Abstract
Management of patients with hepatic failure and liver-based metabolic disorders is complex and expensive. Hepatic failure results in impaired coagulation, altered consciousness and cerebral function, a heightened risk of multiple organ system failure, and sepsis [1]. Such manifold problems are only treatable today and for the foreseeable future by transplantation. In fact, whole or auxiliary partial liver transplantation is often the only available treatment option for severe, even if transient, hepatic failure. Patients with life-threatening liver-based metabolic disorders similarly require organ transplantation even though their metabolic diseases are typically the result of a single enzyme deficiency, and the liver otherwise functions normally. For all of the benefits it may confer, liver transplantation is not an ideal therapy, even for severe hepatic failure. More than 17,000 patients currently await liver transplantation in the United States, a number that seriously underestimates the number of patients that need treatment [2], as it has been estimated that more than a million patients could benefit from transplantation [3]. Unfortunately, use of whole liver transplantation to treat these disorders is limited by a severe shortage of donors and by the risks to the recipient associated with major surgery [4].
INTRODUCTION
The challenge of treating life-threatening liver disease by transplantation of isolated hepatocytes
Management of patients with hepatic failure and liver-based metabolic disorders is complex and expensive. Hepatic failure results in impaired coagulation, altered consciousness and cerebral function, a heightened risk of multiple organ system failure, and sepsis [1]. Such manifold problems are only treatable today and for the foreseeable future by transplantation. In fact, whole or auxiliary partial liver transplantation is often the only available treatment option for severe, even if transient, hepatic failure. Patients with life-threatening liver-based metabolic disorders similarly require organ transplantation even though their metabolic diseases are typically the result of a single enzyme deficiency, and the liver otherwise functions normally. For all of the benefits it may confer, liver transplantation is not an ideal therapy, even for severe hepatic failure. More than 17,000 patients currently await liver transplantation in the United States, a number that seriously underestimates the number of patients that need treatment [2], as it has been estimated that more than a million patients could benefit from transplantation [3]. Unfortunately, use of whole liver transplantation to treat these disorders is limited by a severe shortage of donors and by the risks to the recipient associated with major surgery [4].
Hepatocyte transplantation holds great promise as an alternative to organ transplantation for the treatment of liver diseases, and numerous studies in rodents indicate that transplants consisting of isolated liver cells can correct various metabolic deficiencies of the liver and can reverse hepatic failure [5–17]. The transplant procedure, which involves injection of isolated hepatocytes into the liver or spleen, is far less invasive than transplantation of the whole liver and could be performed on severely ill patients with relatively low risk. In the presence of normal host liver architecture, the transplanted cells integrate into the host liver, providing considerable restorative potential [18]. Because the native liver is not removed, the transplanted hepatocytes need only improve some of the functions of the failing liver and need not replace all hepatic functions.
Although clinical trials of hepatocyte transplantation have demonstrated the long-term safety of the procedure, only partial correction of metabolic disorders has been achieved, and the degree to which donor hepatocytes have restored failing livers has not generally been adequate to circumvent the need for organ replacement [19–27]. While hepatocyte transplantation can be employed safely in humans, its applicability remains limited by a number of issues, some of which include: (1) a critical shortage of donor organs and hepatocytes for transplantation, (2) relatively poor initial and long-term hepatocyte engraftment that limits successful treatment of chronic diseases, such as liver-based metabolic deficiencies, and (3) the lack of a clinically relevant animal model of acute liver failure that could be used to accurately predict the efficacy of new therapies in treating this process.
The challenge of treating acute liver failure
Several thousand cases of acute liver failure occur each year in the United States. Approximately 40% of patients with advanced symptoms survive the acute episode with only medical management. In these cases, regeneration of the native liver makes orthotopic liver transplantation unnecessary. Unfortunately, there is no effective means to distinguish between patients who will survive without transplantation from those who will not. Support options exist for patients with acute renal or cardiovascular insufficiency, obviating the need for transplantation. Unfortunately no effective support exists for patients with liver failure.
Several factors have hindered the development of innovative therapies for treating patients with fulminant liver failure. Despite the availability of numerous surgical and pharmacologic-based animal models of acute hepatic failure, none recapitulate clinical hepatic failure to the point where the efficacy of cell transplantation or liver assist devices could be predicted in patients [28,29]. The severity of liver dysfunction requires that the transplanted hepatocytes function immediately, but the lack of a clinically relevant disease model means that the number of cells that would need to engraft and function immediately to reverse hepatic failure remains essentially unknown. Since clinical experience with auxiliary orthotopic liver transplant for acute hepatic failure indicates that native liver recovery may take 6 months to a year, if ever [30], liver recovery in any representative animal model needs to take at least a week, if not more. The highly variable natural history and the numerous etiologies of acute liver failure have also made assessment of the success of novel interventions in this patient population difficult. Thus, while extracorporeal liver assist devices and hepatocyte transplantation have been applied to the treatment of acute hepatic failure, neither approach has reliably resulted in reversal of hepatic failure to the point where organ transplantation can be avoided [31].
For hepatocyte transplantation, interpretation of its potential has been further confounded by the wide range in the numbers and types of cells transplanted, the sites where cells have been infused, and spontaneous recovery rates approaching 40% [21,27,32–39] It is possible that enrollment of patients in a multi-institutional standardized treatment protocol would help delineate the potential that transplantation of hepatocytes might have for the treatment of fulminant liver failure, and would help identify any hurdles to its successful application. Such a standardized protocol could focus on trying to infuse 1–2 × 108 viable hepatocytes per kilogram through the portal vein for engraftment in the liver. Even this may be difficult to accomplish in a multi-center trial, as the coagulopathy associated with acute liver failure makes access to the portal circulation challenging. Fresh or cryopreserved hepatocytes could be considered for such a trial, but because cryopreserved cells have been shown to engraft less well than fresh hepatocytes (discussed later in this review), an aliquot of each population would need to be characterized in an identical fashion, for comparison, for in vitro activity, plating efficiency, and engraftment potential in immune deficient hosts.
The challenge of treating chronic liver disease resulting from cirrhosis
Hepatocyte transplantation for end-stage liver disease is even more problematic. Abnormalities in hepatic architecture contribute to decrease in liver function, and transplantation of hepatocytes into the portal vein of a cirrhotic liver can generate severe portal hypertension. Furthermore, it is not clear that donor hepatocytes can function for any sustained period of time to improve hepatic failure in the abnormal cirrhotic environment [24,40]. Animal studies suggest that transplantation into the spleens of rats with decompensated liver cirrhosis can improve liver function, and prolong survival [41,42]. Unfortunately, transplanted hepatocytes provided only transient function. Hepatocyte transplantation in humans with end-stage cirrhosis has not resulted in even this level of success, but only anecdotal improvement in some parameters of liver function [24,27,40,43,44]. One explanation may be that hepatocytes were infused through the splenic artery in clinical studies, rather than by the direct splenic puncture approach used in the laboratory. The route of hepatocyte delivery into the spleen has been shown to dramatically influence hepatocyte engraftment and function [45]. Treatment of chronic liver failure might benefit in the future from a new technology called organ de-cellularization, where the cells from a donor organ are removed, leaving intact the complex mixture of structural and functional proteins that constitute the ECM. A de-cellularized human or animal liver could serve as a biologic, architecturally normal scaffold for transplanted cells [46,47]. The scaffold, re-populated with donor hepatocytes and non parenchymal cells, might then be vascularized through the portal circulation as an engineered internal auxiliary liver graft [48]. Since hepatocyte transplantation in chronic liver disease will leave the native cirrhotic liver in place, even if successful at improving liver function, it will still leave unresolved the management of coexisting portal hypertension and the risk of developing hepatocellular carcinoma in the native liver.
The challenge of hepatocyte engraftment and treatment of liver-based metabolic deficiencies
In the long-term, transplanted hepatocytes appear to survive poorly in naïve normal and in immune deficient hosts [20,49,50]. They do, however, survive well in hosts with some forms of liver disease [51–53], and when native liver cell expansion is inhibited by exogenous interventions [54,55]. These observations suggest that some homeostatic mechanism controls the number of surviving donor hepatocytes over time. Graft survival, thus, could be limited by a host cell survival advantage over donor hepatocytes. This situation would be similar to that seen in allogeneic bone marrow transplantation, where the host must undergo a preparative regimen to create an environment conducive to long-term engraftment. Preparative irradiation induces apoptosis of host bone marrow cells and makes room for donor cell engraftment [56], allowing macrochimerism to take place following infusion of donor hematopoietic stem cells. Therefore, the use of hepatocytes for the treatment of liver-based metabolic disease may inevitably fail unless conditions can be established that will allow the enduring survival of hepatocyte transplants, as observed in some forms of liver damage.
Liver-directed radiation has been shown to facilitate repopulation of the native liver by transplanted hepatocytes when it is combined with a hepatic proliferation stimulus [57]. In fact, it has been shown that providing only the hepatic proliferation stimulus results in mild enhancement of hepatocyte engraftment for up to 16 weeks in non-human primates [58]. This is especially important since the number of donor cells that can be safely transplanted into the liver at any one time via the portal vein is small, usually less than 1% of the liver mass. Transplantation of a larger cell mass leads to either severe portal hypertension or translocation of cells out of the liver into the systemic circulation, leading to embolization of cells into the lungs [7,32]. Liver-directed radiation-based preparative regimens inhibit host hepatocyte proliferation and induce post-mitotic hepatocyte death, making “room” for donor hepatocytes to preferentially proliferate and repopulate the irradiated host liver [57,59]. This strategy has been employed to completely correct rodent models of hereditary metabolic deficiencies of the liver corresponding to Crigler-Najjar syndrome and primary hyperoxaluria [60,61]. Unfortunately, donor hepatocyte engraftment, survival, and repopulation studies greater than two years cannot be achieved in rodents, and pre-clinical studies in large animals would be helpful to confirm the safety and efficacy of such a strategy. For diseases that may require fairly limited replacement of the host liver by transplanted donor hepatocytes, reversible partial portal vein embolization may result in adequate donor cell function without the additional risks associated with conditioning the recipient liver with irradiation, although the long-term efficacy of this strategy has not yet been demonstrated.
The risk of modest doses of liver irradiation in infants using the above strategy should be low based on the experience in treating infants with symptomatic liver hemangiomas, and long-term follow-up studies in Wilm’s tumor patients. From the 1950s to 1980s, several reports were published concerning infants (approximately 20) treated with radiation therapy for symptomatic liver hemangiomas that demonstrated that radiation could be safely administered from a single dose of 7 Gy to fractionated doses of up to 50 Gy to portions of the liver. The age of these patients ranged from 1 day to 1 year. Thus, one-third of the normal liver volume may tolerate radiation doses as high as 80–100 Gy without compromising normal liver function. Furthermore, radiation-induced secondary liver cancer has not generally been reported in patients that receive radiation therapy involving external beam treatment [62–66]. In the National Wilm’s Tumor Study, where 2438 patients were followed over 14,381 person-years, only four cases of hepatocellular carcinoma were reported in long-term follow-up, and this was only in patients who received radiation doses ≥35 Gy to the right lobe of the liver. Three out of four of those patients also received chemotherapy, which could have contributed to the second malignancies.
In Gunn rats, an animal model for the hyperbilirubinemia associated with human Crigler-Najjar syndrome type I, pre-conditioning with focused lobar irradiation to as little as 35% of the liver mass prior to allogeneic hepatocyte transplantation results in complete correction of the liver-based metabolic disorder [67]. Thus, for clinical application, it should be possible to radiate a portion of the liver in a range lower than the threshold to induce significant liver injury in order to augment engraftment and replacement of the host liver with donor hepatocytes. Since liver-directed radiation therapy can be safely administered in the clinic using 3-D conformal and intensity-modulated radiation therapy (IMRT) techniques, and can be easily adapted to selectively irradiate part of the liver or a liver lobe without collateral injury to surrounding structures, it should be possible to design safe and clinically effective strategies for irradiating the host liver, and engrafting and expanding donor hepatocytes there.
The shortage of human donors
A major limitation to the clinical application of hepatocyte transplantation has been the lack of an abundant source of human hepatocytes. Hepatocytes are primarily obtained from livers rejected for orthotopic liver transplantation, and unused segments of donor livers used for pediatric recipients [68]. But, these sources do not begin to approach the potential numbers needed to treat all patients that might potentially benefit from hepatocyte transplantation.
One exciting new source of human hepatocytes may be livers from non-heart-beating donors. A recent study documented that these livers, of dubious quality for whole liver transplantation, can generate hepatocytes with acceptable viability and quality [69]. The primary limitation to utilizing these whole organs is the risk of long-term biliary and vascular complications, issues that are of little consequence when only hepatocyte yield and viability are critical.
The impact of the scarcity of livers might be alleviated, to some extent, by cryopreservation of isolated cells, allowing harvest and use of hepatocytes in the absence of an immediately available recipient. To this point, cryopreserved hepatocytes have not proven reliably capable of engrafting and functioning. Some children with urea cycle disorders have been treated with some possible success using cryopreserved hepatocytes [70]; however, the quality of lots of cryopreserved cells and their ability to engraft after thawing has not been shown to be consistent by others [71–76].
Hepatocytes might also be obtained by expansion and differentiation of stem cells. While this possibility has generated enthusiasm, it remains some distance from application. Embryonic stem cells and induced pluripotent stem cells can be coaxed to exhibit some functions of hepatocytes by sequential culture in transcription and growth factors, and sorting to enrich for cells with hepatocyte-specific characteristics [77,78]. This is difficult to accomplish in large numbers and there is still a significant risk of contamination of the enriched population with cells having the potential to form tumors.
The ideal source of stem cells would be derived from the subject to be treated. Such stem cells, referred to as induced pluripotent stem cells (iPS cells), have been created by transducing skin cells, or any of a number of other differentiated cell sources, with transcription factors that transform them into cells that have characteristics and differentiation potential nearly identical to human embryonic stem cells (ES cells) [79,80]. While there is much enthusiasm for the potential use of stem cell-derived hepatocytes, generation of a sufficient mass of functional hepatocytes for treatment of liver failure from autologous cells derived from iPS cells would require a period of weeks for expansion, differentiation, selection, and testing to exclude contamination by tumorogenic precursors, far too long to address the problem of acute hepatic failure. In addition, autologous hepatocytes would require genetic manipulation to treat a metabolic disease and such manipulation might result in changes that could increase cancer risk. Thus, numerous hurdles and unresolved risks make this source of hepatocytes unlikely to be useful for clinical transplantation in the near future.
Finally, xenotransplantation of hepatocytes could address many of the challenges of treating liver disorders. It is not limited by the availability of donors, could be performed repeatedly if needed, and may be more effective than allotransplantation for the treatment of viral hepatitis, since xenogeneic hepatocytes do not appear susceptible to infection by human hepatitis viruses [81,82]. Hepatocyte xenotransplantation has been performed with some success in small animals with hypercholesterolemia [83]. In addition, porcine hepatocytes have been shown to secrete albumin for periods of months in naïve non-human primates [49], where the enduring survival and function of the grafts was achieved using a “conventional” immune suppression regimen, and did not require the use of donor pigs genetically engineered with disrupted synthesis of Galα1-3Gal, or expressing proteins that inhibit activation of complement by bound antibodies [84–90]. Importantly, rats transplanted with hepatocyte xenografts had improved indices of coagulation, less encephalopathy, and survived longer than cirrhotic rats that did not receive hepatocyte xenografts [42], and the porcine hepatocytes engrafted and corrected liver failure for nearly two months without the need for immune suppression. The hepatocyte xenografts caused the rodent recipients in liver failure to be sensitized, but the immune response did not damage already engrafted cells. Thus, the need for immune suppression following hepatocyte transplantation in liver failure could be extremely low. Development of a hepatocyte xenotransplantation program in patients would need to be initiated with caution, as the possibility of transferring an infection across species from the donor animal to man could result in a significant public health concern.
Unfortunately, in the absence of clinically relevant models for human liver disease in non-human primates [91,92], it has so far not been possible to predict the extent to which a hepatocyte xenograft would restore hepatic function in humans. Baboons transplanted with livers derived from transgenic pigs expressing the human complement decay accelerating factor supported the recipient’s life for eight days, and clotting parameters reached nearly normal levels within two days of transplantation [93]. Thus, since transplantation of isolated hepatocytes into the liver is much less invasive than whole liver transplantation and the immunologic barrier appears lower, hepatocyte xenotransplantation could become the preferred treatment for conditions in which some liver function persists or can recover, such as in fulminant liver failure, when immediate availability of donor hepatocytes could be a decisive factor in the patient’s outcome.
SUMMARY
Treatment of patients with liver disease by hepatocyte transplantation has expanded dramatically over the last decade, especially for treatment of patients with liver-based metabolic disorders. While much progress has been made, full realization of its potential has not been reached. Treatment of acute liver failure has been hampered by a number of factors, but the efficacy of hepatocyte transplantation in treating this entity could be better determined through a multi-institutional trial using a uniform and standardized treatment strategy. The barriers to treating chronic liver failure resulting from cirrhosis are more extensive. Novel strategies are being developed to safely precondition patients with liver-directed radiation therapy in order to enhance donor hepatocyte survival, long-term engraftment and improve treatment of patients with life-threatening liver-based genetic deficiencies. This work could soon be translated to the clinic. Once hepatocyte transplantation has been shown to effectively replace organ transplantation for a portion of patients with liver failure and life-threatening liver metabolic diseases, it is likely that multiple novel sources of donor hepatocytes will be developed, making cell therapy available and effective for a broad population of patients with liver disorders.
Box 1. Key Messages.
HEPATOCYTE TRANSPLANTATION FOR HEPATIC FAILURE
Acute Fulminant Liver Failure
Effective as a bridge to organ transplantation
Barriers:
Critical shortage of hepatocytes for transplantation
Large animal models of acute hepatic failure that mimic human disease in man lacking
The number of hepatocytes needed to treat fulminant liver failure unknown
Risk of bleeding is significant with percutaneous access to the portal vein for cell infusion
Workable extrahepatic site for clinical hepatocyte transplantation not yet identified
Possible areas for progress:
In the absence of clinically relevant large animal models of acute hepatic failure, multi-center studies needed to accumulate experience in treating this process
Chronic Liver Failure from Cirrhosis
Clinically only anecdotal improvement in some liver functions reported
Barriers:
Animal studies indicate hepatocyte transplantation may provide only short-term benefit
Workable extrahepatic site for clinical hepatocyte transplantation not yet identified
Fibrosis in the space of Disse limits engraftment of hepatocytes into the liver plates
Possible areas for progress:
Additional laboratory research needed
Box 2. Key Messages.
HEPATOCYTE TRANSPLANTATION FOR METABOLIC LIVER DISEASE
Partial correction of a number of metabolic liver disorders has been reported in patients
Barriers:
Evidence of long-term engraftment and function by transplanted cells lacking
Possible areas for progress:
In animals, complete correction of metabolic liver disease by hepatocyte transplant has been reported following radiation of the liver combined with a hepatic proliferation signal. Translation of this strategy to patients requires investigation.
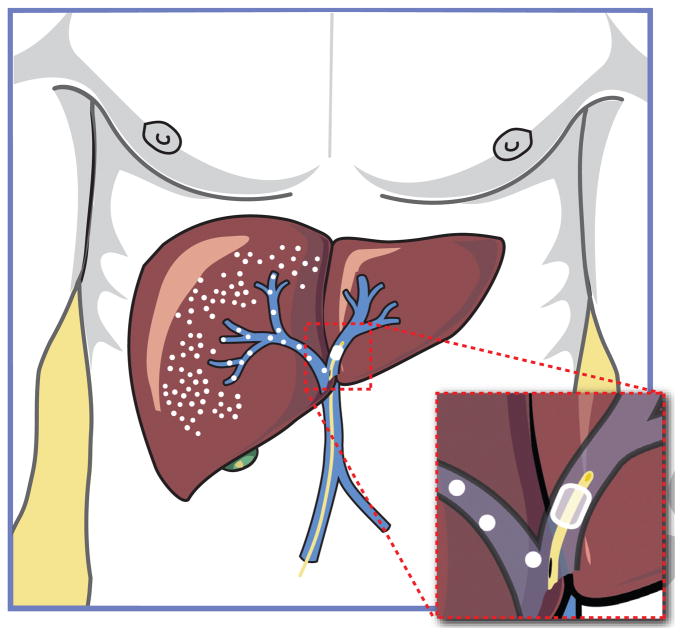
Fig. 1. Partial portal vein occlusion.
Laboratory studies indicate that transient occlusion of the portal circulation can enhance donor hepatocyte engraftment by providing a proliferation signal to donor cells, with or without conditioning of the recipient liver by partial irradiation. Transient occlusion of the left portal venous system is shown, allowing transplantation into the right lobe of the liver. A 6 Fr compliant balloon is positioned in the left portal vein just beyond the bifurcation. It is inflated so as to occlude the left portal vein but allow transportal infusion of cells into the right lobe of the liver through the side port of the endovascular sheath.
Acknowledgments
This work was supported by NIH grants HL52297 (JLP), DK048794, and AI049472 (IJF).
Abbreviations
- ECM
extra-cellular matrix
- ES cells
embryonic stem cells
- IMRT
intensity-modulated radiation therapy
- iPS cells
induced pluripotent stem cells
Footnotes
Financial disclosure: The authors declare no funding from industries or conflict of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42(Suppl 1):S100–107. doi: 10.1016/j.jhep.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Lopez PM, Martin P. Update on liver transplantation: indications, organ allocation, and long-term care. Mt Sinai J Med. 2006 Dec;73(8):1056–1066. [PubMed] [Google Scholar]
- 3.Hagmann M. Biomedicine. New genetic tricks to rejuvenate ailing livers. Science. 2000 Feb 18;287(5456):1185–1187. doi: 10.1126/science.287.5456.1185. [DOI] [PubMed] [Google Scholar]
- 4.Merion RM. When is a patient too well and when is a patient too sick for a liver transplant? Liver Transpl. 2004 Oct;10(10 Suppl 2):S69–73. doi: 10.1002/lt.20265. [DOI] [PubMed] [Google Scholar]
- 5.De Vree JM, Ottenhoff R, Bosma PJ, Smith AJ, Aten J, Oude Elferink RP. Correction of liver disease by hepatocyte transplantation in a mouse model of progressive familial intrahepatic cholestasis. Gastroenterology. 2000 Dec;119(6):1720–1730. doi: 10.1053/gast.2000.20222. [DOI] [PubMed] [Google Scholar]
- 6.Demetriou AA, Whiting JF, Feldman D, Levenson SM, Chowdhury NR, Moscioni AD, et al. Replacement of liver function in rats by transplantation of microcarrier-attached hepatocytes. Science. 1986 Sep 12;233(4769):1190–1192. doi: 10.1126/science.2426782. [DOI] [PubMed] [Google Scholar]
- 7.Fox IJ, Roy-Chowdhury J. Hepatocyte transplantation. J Hepatol. 2004 Jun;40(6):878–886. doi: 10.1016/j.jhep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Groth CG, Arborgh B, Bjorken C, Sundberg B, Lundgren G. Correction of hyperbilirubinemia in the glucuronyltransferase-deficient rat by intraportal hepatocyte transplantation. Transplant Proc. 1977 Mar;9(1):313–316. [PubMed] [Google Scholar]
- 9.Kobayashi N, Fujiwara T, Westerman KA, Inoue Y, Sakaguchi M, Noguchi H, et al. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science. 2000 Feb 18;287(5456):1258–1262. doi: 10.1126/science.287.5456.1258. [DOI] [PubMed] [Google Scholar]
- 10.Kocken JM, Borel Rinkes IH, Bijma AM, de Roos WK, Bouwman E, Terpstra OT, et al. Correction of an inborn error of metabolism by intraportal hepatocyte transplantation in a dog model. Transplantation. 1996 Aug 15;62(3):358–364. doi: 10.1097/00007890-199608150-00010. [DOI] [PubMed] [Google Scholar]
- 11.Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, et al. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976 May 28;192(4242):892–894. doi: 10.1126/science.818706. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro J, Nordlinger B, Ballet F, Cynober L, Coudray-Lucas C, Baudrimont M, et al. Intrasplenic hepatocellular transplantation corrects hepatic encephalopathy in portacaval-shunted rats. Hepatology. 1992 Jan;15(1):12–18. doi: 10.1002/hep.1840150104. [DOI] [PubMed] [Google Scholar]
- 13.Selden C, Calnan D, Morgan N, Wilcox H, Carr E, Hodgson HJ. Histidinemia in mice: a metabolic defect treated using a novel approach to hepatocellular transplantation. Hepatology. 1995 May;21(5):1405–1412. [PubMed] [Google Scholar]
- 14.Sommer BG, Sutherland DE, Simmons RL, Najarian JS. Hepatocellular transplantation for experimental ischemic acute liver failure in dogs. J Surg Res. 1980 Oct;29(4):319–325. doi: 10.1016/0022-4804(80)90064-5. [DOI] [PubMed] [Google Scholar]
- 15.Sutherland DE, Numata M, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation in acute liver failure. Surgery. 1977 Jul;82(1):124–132. [PubMed] [Google Scholar]
- 16.Wiederkehr JC, Kondos GT, Pollak R. Hepatocyte transplantation for the low-density lipoprotein receptor-deficient state. A study in the Watanabe rabbit. Transplantation. 1990 Sep;50(3):466–471. doi: 10.1097/00007890-199009000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida Y, Tokusashi Y, Lee GH, Ogawa K. Intrahepatic transplantation of normal hepatocytes prevents Wilson’s disease in Long-Evans cinnamon rats. Gastroenterology. 1996 Dec;111(6):1654–1660. doi: 10.1016/s0016-5085(96)70029-x. [DOI] [PubMed] [Google Scholar]
- 18.Ponder KP, Gupta S, Leland F, Darlington G, Finegold M, DeMayo J, et al. Mouse hepatocytes migrate to liver parenchyma and function indefinitely after intrasplenic transplantation. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1217–1221. doi: 10.1073/pnas.88.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhawan A, Mitry RR, Hughes RD, Lehec S, Terry C, Bansal S, et al. Hepatocyte transplantation for inherited factor VII deficiency. Transplantation. 2004 Dec 27;78(12):1812–1814. doi: 10.1097/01.tp.0000146386.77076.47. [DOI] [PubMed] [Google Scholar]
- 20.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998 May 14;338(20):1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 21.Habibullah CM, Syed IH, Qamar A, Taher-Uz Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation. 1994 Oct 27;58(8):951–952. doi: 10.1097/00007890-199410270-00016. [DOI] [PubMed] [Google Scholar]
- 22.Horslen SP, Fox IJ. Hepatocyte transplantation. Transplantation. 2004 May 27;77(10):1481–1486. doi: 10.1097/01.tp.0000113809.53415.c2. [DOI] [PubMed] [Google Scholar]
- 23.Horslen SP, McCowan TC, Goertzen TC, Warkentin PI, Cai HB, Strom SC, et al. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics. 2003 Jun;111(6 Pt 1):1262–1267. doi: 10.1542/peds.111.6.1262. [DOI] [PubMed] [Google Scholar]
- 24.Mito M, Kusano M, Kawaura Y. Hepatocyte transplantation in man. Transplant Proc. 1992 Dec;24(6):3052–3053. [PubMed] [Google Scholar]
- 25.Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, et al. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002 Jan 26;359(9303):317–318. doi: 10.1016/S0140-6736(02)07529-3. [DOI] [PubMed] [Google Scholar]
- 26.Sokal EM, Smets F, Bourgois A, Van Maldergem L, Buts JP, Reding R, et al. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003 Aug 27;76(4):735–738. doi: 10.1097/01.TP.0000077420.81365.53. [DOI] [PubMed] [Google Scholar]
- 27.Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis. 1999;19(1):39–48. doi: 10.1055/s-2007-1007096. [DOI] [PubMed] [Google Scholar]
- 28.Newsome PN, Plevris JN, Nelson LJ, Hayes PC. Animal models of fulminant hepatic failure: a critical evaluation. Liver Transpl. 2000 Jan;6(1):21–31. doi: 10.1002/lt.500060110. [DOI] [PubMed] [Google Scholar]
- 29.Terblanche J, Hickman R. Animal models of fulminant hepatic failure. Dig Dis Sci. 1991 Jun;36(6):770–774. doi: 10.1007/BF01311235. [DOI] [PubMed] [Google Scholar]
- 30.Sudan DL, Shaw BW, Jr, Fox IJ, Langnas AN. Long-term follow-up of auxiliary orthotopic liver transplantation for the treatment of fulminant hepatic failure. Surgery. 1997 Oct;122(4):771–777. doi: 10.1016/s0039-6060(97)90086-6. discussion 777–778. [DOI] [PubMed] [Google Scholar]
- 31.Rozga J. Liver support technology--an update. Xenotransplantation. 2006 Sep;13(5):380–389. doi: 10.1111/j.1399-3089.2006.00323.x. [DOI] [PubMed] [Google Scholar]
- 32.Bilir BM, Guinette D, Karrer F, Kumpe DA, Krysl J, Stephens J, et al. Hepatocyte transplantation in acute liver failure. Liver Transpl. 2000 Jan;6(1):32–40. doi: 10.1002/lt.500060113. [DOI] [PubMed] [Google Scholar]
- 33.Fisher RA, Bu D, Thompson M, Tisnado J, Prasad U, Sterling R, et al. Defining hepatocellular chimerism in a liver failure patient bridged with hepatocyte infusion. Transplantation. 2000;69:303–307. doi: 10.1097/00007890-200001270-00018. [DOI] [PubMed] [Google Scholar]
- 34.Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47(4):1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mas VR, Maluf DG, Thompson M, Ferreira-Gonzalez A, Fisher RA. Engraftment measurement in human liver tissue after liver cell transplantation by short tandem repeats analysis. Cell Transplant. 2004;13(3):231–236. doi: 10.3727/000000004783983945. [DOI] [PubMed] [Google Scholar]
- 36.Ott M, Barthold M, Alexandrova K. Clinical applications of human hepatocytes isolated under CGMP conditions. 40th Annual Meeting of the European Association for the Study of the Liver; 2005. [Google Scholar]
- 37.Soriano HE. Clinical Trials of Liver Cell Transplantation in Children with Liver Failure. Cell Transplant Society 10th Anniversary Congress; 2001. [Google Scholar]
- 38.Soriano HE, Wood RP, Kang DC. Hepatocellular transplantation in children with fulminant liver failure. Hepatology. 1997;30:239A. [Google Scholar]
- 39.Sterling RK, Fisher RA. In: Liver Transplantation: Living Donor, Hepatocyte, and Xenotransplantation. Gish R, Sanders WB, editors. Philadelphia: 2001. [DOI] [PubMed] [Google Scholar]
- 40.Mito M. Hepatocyte transplantation in man. Cell transplantation. 1993;2:65. [Google Scholar]
- 41.Kobayashi N, Ito M, Nakamura J, Cai J, Gao C, Hammel JM, et al. Hepatocyte transplantation in rats with decompensated cirrhosis. Hepatology. 2000;31(4):851–857. doi: 10.1053/he.2000.5636. [DOI] [PubMed] [Google Scholar]
- 42.Nagata H, Ito M, Cai J, Edge AS, Platt JL, Fox IJ. Treatment of cirrhosis and liver failure in rats by hepatocyte xenotransplantation. Gastroenterology. 2003 Feb;124(2):422–431. doi: 10.1053/gast.2003.50065. [DOI] [PubMed] [Google Scholar]
- 43.Khan AA, Habeeb A, Parveen N, et al. Peritoneal transplantation of human fetal hepatocytes for the treatment of acute fatty liver of pregnancy: A case report. TropGastroenterol. 2004;25:141–143. [PubMed] [Google Scholar]
- 44.Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, et al. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63(4):559–569. doi: 10.1097/00007890-199702270-00014. [DOI] [PubMed] [Google Scholar]
- 45.Nagata H, Ito M, Shirota C, Edge A, McCowan TC, Fox IJ. Route of hepatocyte delivery affects hepatocyte engraftment in the spleen. Transplantation. 2003;76(4):732–734. doi: 10.1097/01.TP.0000081560.16039.67. [DOI] [PubMed] [Google Scholar]
- 46.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007 Sep;28(25):3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 47.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008 Feb;14(2):213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 48.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ re-engineering: development of a transplantable recellularized liver graft using decellularized liver matrix. Nature Medicine. 2010 doi: 10.1038/nm.2170. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagata H, Nishitai R, Shirota C, Zhang JL, Koch CA, Cai J, et al. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology. 2007 Jan;132(1):321–329. doi: 10.1053/j.gastro.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Nishitai R, Plummer TB, Platt JL. Detection of albumin synthesis in transplanted porcine hepatocytes in mice. Liver Transpl. 2002 Oct;8(10):972–974. doi: 10.1053/jlts.2002.35172. [DOI] [PubMed] [Google Scholar]
- 51.Overturf K, al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997 Nov;151(5):1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 52.Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Replacement of diseased mouse liver by hepatic cell transplantation. Science. 1994 Feb 25;263(5150):1149–1152. doi: 10.1126/science.8108734. [DOI] [PubMed] [Google Scholar]
- 53.Rhim JA, Sandgren EP, Palmiter RD, Brinster RL. Complete reconstitution of mouse liver with xenogeneic hepatocytes. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4942–4946. doi: 10.1073/pnas.92.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo D, Fu T, Nelson JA, Superina RA, Soriano HE. Liver repopulation after cell transplantation in mice treated with retrorsine and carbon tetrachloride. Transplantation. 2002 Jun 15;73(11):1818–1824. doi: 10.1097/00007890-200206150-00020. [DOI] [PubMed] [Google Scholar]
- 55.Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, et al. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998 Jul;153(1):319–329. doi: 10.1016/S0002-9440(10)65574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peters LJ, Withers HR, Cundiff JH, Dicke KA. Radiobiological considerations in the use of total-body irradiation for bone-marrow transplantation. Radiology. 1979 Apr;131(1):243–247. doi: 10.1148/131.1.243. [DOI] [PubMed] [Google Scholar]
- 57.Guha C, Sharma A, Gupta S, Alfieri A, Gorla GR, Gagandeep S, et al. Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation. Cancer Res. 1999 Dec 1;59(23):5871–5874. [PubMed] [Google Scholar]
- 58.Dagher I, Nguyen TH, Groyer-Picard MT, Lainas P, Mainot S, Guettier C, et al. Efficient hepatocyte engraftment and long-term transgene expression after reversible portal embolization in nonhuman primates. Hepatology. 2009 Mar;49(3):950–959. doi: 10.1002/hep.22739. [DOI] [PubMed] [Google Scholar]
- 59.Yamanouchi K, Zhou H, Roy-Chowdhury N, Macaluso F, Liu L, Yamamoto T, et al. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology. 2009 Jan;49(1):258–267. doi: 10.1002/hep.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guha C, Parashar B, Deb NJ, Garg M, Gorla GR, Singh A, et al. Normal hepatocytes correct serum bilirubin after repopulation of Gunn rat liver subjected to irradiation/partial resection. Hepatology. 2002 Aug;36(2):354–362. doi: 10.1053/jhep.2002.34516. [DOI] [PubMed] [Google Scholar]
- 61.Guha C, Yamanouchi K, Jiang J, Wang X, Roy Chowdhury N, Santana A, et al. Feasibility of hepatocyte transplantation-based therapies for primary hyperoxalurias. Am J Nephrol. 2005 Mar–Apr;25(2):161–170. doi: 10.1159/000085408. [DOI] [PubMed] [Google Scholar]
- 62.Mettler FA, Upton AC, Hendee W. Medical effects of ionizing radiation. Philadelphia: Saunders; 2008. [Google Scholar]
- 63.Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol. 2005 Oct;15(4):279–283. doi: 10.1016/j.semradonc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 64.Kovalic JJ, Thomas PR, Beckwith JB, Feusner JH, Norkool PA. Hepatocellular carcinoma as second malignant neoplasms in successfully treated Wilms’ tumor patients. A National Wilms’ Tumor Study report. Cancer. 1991 Jan 15;67(2):342–344. doi: 10.1002/1097-0142(19910115)67:2<342::aid-cncr2820670204>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 65.Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995 Mar 30;31(5):1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 66.Order SE, Donaldson SS. Radiation therapy of benign diseases. 2. New York: Springer; 2003. [Google Scholar]
- 67.Zhou H, Ding J, Avsar Y, Wang X, Roy-Chowdhury J, Roy-Chowdhury N, et al. Repopulation of individual liver lobes by transplanted hepatocytes using regiospecific hepatic irradiation cures jaundice in the Gunn rat model of Crigler-Najjar Syndrome type I. Hepatology. 2009;50(4):303A. [Google Scholar]
- 68.Mitry RR, Dhawan A, Hughes RD, Bansal S, Lehec S, Terry C, et al. One liver, three recipients: segment IV from split-liver procedures as a source of hepatocytes for cell transplantation. Transplantation. 2004;77(10):1614–1616. doi: 10.1097/01.tp.0000122224.98318.19. [DOI] [PubMed] [Google Scholar]
- 69.Hughes RD, Mitry RR, Dhawan A, Lehec SC, Girlanda R, Rela M, et al. Isolation of hepatocytes from livers from non-heart-beating donors for cell transplantation. Liver Transpl. 2006;12(5):713–717. doi: 10.1002/lt.20732. [DOI] [PubMed] [Google Scholar]
- 70.Meyburg J, Das AM, Hoerster F, Lindner M, Kriegbaum H, Engelmann G, et al. One liver for four children: first clinical series of liver cell transplantation for severe neonatal urea cycle defects. Transplantation. 2009 Mar 15;87(5):636–641. doi: 10.1097/TP.0b013e318199936a. [DOI] [PubMed] [Google Scholar]
- 71.Nishitai R, Koch CA, Ogata K, Knudsen BE, Plummer TB, Butters KA, et al. Toward the survival and function of xenogeneic hepatocyte grafts. Liver Transpl. 2005 Jan;11(1):39–50. doi: 10.1002/lt.20305. [DOI] [PubMed] [Google Scholar]
- 72.Terry C, Hughes RD, Mitry RR, Lehec SC, Dhawan A. Cryopreservation-induced nonattachment of human hepatocytes: role of adhesion molecules. Cell transplantation. 2007;16(6):639–647. doi: 10.3727/000000007783465000. [DOI] [PubMed] [Google Scholar]
- 73.Diener B, Utesch D, Beer N, et al. A method for the cryopreservation of liver parenchymal cells for studies of xenobiotics. Cryobiology. 1993;30:116–127. doi: 10.1006/cryo.1993.1011. [DOI] [PubMed] [Google Scholar]
- 74.Hengstler JG, Utesch D, Steinberg P, Platt KL, Diener B, Ringel M, et al. Cryopreserved primary hepatocytes as a constantly available in vitro model for evaluation of human and animal drug metabolism and enzyme induction. Drug Metab Rev. 2000;32:81–118. doi: 10.1081/dmr-100100564. [DOI] [PubMed] [Google Scholar]
- 75.Puppi J, Dhawan A. Human hepatocyte transplantation overview. Methods MolBiol. 2009;481:1–16. doi: 10.1007/978-1-59745-201-4_1. [DOI] [PubMed] [Google Scholar]
- 76.Tanaka K, Soto-Gutierrez A, Navarro-Alvarez N, Rivas-Carrillo JD, Jun HS, Kobayashi N. Functional hepatocyte culture and its application to cell therapies. Cell Transplant. 2006;15(10):855–864. doi: 10.3727/000000006783981332. [DOI] [PubMed] [Google Scholar]
- 77.Basma H, Soto-Gutierrez A, Yannam GR, Liu L, Ito R, Yamamoto T, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009 Mar;136(3):990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2009 Oct 1; doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009 May 8;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 81.Michaels MG, Lanford R, Demetris AJ, Chavez D, Brasky K, Fung J, et al. Lack of susceptibility of baboons to infection with hepatitis B virus. Transplantation. 1996 Feb 15;61(3):350–351. doi: 10.1097/00007890-199602150-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Platt JL. New directions for organ transplantation. Nature. 1998 Apr 30;392(6679 Suppl):11–17. doi: 10.1038/32023. [DOI] [PubMed] [Google Scholar]
- 83.Gunsalus JR, Brady DA, Coulter SM, Gray BM, Edge AS. Reduction of serum cholesterol in Watanabe rabbits by xenogeneic hepatocellular transplantation. Nat Med. 1997 Jan;3(1):48–53. doi: 10.1038/nm0197-48. [DOI] [PubMed] [Google Scholar]
- 84.Fischel RJ, Matas AJ, Platt JL, Perry E, Noreen H, Shumway SJ, et al. Cardiac xenografting in the pig-to-rhesus monkey model: manipulation of antiendothelial antibody prolongs survival. J Heart Lung Transplant. 1992 Sep–Oct;11(5):965–973. discussion 973–964. [PubMed] [Google Scholar]
- 85.Katopodis AG, Warner RG, Duthaler RO, Streiff MB, Bruelisauer A, Kretz O, et al. Removal of anti-Galalpha1,3Gal xenoantibodies with an injectable polymer. J Clin Invest. 2002 Dec;110(12):1869–1877. doi: 10.1172/JCI200216526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004 May 11;101(19):7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuwaki K, Knosalla C, Moran K, Alt A, Katopodis AG, Duthaler RO, et al. Reduction of anti-Galalpha1,3Gal antibodies by infusion of types 2 and 6 gal trisaccharides conjugated to poly-L-lysine. Xenotransplantation. 2004 Mar;11(2):210–215. doi: 10.1046/j.1399-3089.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 88.Lin SS, Weidner BC, Byrne GW, Diamond LE, Lawson JH, Hoopes CW, et al. The role of antibodies in acute vascular rejection of pig-to-baboon cardiac transplants. J Clin Invest. 1998 Apr 15;101(8):1745–1756. doi: 10.1172/JCI2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Platt JL. Genetic modification of xenografts. Curr Top Microbiol Immunol. 2003;278:1–21. doi: 10.1007/978-3-642-55541-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005 Jan;11(1):32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 91.Tsukamoto H, Matsuoka M, French SW. Experimental models of hepatic fibrosis: a review. Semin Liver Dis. 1990 Feb;10(1):56–65. doi: 10.1055/s-2008-1040457. [DOI] [PubMed] [Google Scholar]
- 92.Walker CM. Comparative features of hepatitis C virus infection in humans and chimpanzees. Springer Semin Immunopathol. 1997;19(1):85–98. doi: 10.1007/BF00945027. [DOI] [PubMed] [Google Scholar]
- 93.Ramirez P, Chavez R, Majado M, Munitiz V, Munoz A, Hernandez Q, et al. Life-supporting human complement regulator decay accelerating factor transgenic pig liver xenograft maintains the metabolic function and coagulation in the nonhuman primate for up to 8 days. Transplantation. 2000 Oct 15;70(7):989–998. doi: 10.1097/00007890-200010150-00001. [DOI] [PubMed] [Google Scholar]