Abstract
Objectives
Relationships between non-use of highly active anti-retroviral therapy (HAART), race/ethnicity, violence, drug use and other risk factors are investigated using qualitative profiles of five risk factors (unprotected sex, multiple male partners, heavy drinking, crack, cocaine or heroin use, and exposure to physical violence) and association of the profiles and race/ethnicity with non-use of HAART over time.
Methods
A Hidden Markov Model (HMM) was used to summarize risk factor profiles and changes in profiles over time in a longitudinal sample of HIV-infected women enrolled in the Women's Interagency HIV Study (WIHS) with follow-up from 2002 to 2005 (N=802).
Results
Four risk factor profiles corresponding to four distinct latent states were identified from the five risk factors. Trajectory analysis indicated that states characterized by high probabilities of all risk factors or by low probabilities of all risk factors were both relatively stable over time. Being in the highest risk state did not significantly elevate the odds of HAART non-use (OR: 1.05; 95% CI: 0.6-1.8). However, being in a latent state characterized by elevated probabilities of heavy drinking and exposure to physical violence, along with slight elevations in three other risk factors, significantly increased odds of HAART non-use (OR: 1.4; 95% CI: 1.1-1.9).
Conclusions
The research suggests that HAART use might be improved by interventions aimed at women who are heavy drinkers with recent exposure to physical violence and evidence of other risk factors. More research about the relationship between clustering and patterns of risk factors and use of HAART is needed.
Introduction and Background
Since introduction of HAART in 1996, HIV/AIDS-related morbidity and mortality rates have declined dramatically (Palella et al., 1998). However, between 1993 and 2001 AIDS-related deaths declined at a slower rate among blacks than among whites (Levine et al., 2007). There is also growing evidence of lower rates of use of ART by women of color compared to other women (Cohen et al., 2004). One possible explanation is difference in access to quality medical care and antiretroviral therapy (ART). In addition, prior research has documented that risk factors, such as alcohol abuse and depression, that may vary over time are associated with HAART nonuse in medically eligible women (Lazo et al., 2007). However, many risk factors are concomitant suggesting that they may cluster and, depending on the particular combination, may be a proxy for an underlying unobserved or latent state that gives rise both to the risk factors and to non-use of HAART. Few studies have attempted to summarize multiple risk factors that are potentially clustered as latent states or to examine transitions among such states dynamically over time. Instead, previous investigations of risk factors linked to HAART use have modeled concomitant factors, such as alcohol abuse, drug use, and violence, as separate main effects rather than as clustered observable facets of a common unobserved individual trait or state.
In the present study, data from the Women's Interagency HIV Study (WIHS) were used to investigate the association between non-use of HAART and latent states characterized by clusters of risk factors among HIV-infected women using an extended version of the multivariate discrete Hidden Markov Model (HMM) (MacDonald & Zucchini, 1997; Rijmen, Ip, Rapp, & Shaw, 2008). Advancing our understanding of these relationships is intended to develop a more nuanced understanding of risk factors that impede effective HIV treatment among women and to understand better the interplay between risk factor clusters and racial/ethnic disparities in HAART use among medically eligible women. Findings may also assist in better understanding of the mechanisms by which clusters of risk factors contribute to non-use of HAART and disparities in HIV/AIDS care.
Previous Research on Risk Factors Associated with HAART Non-Use
Previous studies of HIV/AIDS care have documented significant differences in HAART use by gender and race/ethnicity (Cook et al., 2002b; Cohen et al., 2004; Shapiro et al., 1999; Andersen et al., 2000; Cunningham et al., 2000; Cohen et al., 2004; Lillie-Blanton et al., 2009), by drug use (Cook et al., 2002b; Cook et al., 2007) and by history of physical abuse (Cohen et al., 2004). Several studies provide evidence that risk factors may cluster. Physical abuse and substance abuse have been associated with sexual risk factors such as unprotected sex and having multiple partners that have in turn been linked to HAART use. Studies have found that lower medication adherence rates and beliefs about reduced infectiousness among women on HAART were associated with reduced condom use (Wilson et al., 2002, Wilson, 2001). In addition, studies have linked illicit drug use to inconsistent condom use and an increased number of sex partners (Skurnick, Abrams, Kennedy, Valentine, & Cordell, 1998; Clark, Kissinger, Bedimo, Dunn, & Albertin, 1997; Novotna et al., 1999). While independent effects of drug use and physical abuse on medical care use by HIV-infected people have been documented (Cohen et al., 2004; Cook et al., 2002b; Palacio et al., 2004), relatively little is known about combinations or clusters of risk factors that are associated with care obtained by HIV-infected medically eligible women.
We employed an extended version of the multivariate discrete HMM to capture the complexity of possible complex patterns of concomitant factors (i.e., illicit drug use, heavy alcohol use, multiple male partners, unprotected anal or vaginal sex, and exposure to physical violence) and to model the underlying unobserved dynamic process that may give rise to complex observed risk factor patterns that vary over time and are associated with HAART non-use. Unlike trajectory analysis approaches that have employed latent growth curve analysis or growth mixture models to model changes in a single continuous outcome over time (Smith & Ecob, 2007; Lee & Thompson, 2008; Martino, Ellickson, Klein, McCaffrey, & Edelen, 2008; Colman, Ploubidis, Wadsworth, Jones, & Croudace, 2007; Duncan et al., 2007), our approach allowed us to identify complex patterns of risk factors that change, possibly nonlinearly, over time. (See Ip et al, forthcoming, for a more technical explanation of the HMM model as used in this application). Because different latent states characterized by multiple observed binary risk factor indicators are not necessarily linearly ordered, the HMM method provides a more flexible approach for assessing the dynamics of risk factors among HIV-infected women.
Methods
Study population
We used data from the WIHS, an ongoing prospective cohort study that recruited women into six study centers: Brooklyn, Bronx/Manhattan, Washington D.C., Chicago, Southern California, and San Francisco. A standardized interview-based survey was used at semiannual visits to collect information on demographics, medical history, use of antiretroviral medications and other measures of psychosocial history. Detailed information on study methodology, quality assurance, and baseline characteristics of the enrollees have been reported previously (Barkan et al., 1998; Bacon et al., 2005).
The study enrolled 2054 HIV-infected women in 1994-95, and 737 HIV-infected women during the second wave of enrollment (Bacon et al 2005: Table 5). Study participants enrolled at the two time periods did not differ significantly in terms of race/ethnicity, income, education. However, by design the second wave was targeted to enrollees who were younger in age, HAART naïve, or HAART using and therefore had higher CD4 cell counts on average than women enrolled in 1994-1995 (Bacon et al, 2005). Follow-up interviews of all women were conducted at 6-month intervals. Because physical violence was only assessed annually, the analysis employed data from annual visits. In addition, women enrolled at two California sites were excluded from analysis because data on physical violence were not collected from them. Study protocols were reviewed and approved by the institutional review boards at the various study sites.
The study population was limited to the subset of HIV-infected women who self-identified as white, African American/Black, or Hispanic/Latina and who were ever clinically eligible for HAART between study enrollment and visit 23 (October 1, 2005 – March 31, 2006) based on criteria used in a prior study of WIHS participants of either: (a) current use of HAART or (b) CD4+ count <350 cells/μL or (c) HIV RNA >50,000 copies/ml (Cohen et al., 2004).
Analysis was restricted to data collected between 2002 and 2005 in order to include HIV-infected women from both waves. Within a sample of 1127 women at visit 16 with partial data that spanned 4 years from even-numbered visits 16 (2002) to 22 (2005), data on 857 women were available in all four years. Of these, 55 were excluded due to HAART ineligibility. Five risk factors at each time point and four time points or years in total yielded a total of twenty possible time-risk factor indicators for each of the 802 women.
Dependent Variable
The major outcome was self-reported HAART use since last visit (yes or no). We used an indicator of HAART that was constructed by WIHS, using guidelines published by the Department of Health and Human Services. The WIHS defined HAART based on guidelines in place at the time of each study visit.1 Non-HAART combination antiretroviral therapy, monotherapy, and no therapy were all grouped in the HAART non-use category. It is important to stress that the outcome employed (non-use) is consistent with previous research (Cohen et al, 2004). However, it in no way measures either access or treatment adherence. The term “non-use” is used to denote women who reported that their main reason for not taking HAART was that their physician did not prescribe it (33.3% of the sample in 2005) as well as women who reported other reasons for not taking HAART (Lillie-Blanton et al, In Press). In addition, our methodology will assign a woman who used HAART only once during the recall period to the use category.
Laboratory measures
At each semi-annual visit, HIV-infected women had blood drawn and tested for HIV RNA quantification and CD4+ cell count. For multivariate analyses, log10 transformation was used to estimate the effect of a ten-fold change in HIV RNA and log2 to estimate the effect of a two-fold change in CD4+ cell count on non-use. Hepatitis C antibody status, measured at the baseline WIHS visit, was categorized as positive or negative.
Risk Factors
Five risk factor indicators were based on responses to questions about the six months prior to interview. A binary indicator of illicit drug use was derived from the woman's positive response to a question about crack, cocaine or heroin use since last visit. A binary indicator that the woman met the NIAAA criterion for classification as a heavy drinker (>13 drinks per week) since last visit was set equal to 1 (Dufour, 1999). Two binary indicators of sexual risk factors were created. One indicator was set equal to one if the woman reported ≥ 2 male partners within 6 months of interview. The other was set equal to one if she reported unprotected vaginal sex or unprotected anal sex ≥ 1 times in the six months prior to interview. A binary indicator of recent physical violence was set equal to one if the woman reported that she had “experienced serious physical violence since last visit” or if she indicated that she had experienced “partner violence”, “family/friend violence”, and/or “stranger/acquaintance violence” within six months of interview.
Latent states were characterized by patterns of the conditional probabilities of five observed binary indicators of risk that could occur in any combination in the six months prior to interview: 1) experienced any physical violence by a spouse, partner, family member, acquaintance or stranger; 2) having more than one male partner; 3) use of crack, cocaine, or heroin; 4) heavy drinking defined as >13 drinks/week; and 5) unprotected vaginal or anal sex with a male. For example, at any given follow up, a woman might report all five risk factors (and be coded a 1 on each of them) or she might report none of the factors (and be coded a 0 on each). Alternatively, she might report any combination of the five factors yielding 32 (25) possible unique combinations of risk factors.
Other measures
Socio-demographic and health factors assessed in the multivariate analysis included race/ethnicity (mutually exclusive categories of white non-Hispanics; black non-Hispanics; and Hispanics), age in years at the study visit, poverty (family income at baseline based on federal poverty level), years of education, full-time employment (yes/no at baseline), and depressive symptoms (a binary indicator of a score of 23 or higher on the Center for Epidemiological Studies Depression (CES-D) Scale) (Radloff, 1977; Cook et al., 2002a).
Health insurance coverage was classified into mutually exclusive categories: uninsured, private, Medicare/other, and Medicaid/MediCal. Categories were constructed using a classification hierarchy that is used by the Kaiser Commission on Medicaid and Uninsured and the Urban Institute when analyzing data from the Current Population Survey. All participants who reported Medicaid coverage were assigned to the Medicaid category. The remaining respondents were assigned to private; Medicare; military-related coverage such as CHAMPUS; other (e.g. student, no type specified) in that order (Kaiser Commission on Medicaid and the Uninsured, 2008). Because of the small number of respondents with CHAMPUS, respondents with private coverage or CHAMPUS were collapsed into a single category. Only women who reported no public or private source of insurance were classified as uninsured. Women who identified the AIDS Drug Assistance Program (ADAP) of the Ryan White Care Act as their only resource for paying for medications were classified as uninsured since ADAP is not a public or private health insurance plan with a defined benefit package beyond paying for medications. We included a binary indicator of ADAP for any woman who reported enrollment regardless of insurance status.
Hidden Markov Model
To model the state characterized by the five risk factor indicators we used the multiple indicator HMM (MacDonald et al., 1997; Rijmen et al., 2008). Under an HMM, a categorical or dichotomous latent variable is incorporated to account for qualitatively distinct patterns of the entire set of risk factors. To capture the serial correlation of risks over time, it is assumed that the latent state of a woman at time t + 1 depends upon her latent state at time t. The observed risk factors are the observable manifestations of the unobservable latent states. A woman is in an unobserved state during time, t. It gives rise to observed outcomes which she reports at the end of time, t. The factors don't necessarily all happen at once, but they all happen during a given time period that extends from the previous visit to the current visit. The actual combination of indicators observed at each time are a function of the true underlying, but unobserved, state of each woman as well as measurement error. The unobserved or latent states, then, give rise to the true, unobservable, underlying probabilities that women exhibit various combinations of the five observed risk factors.
In fitting HMM models, different numbers of latent states are possible and determining the optimal number of states for a given application is critical. Following an approach (Huang & Bandeen-Roche, 2004) that employed the Bayesian Information Criterion (BIC) (Schwarz, 1978) we determined the optimal number of states by fitting a range of HMMs, each with a different number of states, to the data. This procedure allowed us to identify the model that yielded the optimal (minimum) BIC value and the optimal number of latent states.
In addition to being in a latent state, characterized by a unique pattern of probabilities of observed risk factors at each follow up, each woman has a trajectory over time through the latent space, i.e. she can move from one risk profile to another or remain in the same profile over time. A Hidden Markov process models the woman's trajectory through states over time using transition probabilities that describe the woman's probability of remaining in the same state or transitioning to another state. To obtain a woman's most likely trajectory, we applied Viterbi's algorithm (Viterbi, 1967) to evaluate the globally optimal solution. The Viterbi algorithm calculates the most likely latent state sequence of the HMM given an observation sequence.
The impact of time-varying risk factors on HAART non-use was investigated using two regression-based methods. The first method used the underlying latent state of an individual at each time point, derived from the HMM approach, as predictor. This allowed us to investigate whether or not several risk factors that had been demonstrated in previous research to be individually associated with HAART use and non-use might actually proxy an underlying individual state that is associated with HAART non-use. For comparison, the second method used each of the several identified risk factors as a main effect. We used generalized estimating equations (GEE) (Liang & Zeger, 1986) with logit link function and exchangeable correlation to control for correlation among repeated observations on the same individual. The GEE models were estimated using PROC GENMOD, SAS version 9.1.3(SAS Institute, 2005).
Results
Participant characteristics at visit 16, shown as means or percentages, are provided in Table 1. The sample was predominantly African American (67%). The majority was unmarried and not living with a partner (72%). A sizable proportion (38%) did not graduate from high school, nearly half (49%) were between 40 and 49 years old, and 66% were unemployed. Two thirds (66%) were covered by Medicaid while 16% were privately insured and 13% were uninsured. A quarter of the women (25%) had probable depression scores as measured by the CES-D (23 or greater); 4% reported recent physical violence; 9% reported current crack, cocaine or heroin use; roughly 4% were classified as heavy drinkers; 10% reported more than 1 male partner; and 30% reported unprotected vaginal or anal sex ≥ 1 times in the six months prior to interview.
Table 1.
Characteristics of the 802 HIV-infected women from the Chicago, New York City, and Washington, D.C. Women's Interagency HIV Study.
| Mean/Percent | |
|---|---|
| Race | |
| White | 09.6% |
| Black | 67.1% |
| Latino | 23.3% |
| Marital Status | |
| Married or living with a partner | 27.8% |
| Never married | 38.0% |
| Separated, divorced, annulled, widowed | 34.2% |
| Education | |
| Did not graduate HS | 38.3% |
| HS grad | 30.4% |
| Some college | 25.0% |
| College grad | 06.4% |
| Age at visit | |
| 39 years old or younger | 36.3% |
| 40 – 49 years old | 49.1% |
| 50 years old or older | 14.6% |
| Employment | |
| Currently employed | 33.7% |
| Not currently employed | 66.3% |
| Income | |
| Average household income/year | $19,369 (sd: 20,961) |
| Insurance coverage | |
| Uninsured | 13.1% |
| Private/CHAMPUS | 15.6% |
| Medicaid | 66.0% |
| Medicare/Student/Other/Unknown | 03.9% |
| Depression score 23 or greater | 25.1% |
| Risk Factors in previous six months | |
| Any experience of physical violence | 04.0% |
| Any use of crack, cocaine, heroin | 08.7% |
| Heavy drinker | 03.7% |
| More than one male partner | 09.7% |
| Unprotected sex one or more times | 29.8% |
as of visit 16 (2002)
Based on the BIC criterion, the 4-state model was selected. Figure 1 displays the risk factor profiles associated with each of the four states. Each bar shows the conditional probability of a specific risk factor within each state. Longer bars indicate higher probabilities. The prevalence rates of the states at the first time point (visit 16) are respectively 8%, 29%, 18% and 45% percent.
Figure 1.
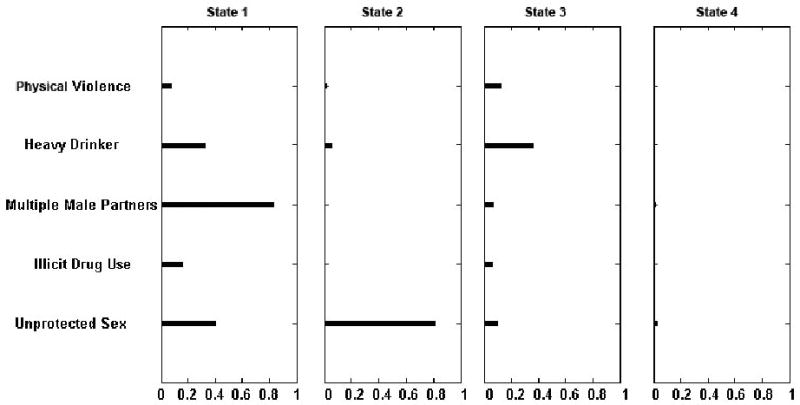
Risk patterns for the 4-state model for the 802 HIV-infected women from the Chicago, New York City, and Washington, D.C. Women's interagency HIV Study.
State 1, the leftmost column in Figure 1, demonstrated relatively high probabilities of all five risks with multiple male partners demonstrating the highest conditional probability (.80) and with the probabilities of heavy drinking and unprotected sex at about .45. Conditional probability of illicit drug use is also elevated (∼.15) as is probability of recent physical violence (.08). Women in State 2 demonstrated a high probability of unprotected sex (.80), but only slightly elevated probability of heavy drinking (<.05) and no elevated probabilities of other risks. Women in State 3 demonstrated elevated probabilities of all five risks, but in different magnitudes from those seen in State 1. Probabilities of drug use, multiple male partners, and unprotected vaginal sex, while slightly elevated, were much lower than the probabilities seen in State 1, whereas probabilities of heavy drinking and recent physical violence were slightly higher. State 4 demonstrated very low probabilities of all five risk factors.
Table 2 provides transition probabilities from one state to another over time. The first row indicates the probability of being in a given state during the first time period. The diagonal entries in the other rows show the probability of remaining in each state (leftmost column) in each time. Other row probabilities indicate the probability of transition from the state indicated by the row header to any other state (column within the same row) in the next time period. As can be seen, State 4 is the most stable (ρ=.98). States 1 and 2 were also fairly stable (ρ=.89 and ρ=.89), while State 3 appears to be the most volatile with the lowest probability that an individual will remain in it in the next time period (ρ =.73). Women in states 2, 3 and 4 had extremely low probabilities of transitioning into State 1. Women in States 2 and 3 who transitioned out were most likely to transition to State 4 (ρ =.10 and ρ =.21 respectively), the state characterized by very low probabilities of all 5 risk factors.
Table 2.
Transition Probability Table for the 802 HIV-infected women from the Chicago, New York City, and Washington, D.C. Women's Interagency HIV Study.
| State 1 | State 2 | State 3 | State 4 | ||
|---|---|---|---|---|---|
| Initial State Probabilities | 0.08 | 0.29 | 0.18 | 0.45 | |
| State Transition Probabilities | State 1 | 0.89 | 0.06 | 0.00 | 0.05 |
| State 2 | 0.01 | 0.89 | 0.00 | 0.10 | |
| State 3 | 0.00 | 0.06 | 0.73 | 0.21 | |
| State 4 | 0.00 | 0.01 | 0.01 | 0.98 | |
Using the global optimization routine, we obtained frequency distributions of the many observed trajectories. Out of a total of 802 observable trajectories with 17 unique patterns, 680 (85%) showed a stable pattern of not changing to another state at any time point. Of these, 362 (45%) trajectories remained in State 4 over the 4 year period while 51 (6%) remained in State 1 for the duration. Of the remaining trajectories, the three most frequently occurring trajectories were 2→2→2→2, (22.8%), 3→3→3→3 (10.5%), 2→4→4→4(3.6%), 2→2→4→4 (2.9%), and 3→3→4→4 (2.9%).
Longitudinal Analysis of Risk Factor Profiles and HAART Use
In both GEE models (Table 3), elevated viral load was associated with statistically significant elevations in the odds of HAART non-use (OR: 22.1 and 24.2; 95% CI: 14.1-34.5 and 15.6-37.6), while being 50 years or older was associated with statistically significant decreases in the odds of HAART non-use (OR: 0.6; 95% CI: 0.4-0.9). Women enrolled in 1994-1995 were significantly less likely not to have used HAART during the study period (OR: 0.7; 95% CI (0.5-0.96) compared to women enrolled in 2001-2002. Regardless of model specification, the log odds ratio for African American women not using HAART over the 4 year study period was elevated above that of white women (OR: 1.3; 95% CI: 0.7-2.2), but not statistically different at conventionally accepted thresholds. Enrollment in ADAP reduced the log odds of HAART non-use (OR: 0.4; 95% CI: 0.3-0.6), while privately insured and uninsured women were at increased risk of HAART non-use when compared to women covered by Medicaid (OR: 1.9; 95% CI: 1.3-2.8 and OR: 2.1; 95% CI: 1.6-3.0 respectively) regardless of model specification.
Table 3.
GEE estimates of Risk Factor Effects on HAART Non-Use for the 802 HIV-infected women from the Chicago, New York City, and Washington, D.C. Women's Interagency HIV Study.
| Main Effects Model | Latent State Model | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |||
| WIHS Wave 2 (enrolled 2001-2002 | 1.00 | |||||
| WIHS Wave 1 (enrolled 1994-95) | 0.70 | 0.50 | 0.96 | 0.70 | 0.51 | 0.96 |
| Log2CD4 Count | 1.01 | 0.97 | 1.05 | 1.01 | 0.97 | 1.05 |
| Log10HIV Viral Load | 22.08 | 14.14 | 34.48 | 24.22 | 15.62 | 37.58 |
| White | 1.00 | 1.00 | ||||
| African American | 1.27 | 0.74 | 2.19 | 1.29 | 0.75 | 2.21 |
| Latina | 1.05 | 0.57 | 1.93 | 1.04 | 0.57 | 1.91 |
| Age <40 | 1.00 | 1.00 | ||||
| Age 40-49 | 0.77 | 0.58 | 1.01 | 0.78 | 0.59 | 1.02 |
| Age 50+ | 0.63 | 0.43 | 0.92 | 0.65 | 0.44 | 0.96 |
| > 200% FPL at Program Intake | 1.00 | 1.00 | ||||
| < 100% FPL at Program Intake | 0.79 | 0.53 | 1.17 | 0.82 | 0.56 | 1.21 |
| 100%-200% of FPL | 0.88 | 0.59 | 1.31 | 0.92 | 0.62 | 1.36 |
| <HS | 0.94 | 0.66 | 1.34 | 0.92 | 0.65 | 1.30 |
| HS Graduate | 1.00 | 1.00 | ||||
| Some College | 0.84 | 0.58 | 1.22 | 0.85 | 0.59 | 1.24 |
| College Graduate | 0.62 | 0.30 | 1.27 | 0.61 | 0.30 | 1.24 |
| Employed | 1.04 | 0.85 | 1.27 | 1.00 | 0.82 | 1.22 |
| Medicaid | 1.00 | 1.00 | ||||
| No Health Insurance | 2.16 | 1.55 | 3.01 | 2.14 | 1.54 | 2.98 |
| Private Health Insurance | 1.93 | 1.33 | 2.79 | 1.94 | 1.34 | 2.80 |
| Medicare/Student/Other | 1.19 | 0.69 | 2.04 | 1.25 | 0.74 | 2.11 |
| Enrolled in ADAP | 0.42 | 0.29 | 0.61 | 0.41 | 0.29 | 0.60 |
| Hepatitis C Antibody Positive | 1.22 | 0.87 | 1.70 | 1.23 | 0.88 | 1.71 |
| Depression/CESD 23+ | 0.95 | 0.77 | 1.16 | 0.95 | 0.78 | 1.16 |
| Risk Factors in Previous Six Monthsa | ||||||
| Any experience of physical violence | 0.78 | 0.47 | 1.31 | |||
| Any crack, cocaine, heroin | 1.33 | 0.89 | 1.99 | |||
| >13 drinks/week | 1.52 | 0.92 | 2.51 | |||
| >1 Sexual partner | 1.06 | 0.75 | 1.48 | |||
| Unprotected anal or vaginal sex | 1.19 | 0.98 | 1.45 | |||
| Most likely latent state in last 6 months | ||||||
| State 1* | 1.05 | 0.63 | 1.75 | |||
| State 2* | 0.88 | 0.61 | 1.26 | |||
| State 3* | 1.43 | 1.08 | 1.88 | |||
| State 4 | 1.00 | |||||
Site-specific fixed effects were also included in the models
The woman's most probable state in a given time period
Of the five risk factor main effects, none demonstrated odds that are statistically significantly elevated, but three demonstrated both higher odds and 95% confidence intervals that barely cover one with relatively high upper bounds. The elevated ORs in combination with the relatively small cell sizes suggest the possibility of a Type II error for two of these (>13 drinks/week within past six months (OR: 1.5; 95% CI: 0.9-2.5; any crack, cocaine, heroin use in within 6 months of interview (OR: 1.3; 95% CI (.9-2.0)).
When binary indicators of the woman's most likely latent state were used instead of individual risk factor main effects, it is notable that other parameter estimates in the model remained relatively stable. Only state 3 represents elevated risk of HAART non-use relative to State 4 (OR: 1.4; 95% CI: 1.1-1.9). The results for state 3, characterized by high probability of heavy drinking along with elevated probability of recent physical violence and somewhat smaller but still evident probabilities of the other three risk behaviors are consistent with the main effect OR for heavy drinking. The risk profile for state 3 suggests that heavy drinking clusters with slight elevations in the other four risk factors. It should also be noted that state 3 was the most volatile of the four latent states as evidenced by the relatively low probability of remaining in it (.73) from one time period to the next.
Conclusions and Discussion
The results provide evidence that risk factors that influence HAART use in HIV-infected women may be multi-dimensional, complex and dynamic. The four latent states or risk profiles consisted of one characterized by high probabilities of all five risk factors (state 1), another characterized by elevated probabilities of heavy drinking and exposure to violence and slight elevations in probabilities of the remaining three risk factors (state 3), one with high probability of unprotected sex (state 2), and a fourth profile with low probabilities of all five risk factors (state 4). The probabilities of transitioning to State 1, the profile with high probabilities of all risk factors, from all other risk profiles were quite low or zero.
GEE analysis indicated that the only risk profile associated with non-use of HAART was state 3, characterized primarily by elevated probabilities of heavy drinking and recent physical violence. The other three individual risk factors (crack, cocaine, heroin use; unprotected sex; and multiple male partners) had conditional probabilities that were relatively low in this latent state. At the same time, the effects of other covariates such as race and insurance remained relatively stable regardless of model specification.
The relative stability of the HMM states indicated by the transition probabilities suggests that there may be little to be gained by modeling risk factors dynamically in a sample of women demographically similar to this population of HIV-infected women. However, future research may find that there is more variation in risk factors over time among younger women, which could result in more informative HMM results.
By far the most significant factor for non-use of HAART was an elevated HIV viral load, in the main effects and latent state models. This extremely strong association is likely because women who are medically eligible for HAART use and are taking HAART have a reduced viral load as a result of the effective therapy. Conversely, women who are medically eligible for HAART, but not taking it have unsuppressed (and in some cases very high) HIV viral loads. The women who are not taking HAART are a mixed group that represents those who never took HAART and those who may have taken HAART in the past and for one reason or another discontinued use. It is important to remember that when women who were eligible, but not taking HAART in 2005, were asked to give their main reason for not taking any antiretroviral medications, the most common reason given was that “my doctor did not prescribe them” (33.3%). The next two most frequent reasons were CD4 count was too high/viral load was too low (18.6%) and that it was a personal decision to wait (13.6%).
The results of the present study indicate a link between HAART non-use and a risk cluster characterized by heavy drinking accompanied by elevated probabilities of recent physical violence and three other risk factors that justifies further investigation. The elevated risk of not using HAART associated with State 3 suggests that whatever its causal origin, it is not likely related to a woman's race/ethnicity since the analysis controlled for this factor. Further investigation of the role of heavy drinking and the reason for the observed clustering of it with elevated probability of recent physical violence and slightly elevated probabilities of the other three risk factors should be undertaken. Findings on the link between alcohol use and non-use of HAART are consistent with several studies mostly of HIV-infected men (Samet, Horton, Meli, Freedberg, & Palepu, 2004; Tucker, Burnam, Sherbourne, Kung, & Gifford, 2003; Palepu, Horton, Tibbetts, Meli, & Samet, 2004). However, the finding has not been widely reported in studies of HIV-infected women.
The general tendency in this sample was for women, regardless of race/ethnicity, to move over time into a lower risk state, state 4, reducing or eliminating risk factors that have been linked in previous research to HAART non-use. There is no way to know if this is a general trend among HIV-infected women or specific to those who participated in this longitudinal study, but it warrants further investigation.
In conclusion, identification of State 3, characterized by a high conditional probability of heavy drinking and lower but still measurably elevated probabilities of recent physical violence and the other three risk factors, suggests a more nuanced profile of women at risk for non-use of HAART. State 3 is distinct from the state with the highest probabilities of all risk factors (State 1) and suggests there may be more than one causal link between heavy drinking and HAART non-use. However, State 3 was also the least stable state with relatively high probability of transitioning to the lowest risk state in any given time period. A better understanding of the role of heavy drinking, risk factors that may co-occur with it, and the determinants of transition to State 4 may aid service providers in improving HAART use. Future research should be directed to investigation of risk factor clusters, especially those associated with heavy drinking, in younger HIV-infected women, the trajectories associated with them, and the causal mechanisms, if any, of their impact on HAART use and non-use.
Acknowledgments
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Institute on Drug Abuse, the National Cancer Institute, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The National Institute on Drug Abuse (R21 DA022971-01) provided primary funding for this study. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
We especially wish to acknowledge Richard Hopley for his responsiveness and meticulous computer programming throughout this project and to thank Joan Beane for her very competent assistance in manuscript preparation. Finally, and most importantly, we gratefully acknowledge the women who participated in the WIHS.
Footnotes
Disclosure: None of the authors have financial interests or relationships to disclose.
The guidelines published in 1998 defined HAART as involving treatment with 2 or more nucleotide reverse transcriptase inhibitors(NRTI) and a protease inhibitor (PI) or in combination with a nonnucleotide reverse transcriptase inhibitors (NNRTI).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alison Snow Jones, Email: Alison.S.Jones@drexel.edu, Department of Health Management & Policy; Drexel University School of Public Health; 1505 Race Street, MS 1035; Philadelphia, PA 19102; (t) 215-762-2568; (f) 215-762-8846.
Marsha Lillie-Blanton, Email: lblanton@gwu.edu, George Washington University School of Public Health and Health Services; 2021 K St. N.W. Suite 800; Wash, D.C. 20006; (t) 202-329-1824; (f) 202-530-2336.
Valerie E. Stone, Email: vstone@partners.org, Department of Medicine; Massachusetts General Hospital; Harvard Medical School; Boston, Massachusetts; mailing address: Massachusetts General Hospital; 55 Fruit St., Bulfinch 130; Boston, MA 02114; (t) 617-726-7708; (f) 617-726-3838.
Edward H. Ip, Email: eip@wfubmc.edu, Department of Biostatistical Sciences; Division of Public Health Sciences; Wake Forest University School of Medicine; Medical Center Blvd; Winston-Salem, NC 27157; (t) 336-716-9833; (f) 336-716-6427.
Qiang Zhang, Email: qizhang@wfubmc.edu, Department of Biostatistical Sciences; Division of Public Health Sciences; Wake Forest University School of Medicine; Medical Center Blvd; Winston-Salem, NC 27157; (t) 336-716-5141; (f) 336-716-6427.
Tracey E. Wilson, Email: Tracey.Wilson@downstate.edu, Department of Community Health Sciences; SUNY Downstate Medical Center; 450 Clarkson Avenue, Box 43; Brooklyn, NY 11203; (t) 718-270-2105; (f) 718-270-3386.
Mardge H. Cohen, Email: mardge.cohen@gmail.com, Departments of Medicine, Stroger Hospital and Rush University, Chicago IL; 50 Parley Avenue, Jamaica Plain, MA 02130; (t) 312-925-5660; (f) None.
Elizabeth T. Golub, Email: egolub@jhsph.edu, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health; 615 N. Wolfe Street, E-7636 Baltimore, MD 21205; (t) 410-502-2502; (f) 410-955-7587.
Nancy A. Hessol, Email: Nancy.Hessol@ucsf.edu, University of California, San Francisco; 405 Irving Street, 2nd Floor; San Francisco, CA 94122; (t) 415-502-6281; (f) 415-476-8528.
Reference List
- Andersen R, Bozzette S, Shapiro M, St C P, Morton S, Crystal S, et al. Access of vulnerable groups to antiretroviral therapy among persons in care for HIV disease in the United States. HCSUS Consortium. HIV Cost and Services Utilization Study. Health Services Research. 2000;35:389–416. [PMC free article] [PubMed] [Google Scholar]
- Bacon MC, von WV, Alden C, Sharp G, Robison E, Hessol N, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clinical and Diagnostic Laboratory Immunology. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- Clark RA, Kissinger P, Bedimo AL, Dunn P, Albertin H. Determination of factors associated with condom use among women infected with human immunodeficiency virus. International Journal of STD and AIDS. 1997;8:229–233. doi: 10.1258/0956462971919976. [DOI] [PubMed] [Google Scholar]
- Cohen MH, Cook JA, Grey D, Young M, Hanau LH, Tien P, et al. Medically eligible women who do not use HAART: the importance of abuse, drug use, and race. American Journal of Public Health. 2004;94:1147–1151. doi: 10.2105/ajph.94.7.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman I, Ploubidis GB, Wadsworth ME, Jones PB, Croudace TJ. A longitudinal typology of symptoms of depression and anxiety over the life course. Biological Psychiatry. 2007;62:1265–1271. doi: 10.1016/j.biopsych.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Cook JA, Cohen MH, Burke J, Grey D, Anastos K, Kirstein L, et al. Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. Journal of Acquired Immune Deficiency Syndromes. 2002a;30:401–409. doi: 10.1097/00042560-200208010-00005. [DOI] [PubMed] [Google Scholar]
- Cook JA, Cohen MH, Grey D, Kirstein L, Burke J, Anastos K, et al. Use of highly active antiretroviral therapy in a cohort of HIV-seropositive women. American Journal of Public Health. 2002b;92:82–87. doi: 10.2105/ajph.92.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA, Grey DD, Burke-Miller JK, Cohen MH, Vlahov D, Kapadia F, et al. Illicit drug use, depression and their association with highly active antiretroviral therapy in HIV-positive women. Drug and Alcohol Dependence. 2007;89:74–81. doi: 10.1016/j.drugalcdep.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WE, Markson LE, Andersen RM, Crystal SH, Fleishman JA, Golin C, et al. Prevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the united states. HCSUS Consortium. HIV Cost and Services Utilization. Journal of Acquired Immune Deficiency Syndromes. 2000;25:115–123. doi: 10.1097/00042560-200010010-00005. [DOI] [PubMed] [Google Scholar]
- Dufour MC. What is moderate drinking? Defining “drinks” and drinking levels. Alcohol Research & Health. 1999;23:5–14. [PMC free article] [PubMed] [Google Scholar]
- Duncan AE, Bucholz KK, Neuman RJ, Agrawal A, Madden PA, Heath AC. Clustering of eating disorder symptoms in a general population female twin sample: a latent class analysis. Psychological Medicine. 2007;37:1097–1107. doi: 10.1017/S0033291707000505. [DOI] [PubMed] [Google Scholar]
- Huang GH, Bandeen-Roche K. Building an identifiable latent class model with covariate effects on underlying and measured variables. Psychometrika. 2004;69:5–32. [Google Scholar]
- Ip EH, Snow Jones A, Heckert DA, Zhang Q, Gondolf ED. Latent Markov Model for Analyzing Temporal Configuration for Violence Profiles and Trajectories in a Sample of Batterers. Sociological Methods and Research forthcoming. [Google Scholar]
- Kaiser Commission on Medicaid and the Uninsured. The uninsured: a primer. Henry J Kaiser Family Foundation 2008 see data notes, pg. 32. [Google Scholar]
- Lazo M, Gange SJ, Wilson TE, Anastos K, Ostrow DG, Witt MD, et al. Patterns and predictors of changes in adherence to highly active antiretroviral therapy: Longitudinal study of men and women. Clinical Infectious Diseases. 2007;45:1377–1385. doi: 10.1086/522762. [DOI] [PubMed] [Google Scholar]
- Lee BR, Thompson R. Examining Externalizing Behavior Trajectories of Youth in Group Homes: Is there Evidence for Peer Contagion. Journal of Abnormal Child Psychology. 2008 doi: 10.1007/s10802-008-9254-4. [DOI] [PubMed] [Google Scholar]
- Levine RS, Briggs NC, Kilbourne BS, King WD, Fry-Johnson Y, Baltrus PT, et al. Black-White mortality from HIV in the United States before and after introduction of highly active antiretroviral therapy in 1996. American Journal of Public Health. 2007;97:1884–1892. doi: 10.2105/AJPH.2005.081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal Data-Analysis Using Generalized Linear-Models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lillie-Blanton M, Stone VE, Snow Jones A, Levi J, Golub ET, Cohen MH, et al. Association of Race, Substance Abuse, and Health Insurance in Use of Highly Active Antiretroviral Therapy Among HIV-infected Women, 2005. American Journal of Public Health. doi: 10.2105/AJPH.2008.158949. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald IL, Zucchini W. Hidden Markov and other models for discrete-valued time series. Chapman & Hall; London: 1997. [Google Scholar]
- Martino SC, Ellickson PL, Klein DJ, McCaffrey D, Edelen MO. Multiple trajectories of physical aggression among adolescent boys and girls. Aggressive Behavior. 2008;34:61–75. doi: 10.1002/ab.20215. [DOI] [PubMed] [Google Scholar]
- Novotna L, Wilson TE, Minkoff HL, McNutt LA, DeHovitz JA, Ehrlich I, et al. Predictors and risk-taking consequences of drug use among HIV-infected women. Journal of Acquired Immune Deficiency Syndromes. 1999;20:502–507. doi: 10.1097/00042560-199904150-00014. [DOI] [PubMed] [Google Scholar]
- Palacio H, Li XH, Wilson TE, Sacks H, Cohen MH, Richardson J, et al. Healthcare use by varied highly active antiretroviral therapy (HAART) strata: HAART use, discontinuation, and naivety. Aids. 2004;18:621–630. doi: 10.1097/00002030-200403050-00006. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. New England Journal of Medicine. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Palepu A, Horton NJ, Tibbetts N, Meli S, Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: the impact of substance abuse treatment. Addiction. 2004;99:361–368. doi: 10.1111/j.1360-0443.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- Radloff IS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rijmen F, Ip E, Rapp S, Shaw E. Qualitative longitudinal analysis of symptoms in patients with primary or metastatic brain tumors. Journal of Royal Statistical Society A. 2008;171:739–753. doi: 10.1111/j.1467-985x.2008.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcoholism-Clinical and Experimental Research. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- SAS Institute. PROC GENMOD SAS vs 9.1.3 [Computer software] Cary, NC: 2005. [Google Scholar]
- Schwarz G. Estimating Dimension of A Model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- Shapiro MF, Morton SC, McCaffrey DF, Senterfitt JW, Fleishman JA, Perlman JF, et al. Variations in the care of HIV-infected adults in the United States -Results from the HIV Cost and Services Utilization Study. JAMA:Journal of the American Medical Association. 1999;281:2305–2315. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- Skurnick JH, Abrams J, Kennedy CA, Valentine SN, Cordell JR. Maintenance of safe sex behavior by HIV-serodiscordant heterosexual couples. AIDS Education and Prevention. 1998;10:493–505. [PubMed] [Google Scholar]
- Smith DJ, Ecob R. An investigation into causal links between victimization and offending in adolescents. The British Journal of Sociology. 2007;58:633–659. doi: 10.1111/j.1468-4446.2007.00169.x. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. American Journal of Medicine. 2003;114:573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- Viterbi AJ. Error bounds for convolutional codes and an asymptotically optimal decoding algorithm. IEEE Transaction, Information Theory. 1967;13:260–269. [Google Scholar]
- Wilson TE. Sexual and reproductive behavior of women with HIV infection. Clinical Obstetrics and Gynecology. 2001;44:289–299. doi: 10.1097/00003081-200106000-00014. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Barron Y, Cohen M, Richardson J, Greenblatt R, Sacks HS, et al. Adherence to antiretroviral therapy and its association with sexual behavior in a national sample of women with human immunodeficiency virus. Clinical Infectious Diseases. 2002;34:529–534. doi: 10.1086/338397. [DOI] [PubMed] [Google Scholar]


