment and baseline abstinence status, patients on either treatment decreased use over time. Furthermore, individuals who had achieved abstinence at baseline had an overall 70% lower proportion of days with cocaine use compared to those who had not achieved abstinence at baseline. The interaction between time and treatment interaction was explored in the model and found to be not statistically significant, and was excluded in the final model.
Figure 4.
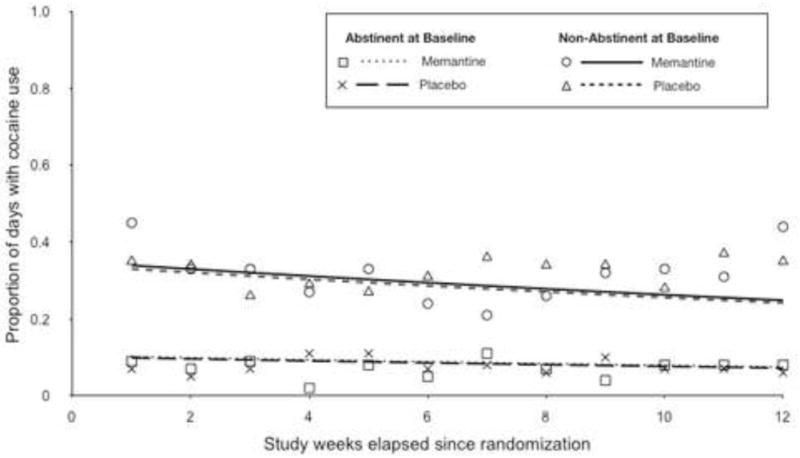
Proportion of days with cocaine use, by treatment and abstinence at baseline. Plot shows observed group means (symbols) and GEE fitted lines.
We have also conducted analyses examining the effects of gender and co-occuring alcohol use disorder on the primary outcome which showed that neither was a predictor of cocaine use. Further, results confirmed that there was no treatment effect of memantine, and that there existed no interaction between treatment and gender and between treatment and alcohol use disorder.
3.5. Secondary Outcome Measures
Sustained Abstinence
Among the thirty-six participants who had achieved abstinence at baseline, thirty participants (83.3%) relapsed into use at some point during the trial. Specifically, 15 of the 19 participants on memantine, and 15 of the 17 on placebo relapsed. The median time of first relapse among participants on memantine was two weeks, whereas that among participants in the placebo groups was three weeks. The difference in the time to relapse between the two treatment groups was not significant (X2(1)=0.97, p=0.32). Nonetheless, despite relapse, the majority of participants who had achieved abstinence at baseline were able to achieve sustained abstinence (i.e. three consecutive weeks of non cocaine use) at some point of the trial. Specifically, 69%of the participants (n=11) in the memantine group and 53% of the participants (n=10) in the placebo group achieved abstinence during the trial. Among participants who had not achieved abstinence at baseline, 19% of the participants (n=4) in the memantine group achieved abstinence, and 13% of the participants (n=3) in the placebo group achieved abstinence. Logistic regression analysis indicated that the probability of abstinence did not differ by treatment group (X2(1)= 0.29, p=0.59), yet as the above proportions indicate, baseline abstinence (X2(1)=6.74, p=0.01) served as a significant predictor of abstinence during the trial. No significant interaction between treatment and baseline abstinence was found (X2(1)= 0.05, p=0.83).
Cocaine Craving
Analyses testing participants’ self-reported weekly proportion of days with craving did not reveal significant changes between the two treatment groups (X2(1)=0.41, p=0.52) or over time (X2(1)=0.01, p=0.91) and baseline abstinence did not predict craving during the trial (X2(1)=0.71, p=0.40). However, a significant effect of craving at baseline (X2(1)=15.2, p<0.0001) suggested that a higher weekly proportion of craving before the trial start predicted a higher craving percentage during the trial.
CGI
Analysis of CGI severity scores indicated that there were no significant treatment (F1,78=0.08, p=0.78) or time effects (F1,635=1.95, p=0.16) on cocaine dependence severity during the trial. However, baseline severity score (F1,78=44.12, p<0.0001) was shown to be a significant predictor of cocaine dependence severity during the trial. Furthermore, a significant baseline by time interaction (F1,635=4.35, p=0.04) suggests that patients with more severe cocaine dependence at baseline experienced a greater decrease in severity over time compared to patients with less severe dependence at baseline, consistent with a floor effect.
3.6. Medication Adherence
The number of used capsules was assessed using a structured calendar-based interview and was quantified as the proportion of used capsules during the 12-week of the treatment trial. Results showed that adherence was not significantly different between the two treatment groups (F1,79=1.92, p=0.17; mean 0.92, SD=0.06 in placebo and mean 0.90, SD=0.08 in memantine groups). Medication adherence was also measured using fluorescent tests for riboflavin. We compared the proportions of fluoresced urine samples collected between the two groups and found that participants on placebo had a significantly higher mean proportion of fluoresced urine samples than the participants on memantine (0.87, SD=0.15 vs. 0.76, SD=0.24, t=2.52, df=79, p=0.02. Furthermore, blood samples for memantine levels were collected at weeks 4, 8 and 12 and at least 1 sample was obtained from 46% of the subjects randomized to memantine. Mean memantine blood level across all three measurements for the group was 109 mcg/ml (SD 80.1, range 2 to 339). These averaged levels are less than half of what would be expected with chronic maintenance on memantine suggesting moderate levels of medication non-adherence in this study.
To assess if medication adherence had any effects on the primary outcome, similar models were analyzed after adjusting for the proportion of used capsules. Results indicated that medication adherence (X2(1)=0.91, p=0.34) had no significant effects on the proportion of days with of cocaine use and no significant difference was observed between the two treatment groups. Interestingly, patients who had higher blood level of memantine had lower proportion of days with cocaine use (X2(1)=3.20, p=0.07) than those with lower blood memantine levels.
3.7. Adverse Effects
The number of patients who reported treatment-emergent adverse events (AEs) are summarized in Table 2. There was no significant difference in the frequency of reported AEs between memantine and placebo group. Nine AEs reported by the memantine-treated patients were considered to be moderate in severity, though only one was considered to be definitely related to memantine. In response, the dose of memantine was reduced in two patients, and medication was discontinued in one patient, while no action was taken in the remaining cases. There were no patients who reported discontinuing the study because of AEs.
Table 2.
Summary of Adverse Events reported in greater than 5% of patients.
| Number (%) of patients |
||
|---|---|---|
| Placebo (N=42) | Memantine (N=39) | |
| Number of Patients with SAE’s* | 2 | 0 |
| Number of Patients who were removed from trial because of AEs* | 0 | 2 |
| Number of Patients with at least 1 TEAE* | 28 (66.7) | 24 (61.5) |
| TEAEs* | ||
| Headache | 7 (16.7) | 5 (12.8) |
| Insomnia | 6 (14.3) | 3 (7.7) |
| Muscle Aches | 5 (11.9) | 2 (5.1) |
| Chills | 3 (7.1) | 0 (0) |
| Fever | 3 (7.1) | 0 (0) |
| Light Headed | 3 (7.1) | 4 (10.3) |
| Anxiety | 2 (4.8) | 2 (5.1) |
| Backache | 2 (4.8) | 3 (7.7) |
| GI Upset | 2 (4.8) | 2 (5.1) |
| Nausea | 2 (4.8) | 2 (5.1) |
| Drowsiness | 1 (2.4) | 3 (7.7) |
| Rash | 1 (2.4) | 2 (5.1) |
| Diarrhea | 0 (0) | 2 (5.1) |
AE, adverse event, SAE, Serious Adverse Event; TEAE, treatment-emergent adverse event.
No significant differences were detected between treatment conditions.
There were three Serious Adverse Events (SAEs) that occurred in this study, one occurred in patient prior to randomization (intestinal blockage), and two occurred in patients randomized to placebo (abdominal pain, finger fracture). Two patients, both randomized to the memantine treatment arm, were removed from the study by investigators due to psychiatric worsening (worsening of depressive symptoms warranting initiation of antidepressant treatment).
Review of laboratory and ECG parameters revealed no clinically remarkable changes from baseline. There were no differences between treatment groups at the end of treatment in terms of weight, blood pressure (SBP and DBP), heart rate, respiration, and oral temperature.
4. Discussion
The results of this randomized, placebo-controlled trial suggest that memantine (40 mg/d) combined with weekly individual therapy, is not broadly effective for the outpatient treatment of cocaine dependence. There were no significant differences between the memantine and placebo treatment groups in the likelihood of cocaine use, rates of achieving abstinence, retention in treatment, CGI ratings, or the level of cocaine craving during the 12 weeks of the trial. Participants who had achieved abstinence during the two-week lead-in had significantly better drug-use outcomes throughout the trial, despite a treatment retention that was no different from that of participants who had not achieved abstinence at baseline. Overall, memantine appeared to be well tolerated in this population, with a frequency of adverse effects comparable to that of placebo, although a dose reduction of memantine was warranted in a few cases.
Our findings stand in contrast to those preclinical studies that showed the effectiveness of NMDA receptor antagonists in preclinical models of cocaine dependence (Bespalov et al., 2000a; Hyytia et al., 1999; Kotlinska and Biala, 2000; Maldonado et al., 2007; Newman and Beardsley, 2006). Nonetheless, unlike preclinical studies, results from this study are consistent with findings obtained in human laboratory with cocaine-dependent volunteers, where memantine did not affect cocaine self-administration or craving (Collins et al., 2006, 2007; Collins et al., 1998).
There are several possible reasons for the observed lack of memantine’s efficacy in cocaine dependent individuals. Based on the results of preclinical studies, we hypothesized that memantine would be more effective in preventing relapse in participants who had achieved initial abstinence rather than in reducing use in active users. However in the present trial, participants who had achieved abstinence during the lead-in remained abstinent throughout the trial and there was little relapse-like behavior for a medication to address. Due to this floor effect, relapse-prevention treatment response was less likely to be seen in the memantine group. Possibly a longer treatment period or a long-term post-treatment follow up might permit the observation of memantine’s effect in preventing relapse. Similarly, lack of a medication effect was observed in participants who had not achieved abstinence during the lead-in period. It was observed that there was gradual decrease in cocaine use over time, but this trend was similar in both the memantine and placebo treatment groups. Participants who did not achieve abstinence early in treatment continued using at the completion of treatment, suggesting that the potential still existed for the medication to have beneficial effects. Perhaps continuing the CM behavioral strategy throughout the 12 weeks of the trial could permit initiation of abstinence in greater number of patients and the emergence of a medication effect, as it has been observed previously with medication enhancing dopaminergic transmission (Schmitz et al., 2008). Inability to detect memantine’s efficacy could also be related to a small sample size, although the repeated quantitative and qualitative measures of drug-use behavior added efficiency to the analyses.
There might be other reasons for the failure of this clinical trial to confirm the results of positive preclinical studies. First, medication that reduces cocaine intake in a homogenous, inbred group of laboratory animals may be effective only in a sub-sample of individuals with cocaine dependence. Such an effect may be difficult to detect in standard clinical trials with heterogeneous patient populations and could only be detected using sample sizes powered for detection of small effect sizes or in a preselected, homogenous patient population. In the case of memantine, perhaps participants who are most likely to benefit from medication are those who were able to achieve substantial abstinence but continue to experience craving and are at risk for relapse. Second, preclinical models of drug addiction in rodents may have excellent construct validity but may not be adequate to predict clinically effective medication, i.e. they lack broad predictive validity (Haney, 2009). Experience with clinical trials of medications for cocaine dependence that showed preclinical efficacy has been generally disappointing as a predictor of any clinical efficacy to date (Vocci and Ling, 2005). This suggests that more preparatory work may be needed, probably including testing using human laboratory in addition to preclinical models, before launching clinical medication efficacy trials.
The target dose of memantine selected for this trial should have been adequate to achieve the hypothesized pharmacological effect. However, the detected serum memantine levels were lower than expected suggesting that many patients were not adherent to medication, in contrast to self-reported high levels of medication taking. Similar underreporting of medication adherence is often encountered in addiction pharmacotherapy trials (Mooney et al., 2004; Schmitz et al., 2008). Reduced levels of medication adherence undermine the power of a study to detect a medication effect. The association observed between higher serum levels of memantine and better drug use outcome is suggestive of an effect of medication that might have been detected, compared to placebo, had there been better overall adherence. However, blood levels and adherence may simply reflect motivation and better outcome in more motivated patients, rather than any specific effect of medication. This suggests that collecting objective measures of adherence, such as serum levels, is important for the interpretation of study findings, and that improved strategies to enhance medication adherence are needed in clinical trials of medications for substance dependence.
In the present study 44% of actively-using individuals become abstinent with the implementation of high-value CM intervention during the first 2 weeks of treatment. This abstinence rate may seem low, but in fact it is consistent with abstinence rates (25–50%) reported at the outset of treatment across studies for this type of behavioral intervention (Higgins et al., 1994; Moeller et al., 2007; Schmitz et al., 2008). Thus, while the behavioral intervention is highly effective compared to control treatments, there is still plenty of room for improvement and for a medication to exert a beneficial effect in combination with behavioral treatment.
A methodological innovation of this trial was the inclusion of a lead-in period with an intensive behavioral intervention prior to randomization. The purpose of this design feature is to resolve the sample into subgroups: an early-responder subgroup with a good prognosis, and a subgroup resistant to behavioral treatment. Such initial abstinence has been shown to be a strong predictor of subsequent abstinence during treatment (Alterman et al., 1997; Bisaga et al., 2005; Ehrman et al., 2001; Kampman et al., 2002; Preston et al., 1998), and as such it is useful as a stratification factor in the randomization and as a covariate in analyses of treatment effects. This design allows exploration of whether a medication is exerting its effect primarily through relapse prevention, in which case medication-placebo differences would be observed mainly among the subgroup that is abstinent at the time or randomization, or through facilitation of abstinence induction, in which case the medication-placebo difference would reside mainly among those still using actively at randomization (Bisaga et al., 2005).
Participants who did not reduce drug use in response to intensive behavioral therapy may arguably be most in need of a medication to address the underlying pathophysiology, perhaps a deficit in brain reward system responsivity (Martinez et al., 2007). Future studies should seek to biologically characterize the subgroup of cocaine dependent patients that fails to rapidly respond to behavioral treatment in an effort to understand the mechanism and to assist with the design of targeted pharmacotherapies.
Finally, this design models a clinical approach in which a medication intervention is used to build upon a behavioral intervention with already established efficacy. A common concern is that a strong platform of behavioral treatment might “overwhelm” and weaken the ability of a placebo-controlled trial to detect a medication effect (Covey et al., 2002). However, sustained abstinence in response to such behavioral interventions is still only 40% to 50% in most trials with a large subgroup of patients that are resistant to even an intensive behavioral treatment. This would be the group that would most benefit from an effective medication. The medication and behavioral treatment may have additive or synergistic effects such that the ability of a trial to detect a medication effect may actually be enhanced by the behavioral platform. Accordingly, several recent trials suggest that monoaminergic medications, including desipramine (Kosten et al., 2003), bupropion (Poling et al., 2006) and l-dopa (Schmitz et al., 2008) may enhance the effectiveness of voucher incentive behavioral therapy.
In summary, the present findings did not support the efficacy of memantine as a treatment for cocaine dependence, despite the strong preclinical support for the beneficial effects of glutamatergic NMDA receptor antagonists. Additional research is warranted to improve the methodology of clinical medication development at the same time as new pharmacological targets and strategies are pursued.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alterman AI, Kampman K, Boardman CR, Cacciola JS, Rutherford MJ, McKay JR, Maany I. A cocaine-positive baseline urine predicts outpatient treatment attrition and failure to attain initial abstinence. Drug Alcohol Depend. 1997;46:79–85. doi: 10.1016/s0376-8716(97)00049-5. [DOI] [PubMed] [Google Scholar]
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Zvartau EE, Beardsley PM, Balster RL. Effects of NMDA receptor antagonists on cocaine-conditioned motor activity in rats. Eur J Pharmacol. 2000a;390:303–311. doi: 10.1016/s0014-2999(99)00927-9. [DOI] [PubMed] [Google Scholar]
- Bespalov AY, Zvartau EE, Balster RL, Beardsley PM. Effects of N-methyl-D-aspartate receptor antagonists on reinstatement of cocaine-seeking behavior by priming injections of cocaine or exposures to cocaine-associated cues in rats. Behav Pharmacol. 2000b;11:37–44. doi: 10.1097/00008877-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Nunes EV. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend. 2006;81:267–274. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Vosburg SK, Nunes EV. Utility of lead-in period in cocaine dependence pharmacotherapy trials. Drug Alcohol Depend. 2005;77:7–11. doi: 10.1016/j.drugalcdep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Comer SD, Ward AS, Popik P, Kleber HD, Fischman MW. The NMDA antagonist memantine attenuates the expression of opioid physical dependence in humans. Psychopharmacology. 2001;157:1–10. doi: 10.1007/s002130100739. [DOI] [PubMed] [Google Scholar]
- Blokhina EA, Kashkin VA, Zvartau EE, Danysz W, Bespalov AY. Effects of nicotinic and NMDA receptor channel blockers on intravenous cocaine and nicotine self-administration in mice. Eur Neuropsychopharmacol. 2005;15:219–225. doi: 10.1016/j.euroneuro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Carroll KM. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. National Institute on Drug Abuse; Rockville, MD: 1998. [Google Scholar]
- Collins ED, Vosburg SK, Ward AS, Haney M, Foltin RW. Memantine increases cardiovascular but not behavioral effects of cocaine in methadone-maintained humans. Pharm Biochem Behav. 2006;83:47–55. doi: 10.1016/j.pbb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Collins ED, Vosburg SK, Ward AS, Haney M, Foltin RW. The effects of acute pretreatment with high-dose memantine on the cardiovascular and behavioral effects of cocaine in humans. Exp Clin Psychopharmacol. 2007;15:228–237. doi: 10.1037/1064-1297.15.3.228. [DOI] [PubMed] [Google Scholar]
- Collins ED, Ward AS, McDowell DM, Foltin RW, Fischman MW. The effects of memantine on the subjective, reinforcing and cardiovascular effects of cocaine in humans. Behav Pharmacol. 1998;9:587–598. doi: 10.1097/00008877-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F, Rivelli S, Stage K. A randomized trial of sertraline as a cessation aid for smokers with a history of major depression. Am J Psychiatry. 2002;159:1731–1737. doi: 10.1176/appi.ajp.159.10.1731. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, Muenz LR, Thase ME, Weiss RD, Gastfriend DR, Woody GE, Barber JP, Butler SF, Daley D, Salloum I, Bishop S, Najavits LM, Lis J, Mercer D, Griffin ML, Moras K, Beck AT. Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Arch Gen Psychiatry. 1999;56:493–502. doi: 10.1001/archpsyc.56.6.493. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Cornish JW. Results of a baseline urine test predict levels of cocaine use during treatment. Drug Alcohol Depend. 2001;62:1–7. doi: 10.1016/s0376-8716(00)00137-x. [DOI] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders - patient edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Haney M. Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addict Biol. 2009;14:9–21. doi: 10.1111/j.1369-1600.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes RJ, Vorel SR, Spector J, Liu X, Gardner EL. Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine-seeking behavior in the rat. Psychopharmacology. 2003;168:75–83. doi: 10.1007/s00213-002-1328-3. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Backstrom P, Liljequist S. Site-specific NMDA receptor antagonists produce differential effects on cocaine self-administration in rats. Eur J Pharmacol. 1999;378:9–16. doi: 10.1016/s0014-2999(99)00446-x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, Rukstalis M, Alterman AI, Pettinati H, Weinrieb RM, O’Brien CP. Cocaine withdrawal severity and urine toxicology results from treatment entry predict outcome in medication trials for cocaine dependence. Addict Behav. 2002;27:251–260. doi: 10.1016/s0306-4603(01)00171-x. [DOI] [PubMed] [Google Scholar]
- Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lepine JP. New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol. 2008;11:425–438. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- Kosten T, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, Stine S, Gonzalez G, Gonsai K. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Biala G. Memantine and ACPC affect conditioned place preference induced by cocaine in rats. Pol J Pharmacol. 2000;52:179–185. [PubMed] [Google Scholar]
- Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, Tsoy M, Bespalov A, Slavina TY, Grinenko AA, Petrakis IL, Pittman B, Gueorguieva R, Zvartau EE, Krystal JH. Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am J Psychiatry. 2007;164:519–523. doi: 10.1176/ajp.2007.164.3.519. [DOI] [PubMed] [Google Scholar]
- Maldonado C, Rodriguez-Arias M, Castillo A, Aguilar MA, Minarro J. Effect of memantine and CNQX in the acquisition, expression and reinstatement of cocaine-induced conditioned place preference. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:932–939. doi: 10.1016/j.pnpbp.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Steinberg JL, Green CM, Reist C, Lai LY, Swann AC, Grabowski J. Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse. 2007;33:367–378. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Sayre SL, Green C, Rhoades H, Schmitz JM. Comparing measures of medication taking in a pharmacotherapy trial for cocaine dependence. Addict Dis Treat. 2004;3:165–173. [Google Scholar]
- Newman JL, Beardsley PM. Effects of memantine, haloperidol, and cocaine on primary and conditioned reinforcement associated with cocaine in rhesus monkeys. Psychopharmacology. 2006;185:142–149. doi: 10.1007/s00213-005-0282-2. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Rammes G, Danysz W. Pharmacodynamics of Memantine: An Update. Current Neuropharmacol. 2008;6:55–78. doi: 10.2174/157015908783769671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, Kampman KM, Lynch KG, Suh JJ, Dackis CA, Oslin DW, O’Brien CP. Gender differences with high-dose naltrexone in patients with co-occurring cocaine and alcohol dependence. J Subst Abuse Treat. 2008;34:378–390. doi: 10.1016/j.jsat.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, Martell B, Kosten TR. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63:219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Higgins ST, Brooner RK, Montoya I, Schuster CR, Cone EJ. Cocaine use early in treatment predicts outcome in a behavioral treatment program. J Consult Clin Psychol. 1998;66:691–696. doi: 10.1037//0022-006x.66.4.691. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Schuster CR, Cone EJ. Assessment of cocaine use with quantitative urinalysis and estimation of new uses. Addiction. 1997;92:717–727. [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Wong CJ, Epstein DH. Shaping cocaine abstinence by successive approximation. J Consult Clin Psychol. 2001;69:643–654. doi: 10.1037//0022-006x.69.4.643. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Mooney ME, Moeller FG, Stotts AL, Green C, Grabowski J. Levodopa pharmacotherapy for cocaine dependence: choosing the optimal behavioral therapy platform. Drug Alcohol Depend. 2008;94:142–150. doi: 10.1016/j.drugalcdep.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, De La Garza R, Newton T, Ling W. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC. World Drug Report 2009. United Nations; New York: 2009. United Nations Office on Drugs and Crime. [Google Scholar]
- Vocci F, Ling W. Medications development: successes and challenges. Pharmacol Therapeutics. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Somoza E, Sarid-Segal O, Goldsmith RJ, Harrer JM, Coleman FS, Kahn R, Osman S, Mezinskis J, Li SH, Lewis D, Afshar M, Ciraulo DA, Horn P, Montgomery MA, Elkashef A. A double-blind, placebo-controlled trial of reserpine for the treatment of cocaine dependence. Drug Alcohol Depend. 2007;91:205–212. doi: 10.1016/j.drugalcdep.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]