Summary
Cerebral hypothermia reduces brain injury and improves behavioral recovery after hypoxia–ischemia (HI) at birth. However, using current enrolment criteria many infants are not helped, and conversely, a significant proportion of control infants survive without disability. In order to further improve treatment we need better biomarkers of injury. A ‘true’ biomarker for the phase of evolving, ‘treatable’ injury would allow us to identify not only whether infants are at risk of damage, but whether they are still able to benefit from intervention. Even a less specific measure that allowed either more precise early identification of infants at risk of adverse neurodevelopmental outcome would reduce the variance of outcome of trials, improving trial power while reducing the number of infants unnecessarily treated. Finally, valid short-term surrogates for long term outcome after treatment would allow more rapid completion of preliminary evaluation and thus allow new strategies to be tested more rapidly. Experimental studies have demonstrated that there is a relatively limited ‘window of opportunity’ for effective treatment (up to about 6–8 h after HI, the ‘latent phase’), before secondary cell death begins. We critically evaluate the utility of proposed biochemical, electronic monitoring, and imaging biomarkers against this framework. This review highlights the two central limitations of most presently available biomarkers: that they are most precise for infants with severe injury who are already easily identified, and that their correlation is strongest at times well after the latent phase, when injury is no longer ‘treatable’. This is an important area for further research.
Keywords: Asphyxia, Biomarkers, Brain metabolism, Electroencephalogram, Hypoxic–ischemic encephalopathy, Near-infrared spectroscopy
Introduction
The seminal discovery about perinatal hypoxia–ischemia (HI) in the last century was that although some brain injury can occur during a sufficiently prolonged/severe episode of HI, in many cases damage actually continued to evolve for hours after resuscitation, during the recovery period.1,2 This evolution offered the tantalizing prospect that there might be a ‘window of opportunity’ to provide treatment to reduce or prevent injury. This potential has been confirmed by the finding that hypothermia can significantly reduce neurodevelopmental disability in infants with acute moderate–severe HI encephalopathy (HIE) at birth (e.g. as highlighted by the systematic meta-analysis by P.S. Shah in this issue of Seminars and by Edwards et al.3). However, these data also clearly show both that protection is only partial, so that many infants still die or have disabilities at 18 months of age. Conversely, approximately a third or more of infants receiving conventional normothermic care in the major trials survived without severe disability.4–6
Clearly, this is not an ideal base from which to further improve outcomes. As for any active intervention, all parents and clinicians would greatly prefer to deliver therapeutic hypothermia only to infants who would benefit from it. Even more importantly, the implication for future studies of improved treatment strategies is that the combination of limited precision plus the reduced rate of adverse outcome with current hypothermic treatment markedly reduces trial power, and so we will need trials that are at least an order of magnitude larger than previous randomized trials of hypothermia against normothermia.7 Thus, it will be important to more precisely target infants who will go to develop clinically significant injury without treatment and determine whether they are ‘treatable’, i.e. whether they are likely to benefit from hypothermia or other interventions. A further hindrance to progress is that currently several years are needed after treatment before neurodevelopmental outcome can be evaluated.
These considerations show why better ‘biomarkers’, biological markers to better quantify the severity of the initial HI insult, and to rapidly determine prognosis before treatment (risk of bad outcome) and then after treatment (valid early surrogates for long term neurodevelopmental outcome) would be of huge benefit for further trials. Even better would be a biomarker for the biological processes involved in the evolving brain injury, since this would both allow us to identify infants who were ‘treatable’, and to provide immediate feedback on whether the intervention was modifying the course of injury.
This chapter dissects the evidence for some of biomarkers already in use and the potential of novel biomarkers to contribute to refinement of therapeutic hypothermia for treatment of infants after HI at birth. We will particularly focus on their potential to answer the central questions: (1) Will the insult cause injury: (2) Are we still in time to treat? The potential for biomarkers such as magnetic resonance imaging (MRI) and electroencephalogram (EEG) to provide robust surrogate outcomes is addressed in detail by others in this issue of Seminars (D. Azzopardi and A.D. Edwards, and M. Thoresen, respectively).
Timing: why is it so critical?
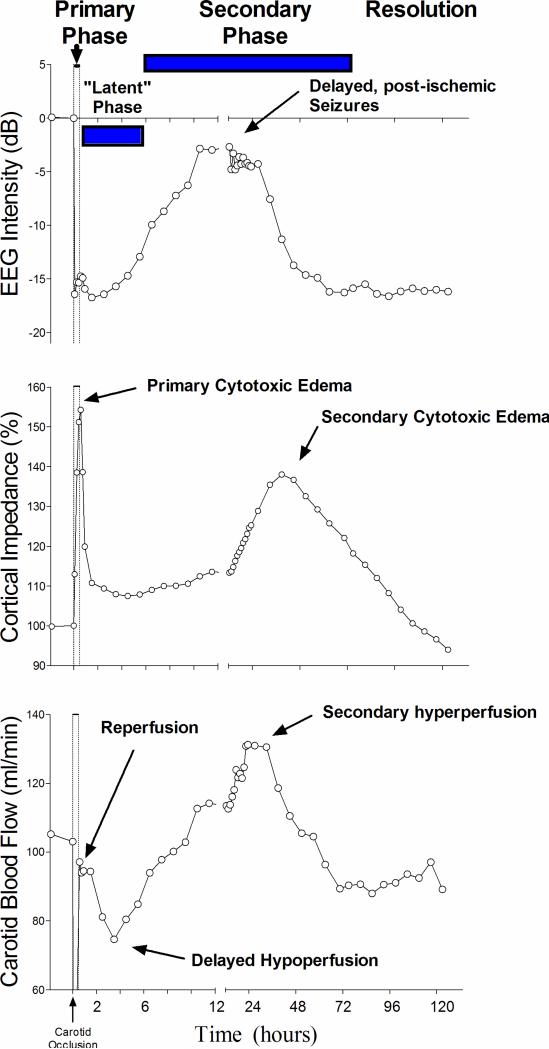
Experimental and clinical studies have shown that while brain cells may die during a sufficiently profound or prolonged episode of HI with primary cerebral energy failure (the ‘primary phase’ of injury, Fig. 1), even after surprising severe insults,8,9 many cells show initial partial or complete recovery (in a ‘latent’ phase). However, this recovery is only transient, and may be followed by deterioration secondarily, with failure of oxidative metabolism (the ‘secondary phase’ of injury). This failure is associated with cytotoxic edema, seizures, cerebral hyperperfusion and ultimately cell death.7 For single, acute insults the secondary phase may start as late as 6–8 h after the end of the insult (Figs 1 and 2).7 Consistent with the experimental pattern, clinical neurodevelopmental outcomes at 1 and 4 years of age are closely correlated with the severity of the secondary failure of oxidative metabolism at 15 h.10
Figure 1.
Illustration of the pathophysiological phases of injury after 30 min of global cerebral ischemia in fetal sheep; data derived from Gunn et al.97 The phases of injury include the immediate reperfusion period lasting about 30 min, during which cellular energy metabolism is restored, with resolution of the acute hypoxic depolarization and cell swelling. This is followed by a latent phase starting about 30–45 min after reperfusion, lasting for up to 6–15 h, in which oxidative cerebral energy metabolism normalizes but electroencephalogram (EEG) activity remains depressed, often with a delayed period of reduced cerebral blood flow. The ‘latent’ phase appears to correspond with the practical window of opportunity for effective neuroprotection. Following the latent phase there is secondary deterioration with delayed seizures and cytotoxic edema as shown by increased tissue impedance, increased blood flow, extracellular accumulation of potential cytotoxins (such as the excitatory neurotransmitters), and about 6–15 h after the asphyxia, failure of oxidative metabolism and damage.7 The acute changes in this phase may take 3 days or more to resolve.
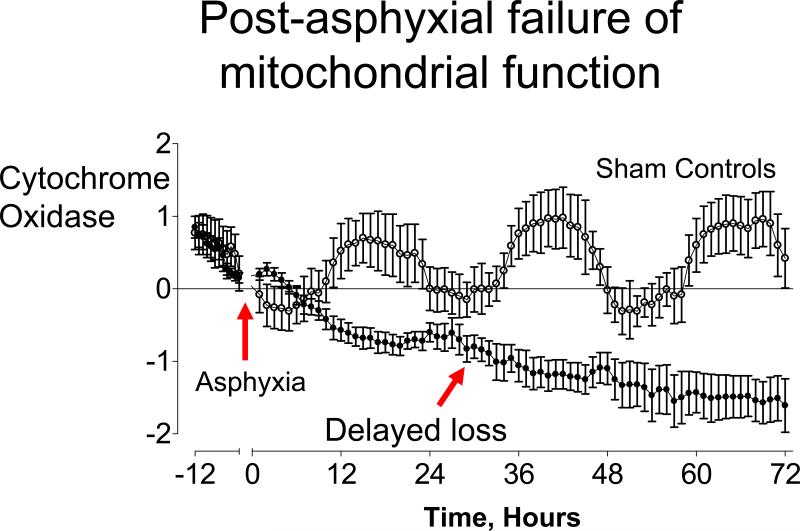
Figure 2.
Evidence of delayed mitochondrial dysfunction after severe hypoxia/ischemia in preterm fetal sheep: time sequence of concentration changes in fetal cytochrome oxidase (CytOx) measured by near-infrared spectroscopy before and after asphyxia induced by 25 min of umbilical cord occlusion (•, n = 7) or sham occlusion (○, n = 7) in preterm fetal sheep. Occlusion data not shown. Data are mean ± SEM hourly averages; derived from Bennet et al.61
[Typesetter: Fig. 2: delete heading above graph. Red arrows: change to black.]
Although we do not know precisely when injury ceases to be ‘treatable’, the preponderance of experimental evidence to date suggests that once the secondary deterioration has started, for practical purposes cells are becoming irreversibly injured.2 Experimental studies of cerebral hypothermia, for example, found that treatment efficacy was progressively lost the longer the initiation of treatment was delayed, and that there was no significant protection if cooling was started after the onset of delayed seizures.7 Based on these experimental data, clinical trials of hypothermia have required that infants are recruited within 6 h after birth.4,6 We also know from experimental studies that hypothermia, at least, is ineffective unless cooling is continued until the end of the secondary phase, well after the latent phase. Although the optimal duration of treatment is still unclear, cooling typically seems to need to be continued for at least 48–72 h for sustained neuroprotection.7 This suggests that whereas cellular stabilization may occur in the latent phase, true repair occurs over longer periods of time.
Why is treatment within 6 h after birth not always effective?
In real life, unlike the in laboratory, insults are not always clearly defined. First, injury may begin many hours before birth,11 and often involves repeated or prolonged exposure to asphyxia.12,13 Thus, injury may already be evolving at the time of birth, leading to a very short or even no latent phase for hypothermia to be of benefit. Finally, to confuse the picture further, some infants go into labor already injured from an insult much earlier in gestation.14,15 However, the majority of cases of acute HI encephalopthy are associated with acute cerebral injury, and therefore are at least potentially treatable.16
Further, the duration of the latent phase is modulated by the severity of the insult, such that the more severe the insult, the shorter the injury, the faster the transition to irreversible death and the shorter the potential window of opportunity for treatment.8,9,17 Indeed, some cells are so injured that they will never fully restore mitochondrial function.18 Thus, although we cannot yet define this group specifically, in principle there must be a cohort of infants are who are too severe to treat.
Has an infant been exposed to an HI insult?
The most ‘classic’ biomarkers are those for exposure to HI. Exposure to HI around the time of birth may be inferred from some combination of the presence of non-reassuring fetal heart changes, an oxygen debt (increased base deficit and blood lactate values) on cord blood gases, and need for resuscitation (i.e. Apgar score), all of which are easily and routinely documented.4,6,11 Unfortunately, non-reassuring heart rate changes are well known to have a very low positive predictive value for neural injury.19 For example, Murray et al. have shown that among infants who develop encephalopathy after birth, those with initially normal fetal heart rate tracings followed by acute ‘sentinel’ events before birth did develop more severe encephalopathy than infants with other patterns such as a progressive deterioration in heart rate pattern – but only a minority (11.5%) of infants with encephalopathy showed this pattern.20
Total oxygen debt, including base deficit (BD) and fetal lactate concentrations, should be a direct measure of anaerobic metabolism. In practice, they show a rather broad, imprecise relationship with neonatal encephalopathy. For example, profound acidosis (BD >18 mmol/L at 30 min of life) was associated with moderate–severe encephalopathy in nearly 80% of patients,21 and no cases occurred with BD <10–12 mmol/L.21,22 Between these extremes, however, outcomes are rather variable. Low et al., for example, found that fewer than half of babies born with cord blood BD >16 mmol/L (and pH <7.0) developed significant encephalopathy, and that encephalopathy still occurred, although at low frequency (~10% of cases), in cases with moderate metabolic acidosis of between 12 and 16 mmol/L.22 Thus, infants at either extreme are correctly identified with reasonable precision (i.e. no versus severe HIE), but not the intermediate group.
These findings denote that while exposure to lack of oxygen is necessary to cause injury, it is not sufficient. The healthy fetus has a remarkable ability to adapt to profound and often prolonged asphyxia, and to frequently tolerate such insults without injury.2,23 This is in large part due to the far greater anerobic neural and cardiac tolerance of the fetus of all mammalian species studied compared with adults,24,25 coupled with the ability to mount a timely and coordinated cardiovascular and metabolic defense to hypoxia.2,26 Thus, both physiological adaptation which allowed the fetus to survive uninjured, and failure of that adaptation that ultimately leads to injury, may be accompanied by a substantial oxygen debt.11
The need for resuscitation at birth is bedeviled by similar limitations. The National Institute of Child Health and Human Development (NICHD) Neonatal Research Network hypothermia trial has reported recently demonstrated, for example, that severely depressed Apgar scores (scores of 0–2) at 10 min of age can identify infants at high risk (76–82%) of disability or death well before more specific evaluations are available for infants with HIE.27 However, only 27% of infants who were recruited to the NICHD trial with moderate or severe HIE had Apgar scores of 0–2.
Possible biochemical markers
During and after exposure to HI, a variety of biochemical markers are elevated in body fluids, are reasonably easy to access, and to measure.28 Given that many are activated within the brain by hypoxia, a number have been suggested to be useful as sentinel biomarkers for HIE, including S100B, neuron-specific enolase (NSE), activin A, adrenomedullin, and interleukin (IL)-1β, and IL-6.28,29 All of these biochemical candidates are induced after hypoxia. However, it is important to note that they can be elevated in other settings. S100B levels, for example, are markedly increased by intrauterine growth restriction or chronic hypoxia,30 are is common during recovery from HI),31 perinatal infection/inflammation, central and peripheral hypoperfusion (which are is common during recovery from HI),31 perinatal infection/inflammation,32,33 traumatic delivery,34 pre-existing neural injury,35 treatments such as prenatal maternal glucocorticoids and anesthetics,36,37 and preterm gestational age,38 as well as being affected by the sex of the infant.36,37,39 Further, the brain does not appear to be the only source for S100B and NSE, for example, which may be released from a variety of tissues, including the umbilical cord and placenta.40
In a recent meta-analysis serum IL-1β and IL-6, and cerebrospinal fluid IL-1β and NSE measured before 96 h of age were significant predictive of long term neurodevelopmental outcome.29 In single studies, urine lactate, first urine 100B, cord blood IL6, serum non-protein-bound iron, serum CD14 cell, nuclear factor-κβ, serum IL-8, and serum ionized calcium also appeared to predict death or abnormal outcomes.29 Several key limitations need to be kept in mind. First, there have been few long term follow-up studies (>12 months of age) to assess neurodevelopmental disablity.29 This is a particular concern for infants with moderate (stage II) encephalopathy who have variable outcomes.6 Many studies used birth asphyxia criteria (Apgar scores or pH and lactate), or neurodevelopment at one week using the Sarnat and Sarnat assessment scale,41 all of which have limited discrimination for long term outcome.42 This is not a theoretical concern; Nadgyman et al. demonstrated that S100B and creatine kinase (CK-BB) measured at 2 h after birth correlated well with HIE indices at birth,43 but subsequently found that there was no significant correlation with neurodevelopmental outcome at age 20 months.44
Second, a delay of up to 96 h before samples are taken is not meaningful for selection of infants with encephalopathy. The majority of studies of biochemical markers have been based on samples taken after 24 h, i.e. well after the latent phase. Even when early samples were taken, the apparent time course has been rather variable. For example, Nadyman et al. reported that S100B, an astroglial protein that leaks from damaged cells, peaked by 2–6 h after birth asphyxia.43 By contrast, others reported that levels remained elevated for 1–2 days.45–47 Bashir et al. reported that S100B levels in urine were elevated at the first measurement at 4 h, and that levels reached a plateau at 12 h, which was sustained for the following week.48 There are similar data in preterm infants in whom serum levels were elevated at 3 h in all infants including those with mild HIE; values stayed relatively constant in the mild group, but increased over time in the severe group.49 In experimental studies in fetal sheep, moderate hypoxia, which does not cause injury, significantly elevates S100B, and these values are sustained for an hour after the insult.50 By contrast, after asphyxia that produced mild, moderate or severe brain injury, there were no early changes, and elevated levels were found only in two fetuses with moderate and severe changes at 24 h after the insult.51 Collectively, these studies suggest that S100B is actually a marker of impaired oxidative metabolism whether in the primary or secondary phases rather than of the latent phase. Thus, elevated levels late in recovery may provide good prediction of adverse outcomes. This interpretation is consistent with studies of traumatic brain injury in older children.52,53
Finally, whereas many of the biochemical markers do appear to distinguish between no or mild injury and severe injury,29 it is not clear that they are any better at predicting outcome for infants with stage II encephalopathy (moderate–severe) than either acid–base values or indeed just knowing the severity of encephalopathy. In many of these studies, there was considerable overlap in values between groups, and babies could have low levels yet have adverse outcomes and vice versa. Further experimental studies are needed to relate time course changes to the evolution of injury and to final outcome before we can conclude that any of these parameters can improve prediction of evolving injury in a timely manner.
Electrophysiology: electroencephalography
Like blood sampling, the EEG can be readily measured at the bedside. As reviewed in detail by M. Thoresen in the present volume, there is evidence that the combination of EEG amplitude or pattern assessment with clinical assessment of neurological abnormalities may improve specificity for adverse outcome.54 Of particular promise, in the CoolCap trial, infants with the most severe EEG changes as shown by the combination of seizures and severe suppression of background activity at the time of recruitment did not appear to improve after cooling.6 This finding has not yet been replicated, and we must note that the mean incidence of adverse outcome (death or disability at 18 months of age) in infants treated with standard therapy in the CoolCap and TOBY trials of therapeutic hypothermia, which required evidence of moderate–severe suppression of EEG amplitude in the first 6 h of life as part of the recruitment criteria,6,55 was comparable to that in the NICHD trial that used only clinical criteria.4
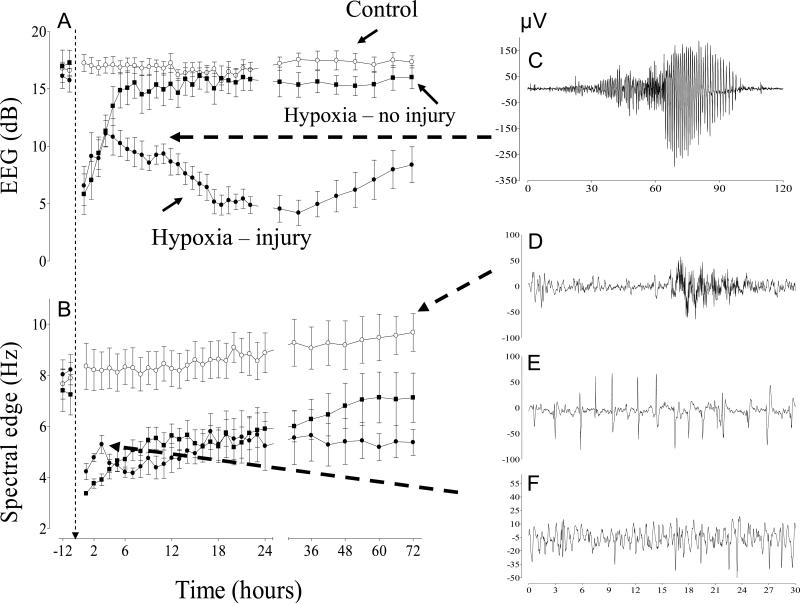
This disappointing outcome most likely reflects experimental and clinical data suggesting that severe suppression of EEG activity in the first 6 h after HI can occur both in subjects with good and bad outcomes.56–58 In fetal sheep, for example, EEG amplitude was suppressed in the first 6 h after asphyxia induced by complete umbilical cord occlusion, regardless of neural outcomes (normal or severely injured) (Fig. 3).58 Both groups then showed a progressive improvement in EEG amplitude, but for different reasons. In the fetuses who sustained no injury, EEG returned to normal baseline values. In the fetuses who developed severe brain injury, the EEG remained highly abnormal, but mean amplitude increased prior to the onset of frank electrographic and clinical seizures.
Figure 3.
(A, B) Time changes in electroencephalogram (EEG) amplitude and spectral edge in 0.6 gestation fetal sheep before and after either sham asphyxia (○, control group), 20 min of asphyxia (■, hypoxia–no injury group), or 30 min of asphyxia (•, hypoxia–injury group) induced by complete umbilical cord occlusion. (C) Example of a large amplitude stereotypic evolving seizure occurring 10 h after the end of asphyxia in the 30 min hypoxia–injury group. (D) Example of a normal EEG showing mixed amplitude and frequencies in the control group. (E, F) Examples of epileptiform transient activity occurring in the first 2 h after the end of asphyxia in the 30 min injury group. Spectral edge transiently increases at this time. No seizures or epileptiform transients were observed in the 20 min hypoxia–no injury group or the control group. Data derived from George et al.58
Thus, suppression of EEG activity alone is insufficient to discriminate between injured and uninjured brain. This is likely to reflect a balance between injury-induced impairment of brain function associated with severe damage and active suppression of brain activity by increased release of neuroinhibitors such as neurosteroids and noradrenaline after moderate insults that helps to improve recovery.59,60 Intriguingly, in the early recovery phase after severe asphyxia in preterm fetal sheep, there was a transient increase in the frequency of EEG activity despite sustained suppression of amplitude (Fig. 3) (George,58 #1500). This rise was associated with a small fall in relative cerebral oxygenation measured with near-infrared spectroscopy,61 and a modest increase in cytochrome oxidase activity similar to that seen during hypoxia,62 consistent with a genuine although small relative increase in brain activity and metabolism. The continuous EEG showed marked EEG transient activity superimposed on a profoundly suppressed background.58,63 This abnormal EEG activity is epileptiform in nature; not the stereotypic evolving seizures seen during the secondary phase of recovery, but rather subtle discrete, fast, sharp, and slow wave spikes; typically high frequency, low amplitude events occurring <400 ms (Fig. 3).64 Their incidence is greatest in the latent phase,63,65–68 and in several studies correlated closely with the severity of neuronal loss in the hippocampus and basal ganglia.58,66,67 In the human newborn epileptiform transients are also documented, but not fully characterized. In preterm infants similar fast epileptiform EEG transients are well-recognized 69,70, and high levels of these transients are associated with adverse neurodevelopmental outcomes.69–72
It is not yet clear whether these transient events are a manifestation of injury or a cause. The finding that suppression of these events is associated with improved outcomes,63,66 despite augmenting them, is associated with increased injury.67 This supports the hypothesis that epileptiform transients are a manifestation of post-hypoxic hyperexcitability of the glutamate receptor, as occurs in immature rodents.73,74 Similarly, in adult models peri-infarct depolarising waves (spreading depression waves) develop after ischemia, and can contribute to expansion of injury by increasing the workload of stressed cells.75 The increasing energy imbalance hastens the failure of the energy status of sick cells, and consequent impairment of cellular homeostasis, ATP production and initiation of cell death processes (secondary energy failure).76
These exciting findings raise the possibility that frequent epileptiform transient activity may be a biomarker of evolving injury in the early recovery phase. This possibility needs to be assessed in focused clinical studies to determine whether these or other features of the EEG are present at all gestational ages after asphyxia, or only in premature infants. If they are, then further evaluation is needed to determine their prognostic reliability in comparison with traditional EEG measures. Finally, we note that other features of the EEG may also have prognostic value. For example, variation of the power spectrum,77 evaluation of non-linear quantitative EEG measures of frequency,78 and long-range correlations in sub-band EEG signals by detrended fluctuation analysis (DFA) may be predictive of outcome and the phase of injury.79 Finally, the diagnostic and prognostic capacity of the EEG may be improved when combined with other markers, such as measures of cerebral oxidative metabolism as discussed below.80
Magnetic resonance spectroscopy and near-infrared spectroscopy
Magnetic resonance spectroscopy (MRS)81 and near-infrared spectroscopy (NIRS)82 can be used to estimate recovery of oxidative metabolism, and thus of mitochondrial function, after birth. MRS is expensive, and few centers at present have this technique available for use within the intensive care unit. Thus in practice its use is hampered by the need to transport sick sedated infants. NIRS can be implemented at the bedside, but provides less direct measures of oxidative metabolism.
[B]Magnetic resonance spectroscopy
Early studies of infants with HIE found that measures of oxidative metabolism such as the ratio of phosphocreatine (PCr) to inorganic phosphate (Pi) fall sharply during HI, are restored during reperfusion and the latent phase, then progressively decline in the secondary phase.83 The severity of the final fall in oxidative metabolism is highly predictive of neurological outcomes at one year.84–86 The central limitation of these findings is that whereas the fall is predictive as early as 6–18 h after birth,87 it again represents a measure of the secondary deterioration and developing cell death,88 well after the realistic window of opportunity for treatment.
More recently, Cady et al.89 reported that in a piglet model of HIE an increase in the ratio between [Pi]/[EPP] (the exchangeable high energy phosphate pool), intracellular pH and intracellular Mg about 2 h after intrapartum HI were associated with severe injury, whereas increased [phosphocreatine]/[EPP] was associated with mild damage. It is unknown whether these parameters predict response to treatment. Nevertheless, these data raise the possibility that in specialist centers (31P) MRS may have potential to help select infants for trials of neuroprotection.89
Near-infrared spectroscopy
Cerebral near-infrared spectroscopy (NIRS) monitoring uses light in the near-infrared region of the spectrum, which is absorbed by oxygenated and deoxygenated hemoglobin (total hemoglobin is an index of cerebral blood volume) and cytochrome oxidase (CytOx), which is the terminal complex of the mitochondrial respiratory chain and generator of ATP.82,90 NIRS can be used at the bedside and is non-invasive,91 but has several important limitations. At present, measurements are qualitative, not quantitative (changes are made relative to an arbitrary baseline), and prone to movement artefact.92 Interpretation of the signals is also an evolving science, particularly with data from new machines which often no longer display independent channels of hemoglobin, but rather present an algorithm weighted average calculation for total oxygen (total oxygen index: TOI) of all vascular beds.82,93 Finally, CytOx, one of the most potentially useful measurements for monitoring cerebral oxidative metabolism, is currently no longer available on clinical machines.93
NIRS has been used to show that during recovery from severe asphyxia there is a brisk restoration of blood volume, oxygenation and oxidative metabolism.62 In the early recovery after asphyxia, CytOx activity returns to normal.61 Following this stability in the latent (early) phase, there is progressive loss of CytOx activity, accompanied by a relative reduction in brain oxygenation extraction consistent with mitochondrial failure (Fig. 2).61 Unfortunately, these data also suggest that loss of CytOx may not be able to be detected until the secondary deterioration is already in progress.94
By contrast with mitochondrial failure, suppression of cerebral metabolic rate for oxygen (CMRO2) in the latent phase occurs rapidly after reperfusion.95 The mechanism and significance of this suppression has been controversial. For example, despite a marked reduction brain blood flow and metabolism, tissue oxygenation may be increased, suggesting active inhibition of brain metabolism.95 Consistent with this finding, there was evidence from recent studies combining NIRS with MRS in newborn piglets after HI that reduced CMRO2 reduction in the latent phase was mediated by a mixture of impaired mitochondrial function and reduced energy demand.96 Whereas suppression of CMRO2 and of EEG amplitude both correlated with duration of cerebral ischemia, the reduction in CMRO2 was more sensitive to milder injuries.80 Thus, the combination of EEG and NIRS monitoring may improve early detection of injury.
Conclusions
The purpose of this review has been to highlight the key attributes needed for any measure or combination of measures to aid in the selection of infants for treatment. Biomarkers should be able to tell us whether an infant needs treatment and, if so, whether brain cells can still be saved; are we in time to treat? This overview of the literature shows that at present none of the proposed biomarkers has been established to be clearly better than the clinical evaluation of HIE. Many of these biomarkers show good correlations with outcomes well after the onset of secondary deterioration, at relatively late time points (e.g. 12 or 24 h or later) and so are of little use for determining whether an infant should be treated. There is a strong need both for detailed studies of the time courses of potential measures after HI, and for studies that discriminate between the effects of exposure to asphyxia per se and neural injury. We speculate that a combination of multiple bedside measures may be needed to detect the critical transition from reversible to irreversible injury and thus allow us to reliably detect infants who are likely to benefit from treatment, at an early enough time that their brain cells can still be saved.
Practice points
None of the proposed biomarkers discussed here has yet been established to be clearly better than clinical evaluation of HIE for recruitment of infants at risk of adverse outcomes.
Many putative biomarkers show a good correlation with outcome, but only after the onset of the secondary deterioration (e.g. 12 or 24 h or even later). In practice this means that they are not useful for determining whether an infant should be treated, although some MRI and MRI parameters are likely to be useful surrogates for neurodevelopmental outcome.
Many proposed biomarkers are strongly associated with adverse outcome after severe HI injury compared with mild or no injury, but are not reliably predictive for outcome after moderate injury.
Research directions
The early and late time-courses of potential biomarkers after HI are still poorly understood.
It is critical to distinguish between the effects of exposure to hypoxia/asphyxia per se and neural injury.
Further long term neurological follow-up of clinical studies and of histopathology findings in experimental studies is vital to validate any apparent short-term predictive benefit of proposed biomarkers.
There is emerging evidence that epileptiform transients in the early recovery phase may be a true biomarker for evolving injury in the latent phase. Clinical and experimental validation is now critical.
Combinations of biomarkers such as NIRS and EEG monitoring may provide complementary parameters that could used to detect the critical transition from reversible to irreversible injury after HI injury.
Acknowledgements
The authors gratefully acknowledge funding support from the Health Research Council of New Zealand, The Auckland Medical Research Foundation, Lottery Health New Zealand, March of Dimes USA, and the US National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bennet L, Dean JM, Gunn AJ. The pathogenesis of preterm brain injury. In: Stevenson DK, Benitz WE, Sunshine P, Druzin ML, editors. Fetal and neonatal brain injury: mechanisms, management, and the risks of practice. 4th edition Cambridge University Press; Cambridge: 2009. pp. 48–57. [Google Scholar]
- 2.Gunn AJ, Bennet L. Timing of injury in the fetus and neonate. Curr Opin Obstet Gynecol. 2008;20:175–81. doi: 10.1097/GCO.0b013e3282f4ef9e. [DOI] [PubMed] [Google Scholar]
- 3.Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. Br Med J. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic–ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 5.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia to improve neurodevelopmental outcome following neonatal encephalopathy. Lancet. 2005;365(9460):663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 7.Gunn AJ, Thoresen M. Hypothermic Neuroprotection. NeuroRx. 2006;3:154–69. doi: 10.1016/j.nurx.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beilharz EJ, Williams CE, Dragunow M, Sirimanne ES, Gluckman PD. Mechanisms of delayed cell death following hypoxic–ischemic injury in the immature rat: evidence for apoptosis during selective neuronal loss. Brain Res Mol Brain Res. 1995;29:1–14. doi: 10.1016/0169-328x(94)00217-3. [DOI] [PubMed] [Google Scholar]
- 9.Geddes R, Vannucci RC, Vannucci SJ. Delayed cerebral atrophy following moderate hypoxia-ischemia in the immature rat. Dev Neurosci. 2001;23:180–5. doi: 10.1159/000046140. [DOI] [PubMed] [Google Scholar]
- 10.Roth SC, Baudin J, Cady E, et al. Relation of deranged neonatal cerebral oxidative metabolism with neurodevelopmental outcome and head circumference at 4 years. Dev Med Child Neurol. 1997;39:718–25. doi: 10.1111/j.1469-8749.1997.tb07372.x. [DOI] [PubMed] [Google Scholar]
- 11.Westgate JA, Wibbens B, Bennet L, et al. The intrapartum deceleration in center stage: a physiological approach to interpretation of fetal heart rate changes in labor. Am J Obstet Gynecol. 2007;197:e1–e11.236. doi: 10.1016/j.ajog.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 12.de Haan HH, Gunn AJ, Williams CE, Heymann MA, Gluckman PD. Magnesium sulfate therapy during asphyxia in near-term fetal lambs does not compromise the fetus but does not reduce cerebral injury. Am J Obstet Gynecol. 1997;176(1 Pt 1):18–27. doi: 10.1016/s0002-9378(97)80005-1. [DOI] [PubMed] [Google Scholar]
- 13.de Haan HH, Gunn AJ, Williams CE, Gluckman PD. Brief repeated umbilical cord occlusions cause sustained cytotoxic cerebral edema and focal infarcts in near-term fetal lambs. Pediatr Res. 1997;41:96–104. doi: 10.1203/00006450-199701000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia–ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–95. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 15.MacLennan A, The International Cerebral Palsy Task Force. Gunn AJ, Bennet L, Westgate JA. A template for defining a causal relation between acute intrapartum events and cerebral palsy: international consensus statement. Br Med J. 1999;319:1054–9. doi: 10.1136/bmj.319.7216.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowan F, Rutherford M, Groenendaal F, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361(9359):736–42. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- 17.Iwata O, Iwata S, Thornton JS, et al. “Therapeutic time window” duration decreases with increasing severity of cerebral hypoxia–ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Res. 2007;1154:173–80. doi: 10.1016/j.brainres.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 18.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia–reperfusion injury. Antioxid Redox Signal. 2008;10:601–19. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- 19.Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334:613–18. doi: 10.1056/NEJM199603073341001. [DOI] [PubMed] [Google Scholar]
- 20.Murray DM, O'Riordan MN, Horgan R, et al. Fetal heart rate patterns in neonatal hypoxic–ischemic encephalopathy: relationship with early cerebral activity and neurodevelopmental outcome. Am J Perinatol. 2009;26:605–12. doi: 10.1055/s-0029-1220774. [DOI] [PubMed] [Google Scholar]
- 21.Wayenberg JL. Threshold of metabolic acidosis associated with neonatal encephalopathy in the term newborn. J Matern Fetal Neonatal Med. 2005;18:381–5. doi: 10.1080/14767050500249916. [DOI] [PubMed] [Google Scholar]
- 22.Low JA, Lindsay BG, Derrick EJ. Threshold of metabolic acidosis associated with newborn complications. Am J Obstet Gynecol. 1997;177:1391–4. doi: 10.1016/s0002-9378(97)70080-2. [DOI] [PubMed] [Google Scholar]
- 23.Bennet LW, JA. Gluckman PD, Gunn AJ. Pathophysiology of asphyxia. In: Levene M, Chervenack FA, editors. Fetal and neonatal neurology and neurosurgery. 4th edition Churchill Livingstone/Elsevier; Philadelphia: 2009. pp. 472–90. [Google Scholar]
- 24.Shelley HJ. Glycogen reserves and their changes at birth and in anoxia. Br Med Bull. 1961;17:137–43. [Google Scholar]
- 25.Dawes GS, Mott JC, Shelley HJ. The importance of cardiac glycogen for the maintenance of life in foetal lambs and newborn animals during anoxia. J Physiol. 1959;146:516–38. doi: 10.1113/jphysiol.1959.sp006208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wassink G, Bennet L, Booth LC, et al. The ontogeny of hemodynamic responses to prolonged umbilical cord occlusion in fetal sheep. J Appl Physiol. 2007;103:1311–17. doi: 10.1152/japplphysiol.00396.2007. [DOI] [PubMed] [Google Scholar]
- 27.Laptook AR, Shankaran S, Ambalavanan N, et al. Outcome of term infants using apgar scores at 10 minutes following hypoxic–ischemic encephalopathy. Pediatrics. 2009;124:1619–26. doi: 10.1542/peds.2009-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gazzolo D, Abella R, Marinoni E, et al. New markers of neonatal neurology. J Matern Fetal Neonatal Med. 2009;22(Suppl 3):57–61. doi: 10.1080/14767050903181468. [DOI] [PubMed] [Google Scholar]
- 29.Ramaswamy V, Horton J, Vandermeer B, et al. Systematic review of biomarkers of brain injury in term neonatal encephalopathy. Pediatr Neurol. 2009;40:215–6. doi: 10.1016/j.pediatrneurol.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Loukovaara M, Teramo K, Alfthan H, et al. Amniotic fluid S100B protein and erythropoietin in pregnancies at risk for fetal hypoxia. Eur J Obstet Gynecol Reprod Biol. 2009;142:115–18. doi: 10.1016/j.ejogrb.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Routsi C, Stamataki E, Nanas S, et al. Increased levels of serum S100B protein in critically ill patients without brain injury. Shock. 2006;26:20–4. doi: 10.1097/01.shk.0000209546.06801.d7. [DOI] [PubMed] [Google Scholar]
- 32.Garnier Y, Frigiola A, Li Volti G, et al. Increased maternal/fetal blood S100B levels following systemic endotoxin administration and periventricular white matter injury in preterm fetal sheep. Reprod Sci. 2009;16:758–66. doi: 10.1177/1933719109335801. [DOI] [PubMed] [Google Scholar]
- 33.Friel LA, Romero R, Edwin S, et al. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med. 2007;35:385–93. doi: 10.1515/JPM.2007.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulpis KH, Margeli A, Akalestos A, et al. Effects of mode of delivery on maternal–neonatal plasma antioxidant status and on protein S100B serum concentrations. Scand J Clin Lab Invest. 2006;66:733–42. doi: 10.1080/00365510600977737. [DOI] [PubMed] [Google Scholar]
- 35.Bokesch PM, Appachi E, Cavaglia M, Mossad E, Mee RB. A glial-derived protein, S100B, in neonates and infants with congenital heart disease: evidence for preexisting neurologic injury. Anesth Analg. 2002;95:889–92. doi: 10.1097/00000539-200210000-00018. table of contents. [DOI] [PubMed] [Google Scholar]
- 36.Bruschettini M, van den Hove DL, Gazzolo D, et al. A single course of antenatal betamethasone reduces neurotrophic factor S100B concentration in the hippocampus and serum in the neonatal rat. Brain Res Dev Brain Res. 2005;159:113–18. doi: 10.1016/j.devbrainres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Vicente E, Tramontina F, Leite MC, et al. S100B levels in the cerebrospinal fluid of rats are sex and anaesthetic dependent. Clin Exp Pharmacol Physiol. 2007;34:1126–30. doi: 10.1111/j.1440-1681.2007.04687.x. [DOI] [PubMed] [Google Scholar]
- 38.Gazzolo D, Vinesi P, Marinoni E, et al. S100B protein concentrations in cord blood: correlations with gestational age in term and preterm deliveries. Clin Chem. 2000;46:998–1000. [PubMed] [Google Scholar]
- 39.Gazzolo D, Michetti F, Bruschettini M, et al. Pediatric concentrations of S100B protein in blood: age- and sex-related changes. Clin Chem. 2003;49(6 Pt 1):967–70. doi: 10.1373/49.6.967. [DOI] [PubMed] [Google Scholar]
- 40.Wijnberger LD, Nikkels PG, van Dongen AJ, et al. Expression in the placenta of neuronal markers for perinatal brain damage. Pediatr Res. 2002;51:492–6. doi: 10.1203/00006450-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 42.Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–21. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 43.Nagdyman N, Komen W, Ko HK, Muller C, Obladen M. Early biochemical indicators of hypoxic–ischemic encephalopathy after birth asphyxia. Pediatr Res. 2001;49:502–6. doi: 10.1203/00006450-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Nagdyman N, Grimmer I, Scholz T, Muller C, Obladen M. Predictive value of brain-specific proteins in serum for neurodevelopmental outcome after birth asphyxia. Pediatr Res. 2003;54:270–5. doi: 10.1203/01.PDR.0000072518.98189.A0. [DOI] [PubMed] [Google Scholar]
- 45.Thorngren-Jerneck K, Alling C, Herbst A, Amer-Wahlin I, Marsal K. S100 protein in serum as a prognostic marker for cerebral injury in term newborn infants with hypoxic ischemic encephalopathy. Pediatr Res. 2004;55:406–12. doi: 10.1203/01.PDR.0000106806.75086.D3. [DOI] [PubMed] [Google Scholar]
- 46.Martins RO, Rotta NT, Portela LV, Souza DO. S100B protein related neonatal hypoxia. Arq Neuropsiquiatr. 2006;64:24–9. doi: 10.1590/s0004-282x2006000100006. [DOI] [PubMed] [Google Scholar]
- 47.Murabayashi M, Minato M, Okuhata Y, et al. Kinetics of serum S100B in newborns with intracranial lesions. Pediatr Int. 2008;50:17–22. doi: 10.1111/j.1442-200X.2007.02506.x. [DOI] [PubMed] [Google Scholar]
- 48.Bashir M, Frigiola A, Iskander I, et al. Urinary S100A1B and S100BB to predict hypoxic ischemic encephalopathy at term. Front Biosci (Elite Ed) 2009;1:560–7. doi: 10.2741/e54. [DOI] [PubMed] [Google Scholar]
- 49.Giuseppe D, Sergio C, Pasqua B, et al. Perinatal asphyxia in preterm neonates leads to serum changes in protein S-100 and neuron specific enolase. Curr Neurovasc Res. 2009;6:110–16. doi: 10.2174/156720209788185614. [DOI] [PubMed] [Google Scholar]
- 50.Giussani DA, Thakor AS, Frulio R, Gazzolo D. Acute hypoxia increases S100beta protein in association with blood flow redistribution away from peripheral circulations in fetal sheep. Pediatr Res. 2005;58:179–84. doi: 10.1203/01.PDR.0000169999.66157.C0. [DOI] [PubMed] [Google Scholar]
- 51.Fujii EY, Kozuki M, Mu J, et al. Correlation of neuron-specific enolase and S100B with histological cerebral damage in fetal sheep after severe asphyxia. Brain Res. 2004;1018:136–40. doi: 10.1016/j.brainres.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 52.Nylen K, Ost M, Csajbok LZ, et al. Serum levels of S100B, S100A1B and S100BB are all related to outcome after severe traumatic brain injury. Acta Neurochir (Wien) 2008;150:221–7. doi: 10.1007/s00701-007-1489-2. discussion 227. [DOI] [PubMed] [Google Scholar]
- 53.Berger RP, Adelson PD, Pierce MC, et al. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J Neurosurg. 2005;103(1 Suppl):61–8. doi: 10.3171/ped.2005.103.1.0061. [DOI] [PubMed] [Google Scholar]
- 54.Shalak LF, Laptook AR, Velaphi SC, Perlman JM. Amplitude-integrated electroencephalography coupled with an early neurologic examination enhances prediction of term infants at risk for persistent encephalopathy. Pediatrics. 2003;111:351–7. doi: 10.1542/peds.111.2.351. [DOI] [PubMed] [Google Scholar]
- 55.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 56.Murray DM, Boylan GB, Ryan CA, Connolly S. Early EEG findings in hypoxic–ischemic encephalopathy predict outcomes at 2 years. Pediatrics. 2009;124:e459–e467. doi: 10.1542/peds.2008-2190. [DOI] [PubMed] [Google Scholar]
- 57.Pezzani C, Radvanyi-Bouvet MF, Relier JP, Monod N. Neonatal electroencephalography during the first twenty-four hours of life in full-term newborn infants. Neuropediatrics. 1986;17:11–18. doi: 10.1055/s-2008-1052492. [DOI] [PubMed] [Google Scholar]
- 58.George S, Gunn AJ, Westgate JA, et al. Fetal heart rate variability and brainstem injury after asphyxia in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2004;287:R925–33. doi: 10.1152/ajpregu.00263.2004. [DOI] [PubMed] [Google Scholar]
- 59.Hirst JJ, Palliser HK, Yates DM, Yawno T, Walker DW. Neurosteroids in the fetus and neonate: potential protective role in compromised pregnancies. Neurochem Int. 2008;52:602–10. doi: 10.1016/j.neuint.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 60.Gunn AJ, Bennet L. Fetal hypoxia insults and patterns of brain injury: insights from animal models. Clin Perinatol. 2009;36:579–93. doi: 10.1016/j.clp.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bennet L, Roelfsema V, Pathipati P, Quaedackers J, Gunn AJ. Relationship between evolving epileptiform activity and delayed loss of mitochondrial activity after asphyxia measured by near-infrared spectroscopy in preterm fetal sheep. J Physiol. 2006;572:141–54. doi: 10.1113/jphysiol.2006.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bennet L, Roelfsema V, Dean J, et al. Regulation of cytochrome oxidase redox state during umbilical cord occlusion in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1569–76. doi: 10.1152/ajpregu.00743.2006. [DOI] [PubMed] [Google Scholar]
- 63.Dean JM, George SA, Wassink G, Gunn AJ, Bennet L. Suppression of post hypoxic–ischemic EEG transients with dizocilpine is associated with partial striatal protection in the preterm fetal sheep. Neuropharmacology. 2006;50:491–503. doi: 10.1016/j.neuropharm.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 64.Scher MS. Controversies regarding neonatal seizure recognition. Epileptic Disord. 2002;4:139–58. [PubMed] [Google Scholar]
- 65.Dean JM, George S, Naylor AS, et al. Partial neuroprotection with low-dose infusion of the 2-adrenergic receptor agonist clonidine after severe hypoxia in preterm fetal sheep. Neuropharmacology. 2008;55:166–74. doi: 10.1016/j.neuropharm.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Bennet L, Dean JM, Wassink G, Gunn AJ. Differential effects of hypothermia on early and late epileptiform events after severe hypoxia in preterm fetal sheep. J Neurophysiol. 2007;97:572–8. doi: 10.1152/jn.00957.2006. [DOI] [PubMed] [Google Scholar]
- 67.Dean JM, Gunn AJ, Wassink G, George S, Bennet L. Endogenous alpha(2)-adrenergic receptor-mediated neuroprotection after severe hypoxia in preterm fetal sheep. Neuroscience. 2006;142:615–28. doi: 10.1016/j.neuroscience.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 68.Dean JM, Gunn AJ, Wassink G, Bennet L. Transient NMDA receptor-mediated hypoperfusion following umbilical cord occlusion in preterm fetal sheep. Exp Physiol. 2006;91:423–9. doi: 10.1113/expphysiol.2005.032375. [DOI] [PubMed] [Google Scholar]
- 69.Scher MS, Bova JM, Dokianakis SG, Steppe DA. Physiological significance of sharp wave transients on EEG recordings of healthy pre-term and full-term neonates. Electroencephalogr Clin Neurophysiol. 1994;90:179–85. doi: 10.1016/0013-4694(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 70.Vecchierini-Blineau MF, Nogues B, Louvet S, Desfontaines O. Positive temporal sharp waves in electroencephalograms of the premature newborn. Neurophysiol Clin. 1996;26:350–62. doi: 10.1016/s0987-7053(97)89149-6. [DOI] [PubMed] [Google Scholar]
- 71.Hughes JR, Guerra R. The use of the EEG to predict outcome in premature infants with positive sharp waves. Clin Electroencephalogr. 1994;25:127–35. doi: 10.1177/155005949402500404. [DOI] [PubMed] [Google Scholar]
- 72.Okumura A, Hayakawa F, Kato T, et al. Abnormal sharp transients on electroencephalograms in preterm infants with periventricular leukomalacia. J Pediatr. 2003;143:26–30. doi: 10.1016/S0022-3476(03)00182-3. [DOI] [PubMed] [Google Scholar]
- 73.Wang C, Jensen FE. Age dependence of NMDA receptor involvement in epileptiform activity in rat hippocampal slices. Epilepsy Res. 1996;23:105–13. doi: 10.1016/0920-1211(95)00086-0. [DOI] [PubMed] [Google Scholar]
- 74.Jensen FE, Wang C, Stafstrom CE, et al. Acute and chronic increases in excitability in rat hippocampal slices after perinatal hypoxia In vivo. J Neurophysiol. 1998;79:73–81. doi: 10.1152/jn.1998.79.1.73. [DOI] [PubMed] [Google Scholar]
- 75.Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81:1065–96. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- 76.Selman WR, Lust WD, Pundik S, Zhou Y, Ratcheson RA. Compromised metabolic recovery following spontaneous spreading depression in the penumbra. Brain Res. 2004;999:167–74. doi: 10.1016/j.brainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 77.Wong FY, Barfield CP, Walker AM. Power spectral analysis of two-channel EEG in hypoxic–ischaemic encephalopathy. Early Hum Dev. 2007;83:379–83. doi: 10.1016/j.earlhumdev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 78.Doyle OM, Greene BR, Murray DM, et al. The effect of frequency band on quantitative EEG measures in neonates with hypoxic–ischaemic encephalopathy. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:717–21. doi: 10.1109/IEMBS.2007.4352391. [DOI] [PubMed] [Google Scholar]
- 79.Jiang D, Wu W, Jia X, et al. Scaling exponents of EEG are related to the temporal process of the therapeutic hypothermia following ischemic brain injury. Conf Proc IEEE Eng Med Biol Soc. 2009;1:2192–5. doi: 10.1109/IEMBS.2009.5334934. [DOI] [PubMed] [Google Scholar]
- 80.Tichauer KM, Elliott JT, Hadway JA, Lee TY, St Lawrence K. Cerebral metabolic rate of oxygen and amplitude-integrated electroencephalography during early reperfusion after hypoxia–ischemia in piglets. J Appl Physiol. 2009;106:1506–12. doi: 10.1152/japplphysiol.91156.2008. [DOI] [PubMed] [Google Scholar]
- 81.Cady EB. Magnetic resonance spectroscopy in neonatal hypoxic–ischaemic insults. Childs Nerv Syst. 2001;17:145–9. doi: 10.1007/s003810000391. [DOI] [PubMed] [Google Scholar]
- 82.van Bel F, Lemmers P, Naulaers G. Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls. Neonatology. 2008;94:237–44. doi: 10.1159/000151642. [DOI] [PubMed] [Google Scholar]
- 83.Robertson NJ, Iwata O. Bench to bedside strategies for optimizing neuroprotection following perinatal hypoxia–ischaemia in high and low resource settings. Early Hum Dev. 2007;83:801–11. doi: 10.1016/j.earlhumdev.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 84.Hope PL, Costello AM, Cady EB, et al. Cerebral energy metabolism studied with phosphorus NMR spectroscopy in normal and birth-asphyxiated infants. Lancet. 1984;2(8399):366–70. doi: 10.1016/s0140-6736(84)90539-7. [DOI] [PubMed] [Google Scholar]
- 85.Azzopardi D, Wyatt JS, Cady EB, et al. Prognosis of newborn infants with hypoxic–ischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1989;25:445–51. doi: 10.1203/00006450-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 86.Martin E, Buchli R, Ritter S, et al. Diagnostic and prognostic value of cerebral 31P magnetic resonance spectroscopy in neonates with perinatal asphyxia. Pediatr Res. 1996;40:749–58. doi: 10.1203/00006450-199611000-00015. [DOI] [PubMed] [Google Scholar]
- 87.Thayyil S, Chandrasekaran M, Taylor A, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics. 2010;125:e382–95. doi: 10.1542/peds.2009-1046. [DOI] [PubMed] [Google Scholar]
- 88.Vannucci RC, Towfighi J, Vannucci SJ. Secondary energy failure after cerebral hypoxia–ischemia in the immature rat. J Cereb Blood Flow Metab. 2004;24:1090–7. doi: 10.1097/01.WCB.0000133250.03953.63. [DOI] [PubMed] [Google Scholar]
- 89.Cady EB, Iwata O, Bainbridge A, Wyatt JS, Robertson NJ. Phosphorus magnetic resonance spectroscopy 2 h after perinatal cerebral hypoxia–ischemia prognosticates outcome in the newborn piglet. J Neurochem. 2008;107:1027–35. doi: 10.1111/j.1471-4159.2008.05662.x. [DOI] [PubMed] [Google Scholar]
- 90.Cooper CE, Cope M, Springett R, et al. Use of mitochondrial inhibitors to demonstrate that cytochrome oxidase near-infrared spectroscopy can measure mitochondrial dysfunction noninvasively in the brain. J Cereb Blood Flow Metab. 1999;19:27–38. doi: 10.1097/00004647-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 91.Peebles DM, Edwards AD, Wyatt JS, et al. Changes in human fetal cerebral hemoglobin concentration and oxygenation during labor measured by near-infrared spectroscopy. Am J Obstet Gynecol. 1992;166:1369–73. doi: 10.1016/0002-9378(92)91606-b. [DOI] [PubMed] [Google Scholar]
- 92.Toet MC, Lemmers PM. Brain monitoring in neonates. Early Hum Dev. 2009;85:77–84. doi: 10.1016/j.earlhumdev.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 93.Greisen G. Is near-infrared spectroscopy living up to its promises? Semin Fetal Neonatal Med. 2006;11:498–502. doi: 10.1016/j.siny.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 94.Peeters-Scholte C, van den Tweel E, Groenendaal F, van Bel F. Redox state of near infrared spectroscopy-measured cytochrome aa(3) correlates with delayed cerebral energy failure following perinatal hypoxia–ischaemia in the newborn pig. Exp Brain Res. 2004;156:20–6. doi: 10.1007/s00221-003-1761-5. [DOI] [PubMed] [Google Scholar]
- 95.Jensen EC, Bennet L, Hunter CJ, Power GG, Gunn AJ. Post-hypoxic hypoperfusion is associated with suppression of cerebral metabolism and increased tissue oxygenation in near-term fetal sheep. J Physiol. 2006;572:131–9. doi: 10.1113/jphysiol.2005.100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Winter JD, Tichauer KM, Gelman N, et al. Changes in cerebral oxygen consumption and high-energy phosphates during early recovery in hypoxic–ischemic piglets: a combined near-infrared and magnetic resonance spectroscopy study. Pediatr Res. 2009;65:181–7. doi: 10.1203/PDR.0b013e31818f06fb. [DOI] [PubMed] [Google Scholar]
- 97.Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–56. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]