Summary
When CHO cells are arrested in S-phase, they undergo repeated rounds of centrosome duplication without cell cycle progression. While the increase is slow and asynchronous, the number of centrosomes in these cells does rise with time. To investigate mechanisms controlling this duplication, we have arrested CHO cells in S-phase for up to 72 hours, and coordinately inhibited new centriole formation by treatment with the microtubule poison colcemid. We find that in such cells, the pre-existing centrosomes remain, and a variable number of foci – containing α/γ-tubulin and centrin 2 – assemble at the nuclear periphery. When the colcemid is washed out, the nuclear-associated foci disappear, and cells assemble new centriole-containing centrosomes, which accumulate the centriole scaffold protein SAS-6, nucleate microtubule asters, and form functional mitotic spindle poles. The number of centrosomes that assemble following colcemid washout increases with duration of S-phase arrest, even though the number of nuclear-associated foci or pre-existing centrosomes does not increase. This suggests that during S-phase, a cryptic generative event occurs repeatedly, even in the absence of new triplet microtubule assembly. When triplet microtubule assembly is restored, these cryptic generative events become realized, and multiple centriole-containing centrosomes assemble.
Introduction
Centrosome duplication in somatic cells is regulated so that the parental centrosome assembles one and only one daughter centrosome during each division cycle (reviewed in Hinchcliffe and Sluder, 2001; Delattre and Gönczy, 2004; Bettencourt-Dias and Glover, 2007). Restricting the centrosome duplication cycle to once per cell cycle is crucial; the loss of this control can lead to abnormal centrosome duplication and tumorigenesis (Sluder and Nordberg, 2004; Nigg, 2007; Basto et al., 2008). At the core of the centrosome lies a pair of microtubule-based structures known as centrioles. Normally, daughter centrioles – called procentrioles – assemble at right angles to each of the pre-existing centrioles (Kuriyama and Borisy, 1981; Kochanski and Borisy, 1990; Tsou and Stearns, 2006). This semi-conservative duplication has implied a patterned mode of centrosome reproduction, whereby the parental centriole generates a template to assemble the procentriole. Such a potential template would spatially restrict the formation of new centrioles, thereby limiting centrosome duplication to once per cell cycle. Several examples of templates have been described, including an annular ring or a looped fiber containing nine electron-dense foci during basal body formation (Dipple 1968; Fulton, 1971; Gould, 1975). Also, acentriolar mouse oocytes contain multivesicular aggregates (MVA), composed in part of 25 nm ring structures which are thought to provide patterning for centrosome assembly (Calarco, 2000). In mammalian cultured cells, a tube structure has been described that reproduces during centrosome duplication (Ou and Rattner, 2000). A central tube that assembles prior to the formation of the procentrioles has also been identified in C. elegans and Drosophila (Pelletier et al., 2006; Rodrigues-Martins, et al., 2007). Finally, the formation of basal bodies in Chlamydomonas is preceded by the assembly of the cartwheel structure (Nakazawa et al., 2007). In all of these later cases, the forerunner of the centriole requires the recruitment of the protein SAS-6 to the parental centrosome (Strnad et al., 2007). Recruitment of SAS-6 to the nascent daughter centriole is also thought to precede the recruitment of centrin-2 (Salisbury et al., 2002), one of the core proteins of the centriole, and one of the earliest to assemble there (Strnad et al., 2007).
Interestingly, in normal (i.e. non-transformed) animal somatic cells, the centrosome can only duplicate once per cell cycle – even if the cell cycle becomes arrested or prolonged – suggesting that centrosome number is controlled by a block to reduplication (Wong and Stearns, 2003). However, in certain cell types, most notably early embryos and certain transformed somatic cells (like Chinese Hamster Ovary “CHO” cells), centrosomes can undergo repeated cycles of duplication without cell cycle progression, if the cell is arrested in S-phase (Sluder et al., 1990; Gard et al., 1990; Balczon et al., 1995; Hinchcliffe et al., 1998, 1999; Khodjakov et al., 2002; Kuriyama et al., 2007, Prosser et al., 2009). In these studies, the number of centrosomes per cell was found to increase, as time spent in S-phase was prolonged (discussed in Rieder and Sluder, 1996). Thus, the normal inhibition of centrosome re-duplication/centrosome amplification can become inactivated during S-phase arrest and the result is an asynchronous increase in centrosome number over time.
Several studies have also shown that centrioles can be experimentally induced to assemble de novo, i.e. new centriole formation in the absence of a pre-existing centriole (Dirksen, 1961; Palazzo et al., 1992), and that this de novo formation requires the conditions of S-phase (Marshall et al., 2001; Khodjakov et al., 2002). If centrosomes are experimentally removed at other times during the cell cycle, de novo centriole formation does not occur (Hinchcliffe et al., 2001; Khodjakov and Rieder, 2001; Marshall et al., 2001), unless the cell cycle can progress into S-phase (Le Terra et al., 2005; Uetake et al., 2007). Importantly, when centrioles in S-phase arrested CHO cells are experimentally destroyed, clouds of pericentriolar material (PCM) form, and the de novo centrioles form inside these clouds (Khodjakov et al., 2002). If in these same cells the microtubule network is disassembled following centrosome ablation, then clouds of PCM will assemble, but de novo centrioles do not. This suggests that the early stages of centrosome assembly can take place in the absence of microtubules, but that microtubules are required to complete the process, to build the nine triplet microtubules of the centrioles, and may play other roles as well (Kuriyama, 1982; Balczon et al., 1999).
If we presume that centrosome duplication can be initiated in the absence of microtubules, then washing out microtubule inhibitors should allow these “precursors” or “templates” to assemble into true centriole-containing centrosomes. If this were carried out in S-phase arrested CHO cells, then perhaps such templates or precursors would undergo repeated generation, accumulating throughout the arrest period. This would result in the formation of multiple centrosomes following washout of the depolymerizing agent. To test these ideas, we have used CHO cells arrested in S-phase with hydroxyurea (HU), and also treated with colcemid to depolymerize the microtubule network. We find that, as expected, colcemid treatment prevents the assembly of triplet microtubules, and there is no increase in the number of centrosomes per cell. However, when the colcemid is washed out, new centrosomes rapidly assemble, and the increase in the resultant number of new centrosomes increases with time spent in S-phase arrest.
Results
Centrosome re-duplication during S-phase arrest is prevented by colcemid
To analyze centrosome re-duplication in S-phase arrested CHO cells, immunofluorescence microscopy was used to count the number of γ-tubulin positive foci (centrosomes) for cells arrested in S-phase for 24, 48 and 72 hours with three separate DNA synthesis inhibitors: aphidicolin, hydroxyurea (HU), and thymidine (Supplemental Figure 1). Previous reports have suggested that extensive clustering of centrosomes is often observed in S-phase arrested cells, and this makes accurate counting of centrosome number difficult (Balczon et al., 1995, 1999). However, individual centrosomes within each cluster were easily discernable with our imaging systems used here. When labeled with anti-γ tubulin these centrosomes appear torus-shaped, similar to “doughnuts”. The number of γ-tubulin positive foci (centrosomes) continued to increase with time under all three of the arrest conditions. At 24 hours, examination of centrosome number under all the three different arrest conditions revealed that the cells contained primarily one-to-four centrosomes. At 48 hours, this had shifted to greater than five centrosomes per cell. By 72 hours, the vast majority of cells arrested under the three conditions contain between five and sixteen centrosomes. Importantly, multinucleate cells were excluded from the cell counts throughout this study. These cells, which arise from failed cytokinesis, are expected to have twice the normal number of centrosomes per cell. Because there is little difference in the effect on repeated centrosome duplication between the various agents used to arrest the cell cycle in S-phase, we chose to use HU for all further experiments.
To examine the effects on centrosome duplication of depolymerizing the microtubule network during prolonged S-phase arrest, S-phase arrested cells were treated with the alkaloid colcemid (Borisy and Taylor, 1967; Kuriyama, 1982). Briefly, asynchronously growing cells were first treated with HU for 12 hours in order to arrest the cell cycle in S-phase. This is a time roughly equal to the duration of the CHO cell cycle (see Durcan et al., 2008a). After 12 hours in HU, cells were treated with HU plus 0.5 μg/ml colcemid (colc) for a further 2, 12, 36, or 60 hours – these points corresponding to 14, 24, 48, and 72 hours of total HU treatment. This concentration of colcemid is sufficient to depolymerize the microtubule network, and to prevent the polymerization of triplet microtubules and the formation of procentrioles, which is inhibited by treatment with 0.1 μg/ml (Kuriyama, 1982). Not surprisingly, after 2 hours of colc treatment, the microtubule network was completely disassembled (Figure 1, A).
Figure 1.
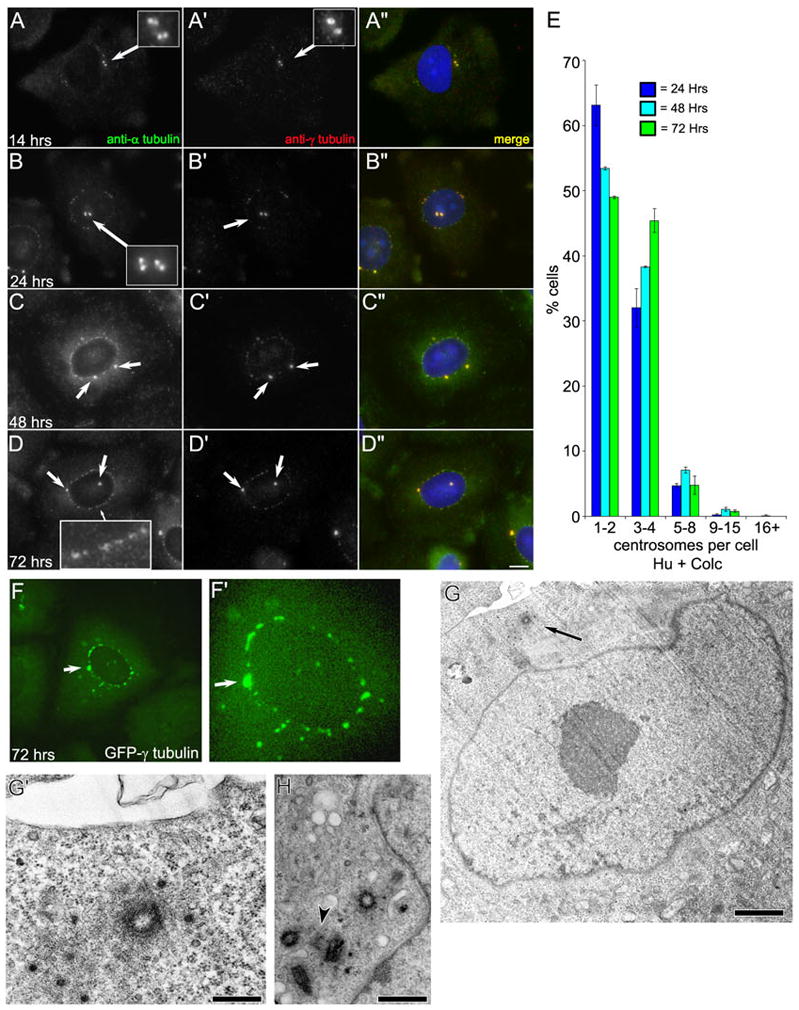
Centrosome duplication does not continue in S phase arrested CHO-K1 cells treated with colcemid. A–D: Immunofluorescent images of CHO-K1 cells labeled with anti-α-tubulin (A, B, C, D) and anti-γ-tubulin (A′, B′, C′, D′). Cells were arrested in S phase for 12 hrs with hydroxyurea and then treated with hydroxyurea and colcemid for a further 2–60 hrs. Times given are from the initial addition of HU. A-A″: After 14 hrs, this cell contains two centrosomes represented by two γ-tubulin foci that colocalize with two α-tubulin foci, representing centrioles (inset). B-B″: After 24 h, the cell contains two centrosomes, each with a pair of centrioles (inset). Nuclear-associated foci, labeled with anti-α and γ-tubulin, are readily visible by this time point. C–D: The irregular nuclear-associated foci persist at the 48 and 72 hr time points. The number of morphologically distinct centrosomes does not increase. E: Quantitation of the number of centrosomes per cell in S phase-arrested CHO-K1 cells treated with HU for 12 hrs, then HU/colc for 12, 36, or 60 hrs. Data is presented as the average number of centrosomes per cell +/− standard deviation for 24, 48, and 72 hrs. Note that the number of centrosomes does not substantially increase throughout the 72 hr time-course. F: Maximum intensity projections of S phase-arrested CHO-K1 cells stably transfected with γ-tubulin-GFP treated with colcemid. Note the irregular nuclear-associated foci are present in the perinuclear region, indicating that these foci are not an artifact of fixation. G: Transmission electron micrograph of a CHO-K1 cell arrested in S phase for 12 hrs with HU, followed by treatment with HU and colcemid for a further 60 hr. Only one centriole is present in this section - arrow (also see high-magnification view in G′). H: Transmission electron micrograph of a different cell from the same experiment showing four complete centrioles, and one pro-centriole (arrowhead). Scale bars: D = 10 μm, G = 0.5 μm, G″ = 0.1 μm, H = 0.2 μm.
When we examined centrosome number in cells treated with HU for 12 hrs, followed by HU/colc for two hrs, we found that they contained predominantly two or four centrosomes; each centrosome consisting of a pair of α-tubulin positive centriole foci surrounded by a cloud of pericentriolar γ-tubulin (Figure 1, A-A′, arrows). In cells treated with HU for 12 hrs, followed by HU/colc for a further 12, 36, or 60 hours (corresponding to 24, 48, and 72 hrs in HU), the cells contained predominantly one-to-four centrosomes – as judged by anti-γ tubulin staining (Figure 1, B′–D′, arrows). Less than 10% of the cells for each of these time points contained greater than five centrosomes (Figure 1, E).
Interestingly, at the 24, 48, and 72 hour time points, many HU/colc-treated cells assembled multiple foci – detected by both anti-α tubulin and anti-γ tubulin antibodies – associated with the outer surface of the nuclear envelope. These foci appear morphologically distinct from the pre-existing centrosomes, being smaller and having less definition (also see Prosser et al., 2009). Although the number of nuclear-associated foci (NAF) was difficult to accurately quantify – even by computer-based “masking” or “count object” methods – they did not appear to increase in number or intensity with the duration in S-phase arrest (not shown).
Live-cell imaging of CHO cells constitutively expressing GFP-γ tubulin revealed that the association of these multiple foci with the nuclear envelope was not a consequence of fixation or antibody labeling (Figure 1, F-F′). Importantly, when CHO cells treated with HU/colc for 72 hrs were examined by serial section electron microscopy, multiple centrioles associated with the nuclear envelope were not observed. Instead individual centrioles, without associated procentrioles were often found (Figure 1G-G′). We did find an example of a cell with four complete centrioles clustered together, that had an associated procentriole (Figure 1, H). However, this cell had been arrested for 72 hrs prior to fixation, suggesting that this procentriole was in the process of forming prior to the addition of colcemid. Together, these observations suggest that new procentrioles are not forming over the 72 hrs of HU/colc treatment.
Next, we examined the distribution of centrin-2 in cells treated with HU and colc. Centrin 2 is a core centrosomal component that is involved in the early events of procentriole formation (Salisbury et al., 2002). When cells from a CHO line constitutively expressing GFP-centrin 2 (CHO-A8 cells: Durcan et al., 2008a) were arrested in S-phase in the presence of colcemid, we found that the centrosomes were positive for centrin, as expected (Figure 2). Interestingly, the centrin-2 observed within the morphologically distinct centrosomes was not restricted to two discrete foci, as would be expected if it were labeling the pair of centrioles (see Durcan et al., 2008a). Instead, we observed the centrosomes containing aggregates of centrin-2 (Figure 2, A–C arrows, also see discussion). We also found that the nuclear associated foci contained large amounts of centrin-2 (Figure 2, B, C). This finding is interesting, because it suggests that these NAF could be centrosome precursors. While they do not contain centrioles, they do accumulate three centrosomal components: α-tubulin, γ-tubulin, and centrin 2.
Figure 2.
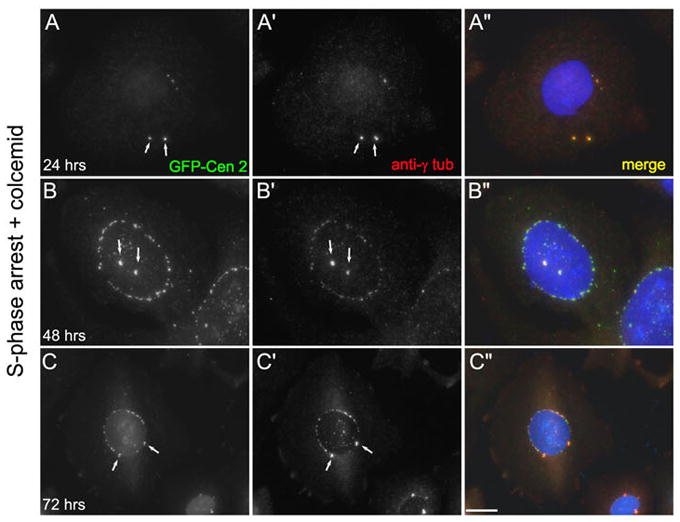
Centrin 2 is a component of nuclear-associated foci. A–C: Images of CHO-A8 cells, which stably express GFP-centrin 2, treated with HU and colcemid. A: At 24 hrs, the cell has two centrosomes (arrows) that are positive for centrin-2 and γ-tubulin. B–C: At 48 and 72hrs, the cells have two centrosomes and numerous nuclear-associated centrosomal foci. Note the presence of irregular perinuclear foci positive for both γ-tubulin and centrin-2. Scale bar = 10 μm.
To test this, we examined the distribution of SAS-6 in CHO cells under our experimental conditions. In addition to centrin 2, the protein SAS-6 has also been shown to be involved in the early events of procentriole formation where it forms a scaffolding structure (Leidel et al., 2005; Strnad et al., 2007). Antibodies against SAS-6 can mark the position of the nascent procentriole. Interphase CHO cells labeled with antibodies against SAS-6 displayed two SAS-6 foci, co-incident with the pair of γ-tubulin labeled centrosomes (Figure 3, A-A″). This characteristic SAS-6 staining pattern on one side of centrosome (the γ-tubulin “doughnut”, that represents the cloud of pericentriolar material predominantly surrounding the mature centriole: Piel et al., 2000) is indicative of the SAS-6 scaffold that forms as part of the developing procentriole (Figure 3, B-B″). Thus, in CHO cells, the characteristic SAS-6 staining pattern is indicative of new procentriole formation.
Figure 3.
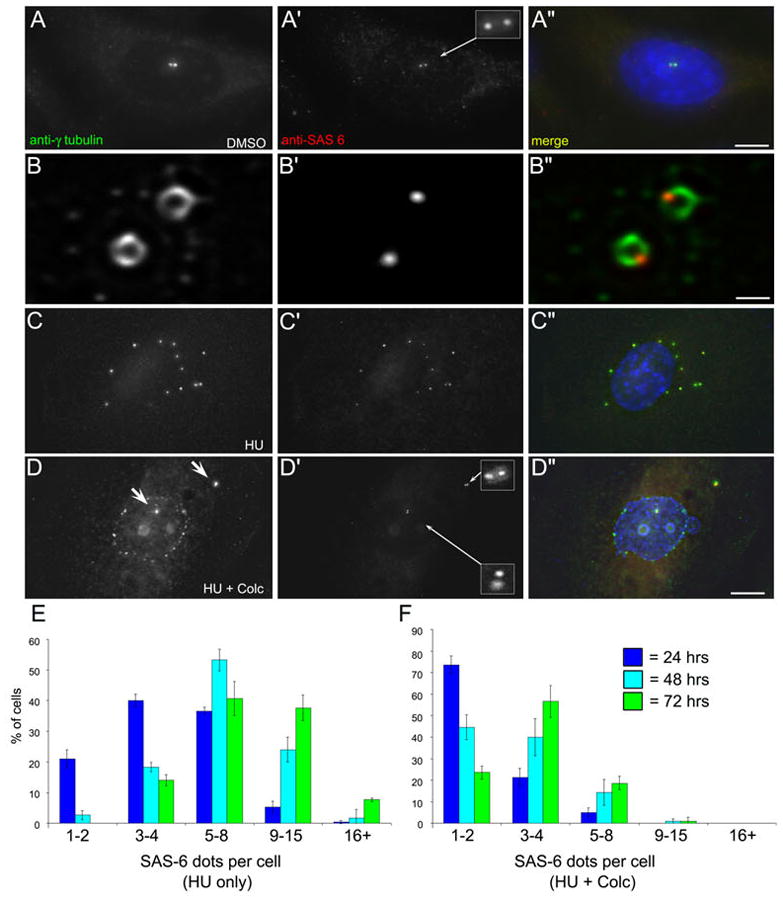
SAS-6 is recruited to the centrosomes, but not nuclear-associated foci in HU/colc-treated cells. A–D: CHO cells labeled with antibodies against γ-tubulin and SAS-6. A-A″ shows an DMSO-control treated interphase cell with two γ-tubulin foci (centrosomes). Each foci has an associated SAS-6 positive foci (inset). B-B″ shows a higher magnification view of γ-tubulin/SAS-6 co-localization at the centrosome. The γ-tubulin is arrayed in a doughnut shape around the mature centriole, with a small focus of SAS-6 delineating the position of the nascent daughter centriole. C-C″ a CHO cell treated with HU for 72 hrs. This cell contains 12 γ-tubulin foci, each with an associated SAS-6 dot. D-D″ shows another CHO cell treated with HU/colc for 72 hrs. Here the cell has two prominent γ-tubulin foci (D, arrows), each with a pair of SAS-6 dots (D′, insets). Note that the nuclear associated foci in D are not recognized by the anit-SAS-6. Scale bars, A″ = 20 μm, B″ = 1 μm, C″ = 10 μm. E: CHO cells treated with HU for 24, 48, and 72 hrs stained with antibodies against SAS-6. The number of SAS-6 positive foci (dots) per cell was counted. Data is presented as the average of three experiments, +/− the standard deviation. F: CHO cells from parallel experiments, treated for 12 hrs with HU, followed by 12, 36, or 60 hrs of HU/colc. The number of SAS-6 dots per cell was counted, and the data presented as above. Note that the number of SAS-6 foci does not significantly increase over 72 hrs.
In cells treated with HU alone, there was a steady increase in the number of SAS-6 positive foci over the 72 hr time course; by 72 hrs the majority of the cells contained five-to-fifteen SAS-6 foci co-incident with the γ-tubulin containing centrosomes (Figure 3, C-C″ and E). However, in cells first treated with HU and then treated with HU/colc, the number of SAS-6 foci did not significantly increase over time. Cells treated for 24 hrs with HU/colc mostly contained two SAS-6 foci, associated with the two pre-existing centrosomes (Figure 3, F and Supplemental Figure 2, B-B″). Those cells treated with HU/colc for either 48 (Figure 3, F and Supplemental Figure 2, E-E″), or 72 hrs contained mainly two-to-four SAS-6 foci (Figure 3, D-D″ and F; also see Supplemental Figure 3, H-H″). Interestingly, the NAF that form in the presence of S-phase arrest and colc treatment were not positive for SAS-6 (Figure 3, D-D″; also see Supplemental Figure 2). This strongly suggests that these NAF are not centrosome precursor structures. Thus, as expected, colcemid treatment prevents the formation of new centrioles during prolonged S-phase arrest. The appearance of the NAF occurs 2–12 hrs after the addition of the colcemid. These irregular foci contain several core centrosomal components: α-tubulin, γ-tubulin, and centrin-2, but do not accumulate SAS-6.
Washing out colcemid from S-phase arrested cells results in the assembly of multiple centrosomes
Even though our measurements demonstrated that the number of centrosomes did not increase with time during treatment with HU/colc, we were interested in determining whether or not there was a cryptic duplication that occurred. One of the advantages of using colcemid to disrupt the microtubule network is that it can be rapidly washed-out of cells, and allow the microtubule network to re-form (Uetake and Sluder 2007, Durcan et al., 2008b). To examine the effects of washing out colcemid, cells were treated with HU/colc for 72 hours as described above. Then the cells on coverslips were quickly washed and transferred into medium containing HU alone. At intervals during the washout, the cells were fixed, labeled with antibodies and analyzed by fluorescence microscopy (Figure 4). As the colcemid washes out of the cells, the multiple NAF become lost from the nuclear envelope. By 35 minutes, short microtubules and microtubule asters are observed in the cytoplasm (Figure 4, C). As the microtubule network reforms, multiple centrosomes are found within the cells (Figure 4, D–E). Thus, the nuclear associated foci that form during S-phase arrest are not persistent structures; their assembly is dependent on both S-phase arrest and a loss of a functional microtubule network.
Figure 4.
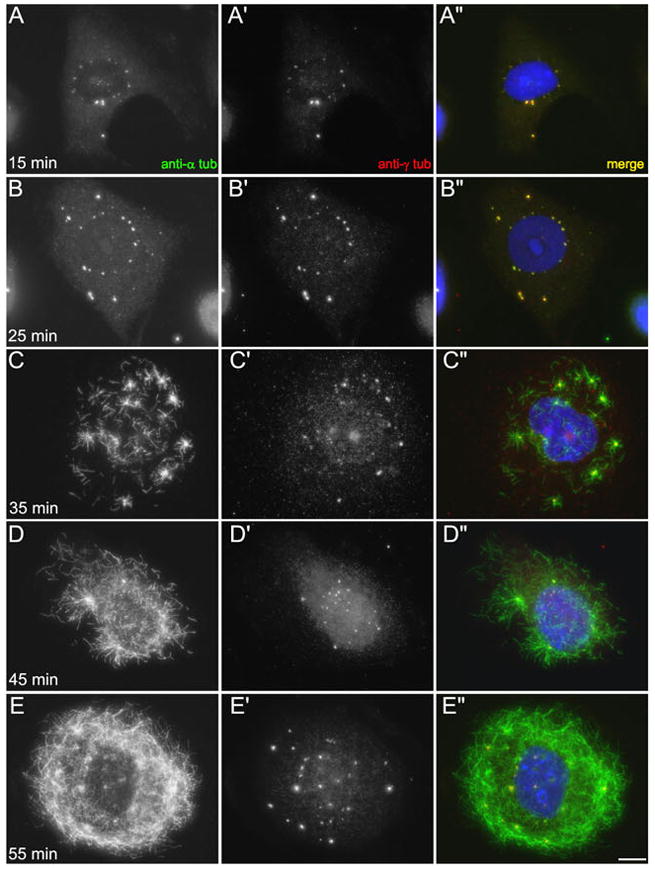
Time-course of colcemid washout in S-phase arrested CHO cells. A–B: 15 and 25 min after colcemid removal, there are multiple γ-tubulin-containing foci around the nucleus and in the cytoplasm. Cytoplasmic microtubules remain absent. C: 35 min after colcemid removal, cytoplasmic microtubules are visible. There are multiple aster-like arrays, and these are γ-tubulin positive. D–E: At 45 and 55 min after colcemid removal, the microtubule network continues to reform. The nuclear-associated foci have dispersed and are no longer visible. Instead, there are multiple γ-tubulin-positive microtubules asters. Scale bar = 10 μm.
To determine whether or not cryptic centrosome duplication events occurred during S-phase arrest in the absence of microtubules, the colcemid was washed out of S-phase cells following 24, 48, and 72 hours of cell cycle arrest (12 hrs HU, followed by 12, 36, or 60 hrs of HU/colc). At each time point, cells were washed for 2 hours (in the continued presence of HU) to allow the microtubule network to re-assemble. Then the number of centrosomes per cell was determined by immunofluorescence microscopy with anti-γ tubulin (Figure 5). Importantly, anti-γ tubulin detected multiple centrosomes; the NAF, which were lost from the cell following colc washout were not part of the γ-tubulin foci counted. The centrosomes in washout cells appeared morphologically normal, and many could nucleate microtubules (Figure 5, B″ inset). Unlike the number of centrosomes in cells treated with HU/colc, which do not increase with time, the final number of centrosomes observed following colcemid washout does indeed progressively increase (Figure 5, D). The fact that cells that have been arrested in S-phase for longer periods assemble greater numbers of centrosomes following colcemid washout strongly suggests that a repeated duplication event has occurred during their arrest. The numbers of centrosomes observed also suggests that these duplication events were slow and asynchronous, just as that observed during prolonged S-phase.
Figure 5.
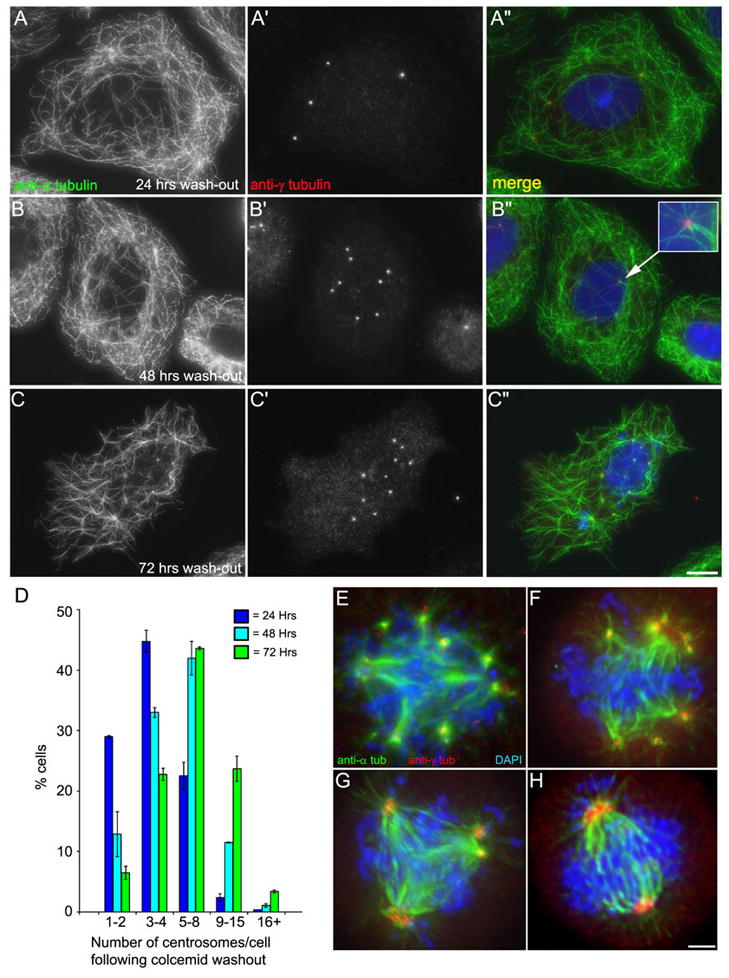
The number of centrosomes that assemble following colcemid washout increases with the duration of cell cycle arrest. A–C: Images of S phase-arrested CHO cells treated with colcemid, followed by colcemid removal 2 hrs prior to fixation. Cells are labeled with anti-α-tubulin and anti-γ-tubulin. A: After 2 hrs of colcemid washout, this cell has re-formed its microtubule network, and contains 4 distinct centrosomes. Note that the nuclear associated foci have dispersed. B: Colcemid washout after 48 hrs arrest, this cell has 8 distinct centrosomes. Note that these centrosomes lie in the center of the re-formed microtubule asters (inset). C: Washout after 72 hrs, the cell has 11 centrosomes. D: Distribution of the average number of centrosomes/cell following 2 hrs colcemid washout for 24, 48, and 72 hrs time points. Note the centrosome number increases with increase in the duration of cell cycle arrest. E–H: Centrosomes formed after colcemid washout assemble functional spindle poles at mitosis. A–D: Images of S phase-arrested CHO cells treated with HU and colcemid for 72 hrs. Both the colcemid and hydroxyurea were removed 12 hrs prior to fixation. Cells were stained with antiα-tubulin and anti-γ-tubulin. E–F: Multiple spindle poles. G: A tripolar spindle with multiple centrosomes per pole. H: A bipolar spindle with multiple centrosomes per pole. Scale bar in C = 10 μm, H = 3 μm.
To determine if these newly formed centrosomes could also function as spindle poles, cells treated for 72 hours with HU/colc were then washed out of both drugs and allowed to progress into mitosis. While many cells that had undergone these extreme treatments remained arrested, multipolar mitotic figures could be identified in these populations (Figure 5, E–H). We note that time-lapse imaging of cells in HU alone or HU/colc revealed that cells do not slip through the S-phase arrest and enter mitosis (not shown). Thus, over time, cells arrested in S-phase without microtubules have the potential to assemble multiple functional centrosomes, and this can only be revealed when the microtubule network is restored, allowing new centrioles to form.
Because SAS-6 is involved in the earliest events of new centriole formation, we examined the localization of SAS-6 in CHO cells following colcemid washout. Cells were treated with HU for 12 hrs, followed by HU/colc for 12, 36, or 72 hrs, and then the colc was washed out for two hours. Washout cells were labeled with antibodies to SAS-6 and γ-tubulin (Figure 6, also see Supplemental Figure 2). Figure 6A–C shows a cell from the 72 hr washout time point. Unlike cells in HU/colc, which contain two-to-four SAS-6 foci, this cell contains 12 SAS-6 foci, each co-incident with a γ-tubulin-containing centrosome (Figure 6, C′). When the number of SAS-6 foci per cell was quantified, we found that there was a steady increase with time in S-phase arrest (Figure 6, D). At 24 hrs, the majority of cells contained less than four SAS-6 foci, whereas at 72 hrs, most of the cells contained five-to-sixteen+ foci (see Supplemental Figure 2). These observations further suggest that multiple, new centrosomes are rapidly forming following colcemid washout.
Figure 6.
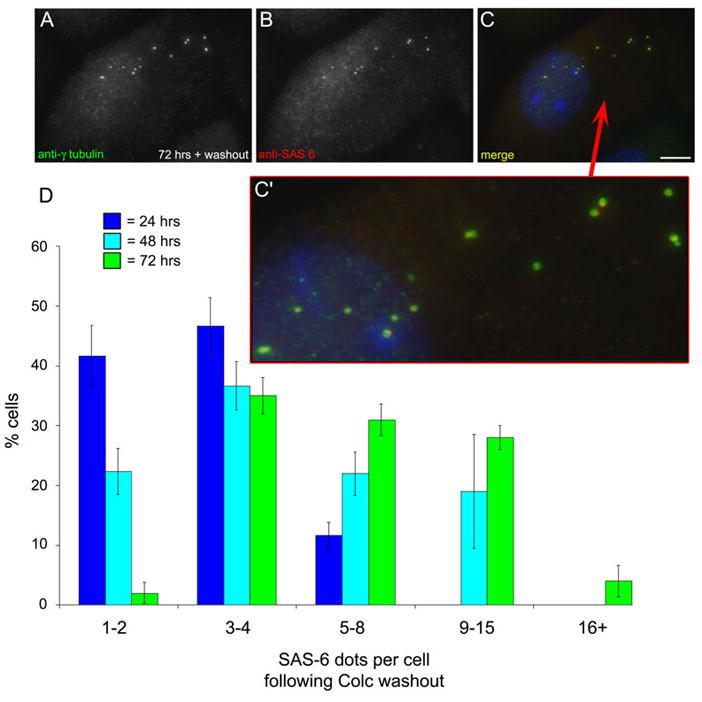
SAS-6 is recruited to multiple centrosomes following colcemid washout. A–C′: CHO cells treated with HU for 12 hrs, followed by treatment with HU/colc for 60 hrs, then the colcemid removed for 2 hrs. Cells were fixed and stained with antibodies against γ-tubulin and SAS-6. The NAF have disassembled, and multiple centrosomes have assembled. Each is co-labeled with anti-SAS-6 (C′). Scale bar = 10 μm. D: The number of SAS-6 foci per cell was quantified for 2 hrs colcemid washout. The average number of SAS-6 positive centrosomes per cell has increased, compared to cells treated with HU/colc and no washout.
To determine the time course for SAS-6 foci formation following colcemid washout, we treated cells for 72 hrs with HU/colc (following our standard protocol), then washed the colc out, and fixed cells at 10 minute intervals, starting at 15 minutes post-washout (Figure 7). Interestingly, we found that multiple SAS-6 foci were visible at the 15-minute time point, well before the NAF had disassembled (Figure 7A-A″). Again, the NAF were not uniformly positive for SAS-6. While in some cases the SAS-6 foci clustered around the prominent γ-tubulin-containing centrosomes, in others, the SAS-6 decorated smaller γ-tubulin foci at the nuclear periphery (Figure 7, A′). However, even following colcemid washout, SAS-6 was not associated with the majority of the NAF that are in the process of disassembling.
Figure 7.
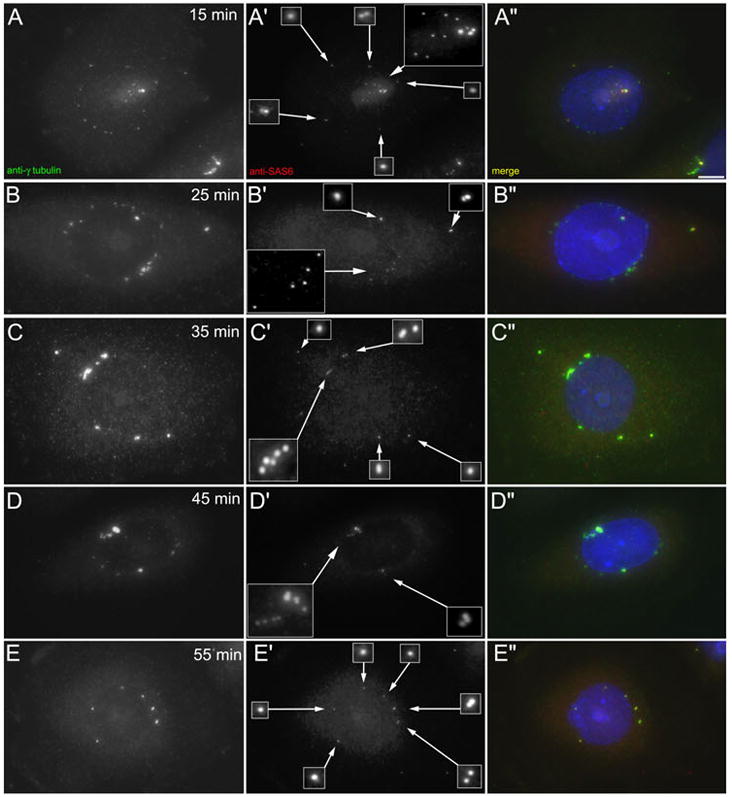
Rapid recruitment of SAS-6 to centrosomes following colcemid washout. A–E: Immunofluorescent images of CHO cells treated with HU for 12 hrs, then HU/colc for a further 60 hrs. The colcemid was then washed out for the times indicated in each frame, and the cells fixed and immuno-labeled with antibodies against γ-tubulin and SAS-6. At T = 15 min (panels A-A″), the SAS-6 is recruited to multiple centrosomes, even before the NAF have disappeared. SAS-6 is localized to the major g-tubulin foci, but also to smaller foci. As the NAF disappear, and the g-tubulin foci become more distinct, these are positive for SAS-6 (C–E).
Finally, we examined the ultrastructure of centrosomes in washout cells by transmission electron microscopy. Cells were treated with HU/colc for 72 hours, as described, and then the colcemid was washed out for two hours. Cells were then fixed, stained, and processed for TEM. Figure 8 shows examples of these cells. By IFM, the cells have re-assembled the microtubule network, the multiple NAF have disappeared, and multiple centrosomes have formed. In the TEM images, there are multiple centrioles per cell; often these are associated with the nuclear envelope (Figure 8, C). In several of the cells examined, there were other interesting structures, in addition to true centrioles. These included immature centrioles and dark-staining foci with a single triplet-microtubule attached (Figure 8, B, large arrows). These dark, electron dense foci appear different from the smaller virus-like particles previously observed in the PCM of CHO cells (Wheatley, 1974; Gould and Borisy, 1977; Khodjakov et al., 2002). The presence of these partial centrioles/centrosomes suggests that these cells have attempted to assemble true centrioles-containing centrosomes, and that this process was incomplete at the time of fixation.
Figure 8.
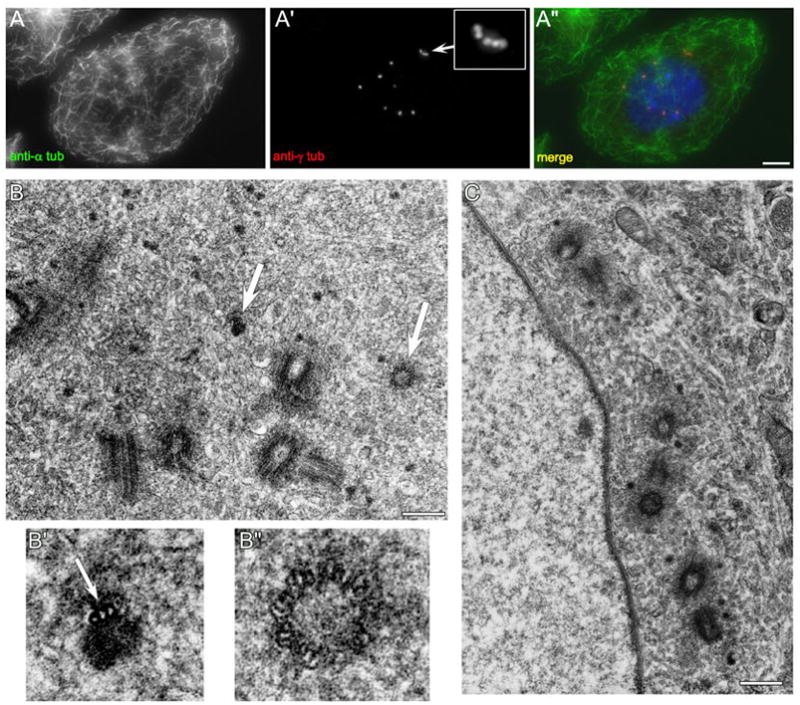
The centrosomes assembled after colcemid washout contain multiple centrioles. CHO cells were treated with HU for 12 hrs, then HU/colc for 60 hrs, followed by washout of colcemid for 2 hrs. A: 2 hrs after colcemid removal, the microtubule network is fully restored, and cells contain multiple centrosomes, including five clustered together (inset). B: These cells contain multiple centrioles. There are five complete centrioles in this section. In addition, there are two incomplete centrosome structures (arrows). B′: Magnified image of an electron dense focus associated with a single triplet microtubule. B″: Magnified image of a small, immature centriole. C: Another cell from the same preparation. There are seven complete centrioles visible in this section. Scale bars: A=10 μm, B, C = 0.4 μm.
Discussion
Here we have addressed the question of whether or not certain key events in the centrosome duplication cycle can be un-coupled from procentriole assembly and elongation. To answer this question, we have used a system, in which S-phase arrested CHO cells assemble multiple centrosomes over time, going from 1–2 centrosomes to over 16 centrosomes in a 72-hour period. During this period, we inhibited microtubule polymerization by the addition of colcemid, which disassembles the interphase microtubule network, as well as inhibiting new procentriole formation. We observed an increase in centrosome number after 72 hrs, but the resultant centrosomes are revealed only when the colcemid is washed out, and new centrioles are allowed to form. Importantly, the number of centrosomes/per increased with the duration of time in S-phase/colcemid, suggesting that a cryptic generative event continues to occur over time. When the colcemid is washed out and microtubules reform, this latent component serves to dictate the assembly of new centriole-containing centrosomes. A key finding is that these new centrosomes recruit SAS-6 only after colcemid washout; the number of SAS-6 foci does not change significantly throughout the 72 hrs of HU/colc treatment nor do the NAF accumulate SAS-6 during the prolonged S-phase arrest. In fact, the number of SAS-6 increases very quickly after removal of the colcemid (within 15 min), at times well before the NAF have disappeared, and well before the γ-tubulin foci resemble the morphologically distinct centrosomes (see Figure 7). SAS-6 has been shown to play a central role in procentriole formation in a wide variety of cell types (Nakazawa et al., 2007 Strnad et al., 2007; Peel et al., 2007). This suggests that the cryptic structures can rapidly assemble into the precursors of centrioles. It is interesting to note that these newly assembled SAS-6 foci are not always in close apposition to the pre-existing centrosomes. While some of the SAS-6 foci are observed around the pre-existing centrosomes, others are located on the nuclear periphery (see Figure 7, A′–C′).
Although the nature and origin of this cryptic generative event remains to be identified, our experiments have suggested several possibilities. One is that the cryptic structures are being generated from the pre-existing centrioles/centrosomes, and then released out into the cell. Such a scenario could account for the multiple SAS-6 foci found at the centrosomes and also along the nuclear periphery. Such a model is also supported by the observation that extra long centrioles in mammalian cells gives rise to greater than normal number of daughter centrioles (Kohlmaier et el., 2009). In this model, the existing centrioles would continuously generate a “seed” or template, which serves to specify and/or initiate the formation of new centrioles following washout of the microtubule poison. Such seeds or templates have been suggested previously (Dippel, 1968, reviewed in Marshall and Rosenbaum, 2003). It is from these structure that the centrioles are thought to assemble (Dirksen, 1991; Loncarek et al., 2007).
However, the question of whether or not such a seed, template or patterning activity is in fact a discreet structure remains to be determined. Recent work has shown that over-expression of Plk4/Sak can induce either the formation of multiple procentrioles forming from a common mother centriole (Habedank et al., 2005; Duensing et al., 2007), or de novo formation of centrioles in the absence of a mother (Bettencourt-Diaz et al., 2005; Peel et al., 2007). It has been argued that Plk4/Sak associated with the mother centriole induces procentriole formation, and that a sufficient concentration of Plk4/Sak can drive assembly in the absence of a mother (Rodrigues-Martins et al., 2007). Rather, new centriole assembly is driven by an accumulation or concentration of Plk4/Sak activity, with or without a pre-existing centriole (reviewed in Loncarek et al., 2007).
However, if the multiple centrosomes observed here arose simply due to an increase in Plk4/Sak levels, then why is there a slow but steady increase in numbers, whereas over-expression of Plk4/Sak leads to a burst of multiple centriole formation? Recent work also suggests that Plk4/Sak levels dramatically decrease during S-phase, due to ubiquitin-mediated proteolysis (Duensing et al., 2007; Cunha-Ferreira et al., 2009; Rogers et al., 2009). In addition, we do not observe multiple procentrioles associated with a single centriole, as seen in cells where Plk4/Sak levels increase (Habedank et al., 2005; Bettencourt-Diaz et al., 2005) or the cycle of ubiquitin-mediated proteolysis has been disrupted, resulting in stabilized Plk4/Sak levels (Duensing et al., 2007; Cunha-Ferreira et al., 2009; Rogers et al., 2009). Thus, it appears unlikely that we are observing Plk4/Sak induced centrosome amplification.
Another possibility is that these cryptic structures are arising de novo. This would be consistent with the model that the cell assembles “precentrioles”, formed from centrin aggregates (LaTerra et al., 2005). Indeed, we do observe that the organization of the centrin-2 within the centrosomes is not as two distinct dots, as normally seen in our CHO-centrin 2-GFP cell line (see Durcan et al., 2008a). However, our observations are different from the de novo centrosome assembly seen in S-phase arrested CHO cells (Khodjakov et al., 2002). In those experiments, multiple centrioles (2–14) were observed to assemble ~24 hours following the laser destruction of the centrosome. In contrast, the number of centrosomes formed here following washout of the colcemid slowly increases with time over 72 hours (Figure 5). Thus, we are not simply observing rapid de novo assembly of multiple centrioles following washout, but rather a gradual increase over time – strongly suggesting a repeated generative event. It is also worth noting that the presence of the pre-existing or resident centriole inhibits de novo formation in CHO cells (Khodjakov et al., 2002), further supporting the idea that de novo duplication is suppressed in our experiments.
The multiple centrosomes we observe could also reflect a slow increase in the total pool of centrosomal subunits available for assembly, rather than the repeated formation of a template. However, evidence from laser ablation studies suggests that CHO cells have sufficient subunits and regulatory molecules to assemble de novo large numbers of centrosomes (14+ centrioles) within 24 hours of S-phase arrest (Khodjakov et al., 2002; LaTerra et al., 2005). Therefore, we conclude that the gradual increase in centrosome number observed following HU/colc washout is not merely due to an increase in the pool of centrosome subunits or regulatory elements. Rather, our findings strongly suggest that a series of latent or cryptic generative events has occurred – albeit lacking proper control and coordination.
In conclusion, our experiments have revealed that a cryptic centrosome generative event repeatedly takes place during S-phase arrest in the absence of microtubules. These generative events result in the production of multiple “seeds” that can recruit core centrosome components and assemble new centrioles, once microtubule polymerization has been restored.
Materials and methods
Unless otherwise noted all reagents were obtained from Sigma Chemical (St. Louis, MO).
Cell culture
Chinese hamster ovary (CHO-K1) cells were obtained from ATCC (Manassas, VA), and cultured in Ham’s F-12, with 10% FCS (Gibco, Grand Island, NY) and 1mg/ml penicillin-streptomycin.
To arrest cells in S-phase, an asynchronous culture of cells were treated with 2 mM hydroxyurea (HU). Alternatively, cells were treated with either 10 μg/ml aphidicolin (Aph) or 2 mM thymidine. To disassemble the microtubule network, 0.5 μg/ml colcemid was added to the cells. To disassemble microtubules during S-phase arrest, asynchronous cells were first treated with 2 mM hydroxyurea (HU) for 12 hours to arrest the cell cycle, and then 1.0 μg/ml colcemid was added for a further 12 hours, 36 hours or 60 hours. To wash out colcemid or HU, cells seeded on coverslips were washed 3x in 37 °C PBS for 30 seconds per wash, then transferred to 10 ml of fresh media for the time specified.
Quantitation of centrosome number was carried out on fixed cells. For each condition and time point, 400 cells were counted from each of three separate experiments. Results are presented as the average of the three experiments with error bars representing the standard deviation.
GFP expressing cells
CHO cells stably expressing γ-tubulin-GFP, and CHO cells expressing GFP-centrin 2 were generated by transfection with either human-γ-tubulin-GFP (pcDNA3-γTGFP: Gift of Alexey Khodjakov, Wadsworth Center, Albany, NY) or human GFP-centrin 2 plasmid (pJLS 148 in Dh5-α cells, gift of Jeff Salisbury, Mayo Clinic, Rochester, MN) using FuGene 6 (Roche, Indianapolis, IN) and selected with 2 mg/ml G 418 as previously described (Durcan et al., 2008b). Resistant cells were sub-cloned in 24-well plates, and screened by fluorescence microscopy. Final colonies were plated as single cells onto a feeder layer of PtK2 cells, re-screened and frozen in LN2. The CHO-γTGFP cells are listed as clone CHO-G8, and the CHO-centrin-2GFP cells are listed as clone CHO-A8.
Fluorescence microscopy
Cells on coverslips were fixed in −20 °C methanol, which preserved the centrin-GFP fluorescence. Fixed cells were labeled with antibodies against γ-tubulin (Sigma), α tubulin (Sigma), or SAS-6 (Santa Cruz Biotechnology, Santa Cruz, CA) followed by 2° antibodies coupled to Alexa dyes (Molecular Probes, Eugene OR). Cells were counter stained with 4′, 6-diamidino-2-phenylindole (DAPI, Sigma). Cells were mounted in 10% PBS, 90% glycerol.
Immunofluorescent images were collected as a Z-series on a DM RXA2 upright microscope, with a 63x 1.4 NA apochromatic CS oil immersion objective (Leica, Bannockburn, IL), and an ORCA-ER CCD camera (Hamamatsu, E. Bridgewater, NJ). Images were captured using Simple-PCI software (Compix, Cranberry Township, PA), and are presented as maximal projections. Contrast adjustment and final assembly of individual panels and three-color overlay images was done using Adobe Photoshop (Mountain View, CA).
Live-cell microscopy
For live imaging, CHO-G8 cells were plated onto bio-cleaned glass coverslips in Imaging Media (Ham’s F-12 w/o phenol red; PromoCell GmbH, Heidelberg, Germany), containing 12mM Hepes, pH 7.2 10% fetal bovine serum) and assembled onto aluminum support slides, as described (Hinchcliffe et al., 2001). Time-lapse images were captured using a Leica DM RXA2 microscope stand, equipped with fluorescence and differential interference optics, enclosed in a custom-made Plexiglas box maintained at 37 °C. Live-cell fluorescence images were captured using a Yokagawa CSU-10 spinning disk confocal head, as modified by McBain Industries (San Diego, CA). Illumination of the fluorescent images was done with a Coherent 488 nm 200 mW “Sapphire” continuous wave optically pumped solid state laser (CW-OPSL); the laser was connected through a fiber optic cable into the excitation port of the spinning disk confocal head, and shuttered through a Ludl MAC5000 shutter controller (described in Hornick et al., 2008). Confocal fluorescent images were taken through a Leica Plan Apo 63x/1.3 NA 37 °C glycerol immersion objective; the detector on the confocal microscope was a Hamamatsu ORCA-AG Digital CCD camera, and images were captured using Simple PCI imaging software, as described (Durcan and Hinchcliffe, 2007).
Electron microscopy
Fixing, embedding and serial sectioning were done as described (Rieder and Cassels, 1999). 85nm sections were cut on a diamond using an RMC MTX ultramicrotome (Boeckeler Instruments, Inc. Tucson, AR). Sections were collected on grids and post stained using saturated Uranyl acetate and Reynold’s lead citrate, and viewed on a Hitachi H600 run at 75kV.
Supplementary Material
A–C: Immunofluorescent images of CHO-K1 cells labeled with anti-α-tubulin and anti-γ-tubulin after treatment with HU for 24, 48, and 72 hrs. Insets: enlarged images of the centrosomal areas. Scale bars: 10 μm. D: Quantitation of the number of centrosomes per cell for CHO-K1 cells arrested in S phase for 24, 48, and 72 hrs. CHO-K1 cells were arrested in S phase with one of the following agents hydroxyurea, aphidicolin or thymidine. Average centrosome number per cell was quantified at 24, 48 and 72 hrs. Note that the number of centrosome rose slowly and continuously throughout the 72 hrs time-course.
A–C: 24 hrs. The cells treated with HU only have amplified centrosomes (A-A′, insets); the cells treated with HU/colc have only two centrosomes, each with a single SAS-6 focus. D–F: 48 hrs. The cells treated with HU/colc have two centrosomes, each with a pair of SAS-6 foci, and the cells have the nuclear associated foci, which are not detected by the SAS-6 antibodies (arrowheads). G–I: 72 hrs. While the HU-only cells have large numbers of centrosomes detected by both anti-γ tubulin and anti-SAS-6, the HU/colc cells have two large γ tubulin-positive centrosomes (H, inset), with a pair of SAS-6 positive foci associated with them (H′, inset). Following washout of the colcemid, these cells have large numbers of centrosomes, with SAS-6 foci associated with them (I″, arrows). Scale bar = 10 μm.
Acknowledgments
We thank Alexey Khodjakov and Jeff Salisbury for their generous gifts of γ-tubulin GFP and centrin 2-GFP respectively, and both JoEllen Welsh and Holly Goodson for critical comments during the course of this work. We also thank Doc Hammer and Jackson Publick for continued scientastic inspiration. JEH is supported by an NIH post-doctoral training grant awarded to Northwestern University (T32 CA080621). This work was supported by research grants from the National Institute of General Medical Science to KTV (R01 GM60560) and EHH (R01 GM072754).
References
- Balczon R, Bao L, Zimmer W, Brown K, Zinkowski RP, Brinkley BR. Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J Cell Biol. 1995;130:105–115. doi: 10.1083/jcb.130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balczon R, Varden CE, Schroer TA. Role for microtubules in centrosome doubling in Chinese hamster ovary cells. Cell Motil Cytoskeleton. 1999;42:60–72. doi: 10.1002/(SICI)1097-0169(1999)42:1<60::AID-CM6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Basto R, Brunk K, Vinadogrova T, Peel N, Franz A, Khodjakov A, Raff JW. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133:1032–1042. doi: 10.1016/j.cell.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol. 2007;8:451–463. doi: 10.1038/nrm2180. [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol. 2005;15:2199–2207. doi: 10.1016/j.cub.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Bobinnec Y, Khodjakov A, Mir LM, Rieder CL, Edde B, Bornens M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J Cell Biol. 1998;143:1575–1589. doi: 10.1083/jcb.143.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy GG, Taylor EW. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967;34:525–533. doi: 10.1083/jcb.34.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarco PG. Centrosome precursors in the acentriolar mouse oocyte. Microsc Res Tech. 2000;49:428–434. doi: 10.1002/(SICI)1097-0029(20000601)49:5<428::AID-JEMT4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Cunha-Ferreira I, Rodrigues-Martins A, Bento I, Riparbelli M, Zhang W, Laue E, Callaini G, Glover DM, Bettencourt-Dias M. The SCF/Slimb ubiquitin ligase limits centrosome amplification through degradation of SAK/PLK4. Curr Biol. 2009;19:43–49. doi: 10.1016/j.cub.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Delattre M, Gönczy P. The arithmetic of centrosome biogenesis. J Cell Sci. 2004;117:1619–1630. doi: 10.1242/jcs.01128. [DOI] [PubMed] [Google Scholar]
- Dippell RV. The development of basal bodies in paramecium. Proc Natl Acad Sci U S A. 1968;61:461–468. doi: 10.1073/pnas.61.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen ER. The presence of centrioles in artificially activated sea urchin eggs. J Biophys Biochem Cytol. 1961;11:244–247. doi: 10.1083/jcb.11.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen ER. Centriole and basal body formation during ciliogenesis revisited. Biol Cell. 1991;72:31–38. doi: 10.1016/0248-4900(91)90075-x. [DOI] [PubMed] [Google Scholar]
- Duensing A, Liu Y, Perdreau SA, Kleylein-Sohn J, Nigg EA, Duensing S. Centriole overduplication through the concurrent formation of multiple daughter centrioles at single maternal templates. Oncogene. 2007;26:6280–6288. doi: 10.1038/sj.onc.1210456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcan TD, Halpin ES, Casaletti L, Vaughan KT, Pierson M, Woods S, Hinchcliffe EH. Centrosome duplication proceeds during mimosine-induced G1 cell cycle arrest. J Cell Physiol. 2008a;215:182–191. doi: 10.1002/jcp.21298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcan T, Halpin E, Rao T, Collins N, Tribble E, Hornick J, Hinchcliffe EH. Tektin 2 is required for central spindle microtubule organization and the completion of cytokinesis. J Cell Biol. 2008b;181:595–603. doi: 10.1083/jcb.200711160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcan TM, Hinchcliffe EH. Digital image files in light microscopy. Methods Cell Biol. 2007;81:315–333. doi: 10.1016/S0091-679X(06)81015-0. [DOI] [PubMed] [Google Scholar]
- Freed E, Lacey KR, Huie P, Lyapina SA, Deshaies RJ, Stearns T, Jackson PK. Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 1999;13:2242–2257. doi: 10.1101/gad.13.17.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton C. Centrioles. In: Beerman C, Reinert J, Ursprung H, editors. Origin and Continuity of Cell Organelles. New York: Springer-Verlag; 1971. pp. 170–213. [Google Scholar]
- Gard DL, Hafezi S, Zhang T, Doxsey SJ. Centrosome duplication continues in cycloheximide-treated Xenopus blastulae in the absence of a detectable cell cycle. J Cell Biol. 1990;110:2033–2042. doi: 10.1083/jcb.110.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RR. The basal bodies of Chlamydomonas reinhardtii: formation from probasal bodies, isolation, and partial characterization. J Cell Biol. 1975;65:65–74. doi: 10.1083/jcb.65.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RR, Borisy GG. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J Cell Biol. 1977;73:601–15. doi: 10.1083/jcb.73.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. The Polo kinase Plk4 functions in centriole duplication. Nat Cell Biol. 2005;7:1140–1146. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe EH, Cassels GO, Rieder CL, Sluder G. The coordination of centrosome reproduction with nuclear events during the cell cycle in the sea urchin zygote. J Cell Biol. 1998;140:1417–1426. doi: 10.1083/jcb.140.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe EH, Li C, Thompson EA, Maller JL, Sluder G. Requirement of Cdk2 - Cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science. 1999;283:851–854. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe EH, Miller FJ, Cham M, Khodjakov A, Sluder G. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science. 2001;291:1547–1550. doi: 10.1126/science.1056866. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe EH, Sluder G. “It takes two to tango”: understanding how centrosome duplication is regulated throughout the cell cycle. Genes Dev. 2001;15:1167–1181. doi: 10.1101/gad.894001. [DOI] [PubMed] [Google Scholar]
- Hornick J, Bader J, Trimble K, Tribble E, Breunig JS, Halpin E, Vaughan K, Hinchcliffe EH. Live-cell analysis of mitotic spindle formation in taxol-treated cells. Cell Motil Cytoskeleton. 2008;65:1–19. doi: 10.1002/cm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. The sudden recruitment of gamma-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J Cell Biol. 1999;146:585–596. doi: 10.1083/jcb.146.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J Cell Biol. 2001;152:237–242. doi: 10.1083/jcb.153.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL, Sluder G, Cassels G, Sibon O, Wang CL. De novo formation of centrosomes in vertebrate cells arrested during S phase. J Cell Biol. 2002;158:1171–1181. doi: 10.1083/jcb.200205102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanski RS, Borisy GG. Mode of centriole duplication and distribution. J Cell Biol. 1990;110:1599–1605. doi: 10.1083/jcb.110.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmaier G, Loncarek J, Meng X, McEwen BF, Mogensen MM, Spektor A, Dynlacht BD, Khodjakov A, Gönczy P. Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr Biol. 2009;19:1012–1018. doi: 10.1016/j.cub.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R, Borisy GG. Centriole cycle in Chinese hamster ovary cells as determined by whole-mount electron microscopy. J Cell Biol. 1981;91:814–821. doi: 10.1083/jcb.91.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R. Effect of colcemid on the centriole cycle in Chinese hamster ovary cells. J Cell Sci. 1982;53:155–171. doi: 10.1242/jcs.53.1.155. [DOI] [PubMed] [Google Scholar]
- Kuriyama R, Terada Y, Lee KS, Wang CL. Centrosome replication in hydroxyurea-arrested CHO cells expressing GFP-tagged centrin2. J Cell Sci. 2007;120:2444–2453. doi: 10.1242/jcs.008938. [DOI] [PubMed] [Google Scholar]
- LaTerra S, English CN, Hergert P, McEwen BF, Sluder G, Khodjakov A. The de novo centriole assembly pathway in HeLa cells: cell cycle progression and centriole assembly/maturation. J Cell Biol. 2005;168:713–722. doi: 10.1083/jcb.200411126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncarek J, Sluder G, Khodjakov A. Centriole biogenesis: a tale of two pathways. Nat Cell Biol. 2007;9:736–738. doi: 10.1038/ncb0707-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF, Vucica Y, Rosenbaum JL. Kinetics and regulation of de novo centriole assembly. Implications for the mechanism of centriole duplication. Curr Biol. 2001;11:308–317. doi: 10.1016/s0960-9822(01)00094-x. [DOI] [PubMed] [Google Scholar]
- Marshall WF, Rosenbaum JL. Tubulin superfamily: giving birth to triplets. Curr Biol. 2003;13:R55–56. doi: 10.1016/s0960-9822(02)01427-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Hayashi K, Nishida E. Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr Biol. 1999;9:429–432. doi: 10.1016/s0960-9822(99)80191-2. [DOI] [PubMed] [Google Scholar]
- Nakazawa Y, Hiraki M, Kamiya R, Hirano M. SAS-6 is a cartwheel protein that establishes the 9-fold symmetry of the centriole. Curr Biol. 2007;17:2169–2174. doi: 10.1016/j.cub.2007.11.046. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Origins and consequences of centrosome aberrations in human cancers. Int J Cancer. 2006;119:2717–2723. doi: 10.1002/ijc.22245. [DOI] [PubMed] [Google Scholar]
- Ou Y, Rattner JB. A subset of centrosomal proteins is arranged in a tubular conformation that is reproduced during centrosome duplication. Cell Motil Cytoskeleton. 2000;47:13–24. doi: 10.1002/1097-0169(200009)47:1<13::AID-CM2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Palazzo RE, Vaisberg E, Cole RW, Rieder CL. Centriole duplication in lysates of Spisula solidissima oocytes. Science. 1992;256:219–221. doi: 10.1126/science.1566068. [DOI] [PubMed] [Google Scholar]
- Peel N, Stevens NR, Basto R, Raff JW. Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr Biol. 2007;17:834–843. doi: 10.1016/j.cub.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier L, O’Toole E, Schwager A, Hyman AA, Müller-Reichert T. Centriole assembly in Caenorhabditis elegans. Nature. 2006;444:619–623. doi: 10.1038/nature05318. [DOI] [PubMed] [Google Scholar]
- Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J Cell Biol. 2000;149:317–330. doi: 10.1083/jcb.149.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser SL, Straatman KR, Fry AM. Molecular dissection of the centrosome overduplication pathway in S-phase-arrested cells. Mol Cell Biol. 2009;29:1760–1773. doi: 10.1128/MCB.01124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Cassels G. Correlative light and electron microscopy of mitotic cells in monolayer cultures. Methods Cell Biol. 1999;61:297–315. doi: 10.1016/s0091-679x(08)61987-1. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt-Dias M. Revisiting the role of the mother centriole in centriole biogenesis. Science. 2007;316:1046–50. doi: 10.1126/science.1142950. [DOI] [PubMed] [Google Scholar]
- Rogers GC, Rusan NM, Roberts DM, Peifer M, Rogers SL. The SCF Slimb ubiquitin ligase regulates Plk4/Sak levels to block centriole reduplication. J Cell Biol. 2009;184:225–239. doi: 10.1083/jcb.200808049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury JL, Sunio KM, Busby R, Springett M. Centrin-2 is required for centriole duplication in mammalian cells. Curr Biol. 2002;12:1287–1292. doi: 10.1016/s0960-9822(02)01019-9. [DOI] [PubMed] [Google Scholar]
- Sluder G, Lewis K. Relationship between nuclear DNA synthesis and centrosome reproduction in sea urchin eggs. J Exp Zool. 1987;244:89–100. doi: 10.1002/jez.1402440111. [DOI] [PubMed] [Google Scholar]
- Sluder G, Miller FJ, Cole R, Rieder CL. Protein synthesis and the cell cycle: centrosome reproduction in sea urchin eggs is not under translational control. J Cell Biol. 1990;110:2025–2032. doi: 10.1083/jcb.110.6.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Nordberg JJ. The good, the bad and the ugly: the practical consequences of centrosome amplification. Curr Opin Cell Biol. 2004;16:49–54. doi: 10.1016/j.ceb.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Sluder G, Rieder CL. Controls for centrosome reproduction in animal cells: issues and recent observations. Cell Motil Cytoskeleton. 1996;33:1–5. doi: 10.1002/(SICI)1097-0169(1996)33:1<1::AID-CM1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Strnad P, Leidel S, Vinogradova T, Euteneuer U, Khodjakov A, Gönczy P. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev Cell. 2007;13:203–213. doi: 10.1016/j.devcel.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou MF, Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006;442:947–951. doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- Uetake Y, Loncarek J, Nordberg JJ, English CN, LaTerra S, Khodjakov A, Sluder G. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J Cell Biol. 2007;176:173–182. doi: 10.1083/jcb.200607073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetake Y, Sluder G. Cell cycle progression without an intact microtubule cytoskeleton. Curr Biol. 2007;17:2081–2086. doi: 10.1016/j.cub.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley DN. Pericentriolar virus-like particles in Chinese hamster ovary cells. J Gen Virol. 1974;24:395–399. doi: 10.1099/0022-1317-24-2-395. [DOI] [PubMed] [Google Scholar]
- Wong C, Stearns T. Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nat Cell Biol. 2003;5:539–544. doi: 10.1038/ncb993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A–C: Immunofluorescent images of CHO-K1 cells labeled with anti-α-tubulin and anti-γ-tubulin after treatment with HU for 24, 48, and 72 hrs. Insets: enlarged images of the centrosomal areas. Scale bars: 10 μm. D: Quantitation of the number of centrosomes per cell for CHO-K1 cells arrested in S phase for 24, 48, and 72 hrs. CHO-K1 cells were arrested in S phase with one of the following agents hydroxyurea, aphidicolin or thymidine. Average centrosome number per cell was quantified at 24, 48 and 72 hrs. Note that the number of centrosome rose slowly and continuously throughout the 72 hrs time-course.
A–C: 24 hrs. The cells treated with HU only have amplified centrosomes (A-A′, insets); the cells treated with HU/colc have only two centrosomes, each with a single SAS-6 focus. D–F: 48 hrs. The cells treated with HU/colc have two centrosomes, each with a pair of SAS-6 foci, and the cells have the nuclear associated foci, which are not detected by the SAS-6 antibodies (arrowheads). G–I: 72 hrs. While the HU-only cells have large numbers of centrosomes detected by both anti-γ tubulin and anti-SAS-6, the HU/colc cells have two large γ tubulin-positive centrosomes (H, inset), with a pair of SAS-6 positive foci associated with them (H′, inset). Following washout of the colcemid, these cells have large numbers of centrosomes, with SAS-6 foci associated with them (I″, arrows). Scale bar = 10 μm.


