Abstract
α-Synuclein (α-syn) is the major component of pathologic inclusions that characterize neurodegenerative disorders such as Parkinson disease, dementia with Lewy body disease, and multiple system atrophy. The present study uses novel phospho-specific antibodies to assess the presence and regulation of phosphorylated Ser87 and Ser129 in α-syn in human brain samples and in a transgenic mouse model of α-synucleinopathies. By immunohistochemistry, α-syn phosphorylated at Ser129, but not at Ser87, was abundant in α-syn inclusions. Under normal conditions, Ser129 phosphorylation, but not Ser87 phosphorylation, was detected at low levels in the soluble biochemical fractions in human α-syn transgenic mice and stably transfected cultured cells. Therefore, a role for Ser87 phosphorylation in α-synucleinopathies is unlikely, and in vitro assays showed that phosphorylation at this site would inhibit polymerization. In vitro studies also indicated that hyperphosphorylation of Ser129 α-syn in pathologic inclusions may be due in part to the intrinsic properties of aggregated α-syn to act as substrates for kinases but not phosphatases. Further studies in transgenic mice and cultured cells suggest that cellular toxicity, including proteasomal dysfunction, increases casein kinase 2 activity, which results in elevated Ser129 α-syn phosphorylation. These data provide novel explanations for the presence of hyperphosphorylated Ser129 α-syn in pathologic inclusions.
Keywords: α-synuclein, Casein kinase, Fibrillation, Parkinson disease, Phosphorylation, PP2C, Proteasome
INTRODUCTION
Parkinson disease (PD) is a neurodegenerative disorder characterized by a loss of dopaminergic neurons in the substantia nigra pars compacta. Pathologic analysis of PD brains reveals intracytoplasmic perikaryal inclusions known as Lewy bodies (LBs) in some of the remaining dopaminergic neurons, as well as similar inclusions in neuronal processes are termed Lewy neurites (1-3). The major component of LBs and Lewy neurites is α-synuclein (α-syn), a 140-amino acid protein that is normally soluble and is localized to neuronal synaptic terminals (1-4). α-Synuclein aberrantly polymerizes into 10- to 15-nm amyloidogenic fibrils that associate to form the Lewy inclusions that contribute to the pathogenesis of PD (1-3). A role for α-syn in the pathophysiology of PD is further supported by the identification in familial forms of PD of several missense mutations in α-syn and short chromosomal duplications and triplications that include the gene for α-syn (5, 6). Furthermore, α-syn is the major component of pathologic inclusions in other neurodegenerative diseases, including dementia with LBs (DLB), LB variant of Alzheimer disease (LBVAD), and multiple systems atrophy (MSA), which are collectively termed α-synucleinopathies (1, 5, 7-11).
Although the initial identification of the α-syn protein in bovine brain was based on the characterization of a neuron-specific phosphoprotein (12-14), the purposes of phosphorylation and the kinases involved are unclear. α-Synuclein is predominantly phosphorylated at serine residues, and the major putative kinases implicated include G-protein coupled receptor kinase (GRK) 5, dual-specificity tyrosine-regulated kinase 1A, casein kinase (CK) 1, and CK2 (15-18).
The major phosphorylation site on α-syn is Ser129 (15, 19, 20), which is located in the negatively charged C-terminal tail of α-syn. A secondary site of Ser87, located near the hydrophobic domain, has been noted in vitro and in cultured cells (15, 17). In human brain samples, α-syn is reportedly only phosphorylated at low levels at Ser129, but this amino acid residue can be hyperphosphorylated in pathologic α-syn inclusions (19-22). α-Synuclein is also phosphorylated at Ser129 in the pathologic inclusions of transgenic mouse models of α-synucleinopathies (19, 21, 23). Furthermore, it has been suggested that phosphorylation of Ser129 in α-syn by CK2 may promote in vitro fibrillation (19) and in situ inclusion formation (24). In other models, however, phosphorylation of Ser129 seems to decrease or not affect inclusion formation (25, 26).
In contrast to Ser129, fewer studies have reported phosphorylation of Ser87 α-syn (15, 17). In vitro, Ser87 in α-syn can be phosphorylated by CKs (15) and dual-specificity tyrosine-regulated kinase 1A (17), and in cultured cells, phosphorylation at Ser87 of α-syn may promote α-syn inclusion formation and decrease cell viability (17).
In this study, we have used in vitro, in situ, and in vivo methods to examine the presence of phospho-Ser129 and phospho-Ser87, the regulation of phosphorylation, and the mechanisms associated with the hyperphosphorylation of Ser129 in pathologic inclusions.
MATERIALS AND METHODS
Antibodies
pSer129 is a novel mouse monoclonal antibody raised against phospho-peptide CAYEMPpSEEGYQ conjugated to maleimide-activated keyhole limpet hemocyanin using methods previously described (27, 28).
Syn102 is a mouse monoclonal antibody recognizing the C-terminus of α-syn and β-syn (27). Syn211 is a mouse monoclonal antibody specific for human α-syn that requires amino acids 121 to 125 (27). SNL-4 is a polyclonal rabbit antibody raised against a synthetic peptide corresponding to amino acids 2 to 12 of α-syn (7, 27). SNL-4 recognizes all LBs and glial cytoplasmic inclusions (GCIs) in cases of PD, DLB, and MSA (7, 27). Syn514 is a mouse monoclonal antibody that recognizes pathologic α-syn (29). Antibody to phospho-Ser87 (pSer87) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). PHF-1 antibody is a mouse monoclonal antibody specific for tau phosphorylated at Ser396 or Ser404 (30). Total tau protein expression was assessed with a combination of the tau phosphorylationYindependent antibodies T14 and T46 (31). Casein kinase 2> antibody was purchased from Upstate Biotechnology (Charlottesville, VA). The CK2A, GRK5, and GRK2 antibodies were purchased from Santa Cruz Biotechnology.
In Vitro Kinase Assays
Recombinant human α-syn (wild-type [WT] and Ser87Ala, Ser129Ala, and Ser87Ala/Ser129Ala mutants) was produced in BL21 Escherichia coli and purified to homogeneity as previously described (32). Five micrograms of each recombinant protein was phosphorylated in vitro by 500 U of commercially available enzyme kinases CK1 (New England Biolabs, Ipswich, MA) and CK2 (New England Biolabs) in buffers provided by the manufacturer. Each kinase reaction was performed at room temperature for 90 minutes in the presence of 200 μmol/L of adenosine triphosphate (ATP). The relative levels of phosphorylation and the specificity of each kinase for the specific serine residues were assessed by performing kinase reactions with [γ-32P]ATP. Reactions were stopped with addition of sodium dodecyl sulfate (SDS) sample buffer and heating to 100°C for 5 minutes. For assessment of 32P incorporation, samples resolved onto 15% SDS/polyacrylamide gels were exposed to a 32P phosphoimaging screen (Molecular Dynamics, Piscataway, NJ), with direct quantification by excision of Coomassie-stained protein bands, followed by scintillation counts.
Western Blot Analysis
Protein samples were resolved by SDS/polyacrylamide gel electrophoresis (15% gels for α-syn, CK1, and CK2 immunoblots or 8% for tau, GRK2, and GRK5 immunoblots), followed by electrophoresis onto nitrocellulose membranes. Membranes were blocked in Tris-buffered saline (TBS) with 5% dry milk and incubated overnight with Syn211, pSer129, or Syn102 in TBS/5% dry milk or pSer87 in TBS/3% bovine serum albumin. For total protein lysates, pSer129 was incubated in TBS/3% bovine serum albumin. Other antibodies were used at manufacturer-suggested specifications. Each incubation was followed by goat anti-mouse-conjugated horseradish peroxidase (Amersham Biosciences, Piscataway, NJ) or goat anti-rabbit horseradish peroxidase (Cell Signaling Technology, Danvers, MA), and immunoreactivity was detected using chemiluminescent reagent (NEN, Boston, MA), followed by exposure on x-ray film.
Immunohistochemistry
Postmortem brain samples from patients with PD, LBVAD, DLB, MSA, or AD and neuropathologically normal controls (Table) were harvested, fixed, and processed for immunohistochemistry as previously described (7, 33). Sequential 6-μm tissue sections were immunostained using the avidin-biotin complex detection system (Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine. Primary antibodies were incubated overnight in Tris with 5% fetal bovine serum. Tissue sections were lightly counterstained with hematoxylin. Co-occurrence within LBs and GCIs was assessed by independent counting of inclusions in adjacent sections.
TABLE.
Demographic Data for Human Brain Samples
| Diagnosis | Sex | Age Range (years) | Postmortem Interval Range (hours) |
|---|---|---|---|
| PD | 8 M; 3 F | 49-97 | 3-21 |
| AD | 2 M; 1 F | 73-75 | 10-20 |
| LBVAD | 9 M; 4 F | 62-86 | 3-22 |
| DLB | 2 M; 1 F | 79-91 | 2-12 |
| MSA | 8 M; 5 F | 43-79 | 8-43 |
| Normal | 2 M; 1 F | 47-89 | 10-12.5 |
AD, Alzheimer disease; DLB, dementia with Lewy bodies; F, female; LBVAD, Lewy body variant of Alzheimer disease; M, male; MSA, multiple systems atrophy; PD, Parkinson disease.
Immunofluorescence
Immunofluorescence analysis of postmortem samples was performed on paraffin-embedded brain sections as previously described (33). Mouse brain tissue was isolated, fixed, and processed as previously described (34). Sections were incubated overnight with primary antibodies, diluted in Tris/5% dry milk or in Tris/5% fetal bovine serum, followed by goat anti-mouse secondary conjugated to Alexa 594 and goat anti-rabbit secondary conjugated to Alexa 488 (Invitrogen, Carlsbad, CA). Sections were postfixed with formalin and coverslipped using Vectashield with 4′-6-diamidino-2-phenylindole mounting medium (Vector Laboratories).
Biochemical Fractionation
Tissue was homogenized in 3 ml/g of high-salt (HS) buffer (50 mmol/L of Tris, 750 mmol/L of NaCl, 5 mmol/L of EDTA, and a cocktail of protease inhibitors and phosphatase inhibitors). The protease inhibitor cocktail contained 1 mmol/L of phenylmethylsulfonyl and 1 mg/ml each of pepstatin, leupeptin, N-tosyl-L-phenylalanyl chloromethyl ketone, N-tosyl-lysine chloromethyl ketone, and soybean trypsin inhibitor; the phosphatase inhibitor cocktail contained 50 mmol/L of NaF, 1 mmol/L of NaVO4, and 1 μmol/L of okadaic acid (OA). Samples were sedimented at 100,000 × g for 20 minutes, and supernatants were removed for analysis. Pellets were rehomogenized in successive buffers, after which each was sedimented, and supernatant was removed: HS containing 1% Triton X-100 (HS/Triton), RIPA (50 mmol/L of Tris, 150 mmol/L of NaCl, 5 mmol/L of EDTA, 1% NP40, 0.5% sodium deoxycholate, and 0.1% SDS), and SDS/urea (8 mol/L of urea, 2% SDS, 10 mmol/L of Tris; pH 7.5). Sodium dodecyl sulfate sample buffer was added, and samples (except for the SDS/urea fractions) were heated to 100°C for 5 minutes prior to Western blot analysis.
In Vitro Fibrillation Assays
For fibrillation assays, samples were diluted to 5 mg/ml in 100 mmol/L of Na acetate, pH 7.4, and were subjected to constant agitation for 24 to 60 hours at 37°C as previously described (32, 35). Samples were sedimented at 100,000 × g for 20 minutes, and the pellet (P) was analyzed in relationship to the supernatant (S) by resolving via SDS/polyacrylamide gel electrophoresis, stained with Coomassie, and quantification by densitometry. The percentage of protein in pellets was calculated as [P / (P + S)] × 100.
Amyloid formation was determined by K114 fluorometry as previously described (36). A fraction of each sample was incubated with K114 (10 μmol/L) in 100 mmol/L of glycine, pH 8.5, and fluorescence signal was measured (λex, 380 nm; λem, 550 nm; cutoff, 530 nm) with a SpectraMax Gemini fluorometer and SoftMax Pro software (Molecular Devices Corp., Sunnyvale, CA).
To determine the effect of phosphorylation on α-syn fibril formation, recombinant WT, Ser87Ala, Ser129Ala, and Ser87Ala/Ser129Ala α-syn were incubated overnight with CK1 in the absence or presence of ATP as previously described. After phosphorylation, CK1 was heat inactivated and removed by centrifugation, and proteins were incubated in CK1 assay buffer, diluted in 100 mmol/L of Na acetate, pH 7.4, and assayed as previously described.
For experiments in which polymerized α-syn was subjected to kinase assays, fibrillized α-syn was centrifuged at 2,200 × g and washed 3 times by gentle trituration in sterile water followed by centrifugation. After isolation, fibrillized α-syn was gently homogenized in water, and protein concentration was determined by bicinchoninic acid protein assay reagent (Pierce Thermo Scientific, Rockford, IL) prior to experimentation.
In Vitro Protein Phosphatase Assays
Recombinant human WT α-syn or tau was incubated with CK2 or CK1, respectively, as previously described. Kinases were heat inactivated at 65°C for 30 minutes, after which the reaction was rehomogenized and aliquoted into protein phosphatase reactions containing 2 μg of α-syn or tau and 1 U of protein phosphatase (PP) 1 (New England Biolabs), PP2A (EMD Biosciences, San Diego, CA), PP2C (EMD Biosciences), λ phosphatase (New England Biolabs), or 0.5 U PP2B (EMD Biosciences), where 1 U is defined as the amount of enzyme that will release 1.0 nmol of [32P]Pi from 32P-labeled phosphorylase per minute at 30°C, pH 7.0. Protein phosphatase 1 and PP2A reactions were performed in a buffer containing 50 mmol/L of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 100 mmol/L of NaCl, 2 mmol/L of dithiothreitol, 0.1 mmol/L of EGTA, and 0.025% Tween-20, pH 7.5. Protein phosphatase 2B, PP2C, and λ reactions were performed in a buffer containing 50 mmol/L of Tris-HCl, 100 mmol/L of NaCl, 2 mmol/L of dithiothreitol, 0.1 mmol/L of EGTA, 0.01% Brij, and 100 μg/ml of bovine serum albumin, pH 7.5. Reactions were supplemented as follows: 1 mmol/L of MnCl2 for PP1, 5 mmol/L of CaCl2 and 3 μmol/L of calmodulin (EMD Biosciences) for PP2B, 10 mmol/L of MgCl2 for PP2C, and 2 mmol/L of MnCl2 for λ. Reactions were incubated overnight at 30°C and were stopped by addition of SDS sample buffer and boiling.
SH-SY5Y Neuroblastoma Cell Culture
SH-SY5Y cells that stably express human WT α-syn or vector control were maintained as previously described (37). The parent cells (CRL-2266) were obtained from the American Type Culture Collection (Manassas, VA). Cultures were treated with the following drugs purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified: 10 μmol/L of CK1 inhibitor D4476 [4-(4-(2,3-dihydrobenzo(1,4)dioxin-6-yl)-5-pyridin-2-yl-1H-imidazol-2-yl)benzamide; EMD Biosciences], 10 or 25 μmol/L of CK2 inhibitors DMAT [a derivative of TBB; 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole (38, 39)], 100 μmol/L of DRB (5,6-dichloro-1-β-D-ribofunanosylbenzimidazole), and 20 μmol/L of TBCA [(E)-3-(2,3,4,5-tetrabiomophenyl)acrylic acid; EMD Biosciences (40)]; 20 nmol/L of OA (EMD Biosciences); 10 μmol/L of FK506; 10 μmol/L of MG132 (Z-Leu-Leu-Leu-al); 25 mmol/L of NH4Cl; 5 μmol/L of lactacystin; 10 μmol/L of MPP+ (1-methyl-4-phenylpyridinium iodide); 500 μmol/L of dopamine; 50 μmol/L of 6-hydroxydopamine; 200 μmol/L of H2O2 (Fisher Scientific, Fair Lawn, NJ); 1 μmol/L of rotenone; 100 μmol/L of arsenite; 2 μmol/L of thapsigargin; and 50 μmol/L of etoposide. Treatments were matched with a vehicle control of 0.1% dimethyl sulfoxide (Fisher Scientific). Samples were retrieved in 1.5× Laemmli sample buffer (75 mmol/L of Tris-HCl, pH 6.8, 3% SDS, 15% glycerol, 3.75 mmol/L of EDTA, pH 7.4) and boiled. Protein concentration was determined using bicinchoninic acid protein assay reagent (Pierce Thermo Scientific, Milwaukee, WI). Western blot analysis was performed on 7 μg of protein per sample for pSer129 and 2 μg of protein per sample for Syn211.
Quantitative Analysis
[γ-32P]ATP incorporation was quantified with Image-Quant software (Molecular Dynamics, Inc., Sunnyvale, CA), and Western blot data were quantified by ImageJ software (National Institutes of Health, Bethesda, MD). Data were analyzed as a fraction or percent of control conditions and compared with vehicle control conditions. When multiple treatments were performed, the percent change was standardized to the initial treatment to determine the effect of the subsequent treatment. All comparisons were completed by 2-way parametric t-tests unless otherwise stated. Multiple comparisons were made by 1-way analysis of variance, and 1-sample comparisons were completed by 1-sample t-test using GraphPad InStat software (San Diego, CA).
RESULTS
In Vitro Phosphorylation of α-Syn and Antibody Specificity
Previous studies have identified 2 potential sites on α-syn for CK1 and CK2 phosphorylation, a major phosphorylation site at Ser129 and a minor site at Ser87 (15). These sites have been implicated in the ability of α-syn to form pathologic inclusions (17, 19-21, 24). To further examine the phosphorylation of α-syn, we produced a novel phospho-specific antibody directed towards phospho-Ser129 (pSer129) and tested a commercially available phospho-Ser87 antibody (pSer87, Santa Cruz Biotechnology).
The specificity of these antibodies was first determined by in vitro phosphorylation of recombinant α-syn protein by CK1 or CK2. Site specificity was confirmed through generation of serine-to-alanine mutations in α-syn (Ser129-Ala, Ser87Ala, and double-mutant Ser87Ala/Ser129Ala). Total phosphorylation of α-syn was assessed by [γ-32P]ATP incorporation for each protein and each kinase reaction (Fig. 1). Coomassie staining of SDS/polyacrylamide gels and immunoblotting with the previously described antibody Syn102 (α-syn) (27) were used to control for protein loading.
FIGURE 1.
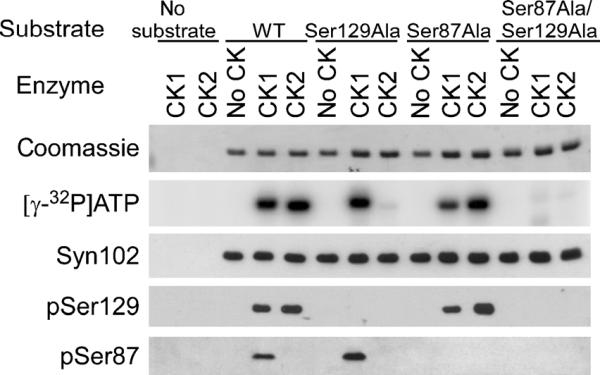
Specificity of novel phospho-α-synuclein (α-syn) antibodies using recombinant α-syn protein. Recombinant wild-type α-syn or α-syn with Ser129Ala and/or Ser87Ala mutations was subjected to in vitro phosphorylation assays by no kinase (no casein kinase [CK]), CK1, or CK2, as described in Materials and Methods section. Equal amounts of each protein were analyzed by phosphoimaging of [γ-32P] adenosine triphosphate (ATP) incorporation and by Western blot analysis with Syn102 (total α-syn), pSer129, or pSer87. Results indicated that CK1 phosphorylated α-syn at Ser87 and Ser129, and CK2 phosphorylated only at Ser129. The immunoblotting data show phosphorylation site specificity of pSer87 and pSer129 antibodies.
Phosphorylation, monitored by [γ-32P]ATP incorporation, was noted only in the presence of CK1 or CK2. The mutant recombinant protein Ser129Ala α-syn incorporated [γ-32P]ATP with CK1, but not with CK2. The Ser87Ala α-syn protein reduced [γ-32P]ATP incorporation with CK1, but not with CK2, and Ser87Ala/Ser129Ala α-syn protein did not incorporate [γ-32P]ATP. These data indicate that CK1 phosphorylates α-syn at both Ser87 and Ser129, whereas CK2 phosphorylates α-syn only at Ser129 in vitro.
Western blot analyses revealed that pSer129 recognized CK1- and CK2-phosphorylated α-syn (WT and mutants), consistent with phosphorylation at Ser129, but not protein containing the Ser129Ala mutation. pSer87 antibody only recognized CK1-phosphorylated α-syn, but not protein containing the Ser87Ala mutation. None of the phosphospecific antibodies recognized nonphosphorylated α-syn even after x-ray film overexposure (data not shown). pSer129 also recognized in vitro phosphorylated recombinant mouse α-syn, whereas the pSer87 antibody did not (data not shown). This is consistent with the lack of a homologous Ser87 phosphorylation site in mouse α-syn (15).
Phosphorylated α-Syn in DLB and MSA Brains
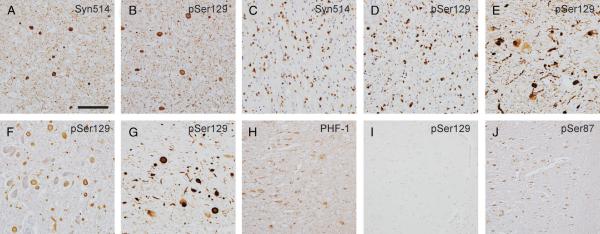
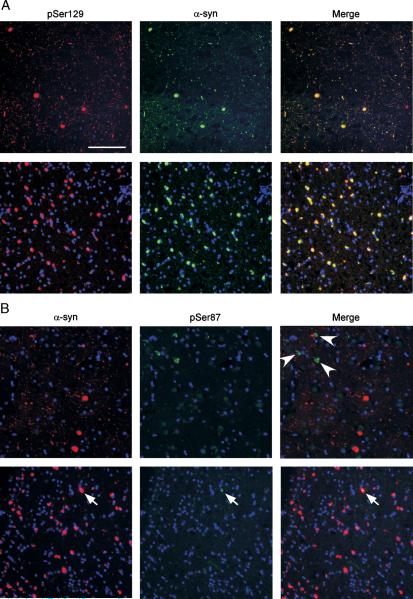
Previous studies have suggested that a high percentage of pathologic α-syn is phosphorylated at Ser129 (19-21). Brain sections from patients with PD, DLB, LBVAD, or MSA were analyzed by immunohistochemistry and double labeling immunofluorescence to ascertain the phosphorylation of α-syn in pathologic inclusions. In tissue sections from patients with PD, DLB, and LBVAD, pSer129 detected abundant LBs, Lewy neurites, and neuroaxonal spheroids (Figs. 2, 3A). Conversely, pSer87 very rarely labeled α-syn pathologic inclusions (Fig. 3B). In double-immunofluorescence studies, pSer129 was compared against antibody SNL-4 (7, 27), and pSer87 was compared against antibody Syn514 (29). Double immunofluorescence between Syn514 and SNL-4 showed total colocalization, supporting that both α-syn antibodies recognized all α-syn inclusions (data not shown). Semiquantitative analyses of adjacent sections of DLB and LBVAD brains revealed that most (98 − 7% SD) LBs recognized by Syn514 also were labeled with pSer129. Similarly, most GCIs (104 ± 4.5% SD) in the brains of MSA patients were robustly labeled with pSer129 (Figs. 2D, 3A). Most GCIs also demonstrated a paucity of staining for pSer87 (Fig. 3B). To verify the specificity of the phospho-α-syn antibodies further, sections from AD patients that, by definition, contain neurofibrillary tangles composed of hyper-phosphorylated tau were immunostained. Neurofibrillary tangles were detected with the anti-phospho-tau antibody PHF-1 (Fig. 2H), but not with pSer129 (Fig. 2I). pSer87, however, stained abundant neurofibrillary tangles in AD (Fig. 2J) and LBVAD (Fig. 3B) cases, suggesting that this antibody cross-reacts with the phospho-tau epitope. This also indicates that the paucity of immunostaining of this antibody for α-syn pathologic inclusions is not due to its inability to react in fixed paraffin-embedded tissue sections.
FIGURE 2.
Immunohistochemistry of pathologic inclusions using phospho-specific antibodies for α-synuclein (α-syn). Brain sections were immunostained with antibodies Syn514 (A, C), pSer129 (B, D-G, I), PHF-1 (H), or pSer87 (J). Antibodies Syn514 and pSer129 recognized abundant α-syn pathologic inclusions in the cingulate cortex of a patient with dementia with Lewy bodies (DLB; A, B), the cerebellum of a patient with multiple systems atrophy (C, D), the substantia nigra of a patient with Parkinson disease (PD; E), the locus ceruleus of a PD patient with the familial Ala53Thr mutation (F), and the substantia nigra of DLB patients (G). Immunostaining of the hippocampus of a patient with Alzheimer disease is shown in (H-J). PHF-1 (H) and pSer87 (J) recognized characteristic neurofibrillary tangles, but pSer129 (I) demonstrated no immunoreactivity with neurofibrillary tangles. Scale bars = (A-G) 100 μm; (H-J) 200 μm.
FIGURE 3.
Double immunofluorescence with α-synuclein (α-syn) and phospho-specific antibodies in brain sections. (A) Tissue sections from the cingulate cortex from a patient diagnosed with Lewy body (LB) variant of Alzheimer disease (LBVAD; top row) or the cerebellum from a patient with multiple systems atrophy (MSA; bottom row) were double labeled with pSer129 (red) and α-syn (SNL-4 antibody; green). Extensive colocalization in LBs, Lewy neurites, and glial cytoplasmic inclusions (GCIs) was observed. (B) Tissue sections from the cingulate cortex from a patient with LBVAD (top row) or the cerebellum from a patient with MSA (bottom row) were double labeled with α-syn (Syn514; red) and pSer87 (green). Most LBs, Lewy neurites, and GCIs labeled with Syn514 were not immunoreactive with pSer87. Only occasional (<1%) GCIs labeled with Syn514 were also labeled with pSer87 (arrow). However, pSer87 labeled neurofibrillary tangle-like inclusions in cases of LBVAD (arrowheads). Scale bar = 100 μm.
Analysis of Phospho-α-Syn by Biochemical Fractionation
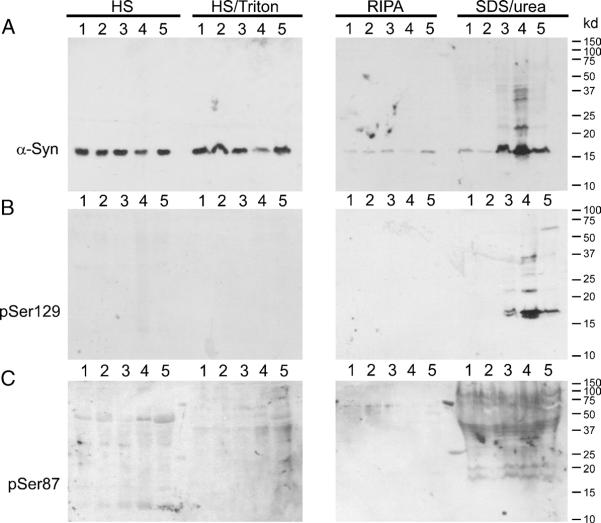
The phosphorylation state of α-syn in human brains was further assessed by biochemical extraction, followed by immunoblotting analysis. Cortices from controls (Samples 1 and 2) and patients with DLB (Samples 3-5) were sequentially extracted with buffers of increasing strength of protein solubility. Consistent with previous studies (11, 28, 41), most α-syn, as observed by the antibody Syn211, was found in the HS and the HS/Triton fractions (Fig. 4A). Triton X-100—insoluble α-syn was predominantly present only in brains from patients with DLB (Samples 3-5), which is a reflection of aggregated α-syn. Furthermore, phosphorylation of α-syn at Ser129 as detected by pSer129 was predominantly present in the SDS/urea fractions of DLB patients (Fig. 4B). Phosphorylation at Ser87 of α-syn was not detected because pSer87 recognized only background immunoreactivity (Fig. 4C).
FIGURE 4.
Sequential extraction and Western blot analysis of brains from patients with dementia with Lewy body (DLB). Sequential extractions of the cingulate cortex from DLB patients were performed using buffers of increasing strength of protein solubility, as described in Materials and Methods section. Samples 1 and 2 were from control cortices, and Samples 3 to 5 were from patients with DLB. (A) Western blot analysis of sequentially extracted fractions with Syn211 (α-synuclein [α-syn]) shows α-syn extracted in soluble fractions (high salt [HS] and HS/Triton) of all cortices. RIPA-insoluble, sodium dodecytl sulfate (SDS)/urea/soluble α-syn was predominantly identified in samples from DLB patients (Samples 3-5). (B) Immunoblotting with pSer129 identified α-syn phosphorylated at Ser129 in only the insoluble fraction of DLB patients. (C) Immunoblotting with pSer87 presented only nonspecific immunoreactivity for all samples; no band corresponding to phosphorylated α-syn could be detected.
Samples of cerebellum from MSA and control patients were similarly analyzed. As with DLB cortical samples, anti-α-syn antibodies (Syn211) recognized α-syn in HS and HS/Triton fractions, with α-syn only detected in the SDS/urea fraction of MSA patients (Fig. 5A). Although 3 MSA patients (Samples 4-6) were analyzed, Patient 4 did not show significant α-syn immunoreactivity in the SDS/urea fraction, which was consistent with the low abundance of GCIs for this patient by immunohistochemistry (data not shown). Phospho-Ser129 immunoreactivity was only detected in the fractions (SDS/urea fractions) containing aggregated α-syn (Fig. 5B), and no immunoreactivity was noted in any samples with pSer87 (Fig. 5C).
FIGURE 5.
Sequential extraction and Western blot analysis of cerebella from patients with multiple systems atrophy (MSA). Sequential extraction of cerebella from control and MSA patients was performed using buffers of increasing strength of protein solubility, as described in Materials and Methods section. Samples 1 to 3 were from control cerebella, and Samples 4 to 6 were from patients diagnosed with MSA. (A) Western blot analysis of sequential extracted fractions with Syn211 (α-synuclein [α-syn]) showed α-syn predominantly in the high-salt (HS) fraction of all samples. α-Synuclein was identified in the sodium dodecyl sulfate (SDS)/urea-soluble fraction of only Samples 5 and 6. The paucity of RIPA-insoluble α-syn in Sample 4 was consistent with a low number of GCIs in this case. (B) Immunoblotting with pSer129 detected phospho-Ser129 in SDS/urea-soluble fractions of Samples 5 and 6. (C) α-Synuclein phosphorylated at Ser87 was not detected in any fractions. *Nonspecific cross-reacting band.
Analysis of α-Syn Phosphorylation in a Mouse Model of Neuronal α-Synucleinopathies
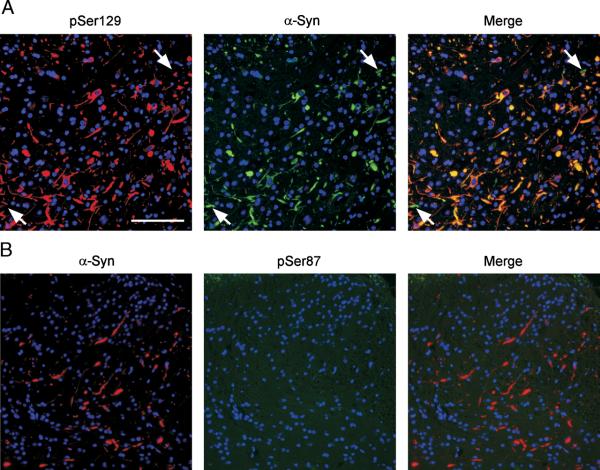
Because the phosphorylation state of proteins can be altered during the postmortem interval, especially due to ongoing phosphatase activity, the phosphorylation state of α-syn was assessed in a transgenic mouse model of α-synucleinopathies. The mice overexpress human Ala53Thr α-syn (transgenic line M83) and exhibit an age-dependent motor phenotype that is associated with the formation of neuronal inclusions (34). Double labeling immunofluorescence demonstrated that most α-syn pathologic inclusions in these animals are phosphorylated at Ser129 (Fig. 6A), although rare inclusions that were not reactive with pSer129 can be observed (Fig. 6A; arrow). Conversely, and consistent with the findings in human α-synucleinopathies, there was a paucity of staining for pSer87 (Fig. 6B).
FIGURE 6.
Double immunofluorescence between α-synuclein (α-syn) and phospho-specific antibodies in Ala53Thr-overexpressing transgenic mice. (A) Tissue sections from the spinal cord of a clinically diseased mouse with transgenic overexpression of human Ala53Thr α-syn (line M83) were double labeled with pSer129 (red) and α-syn (SNL-4 antibody; green). Double immunofluorescence revealed α-syn/containing inclusions that were highly, but not completely, colabeled for α-syn phosphorylated at Ser129. Some pathologic inclusions (G1%) did not colocalize (arrows). (B), Double labeling immunofluorescence with α-syn (Syn514; red) and pSer87 (green) of tissue sections from symptomatic M83 transgenic mice did not identify the presence of α-syn phosphorylated at Ser87 in α-syn inclusions. Scale bar = 100 μm.
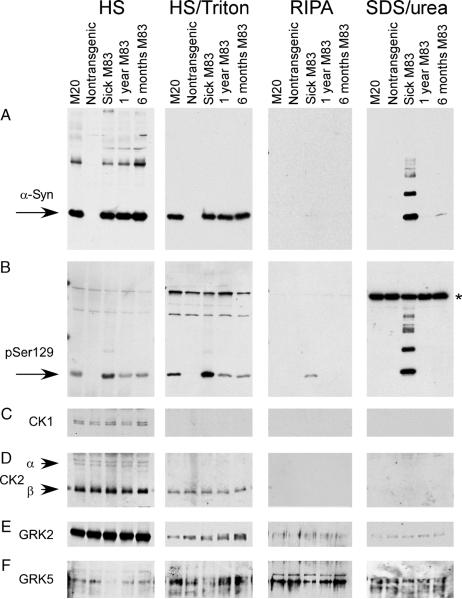
To examine the status of phosphorylation over the course of disease progression, the phosphorylation state of α-syn in the spinal cord of transgenic mice overexpressing WT human α-syn (M20 line), a nontransgenic animal, and 3 M83 mice was further assessed by biochemical fractionation followed by immunoblotting. The M20 transgenic line does not develop pathology, and of the 3 M83 mice, there was 1 sick animal aged 12 months, a nonsick animal aged 12 months, and a younger animal aged 6 months. Similar to our previous data in the spinal cord (34), immunoblotting with the human α-syn-specific antibody Syn211 demonstrated that most α-syns were extracted in the HS and HS/Triton fractions (Fig. 7A), whereas α-syn was observed in the SDS/urea fraction only in the affected M83 mouse (Lane 3). Similar amounts of human α-syn were noted in the soluble fractions of all transgenic mice samples. α-Synuclein phosphorylated at Ser129 was detectable in the soluble fraction of all mice overexpressing α-syn; however, a significant increase in pSer129 immunoreactivity was observed in the soluble fractions of the sick M83 mouse (Fig. 7B). Highly phosphorylated α-syn was also observed in the SDS/urea fraction of the affected mice. The higher molecular mass species detected by Syn211 and pSer129 in the SDS/urea fraction of the affected M83 mouse has previously been demonstrated to be due to ubiquitination (42). No α-syn immunoreactivity was noted with pSer87 in any fraction (data not shown). To begin to ascertain a mechanism that can account for the increase in the phosphorylation of α-syn at Ser129, expression levels of kinases that have been implicated in phosphorylation of Ser129 (CK1, CK2 α and β subunits, GRK2, and GRK5) were assessed. However, no increases in kinase immunoreactivity were noted for any animal (Figs. 7C-F).
FIGURE 7.
Sequential extraction and Western blot analysis for phosphorylated α-synuclein (α-syn) in mouse spinal cord samples. Sequential extraction followed by Western blot analyses were performed on spinal cords from a transgenic mouse expressing human WT α-syn (line M20), a nontransgenic mouse, and transgenic mice expressing human Ala53Thr α-syn (line M83). Two normal-appearing M83 mice at 6 and 12 months of age and a mouse displaying severe paralysis at 12 months of age were analyzed. (A) The antibody Syn211, which is specific for human α-syn, identified α-syn in high-salt (HS) and HS/Triton fractions in all M20 and M83 samples (all mice with transgenic overexpression of α-syn). α-Synuclein was identified in the sodium dodecyl sulfate (SDS)/urea fraction in only the sick M83 animal. (B) Phospho-Ser129 was identified in HS and HS/Triton fractions of all α-syn transgenic mice; however, higher levels of Ser129 phosphorylation were observed in HS and HS/Triton fractions in the sick M83 animal. In the RIPA and SDS/urea fractions, α-syn phosphorylated at Ser129 was only observed in the sick M83 mouse. Western blot analysis was performed with antibodies specific to casein kinase (CK) 1 (C), CK2α and CK2A (D), G-protein coupled receptor kinase (GRK) 2 (E), and GRK5 (F). No differences in kinase expression between any of the mouse samples were noted. *Nonspecific cross-reacting band.
In Vitro Polymerization of Phosphorylated α-Syn
The presence of phospho-Ser129 α-syn in inclusions and the increase in phosphorylation in the soluble fraction from affected M83 mice suggest a role for phosphorylation in inclusion formation. Therefore, in vitro polymerization assays were performed to assess the role of phosphorylation on fibril formation (32, 35). Recombinant human α-syn (WT, Ser129Ala, Ser87Ala, or Ser87Ala/Ser129Ala) was phosphorylated in vitro by CK1, which is the kinase that can phosphorylate both Ser87 and Ser129. After phosphorylation, the proteins were diluted in 100 mmol/L of Na acetate to a final concentration of 5 mg/ml and agitated for approximately 28 hours. Data were analyzed by both sedimentation assays and K114 amyloid fluorometry (Figs. 8A, B). Control experiments were performed in the absence of CK1 (data not shown) or in the absence of ATP (+CK1 - ATP). Both control types produced similar results for WT α-syn and each mutant protein. In contrast, reactions, to which both CK1 and ATP were added, resulted in robust reductions in α-syn fibril formation for WT and Ser129Ala α-syn proteins. Phosphorylation at Ser129 of Ser87Ala α-syn with CK1 resulted in less substantial but still significant reductions in α-syn polymerization. Additional experiments using CK2 to phosphorylate α-syn at Ser129 produced similar results (data not shown). Incubation of Ser87Ala/Ser129Ala α-syn with both CK1 and ATP did not result in significant changes in fibril formation when compared with control reactions. These data suggest that CK1-mediated phosphorylation, especially at Ser87, can inhibit fibril formation.
FIGURE 8.
In vitro polymerization assays using recombinant phosphorylated α-synuclein (α-syn). (A) Kinase assays (casein kinase [CK] 1 and adenosine triphosphate [ATP]) were performed with recombinant wild-type (WT), Ser129Ala, Ser87Ala, or Ser87Ala/ Ser129Ala α-syn. Control conditions, excluding ATP from the reaction mixture (+CK1 - ATP), produced similar results to reactions excluding CK1 (data not shown). Each α-syn reaction at a concentration of 5 mg/ml was agitated at 37°C for 24 to 28 hours. Sedimentation of α-syn was assessed by centrifugation at 100,000 χ g, and the relative amount of α-syn in the supernatants (S) and pellets (P) was visualized by Coomassie blue stain. Graph represents the percentage of α-syn pelleted [P / (P + S)] 100 in each condition. Data represent averages ± standard error of mean (SEM). Decreased fibrillation rates were noted in the presence of both CK1 and ATP for WT, Ser129Ala, and Ser87Ala (comparisons were made between +CK1 + ATP conditions and control conditions in the absence of CK1 or ATP by 1-way analysis of variance). *, p = 0.0002, n = 9; †,p G 0.0001, n = 12; ‡, p = 0.006, n=9. (B) Amyloid formation was determined with K114 fluorometry as described in Materials and Methods section. Data represent averages T SEM. Comparisons between α-syn containing CK1 and ATP were made against the corresponding condition in the absence of ATP. Decreases in K114 fluorometrics were noted for phosphorylation of WT (n = 6) and Ser129Ala (n = 9). *, p = 0.03; †,p < 0.0001. (C) Sedimentation analysis and (D) K114 amyloid fluorometry of nonphosphorylated α-syn or α-syn phosphorylated at Ser87 at a stoichiometry of 0.1 mol/L of phosphate per mole of α-syn. This was accomplished by phosphorylating Ser129Ala α-syn to approximately one third with CK1, inactivating the kinase as described in Materials and Methods section, and mixing the phosphorylated protein at 1.5 mg/ml with nonphosphorylated WT α-syn at 3.5 mg/ml. These reactions were compared with nonphosphorylated Ser129Ala α-syn and WT α-syn incubated in the same proportion (*, p = 0.0002; †,p < 0.0001; n = 12).
These findings involving the phosphorylation of Ser87 were further confirmed by conducting fibrillation experiments with α-syn phosphorylated at a substoichiometric level. α-Synuclein protein was prepared where only 10% of the protein reaction was specifically modified at Ser87. This was accomplished by phosphorylating Ser129Ala α-syn with CK1 (which typically resulted in one third of the protein phosphorylated at Ser87) and adding it to nonmodified WT α-syn so that the final percentage of phosphorylation was 10% in a final reaction concentration of 5 mg/ml. Phosphorylation at Ser87 dramatically inhibited fibril formation compared with nonphosphorylated protein (Figs. 8C, D). Electron microscopy (data not shown) of phospho-Ser87 reactions revealed the presence of rare fibrils, indicating that phosphorylation at Ser87 slowed but did not completely prevent polymerization.
In Vitro Phosphorylation and Dephosphorylation of Fibrillized α-Syn
To characterize the mechanism involved in the hyperphosphorylation of Ser129 α-syn in pathologic inclusions further, the relative ability of soluble and fibrillized α-syn to act as substrates for kinases and phosphatase was analyzed in vitro. Both the soluble and the fibrillized forms of WT α-syn were substrates for CK1 or CK2 (Fig. 9), and the fibrillized form demonstrated a slight increase in phosphorylation relative to soluble (an average increase of 52.5% for CK1 and 20.3% for CK2; n = 3). Additionally, in the fibrillized form, Ser129, but not Ser87, was a substrate for CK1 and CK2 when immunoreactivity with pSer129 and pSer87 was examined. These data indicate that fibrillized α-syn can be a substrate for kinases at Ser129.
FIGURE 9.

Fibrillized α-synuclein (α-syn) is a substrate for casein kinase (CK) 1 or CK2. Recombinant wild-type (WT) α-syn was fibrillized at 37°C at a concentration of 5 mg/ml for 72 hours. Polymerized α-syn was recovered, and both soluble (S) and fibrillized (F) were reacted in vitro with CK1 or CK2. Samples without kinase added were included as controls. Coomassie staining of polyacrylamide gels was used to evaluate overall protein levels, 32P incorporation evaluated overall phosphorylation, and Western blot analyses with pSer129 and pSer87 assessed phosphorylation at specific residues. Both soluble and fibrillized α-syn were substrates for CK1 and CK2. Fibrillation of α-syn resulted in increased 32P incorporation: an average of 52.5% for CK1 and 20.3% for CK2 (n = 3). Immunoblotting with pSer129 and pSer87 indicated phosphorylation at only Ser129 after fibrillation.
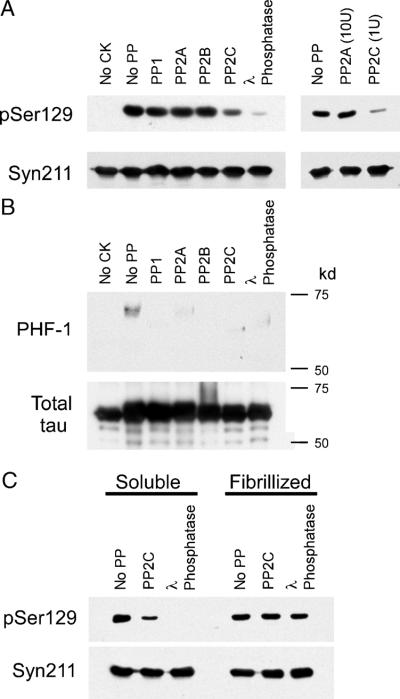
Wild-type α-syn was phosphorylated in vitro with CK2 and then subjected to the major brain protein phosphatases PP1, PP2A, PP2B, and PP2C. Dephosphorylation was also tested with L phosphatase, a nonspecific phosphatase for serine/threonine residues. Reductions of phospho-Ser129 were observed with only PP2C and λ phosphatases (Fig. 10A). Even the use of 10-fold more PP2A activity compared with PP2C did not result in the dephosphorylation of α-syn in vitro. To confirm that each of our phosphatases was active under the conditions used, recombinant tau was phosphorylated in vitro by CK1 and then subjected to each phosphatase (Fig. 10B). Every phosphatase effectively dephosphorylated tau under these conditions, similar to results reported by others (43-47). Therefore, our data suggest that PP2C would likely be the phosphatase involved in phospho-Ser129 regulation.
FIGURE 10.
Dephosphorylation of phospho-Ser129 α-synuclein (α-syn) by phosphatases in vitro. (A) Recombinant wild-type (WT) α-syn was phosphorylated by casein kinase (CK) 2, as described in Materials and Methods section. The kinase was inactivated, and then phosphorylated α-syn was incubated with no protein phosphatase (no PP), PP1, PP2A, PP2B, PP2C, or λ phosphatase. Dephosphorylation of α-syn was monitored by immunoblotting with pSer129. Protein phosphatase 2C and λ phosphatase were able to dephosphorylate Ser129, whereas PP1, PP2A, or PP2B could not. Ten-fold more PP2A than PP2C (right panel) had no effect on levels of phospho-Ser129 α-syn, whereas PP2C can dephosphorylate Ser129 (representative immunoblot of 3 independent experiments). Immunoblotting with Syn211 was used as a loading control and to verify the integrity of the protein throughout experimentation. (B) Recombinant tau protein was phosphorylated by CK1 in vitro and then subjected to PP1, PP2A, PP2C, or λ phosphatase. Immunoblotting with phospho-tau-specific antibody PHF-1 was used to monitor the phosphorylation of tau. (C) Recombinant WT α-syn was phosphorylated in the soluble or the fibrillized form (Fig. 9) and then treated with no PP, PP2C, or λ phosphatase. pSer129 immunoreactivity decreased for soluble α-syn with both PP2C (by 62%; p = 0.04 by 1-sample t-test; n = 3) and λ phosphatase (by 97%; p = 0.001 by 1-sample t-test; n = 3). With fibrillized phosphorylated α-syn, immunoreactivity with pSer129 decreased by only 16% for PP2C (p > 0.05 by 1-sample t-test; n = 3) and 21% for λ phosphatase (p = 0.04 by 1-sample t-test; n = 3). The levels of remaining pSer129 immunoreactivity between the soluble and fibrillized α-syn were significantly different (p = 0.04 for PP2C; p = 0.0001 for λ; n = 3).
Because our data indicate that fibrillized α-syn in pathologic inclusions is phosphorylated at Ser129, we examined whether phosphorylated, fibrillized α-syn can be a substrate for PP2C or λ. Either soluble or fibrillized WT α-syn was phosphorylated by CK2 and then exposed to no phosphatase, PP2C, or λ phosphatase (Fig. 10C). Only soluble α-syn exhibited any substantial decrease in phosphorylation after protein phosphatase exposure. Protein phosphatase 2C resulted in a 62% T 22% SD loss of phosphorylation for soluble but only a 16% T 12% SD loss for fibrillized (p = 0.04; n = 3). More dramatically, λ phosphatase resulted in a 97% T 5% SD loss of phosphorylation for soluble but only a 21% T 7% SD loss for fibrillized (p = 0.0001; n = 3). These data indicate that fibrillized α-syn is not a good substrate for protein phosphatases in vitro.
Regulation of α-Syn Phosphorylation in Cultured Cells
The data from transgenic mice indicated that under pathologic conditions, soluble α-syn can be hyperphosphorylated at Ser129. To examine the mechanisms that can influence the phosphorylation of α-syn Ser129 further, we used SH-SY5Y neuroblastoma cells that were stably transfected with WT human α-syn (37). Cells were treated with CK inhibitors (DMAT for CK2 and D4476 for CK1), with phosphatase inhibitors (OA for PP2A and FK506 for PP2B), and a battery of toxic treatments that affect specific cellular processes.
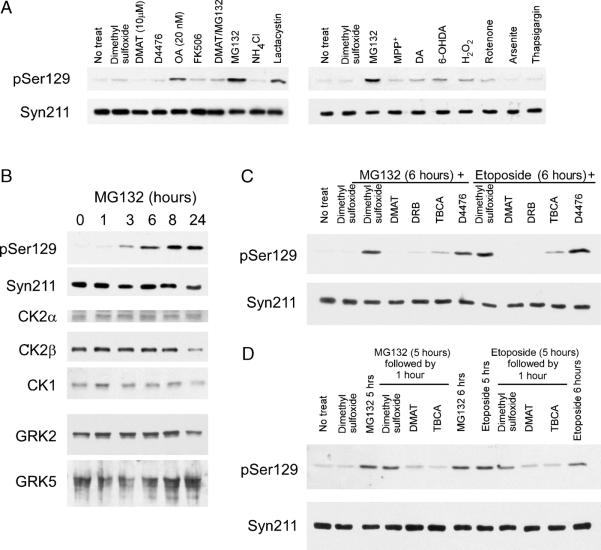
Immunoblotting with pSer129, SH-SY5Y cells presented a very low basal level of phosphorylation at Ser129 (Fig. 11A). After 3 hours of treatment, a partial decrease (~50%) in phospho-Ser129 α-syn was noted with DMAT (10 μmol/L), and no change was noted with D4476. The PP1 and PP2A inhibitor OA resulted in an increase in phosphorylation at a concentration that would be more specific to PP2A (20 nmol/L). The proteosomal inhibitors MG132 and lactacystin, but not the lysosomal inhibitor NH4Cl, resulted in a dramatic increase in the phosphorylation of Ser129 (~10-fold). Slight increases in phospho-Ser129 were observed with dopamine, 6-OHDA, hydrogen peroxide (H2O2), and rotenone, but not MPP+, arsenite, or thapsigargin. These increases were not as substantial as that noted with MG132. No changes in overall α-syn levels were noted with any treatment, and no pSer87 immunoreactivity was noted (data not shown). To examine if the increases in phosphorylation resulting from proteasomal inhibition were dependent on CK2, cultures were treated concurrently with MG132 and DMAT (10 μmol/L). This treatment significantly inhibited the MG132-mediated increase in Ser129 phosphorylation, suggesting that CK2 may be at least partially required for this effect. To assess the kinetics of Ser129 phosphorylation by MG132 further, a time-course analysis with MG132 was performed. Increases in phospho-Ser129 were noted first at 3 hours, increased through 8 hours, and maintained through 24 hours of treatment (Fig. 11B).
FIGURE 11.
Effect of stress conditions on phosphorylation of Ser129 in α-synuclein (α-syn) in SH-SY5Y neuroblastoma cells. (A) Western blot analysis of SH-SY5Y cells treated for 3 hours with 10 μmol/L of DMAT (a derivative of TBB; 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole), 10 μmol/L of D4476 [4-(4-(2,3-dihydrobenzo(1,4)dioxin-6-yl)-5-pyridin-2-yl-1H-imidazol-2-yl) benzamide], 20 nmol/L of okadaic acid (OA), 5 μmol/L of FK506, 10 μmol/L of concurrent DMAT and MG132, 25 mmol/L of NH4Cl, or 5 μmol/L of lactacystin, 10 μmol/L of MG132, 10 μmol/L of MPP+ (1-methyl-4-phenylpyridinium iodide), 500 μmol/L of dopamine (DA), 50 μmol/L of 6-hydroxydopamine (6-OHDA), 200 μmol/L of hydrogen peroxide (H2O2), 1 μmol/L of rotenone, 100 μmol/L of arsenite, and 2 μmol/L of thapsigargin. pSer129 immunoreactivity dramatic increased with MG132 (p = 0.002), lactacystin (p = 0.004), and OA (p = 0.04), but to a lesser extent with DA, 6-OHDA, H2O2, and rotenone. DMAT inhibited the phosphorylation resulting from MG132 treatment (p = 0.003 as compared with MG132 treatment alone; n = 3). No differences in total α-syn immunoreactivity were noted (Syn211 antibody). (B) SH-SY5Y cells were treated with 10 μmol/L of MG132 for 0, 1, 3, 6, 8, or 24 hours. pSer129 immunoreactivity increased with MG132 through 8 hours of treatment. No differences in total α-syn (Syn211 antibody), casein kinase (CK) 2α, CK2β, CK1, G-protein coupled receptor kinase (GRK) 2, or GRK5 expression were noted. (C) SH-SY5Y cells were treated concurrently for 6 hours with 10 μKmol/L of MG132 or 50 μmol/L of etoposide and vehicle (dimethyl sulfoxide) or 1 of the following CK inhibitors: 25 μmol/L of DMAT, 100 μmol/L of DRB (5,6-dichloro-1-A-D-ribofuranosylbenzimidazole), 20 μmol/L of TBCA [(E)-3-(2,3,4,5-tetrabiomophenyl)acrylic acid], or 10 μmol/L of D4476. Increases in phospho-Ser129 α-syn were prevented by the CK2 inhibitors DMAT (p < 0.0001), DRB (p = 0.0001), and TBCA (p = 0.0007), but not the CK1 inhibitor D4476 (each by 1-sample t-test as compared with MG132 and dimethyl sulfoxide; n = 4). (D) SH-SY5Y cells were treated for 5 hours with MG132 or etoposide, and then dimethyl sulfoxide, 25 μmol/L of DMAT, or 20 μmol/L of TBCA was added concomitantly for 1 hour. Representative immunoblots of pSer129 and α-syn indicated a significant decrease in pSer129 immunoreactivity after DMAT (71%; p < 0.0001) or TBCA treatment (84%; p < 0.0001; each by 1-sample t-test as compared with 5 hours of MG132 treatment; n = 4). Similar results are seen with etoposide.
The CK2 regulatory subunit CK2β can be a substrate for the proteasome in other systems (48). Treatment with MG132, however, did not alter CK2β expression over this period (Fig. 11B). Furthermore, no changes in expression levels of CK1, CKα, GRK2, and GRK5 were identified. Therefore, MG132 did not alter expression levels of the kinases.
To examine the kinase specificity of this hyperphosphorylated state induced by proteosomal inhibition, SH-SY5Y cells were treated for 6 hours concurrently with MG132 and either a CK2 inhibitor [DMAT, DRB, or TBCA] or a CK1 inhibitor (D4476). The CK2 inhibitors blocked the MG132-mediated increases in phosphorylation at Ser129 (Fig. 11C), whereas D4476 did not. Similar hyperphosphorylation of α-syn that was blocked by CK2 inhibitors was observed with etoposide, a cellular toxin that increases CK2 activity (49, 50).
These results suggest that proteasome inhibition may increase phosphorylation by increasing activity of CK2, or that phospho-Ser129 α-syn may be a specific substrate for proteosomal degradation. Although phosphorylation by CK2 may promote proteasome-mediated degradation in some systems (51, 52), no change in total α-syn expression or appearance of degradation products was noted in any of these conditions.
To distinguish between increased kinase activity or increased accumulation of the phosphorylated form of α-syn, SH-SY5Y cells were treated with MG132 for 5 hours, followed by concomitant addition of 25 μmol/L of DMAT or 20 μmol/L of TBCA for 1 hour. The short treatment with CK2 inhibitors resulted in a reversal of α-syn hyperphosphorylation (Fig. 11D). These findings indicate that MG132 treatment resulted in the increased CK2 activity that was responsible for hyperphosphorylation of α-syn. Five hours of etoposide treatment also resulted in hyperphosphorylation of α-syn that was reversed by 1 hour of CK2 inhibitor treatment. Therefore, α-syn hyperphosphorylation can result from toxic treatments that increase CK2 activity.
DISCUSSION
The role of α-syn pathologic inclusions in neurodegenerative diseases is the focus of intense research. Although genetic alterations causing point mutations or increased expression are mechanisms that can lead to disease, there is still much to learn regarding the cellular mechanism(s) that regulate inclusion formation. Phosphorylation of α-syn has been suggested as a modification that may influence this process.
Our in vitro data indicate that Ser129 can be phosphorylated by either CK1 or CK2, but Ser87 was modified only by CK1. In cultured cells, it was suggested that phosphorylation at Ser87 of α-syn may promote α-syn inclusion formation (17), but most pathologic inclusions were not stained with an antibody to phospho-Ser87 α-syn. Phosphorylated Ser87 was also not detected in biochemically extracted/soluble fractions or biochemical fractions enriched in pathologic inclusions isolated from human brain tissues or transgenic mouse expressing human α-syn. Using mass spectrometric analysis with complete sequence coverage, Anderson et al (20) also did not detect Ser87 phosphorylation in either soluble fractions or biochemical fractions enriched in aggregated α-syn in control brains or in brains with various α-synucleinopathies. Therefore, there is a lack of evidence for a role of Ser87 phosphorylation in the normal or diseased brains. Nevertheless, our in vitro data indicate that if phosphorylation of Ser87 were to occur, it would inhibit inclusion formation, which would be consistent with the notion that a large charged modification in the middle hydrophobic region of α-syn can suppress fibril formation (35).
In agreement with previous studies (19-21) and using a novel phospho-specific antibody, we observed that α-syn is highly phosphorylated at Ser129 in pathologic inclusions of human brain samples from patients with PD, DLB, or MSA. Hyperphosphorylation of Ser129 also was observed in inclusions of the human A53T α-syn transgenic mouse model of α-synucleinopathies used here, similar to other α-synucleinopathies (19-21, 53). Phosphorylation at Ser129 was not readily detectable in the soluble HS fractions from human brain tissues but was present at low levels in fractions from human α-syn transgenic mouse spinal cord tissue. This is likely a result of the rapid dephosphorylation of soluble α-syn that can occur postmortem (19, 54).
Some previous studies have suggested that phosphorylation at Ser129 may promote α-syn fibril or inclusion formation (19, 24, 55), whereas others have suggested that CK2-mediated phosphorylation of Ser129 α-syn prevents or has no effect on inclusion formation (25, 26). Our studies support the notion that Ser129 phosphorylation does not promote α-syn fibril formation and suggest that hyperphosphorylation of α-syn in inclusions may be due to 3 factors: 1) the ability of fibrillized α-syn to act as a substrate for kinases; 2) the relative inability of fibrillized-phosphorylated α-syn to be dephosphorylated; and 3) the increased activity of kinases associated with stress or toxic conditions as demonstrated in cultured cells. The latter is further supported by the increase in phosphorylation of Ser129 in soluble fractions in the sick human A53T α-syn transgenic mouse, whereas no progressive increase in phospho-Ser129 α-syn was identified in older, asymptomatic mice. Therefore, although we have also observed inclusions highly phosphorylated at Ser129, our studies suggest that fibril and inclusion formation likely occurs prior to phosphorylation, and that this phosphorylation becomes more pronounced as disease progresses.
The studies in cultured cells indicate that several different types of stresses can lead to an increase in Ser129 phosphorylation. Nevertheless, it is intriguing that drugs causing proteasomal inhibition lead to the most robust increases in Ser129 phosphorylation. This is particularly interesting because many studies have linked proteasomal inhibition or dysfunction with PD (56-61). Because some studies have suggested that aggregated α-syn may lead to dysfunction of the proteasome (62-64), aggregation of α-syn itself may contribute to the kinase activity that leads to α-syn hyperphosphorylation.
The increase in Ser129 phosphorylation associated with toxic treatment was predominantly due to CK2 activation, as demonstrated with CK2-specific inhibitors in cultured cells. In addition, the ability of Ser129 to be hyperphosphorylated in situ by CK2 was recapitulated with etoposide, a cellular toxin that is known to increase CK2 activity (49, 50). Increased CK2 activity associated with toxic challenges has been previously reported (55). The involvement of CK2 is further supported by a recent study demonstrating that CK2 is the major kinase involved in phosphorylating Ser129 in brain (54). However, the additive contributions of other kinases cannot be excluded.
In vitro dephosphorylation with the major brain protein phosphatases was used to assess the regulation of α-syn phosphorylation. Our in vitro data support PP2C as an important phosphatase involved in the dephosphorylation of Ser129 α-syn. This finding could not be further tested in situ or in vivo because PP2C-specific inhibitors are not yet readily available. Treatment of cultured cells with 20 nmol/L of OA resulted in increased Ser129 phosphorylation as reported by others (15, 54). Although OA at this concentration should specifically inhibit PP2A, PP2A did not dephosphorylate Ser129 in vitro. Okadaic acid treatment can result in complex effects, and general toxicity associated with PP2A inhibition may explain OA-mediated increases in phosphorylation at Ser129.
Overall, our data support the notion that Ser129 is phosphorylated at relatively low levels during steady state under normal conditions. Phosphorylation by CK2 and dephosphorylation by PP2C indicate that these may be important enzymes that regulate the phosphorylation of α-syn. Furthermore, the hyperphosphorylation of Ser129 α-syn observed in inclusions in α-synucleinopathies may be due to the intrinsic properties of aggregated α-syn to act as substrates for kinases, but not phosphatases, in addition to acting as a marker of cellular toxicity and proteasome dysfunction exhibited by activation of CK2. Therefore, phosphorylation of Ser129 in inclusions seems to provide a marker of the disease state. Moreover, the findings provide an alternative explanation for the high levels of Ser129 phosphorylation in pathologic α-syn inclusions.
ACKNOWLEDGMENTS
The authors thank Drs. John Q. Trojanowski and Virginia M.-Y. Lee (Center for Neurodegenerative Disease Research, University of Pennsylvania) and the families of patients who make this research possible.
This work was supported by Grant No. AG09215 from the National Institute on Aging and Grant No. NS053488 from the National Institute of Neurological Disorders and Stroke. E.A.W. was supported by training Grant No. T32 AG00255 from the National Institute on Aging.
REFERENCES
- 1.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 2.von Bohlen Und HO. Synucleins and their relationship to Parkinson`s disease. Cell Tissue Res. 2004;318:163–74. doi: 10.1007/s00441-004-0921-7. [DOI] [PubMed] [Google Scholar]
- 3.Lee VM, Trojanowski JQ. Mechanisms of Parkinson`s disease linked to pathological alpha-synuclein: New targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Sidhu A, Wersinger C, Vernier P. Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J. 2004;18:637–47. doi: 10.1096/fj.03-1112rev. [DOI] [PubMed] [Google Scholar]
- 5.Forman MS, Lee VM, Trojanowski JQ. Nosology of Parkinson`s disease: Looking for the way out of a quagmire. Neuron. 2005;47:479–82. doi: 10.1016/j.neuron.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson`s disease. Science. 1997;276:2045–47. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 7.Duda JE, Giasson BI, Gur TL, et al. Immunohistochemical and biochemical studies demonstrate a distinct profile of alpha-synuclein permutations in multiple system atrophy. J Neuropathol Exp Neurol. 2000;59:830–41. doi: 10.1093/jnen/59.9.830. [DOI] [PubMed] [Google Scholar]
- 8.Lippa CF, Schmidt ML, Lee VM, et al. Dementia with Lewy bodies. Neurology. 1999;52:893. doi: 10.1212/wnl.52.4.893. [DOI] [PubMed] [Google Scholar]
- 9.Spillantini MG, Schmidt ML, Lee VMY, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 10.Spillantini MG, Crowther RA, Jakes R, et al. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson`s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–8. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 11.Tu PH, Galvin JE, Baba M, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–22. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 12.Nakajo S, Tsukada K, Omata K, et al. A new brain-specific 14-kDa protein is a phosphoprotein. Its complete amino acid sequence and evidence for phosphorylation. Eur J Biochem. 1993;217:1057–63. doi: 10.1111/j.1432-1033.1993.tb18337.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakajo S, Shioda S, Nakai Y, et al. Localization of phosphoneuroprotein 14 (PNP 14) and its mRNA expression in rat brain determined by immunocytochemistry and in situ hybridization. Brain Res Mol Brain Res. 1994;27:81–86. doi: 10.1016/0169-328x(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 14.Shibayama-Imazu T, Okahashi I, Omata K, et al. Cell and tissue distribution and developmental change of neuron specific 14 kDa protein (phosphoneuroprotein 14) Brain Res. 1993;622:17–25. doi: 10.1016/0006-8993(93)90796-p. [DOI] [PubMed] [Google Scholar]
- 15.Okochi M, Walter J, Koyama A, et al. Constitutive phosphorylation of the Parkinson`s disease associated alpha-synuclein. J Biol Chem. 2000;275:390–97. doi: 10.1074/jbc.275.1.390. [DOI] [PubMed] [Google Scholar]
- 16.Arawaka S, Wada M, Goto S, et al. The role of G-proteinYcoupled receptor kinase 5 in pathogenesis of sporadic Parkinson`s disease. J Neurosci. 2006;26:9227–38. doi: 10.1523/JNEUROSCI.0341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim EJ, Sung JY, Lee HJ, et al. Dyrk1A phosphorylates alpha-synuclein and enhances intracellular inclusion formation. J Biol Chem. 2006;281:33250–57. doi: 10.1074/jbc.M606147200. [DOI] [PubMed] [Google Scholar]
- 18.Pronin AN, Morris AJ, Surguchov A, et al. Synucleins are a novel class of substrates for G proteinYcoupled receptor kinases. J Biol Chem. 2000;275:26515–22. doi: 10.1074/jbc.M003542200. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara H, Hasegawa M, Dohmae N, et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–64. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JP, Walker DE, Goldstein JM, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–52. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 21.Kahle PJ, Neumann M, Ozmen L, et al. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3:583–88. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishie M, Mori F, Fujiwara H, et al. Accumulation of phosphorylated alpha-synuclein in the brain and peripheral ganglia of patients with multiple system atrophy. Acta Neuropathol (Berl) 2004;107:292–98. doi: 10.1007/s00401-003-0811-1. [DOI] [PubMed] [Google Scholar]
- 23.Neumann M, Kahle PJ, Giasson BI, et al. Misfolded proteinase KYresistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–39. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith WW, Margolis RL, Li X, et al. Alpha-synuclein phosphorylation enhances eosinophilic cytoplasmic inclusion formation in SH-SY5Y cells. J Neurosci. 2005;25:5544–52. doi: 10.1523/JNEUROSCI.0482-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee G, Tanaka M, Park K, et al. Casein kinase IIYmediated phosphorylation regulates alpha-synuclein/synphilin-1 interaction and inclusion body formation. J Biol Chem. 2004;279:6834–39. doi: 10.1074/jbc.M312760200. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–63. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 27.Giasson BI, Jakes R, Goedert M, et al. A panel of epitope-specific antibodies detects protein domains distributed throughout human alpha-synuclein in Lewy bodies of Parkinson`s disease. J Neurosci Res. 2000;59:528–33. doi: 10.1002/(SICI)1097-4547(20000215)59:4<528::AID-JNR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Giasson BI, Duda JE, Murray IV, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–89. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 29.Duda JE, Giasson BI, Mabon ME, et al. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002;52:205–10. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 30.Otvos L, Jr, Feiner L, Lang E, et al. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;39:669–73. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- 31.Kosik KS, Orecchio LD, Binder L, et al. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988;1:817–25. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- 32.Greenbaum EA, Graves CL, Mishizen-Eberz AJ, et al. The E46K mutation in alpha-synuclein increases amyloid fibril formation. J Biol Chem. 2005;280:7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- 33.Giasson BI, Covy JP, Bonini NM, et al. Biochemical and pathological characterization of Lrrk2. Ann Neurol. 2006;59:315–22. doi: 10.1002/ana.20791. [DOI] [PubMed] [Google Scholar]
- 34.Giasson BI, Duda JE, Quinn SM, et al. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron. 2002;34:521–33. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 35.Giasson BI, Murray IVJ, Trojanowski JQ, et al. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–86. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 36.Crystal AS, Giasson BI, Crowe A, et al. A comparison of amyloid fibrillogenesis using the novel fluorescent compound K114. J Neurochem. 2003;86:1359–68. doi: 10.1046/j.1471-4159.2003.01949.x. [DOI] [PubMed] [Google Scholar]
- 37.Mazzulli JR, Mishizen AJ, Giasson BI, et al. Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci. 2006;26:10068–78. doi: 10.1523/JNEUROSCI.0896-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagano MA, Meggio F, Ruzzene M, et al. 2-Dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole: A novel powerful and selective inhibitor of protein kinase CK2. Biochem Biophys Res Commun. 2004;321:1040–44. doi: 10.1016/j.bbrc.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 39.Pagano MA, Andrzejewska M, Ruzzene M, et al. Optimization of protein kinase CK2 inhibitors derived from 4,5,6,7-tetrabromobenzimidazole. J Med Chem. 2004;47:6239–47. doi: 10.1021/jm049854a. [DOI] [PubMed] [Google Scholar]
- 40.Pagano MA, Poletto G, DiMaira G, et al. Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. Chembiochem. 2007;8:129–39. doi: 10.1002/cbic.200600293. [DOI] [PubMed] [Google Scholar]
- 41.Baba M, Nakajo S, Tu PH, et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson`s disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–84. [PMC free article] [PubMed] [Google Scholar]
- 42.Sampathu DM, Giasson BI, Pawlyk AC, et al. Ubiquitination of alpha-synuclein is not required for formation of pathological inclusions in alpha-synucleinopathies. Am J Pathol. 2003;163:91–100. doi: 10.1016/s0002-9440(10)63633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu F, Grundke-Iqbal I, Iqbal K, et al. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–50. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto H, Hasegawa M, Ono T, et al. Dephosphorylation of fetal-tau and paired helical filamentsYtau by protein phosphatases 1 and 2A and calcineurin. J Biochem (Tokyo) 1995;118:1224–31. doi: 10.1093/oxfordjournals.jbchem.a125011. [DOI] [PubMed] [Google Scholar]
- 45.Gong CX, Grundke-Iqbal I, Iqbal K. Dephosphorylation of Alzheimer`s disease abnormally phosphorylated tau by protein phosphataseY2A. Neuroscience. 1994;61:765–72. doi: 10.1016/0306-4522(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 46.Gong CX, Grundke-Iqbal I, Damuni Z, et al. Dephosphorylation of microtubule-associated protein tau by protein phosphataseY1 and Y2C and its implication in Alzheimer disease. FEBS Lett. 1994;341:94–98. doi: 10.1016/0014-5793(94)80247-5. [DOI] [PubMed] [Google Scholar]
- 47.Gong CX, Singh TJ, Grundke-Iqbal I, et al. Alzheimer`s disease abnormally phosphorylated tau is dephosphorylated by protein phosphataseY2B (calcineurin) J Neurochem. 1994;62:803–6. doi: 10.1046/j.1471-4159.1994.62020803.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhang C, Vilk G, Canton DA, et al. Phosphorylation regulates the stability of the regulatory CK2beta subunit. Oncogene. 2002;21:3754–64. doi: 10.1038/sj.onc.1205467. [DOI] [PubMed] [Google Scholar]
- 49.DeVore RF, Corbett AH, Osheroff N. Phosphorylation of topoisomerase II by casein kinase II and protein kinase C: Effects on enzyme-mediated DNA cleavage/religation and sensitivity to the antineoplastic drugs etoposide and 4′-(9-acridinylamino)methane-sulfon-m-anisidide. Cancer Res. 1992;52:2156–61. [PubMed] [Google Scholar]
- 50.DiMaira G, Brustolon F, Bertacchini J, et al. Pharmacological inhibition of protein kinase CK2 reverts the multidrug resistance phenotype of a CEM cell line characterized by high CK2 level. Oncogene. 2007;26:6915–26. doi: 10.1038/sj.onc.1210495. [DOI] [PubMed] [Google Scholar]
- 51.Franck N, Le SJ, Guguen-Guillouzo C, et al. Hepatitis C virus NS2 protein is phosphorylated by the protein kinase CK2 and targeted for degradation to the proteasome. J Virol. 2005;79:2700–2708. doi: 10.1128/JVI.79.5.2700-2708.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim YS, Lee JY, Son MY, et al. Phosphorylation of threonine 10 on CKBBP1/SAG/ROC2/Rbx2 by protein kinase CKII promotes the degradation of IkappaBalpha and p27Kip1. J Biol Chem. 2003;278:28462–69. doi: 10.1074/jbc.M302584200. [DOI] [PubMed] [Google Scholar]
- 53.Yamada M, Iwatsubo T, Mizuno Y, et al. Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: Resemblance to pathogenetic changes in Parkinson`s disease. J Neurochem. 2004;91:451–61. doi: 10.1111/j.1471-4159.2004.02728.x. [DOI] [PubMed] [Google Scholar]
- 54.Ishii A, Nonaka T, Taniguchi S, et al. Casein kinase 2 is the major enzyme in brain that phosphorylates Ser129 of human alpha-synuclein: Implication for alpha-synucleinopathies. FEBS Lett. 2007;581:4711–17. doi: 10.1016/j.febslet.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi M, Ko LW, Kulathingal J, et al. Oxidative stress-induced phosphorylation, degradation and aggregation of alpha-synuclein are linked to upregulated CK2 and cathepsin D. Eur J Neurosci. 2007;26:863–74. doi: 10.1111/j.1460-9568.2007.05736.x. [DOI] [PubMed] [Google Scholar]
- 56.McNaught KS, Jenner P. Proteasomal function is impaired in substantia nigra in Parkinson`s disease. Neurosci Lett. 2001;297:191–94. doi: 10.1016/s0304-3940(00)01701-8. [DOI] [PubMed] [Google Scholar]
- 57.McNaught KS, Belizaire R, Jenner P, et al. Selective loss of 20S proteasome alphaYsubunits in the substantia nigra pars compacta in Parkinson`s disease. Neurosci Lett. 2002;326:155–58. doi: 10.1016/s0304-3940(02)00296-3. [DOI] [PubMed] [Google Scholar]
- 58.McNaught KS, Jackson T, JnoBaptiste R, et al. Proteasomal dysfunction in sporadic Parkinson`s disease. Neurology. 2006;66:S37–S49. doi: 10.1212/wnl.66.10_suppl_4.s37. [DOI] [PubMed] [Google Scholar]
- 59.Tofaris GK, Razzaq A, Ghetti B, et al. Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem. 2003;278:44405–11. doi: 10.1074/jbc.M308041200. [DOI] [PubMed] [Google Scholar]
- 60.Tofaris GK, Layfield R, Spillantini MG. Alpha-synuclein metabolism and aggregation is linked to ubiquitin-independent degradation by the proteasome. FEBS Lett. 2001;509:22–26. doi: 10.1016/s0014-5793(01)03115-5. [DOI] [PubMed] [Google Scholar]
- 61.Mytilineou C, McNaught KS, Shashidharan P, et al. Inhibition of proteasome activity sensitizes dopamine neurons to protein alterations and oxidative stress. J Neural Transm. 2004;111:1237–51. doi: 10.1007/s00702-004-0167-2. [DOI] [PubMed] [Google Scholar]
- 62.Snyder H, Mensah K, Theisler C, et al. Aggregated and monomeric α-synuclein bind to the S6′ proteasomal protein and inhibit proteasomal function. J Biol Chem. 2003;278:11753–59. doi: 10.1074/jbc.M208641200. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka Y, Engelender S, Igarashi S, et al. Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum Mol Genet. 2001;10:919–26. doi: 10.1093/hmg/10.9.919. [DOI] [PubMed] [Google Scholar]
- 64.Lindersson E, Beedholm R, Hojrup P, et al. Proteasomal inhibition by alpha-synuclein filaments and oligomers. J Biol Chem. 2004;279:12924–34. doi: 10.1074/jbc.M306390200. [DOI] [PubMed] [Google Scholar]