Abstract
Human papillomaviruses (HPV) are established as a major cause of cervical carcinoma. However, causality inference is dependent on prospective evidence showing that exposure predicts risk for future disease. Such evidence is available for squamous cell carcinoma, but not for cervical adenocarcinoma. We followed a population-based cohort of 994 120 women who participated in cytological screening in Sweden for a median of 6.7 years. Baseline smears from women who developed adenocarcinoma during follow-up (118 women with in situ disease and 164 with invasive disease) and their individually matched controls (1434 smears) were analyzed for HPV using PCR. Conditional logistic regression was used to estimate odds ratios (OR) of future adenocarcinoma with 95% confidence intervals (CI). Being positive for HPV 16 in the first cytologically normal smear was associated with increased risks for both future adenocarcinoma in situ (OR 11.0, 95 % CI 2.6–46.8) and invasive adenocarcinoma (OR 16.0, 95 % CI 3.8–66.7), compared to being negative for HPV 16. Similarly, an HPV 18 positive smear was associated with increased risks for adenocarcinoma in situ (OR 26.0, 95 % CI 3.5–192) and invasive adenocarcinoma (OR 28.0, 95 % CI 3.8–206), compared to an HPV 18 negative smear. Being positive for HPV 16/18 in two subsequent smears was associated with an infinite risk of both in situ and invasive adenocarcinoma. In conclusion, infections with HPV 16 and 18 are detectable up to at least 14 years before diagnosis of cervical adenocarcinoma. Our data provide prospective evidence that the association of HPV16/18 with cervical adenocarcinoma is strong and causal.
Keywords: Adenocarcinoma, adenocarcinoma in situ, HPV, cervical cancer, prospective
INTRODUCTION
Incidence rates of cervical adenocarcinoma, which accounts for 10–20 % of all cervical cancers, have increased continuously in developed countries during the last two decades, as opposed to those of squamous cell cervical carcinoma 1–2. This upward trend, noted particularly among women under age 40, has occurred despite extensive cytological Pap smear screening 3–6. Consequently, a deeper understanding of the etiology of cervical adenocarcinoma, and better preventive efforts are urgently called for.
A recent collaborative study indicated that the two histological forms of cervical cancer, squamous cell and adenocarcinoma, share most known risk factors 7, the main one being infection with human papillomaviruses (HPV) 8–9. Certain oncogenic types of HPV, in particular HPV 16 and 18, have been strongly associated with risk of cervical adenocarcinoma in several case-control studies 9. Provided that early detection of HPV DNA is possible, this may offer the best means of preventing the development of adenocarcinoma, since prevention through regular cytological screening has proven to be difficult. Previous studies, however, determined HPV status only at the time of diagnosis, and therefore were unable to establish a temporal association between HPV infection and subsequent development of invasive adenocarcinoma (AC) or its precursor, adenocarcinoma in situ (AIS). To clarify the temporal association between HPV infection and the risks of in situ and invasive adenocarcinoma, we prospectively examined HPV status in repeated smears in a population-based cohort of women screened at least once for cervical cancer over a period of up to 26 years.
METHODS
Participants
Cytological screening with Papanicolaou (Pap) smears was gradually introduced into Sweden, starting in 1967. Since the mid-1970’s, all Swedish women have been invited to screening every 3 or 4 years 10. Virtually all Pap smears have been stored, and computerized records containing all information from the cytological screening are kept in the Swedish National Cervical Screening Register 11 . The Swedish National Cancer Registry, established in 1958, records new diagnoses of both invasive cervical cancer and severe dysplasia or cancer in situ of the cervix. The register is considered to include virtually 100% of all incident cancer cases in Sweden 12.
The source population for this study comprised all Swedish women (994 120) who participated in cervical screening within eight Swedish counties sometime during the period 1969–2002. Using the same study design as in a previous investigation of cervical squamous cell carcinoma in situ 13, we identified in the National Cervical Screening Register a cohort of 968 126 women, whose first registered smear during the study period was classified as cytologically normal. Records from our cohort were then linked to the National Cancer Register to identify all women with a first diagnosis of either AIS or AC after entry in our study.
We identified 121 AIS cases and 174 AC cases. Using case-control pair sampling, one woman - matched on county, date of entry into cohort (+/− 3 months), and age (+/− 1 year) - was randomly selected as an individually matched control for each AIS and AC case woman. For both members of each case-control pair, all smears taken prior to the date of diagnosis of the case were identified and retrieved from the archives. To verify the diagnoses of AIS or AC, the histological specimens from all identified cases were reviewed by an experienced pathologist.
Smear Analyses
Each smear was re-coded and re-labelled to ensure blinding of case-control status during DNA extraction and HPV analysis. Samples belonging to the same case-control pair were included in the same analysis batch. After DNA extraction by methods described elsewhere 14, all smears were analysed for the presence of seven low-risk HPV types (HPV 6, 7, 11, 42, 43, 70, and 90), and 16 high-risk HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73, and 82). We used polymerase chain reaction (PCR) amplification of a consensus region using GP5+/6+ primers 15, followed by detection of biotinylated HPV amplicons by hybridisation to short oligonucleotide probes covalently linked to fluorescence-labeled carboxy-coated polystyrene beads on the Bioplex 200 Luminex system (Biorad, CA, USA), as described previously 16. The amount of amplifiable DNA in the samples was determined by real-time PCR for the housekeeping β-globin gene.
Initially, 1622 smears were identified. We subsequently excluded 113 smears with missing or negative β-globin value, 28 because they were from incomplete case-control pairs, and 47 smears because they were obtained within the year immediately preceding diagnosis as part of diagnostic work-up of the case. Consequently, one AIS case, five AC cases, and seven control women were excluded since they did not have any eligible Pap smears. Another two AIS cases and five AC cases were excluded because the case-control pairs were incomplete or because smears were taken on the same day as diagnosis of the disease. Finally, 1434 smears from 282 (118 AIS and 164 AC) complete case-control pairs, in which each woman had at least one β-globin-positive smear, remained for the statistical analyses.
Statistical Analysis
Due to the matched case-control design, conditional logistic regression was used to estimate odds ratios (ORs). Analyses were conditioned on the case-control pair variable that indicates a matched case-control pair. Cervical cancer being a rare disease, we interpreted the ORs as estimates of relative risk (RR), namely the risk of an outcome (AIS or AC) in women exposed to a specific HPV strain, relative to that in women not exposed to that strain. All low risk HPV types (LRHPV) and high risk HPV types excluding HPV 16 and 18 (non-16/18 HRHPV) were analyzed as combined categories. Pooled risk estimates for these HPV groupings were calculated, as we lacked statistical power to obtain reliable HPV type-specific risk estimates for single types, except for HPV 16 and 18. We also lacked power to assess multiple HPV infections separately. Separate analyses were conducted to assess HPV presence in the first and in the last smear prior to diagnosis for the case. Exposure categories were defined as follows; a) HPV 16 – first/last smear positive for HPV 16; b) HPV 18 – first/last smear positive for HPV 18; c) HPV 16/18 – first/last smear positive for HPV 16 and/or HPV 18; d) non-16/18 HRHPV – first/last smear positive for one or more high risk HPV types other than HPV 16 or HPV 18; e) LRHPV – first/last smear positive for one or more low risk HPV types. Attributable risk proportions were calculated based on ORs obtained from the conditional logistic regression models 17. HPV prevalences among cases and controls were estimated by dividing time prior to diagnosis into seven intervals (two-year intervals up to 14 years prior and one open interval prior to that) and calculating the proportion of HPV-positive smears in each interval; patients could contribute smears to several intervals, however, results of smears from the same patient falling into the same interval were averaged. Prevalences were plotted over median time before diagnosis in each interval, and a loess smoother weighted with the number of observations per interval was added. For studying the effect of persistent HPV16/18 infection on adenocarcinoma risk, we identified all subjects in case-control pairs where both cases and controls had at least two smears prior to diagnosis. Based on the HPV test results of the first two consecutive smears, subjects were classified as either double positive, double negative or mixed, i.e. positive/negative or negative/positive. Data are presented in contingency tables. ORs for exposure categories were estimated with conditional logistic regression. All calculations were done using R version 2.8.1 18.
Informed consent from participating women was not required according to the Karolinska Institute Ethics Review Board which approved the study.
RESULTS
Characteristics of the Participants
The characteristics of the 118 AIS and 164 AC cases and their matched control women are described in Table 1. The AIS cases were younger at time of diagnosis (median age 36, range 20–60 years) compared to the AC cases (median age 43, range 24–88 years). The AIS cases and controls were followed for a median time of about 7 years (range 1–26 years), and the AC cases had a median follow-up time of around 6 years (range 1–21 years). The number of smears registered during follow-up were fairly similar for AIS and AC cases and their controls, with a median of 2 to 3 per woman and a range from 1 to 13 (Table 1).
Table 1.
Characteristics of the participants.
| Adenocarcinoma in situ | Invasive adenocarcinoma | |||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| Number of study subjects | 118 | 118 | 164 | 164 |
| Age at diagnosis1 | 36 (20 – 60) | 36 (19 – 61) | 43 (24 – 88) | 43 (24 – 88) |
| Age at entry | 27 (16 – 57) | 28 (16 – 57) | 36.5 (17 – 82) | 36 (17 – 81) |
| Study time | 7.3 (1 – 26) | 7.45 (1 – 25.9) | 6.1 (1 – 21.1) | 5.9 (1 – 21.3) |
| Number of smears | 373 | 324 | 392 | 345 |
| Median no. of smears per subject | 3 (1 – 13) | 2 (1 – 12) | 2 (1 – 9) | 2 (1 – 8) |
Median age and study time in years (range).
HPV Prevalence
In the first smear at study entry, we detected one or more high risk HPV types in 45% of the AIS cases and 8% of the controls, while the corresponding figures were 40% for AC cases and 10% for controls (Table 2). The two most common types detected in the first smear of cases were HPV 16 and HPV 18. Multiple high risk types were detected in 13% of AIS cases and in 9 % of AC cases, but in only 1% of the control women. Table 2 also shows the HPV prevalence in the last available smear preceding diagnosis of AIS or AIC. HPV 16 and 18 prevalence increased from first to last smear in both AIS and AC cases, whereas the prevalence among controls remained fairly stable (Table 2).
Table 2.
Prevalence of specific HPV types and HPV type groupings in first and last Pap smear for adenocarcinoma in situ, and invasive adenocarcinoma cases and their controls
| Adenocarcinoma in situ | Invasive adenocarcinoma | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First smear | Last smear | First smear | Last smear | ||||||
| HPV Type | Low Risk (LR) or High Risk (HR) | Cases N=118 n (%) | Controls N=118 n (%) | Cases N=118 n (%) | Controls N=118 n (%) | Cases N=164 n (%) | Controls N=164 n (%) | Cases N=164 n (%) | Controls N=164 n (%) |
| HPV 6 | LR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1) |
| HPV 7 | LR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HPV 11 | LR | 0 | 0 | 0 | 1 (1) | 0 | 2 (1) | 1 (1) | 1(1) |
| HPV 16 | HR | 23 (19) | 3 (3) | 35 (30) | 5 (4) | 33 (20) | 3 (2) | 38 (23) | 2 (1) |
| HPV 18 | HR | 26 (22) | 1 (1) | 34 (29) | 0 | 28 (17) | 1 (1) | 38 (23) | 2 (1) |
| HPV 31 | HR | 3 (3) | 0 | 4 (3) | 3 (3) | 5 (3) | 4 (2) | 5 (3) | 3 (2) |
| HPV 33 | HR | 1 (1) | 0 | 2 (2) | 0 | 3 (2) | 0 | 4 (2) | 0 |
| HPV 35 | HR | 1 (1) | 1 (1) | 1 (1) | 0 | 1 (1) | 1 (1) | 1 (1) | 0 |
| HPV 39 | HR | 0 | 1 (1) | 3 (3) | 0 | 0 | 0 | 0 | 0 |
| HPV 42 | LR | 3 (3) | 3 (3) | 1(3) | 3 (3) | 0 | 1 (1) | 0 | 0 |
| HPV 43 | LR | 1(1) | 0 | 1 (1) | 0 | 0 | 1 (1) | 1 (1) | 1 (1) |
| HPV 45 | HR | 4 (3) | 1 (1) | 4 (3) | 1 (1) | 5 (3) | 2 (1) | 8 (5) | 1 (1) |
| HPV 51 | HR | 3 (3) | 0 | 2 (2) | 0 | 2 (1) | 0 | 3 (2) | 0 |
| HPV 52 | HR | 1 (1) | 0 | 2 (2) | 1 (1) | 1 (1) | 0 | 1 (1) | 1 (1) |
| HPV 56 | HR | 0 | 1(1) | 2 (2) | 0 | 1 (1) | 0 | 2 (1) | 1 (1) |
| HPV 58 | HR | 2 (2) | 0 | 1 (1) | 0 | 0 | 2 (1) | 0 | 1 (1) |
| HPV 59 | HR | 2 (2) | 0 | 1 (1) | 0 | 1 (1) | 2 (1) | 3 (2) | 1 (1) |
| HPV 66 | HR | 4 (3) | 0 | 2 (2) | 1 (1) | 5 (3) | 0 | 1 (1) | 1 (1) |
| HPV 68 | HR | 1 (1) | 0 | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| HPV 70 | LR | 0 | 0 | 0 | 0 | 0 | 1 (1) | 2 (2) | 2 (1) |
| HPV 73 | HR | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 0 |
| HPV 82 | HR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HPV 16/18 | HR | 45 (38) | 4 (3) | 63 (53) | 5 (4) | 58 (35) | 4 (2) | 73 (45) | 4 (2) |
| Non-16/18 | HR | 8 (7) | 3 (3) | 11 (9) | 4 (3) | 7 (4) | 9 (5) | 12 (7) | 7 (4) |
| Any LRHPV | LR | 4 (3) | 3 (3) | 2 (2) | 4 (3) | 0 | 5 (3) | 2 (1) | 5 (3) |
| Any HRHPV | HR | 53 (45) | 7 (6) | 74 (63) | 9 (8) | 65 (40) | 13 (8) | 85 (52) | 11 (7) |
| Any HPV | HR or LR | 53 (45) | 10 (8) | 75 (64) | 12 (10) | 65 (40) | 17 (10) | 85 (52) | 15 (9) |
| Multiple HPV types | HR or LR | 15 (13) | 1 (1) | 16 (14) | 2 (2) | 15 (9) | 2 (1) | 17 (10) | 3 (2) |
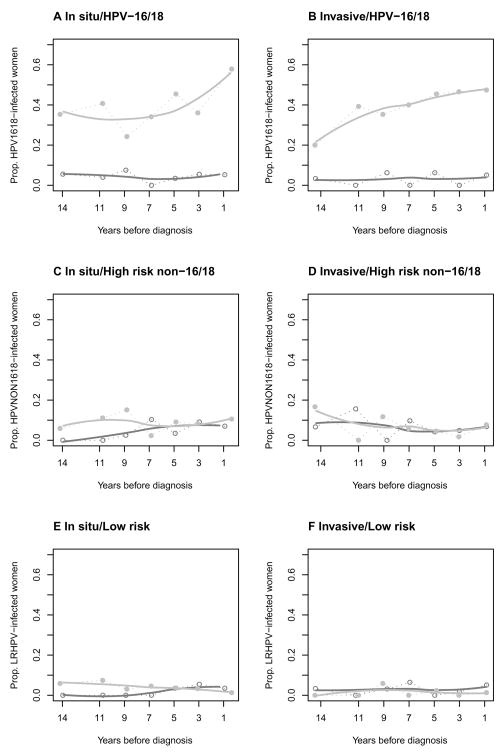
Among AIS cases, the probability of being positive for HPV 16 or 18 increased as time to diagnosis decreased, from about 35% 14 years or more prior to diagnosis to about 57% just before diagnosis (Figure 1A). For AC cases, the probability of HPV 16 or 18 positivity was 20% 14 years or more before diagnosis, and increased to 47% close to diagnosis (Figure 1B). The probability of non-HPV 16/18 high-risk HPV infection in AIS and AC cases was below 15% at the start of follow-up, and did not change appreciably as time of diagnosis approached (Figure 1C and D). Among controls, the prevalence remained stable below 10% throughout the follow-up period (Figure 1–2 A and B). The probability of being infected with low-risk HPV was similar for AIS and AC cases and remained constant over time for both cases and controls (Figure 1E and F).
Figure 1.
A–F. Prevalences of different HPV strains for cases and controls over several time periods prior to diagnosis/end of follow-up. See Methods for details. The grey line represents cases and the black line controls.
Risk Associations
Being positive for HPV in the first cytologically normal smear was associated with almost 11-fold (95% CI 2.6–46.8) increased risk of AIS, while HPV 18 positivity increased the risk 26-fold (95% CI 3.5–192), compared to being negative for the corresponding HPV strain in the first smear (Table 3). The risk association increased from first to last smear for both HPV 16 and 18 (Table 3). The risk of developing AC in relation to infection with HPV 16 or 18 in the first smear was somewhat higher than for AIS (OR 16.0, 95% CI 3.8–66.7 for HPV 16, and OR 28.0, 95% CI 3.8–206 for HPV 18), but the change in risk estimates from first to last smear was less pronounced than for AIS (Table 3). Infections with other high-risk HPV types were not associated with any statistically significant increase in risk of AIS or AC (tables 3 and 4).
Table 3.
Conditional logistic regression showing odds ratios (OR) and 95% confidence interval (CI) of in situ adenocarcinoma among women with HPV positive first and last smear.
| Adenocarcinoma in situ | ||||||
|---|---|---|---|---|---|---|
| First smear | Last smear | |||||
| Positive cases/controls | OR | 95% CI | Positive cases/controls | OR | 95% CI | |
| HPV 16 | 23/3 | 11.0 | 2.6–46.8 | 35/5 | 32.0 | 4.2–227 |
| HPV 18 | 26/1 | 26.0 | 3.5–192 | 34/0 | ∞ | 0-∞ |
| HPV 16 /18 | 45/4 | 14.7 | 4.5–47.2 | 63/5 | 59.0 | 8.2–426 |
| Non-16/18 HRHPV | 8/3 | 3.5 | 0.7–16.8 | 11/4 | 2.8 | 0.9–8.6 |
| LRHPV | 4/3 | 1.3 | 0.3–6.0 | 2/4 | 0.5 | 0.1–2.7 |
Perfect separation, no estimate
HRHPV denotes High Risk HPV and LRHPV Low Risk HPV.
Table 4.
Conditional logistic regression showing odds ratios (OR) and 95% confidence interval (CI) of invasive adenocarcinoma among women with HPV positive first and last smear.
| Invasive adenocarcinoma | ||||||
|---|---|---|---|---|---|---|
| First smear | Last smear | |||||
| Positive cases/controls | OR | 95% CI | Positive cases/controls | OR | 95% CI | |
| HPV 16 | 33/3 | 16.0 | 3.8–66.7 | 38/2 | 19.0 | 4.6–78.8 |
| HPV 18 | 28/1 | 28.0 | 3.8–206 | 38/2 | 19.0 | 4.6–78.8 |
| HPV 16 /18 | 58/4 | 19.0 | 6.0–60.7 | 73/4 | 24.0 | 7.6–76.2 |
| Non-16/18 HRHPV | 7/9 | 0.8 | 0.3–2.1 | 12/7 | 1.7 | 0.7–4.4 |
| LRHPV | 0/5 | 0.8 | 0.3–2.1 | 2/5 | 0.4 | 0.1–2.1 |
HRHPV denotes High Risk HPV and LRHPV Low Risk HPV.
In the studied cohort, the HPV 16 and/or 18 attributable risk proportion (ARP) in the first smear was 36% (95% CI 26–45%) for AIS and 35% (95% CI 26–41%) for AC. The corresponding HPV 16/18 ARP for the last smear prior to diagnosis was 52% (95% CI 43–62%) for AIS and 43% (95% CI 35–51%) for AC.
We identified at least two eligible smears for 75 case-control pairs with AIS and 55 for AC. The average time interval between the smears was 3.0 years for AIS (sd=2.0 years), and 3.1 years for AC (sd=2.3 years), with no significant differences between cases and controls (p=0.44 and p=0.82, respectively). Being positive for HPV 16/18 in the first smear and negative in the second smear conferred ORs 6.0 (95% CI 1.3–26.8) and 2.9 (95% CI 0.1–84.1) for AIS and AC, respectively, compared to being negative in both smears (Table 5). Being negative for HPV 16/18 in the first smear and positive in the second smear conferred OR 7.5 (95% CI 1.7–32.8) and 8.4 (95% CI 0.9–79.7) for AIS and AC, respectively, while all double positive subjects were cases for both forms of the disease (p=4x10−7 for AIS and p=1x10−6 for AC, both corresponding to OR=∞ in conditional logistic regression)(Table 5). We found similar results for exposure to HPV 16 and HPV 18 on their own (data not shown), however, no other strain or combination of strains showed significance, unless HPV 16/18 were included (data not shown).
Table 5.
HPV16/18 infection status for subjects in case-control pairs with at least two smears
| HPV16/18 in first two smears | Adenocarcinoma in situ | Invasive adenocarcinoma | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases (%) | Controls (%) | OR | 95% CIa | p-valueb | Cases (%) | Controls (%) | OR | 95% CIa | p-valueb | |
| − − | 31 (41%) | 70 (93%) | 1.0 | - | - | 30 (54%) | 52 (94%) | 1.0 | - | - |
| +− | 12 (16%) | 3 (4%) | 6.0 | 1.3–26.8 | 5x10−3 | 1 (2%) | 2 (4%) | 2.9 | 0.1–84.0 | 5x10−1 |
| −+ | 15 (20%) | 2 (3%) | 7.5 | 1.7–32.8 | 8x10−4 | 7 (13%) | 1 (2%) | 8.4 | 0.9–79.7 | 2x10−2 |
| ++ | 17 (23%) | 0 (0%) | ∞ | - | 4x10−7 | 17 (31%) | 0 (0%) | ∞ | - | 1x10−6 |
| Total | 75 (100%) | 75 (100%) | 55 (100%) | 55 (100%) | ||||||
based on Wald-statistic
based on likelihood ratio test
DISCUSSION
In this large prospective study, we found that infection with HPV 16 and 18, detected in cytologically normal smears up to at least 14 years before diagnosis, were associated with substantially increased risks of subsequent development of invasive adenocarcinoma (AC) and its precursor, adenocarcinoma in situ (AIS). The risk estimates were generally higher for HPV 18, particularly when they were based on the first eligible smear. Interestingly, the type-specific risk associations did not differ substantially between the two diagnoses, AIS and AC. Double positivity in two subsequent smears conferred an infinite risk increase for both AIS and AC.
We determined HPV status in archival smears, taken repeatedly up to 26 years before the diagnosis of AIS and AC, in cases and control women participating in cytological screening in Sweden. Because of the nested design, our study preserved the validity of the underlying cohort, and our risk estimates are likely to reflect the true risk for the cohort under study. Previous studies have been case series 19–22 where no risk association could be assessed, or case-control studies 7, 9, 23–24, where the temporality of the association could not be addressed, because HPV status was assessed concurrently with disease diagnosis. Because we assessed exposure to HPV up to 26 years before the date of diagnosis of the case (Table 1), our study provides strong evidence in support of the notion that infection with HPV precedes the development of adenocarcinoma of the cervix. Involvement of a substantial proportion of the eligible population in the screening program enhances the generalizability of our findings. If there were more than one smear taken prior to one year before diagnosis we only included the first, since case women had more smears registered during the last year before diagnosis (92 versus 39 smears for control women), probably collected as part of the diagnostic work-up.
One possible limitation of our study is the lack of data on confounding or effect-modifying factors such as oral contraceptive use, smoking, parity, and other sexual transmitted infections 25. However, given the magnitude of the associations with HPV, it is unlikely that adjustment for other factors, themselves modestly associated with risk 7, 9, would have changed our conclusions that HPV 16 and 18 are strongly associated with adenocarcinoma of the cervix. Another possible limitation is the use of archival Pap smears which might lower the sensitivity of our HPV analyses and thus underestimate the true HPV prevalence in our cohort. Most likely, this underestimation would be non-differential for cases and controls, driving the estimated odds ratios towards the null, and also entailing an underestimation of the true attributable risk proportions.
The overall HPV 16/18 prevalence among prospective case women increased as time to diagnosis decreased, reaching a level of about 57% one year before diagnosis (Figure 1A). This estimate is somewhat lower than what has been reported in previous studies 7, 9. Again, this could be due to our estimates being based on archival smears. We observed that the HPV type-specific risks for AIS and AC are not the same as those for squamous cell carcinoma; in contrast to squamous cell carcinoma, HPV18 had a stronger association with AIS and AC than HPV 16, and other HPV types classified as oncogenic for squamous cell cancer were not statistically significantly associated with risk of AIS or AC (tables 3 and 4), These findings are in accordance with several previous studies that have consistently found HPV 18 to be preferentially associated with adenocarcinoma rather than with squamous cell carcinoma of the cervix 7. In contrast to the results of our study, Castellsagué et al. reported an association with high risk types other than HPV 16 and 18 with cervical adenocarcinoma 9. However, these contradictory results should be interpreted cautiously, since both our and their study suffered from low statistical power to investigate associations with HPV types of low prevalence.
The strong link between infection with HPV 16 or18 and malignant transformation of the glandular epithelium of the cervix suggests that screening for these HPV types might be a useful tool for improving the prevention and/or early detection of adenocarcinomas of the cervix, which has proven to be difficult through regular cytological screening 26. It was recently estimated that Swedish women who did not attend to regular cytological screening had a modest 60% increased risk of being diagnosed with cervical adenocarcinoma but a 200% increased risk of squamous cell carcinoma, in comparison to women who took part in the screening 27. Hence, cytological screening entails substantially lower protection against adenocarcinoma than squamous cell cancer of the cervix. Our results point to a strong temporal association between HPV and cervical adenocarcinoma, which has previously not been possible to demonstrate. Detection of HPV 16 and 18 as objective molecular markers of infection could allow diagnosis of cervical adenocarcinomas at an earlier stage in the natural history of the disease. In particular, women positive for HPV 16/18 on two subsequent tests seem to be at very high risk of acquiring the disease. Furthermore, HPV analyses of precursor lesions, namely adenocarcinoma in situ and glandular dysplasia, may help to find women at risk of further progression to invasive disease, and thus improve the prevention of adenocarcinomas.
We conservatively estimate that 35– 52% of all adenocarcinoma cases in our cohort are attributable to infection with HPV 16 or 18. Our results also indicate that the first generation of HPV vaccines, targeting HPV 16 and 18 infections, might prevent at least one third of cervical adenocarcinomas and its precursor lesions in our cohort. Despite the worrying increase in adenocarcinoma rates in the last decades, there are reasons for optimism. Through a combination of continuous public education about transmission of HPV, improved detection of precursor lesions of cervical carcinoma by incorporating HPV testing into the screening programs, and by vaccination against HPV 16 and 18, carcinoma of the cervix may become one of the major forms of cancer that is most preventable on a global scale.
We conclude that infections with HPV 16 and 18, detected at least 14 years prior to diagnosis while no cytological changes are yet evident, are associated with increased risk of developing both in situ and invasive cervical adenocarcinoma. At least one-third of the incident cases of cervical adenocarcinoma should be preventable through the currently available vaccines against HPV.
Novelty and Impact.
This study provides prospective evidence supporting that the strong association of HPV16/18 with cervical adenocarcinoma is indeed of etiological significance. Availability of such evidence is essential for efforts aimed at preventing cervical adenocarcinoma by way of preventing HPV infections. Furthermore, the fact that HPV positivity strongly predicted development of cervical adenocarcinoma also many years into the future further implies that HPV-based cervical screening programs could have a lasting protective effect against this disease.
Acknowledgments
We are grateful for the input provided by Ninoa Malki for database management and Ms Aline Marshall, Ms Carina Eklund and Ms Kia Sjölin in the Joakim Dillner lab for the HPV testing. We would also like to thank Dr. Anders Lindgren for review of histopathological material. The people mentioned in this section all gave their consent to be acknowledged.
Financial disclosure
This work was supported by National Cancer Institute (1R01CA93378-01A1 R01CA111720-01 and R01CA111720-02 and 5R01CA111720-03) and a grant from the Swedish Cancer Society.
Lisen Arnheim Dahlström, Karin Sundström and Carani B Sanjeevi were funded by these grants. All other authors are employed and funded by affiliated university or hospital.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health or the Swedish Cancer Society. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gustafsson L, Ponten J, Zack M, Adami HO. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997 Sep;8(5):755–763. doi: 10.1023/a:1018435522475. [DOI] [PubMed] [Google Scholar]
- 2.Vizcaino AP, Moreno V, Bosch FX, Munoz N, Barros-Dios XM, Borras J, Parkin DM. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int J Cancer. 2000 May 1;86(3):429–435. doi: 10.1002/(sici)1097-0215(20000501)86:3<429::aid-ijc20>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 3.Vizcaino AP, Moreno V, Bosch FX, Munoz N, Barros-Dios XM, Parkin DM. International trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas. Int J Cancer. 1998 Feb 9;75(4):536–545. doi: 10.1002/(sici)1097-0215(19980209)75:4<536::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Visioli CB, Zappa M, Ciatto S, Iossa A, Crocetti E. Increasing trends of cervical adenocarcinoma incidence in Central Italy despite Extensive Screening Programme, 1985–2000. Cancer Detect Prev. 2004;28(6):461–464. doi: 10.1016/j.cdp.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Bray F, Carstensen B, Moller H, Zappa M, Zakelj MP, Lawrence G, Hakama M, Weiderpass E. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomarkers Prev. 2005 Sep;14(9):2191–2199. doi: 10.1158/1055-9965.EPI-05-0231. [DOI] [PubMed] [Google Scholar]
- 6.Bergstrom R, Sparen P, Adami HO. Trends in cancer of the cervix uteri in Sweden following cytological screening. Br J Cancer. 1999 Sep;81(1):159–166. doi: 10.1038/sj.bjc.6690666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ICESCC. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2006 Feb 15;120(4):885–891. doi: 10.1002/ijc.22357. [DOI] [PubMed] [Google Scholar]
- 8.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003 Jan 13;88(1):63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castellsague X, Diaz M, de Sanjose S, Munoz N, Herrero R, Franceschi S, Peeling RW, Ashley R, Smith JS, Snijders PJ, Meijer CJ, Bosch FX. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006 Mar 1;98(5):303–315. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson L, Sparen P, Gustafsson M, Wilander E, Bergstrom R, Adami HO. Efficiency of organised and opportunistic cytological screening for cancer in situ of the cervix. Br J Cancer. 1995 Aug;72(2):498–505. doi: 10.1038/bjc.1995.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparen P. Report from the National Cervical Screening Register. Karolinska Institutet; Stockholm: 2007. [Accessed August 17, 2009]. Cervical Screening in Sweden in 2006. at http://ki.se/content/1/c6/05/05/04/Rapport_2007.pdf. [Google Scholar]
- 12.Cancerregistret. Swedish Cancer Registry. National Board of Health and Welfare; Stockholm: 2008. Cancer Incidence in Sweden 2007. Official Statistics of Sweden. [Google Scholar]
- 13.Ylitalo N, Josefsson A, Melbye M, Sorensen P, Frisch M, Andersen PK, Sparen P, Gustafsson M, Magnusson P, Ponten J, Gyllensten U, Adami HO. A prospective study showing long-term infection with human papillomavirus 16 before the development of cervical carcinoma in situ. Cancer Res. 2000 Nov 1;60(21):6027–6032. [PubMed] [Google Scholar]
- 14.Chua KL, Hjerpe A. Polymerase chain reaction analysis of human papillomavirus in archival cervical cytologic smears. Anal Quant Cytol Histol. 1995 Aug;17(4):221–229. [PubMed] [Google Scholar]
- 15.de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995 Apr;76( Pt 4):1057–1062. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- 16.Soderlund-Strand A, Carlson J, Dillner J. Modified general primer PCR system for sensitive detection of multiple types of oncogenic human papillomavirus. J Clin Microbiol. 2009 Mar;47(3):541–546. doi: 10.1128/JCM.02007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuritz SJ, Landis JR. Attributable risk estimation from matched case-control data. Biometrics. 1988 Jun;44(2):355–367. [PubMed] [Google Scholar]
- 18.Development Core Team R. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2008 URL: http://www.R-project.org.
- 19.Pirog EC, Kleter B, Olgac S, Bobkiewicz P, Lindeman J, Quint WG, Richart RM, Isacson C. Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am J Pathol. 2000 Oct;157(4):1055–1062. doi: 10.1016/S0002-9440(10)64619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson S, Rylander E, Larsson B, Strand A, Silfversvard C, Wilander E. The role of human papillomavirus in cervical adenocarcinoma carcinogenesis. Eur J Cancer. 2001 Jan;37(2):246–250. doi: 10.1016/s0959-8049(00)00376-2. [DOI] [PubMed] [Google Scholar]
- 21.Zielinski GD, Snijders PJ, Rozendaal L, Daalmeijer NF, Risse EK, Voorhorst FJ, Jiwa NM, van der Linden HC, de Schipper FA, Runsink AP, Meijer CJ. The presence of high-risk HPV combined with specific p53 and p16INK4a expression patterns points to high-risk HPV as the main causative agent for adenocarcinoma in situ and adenocarcinoma of the cervix. J Pathol. 2003 Dec;201(4):535–543. doi: 10.1002/path.1480. [DOI] [PubMed] [Google Scholar]
- 22.Tawfik El-Mansi M, Cuschieri KS, Morris RG, Williams AR. Prevalence of human papillomavirus types 16 and 18 in cervical adenocarcinoma and its precursors in Scottish patients. Int J Gynecol Cancer. 2006 May–Jun;16(3):1025–1031. doi: 10.1111/j.1525-1438.2006.00552.x. [DOI] [PubMed] [Google Scholar]
- 23.Bulk S, Berkhof J, Bulkmans NW, Zielinski GD, Rozendaal L, van Kemenade FJ, Snijders PJ, Meijer CJ. Preferential risk of HPV16 for squamous cell carcinoma and of HPV18 for adenocarcinoma of the cervix compared to women with normal cytology in The Netherlands. Br J Cancer. 2006 Jan 16;94(1):171–175. doi: 10.1038/sj.bjc.6602915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chew GK, Cruickshank ME, Rooney PH, Miller ID, Parkin DE, Murray GI. Human papillomavirus 16 infection in adenocarcinoma of the cervix. Br J Cancer. 2005 Nov 28;93(11):1301–1304. doi: 10.1038/sj.bjc.6602855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohan T. The epidemiology of adenocarcinoma of the cervix. In: Rohan T, Shah K, editors. Cervical cancer: from etiology to prevention. Dordrecht: Kluwer Academic Publishers; 2004. pp. 217–234. [Google Scholar]
- 26.Sasieni P, Castanon A, Cuzick J. Screening and adenocarcinoma of the cervix. Int J Cancer. 2009 Mar 4;125(3):525–529. doi: 10.1002/ijc.24410. [DOI] [PubMed] [Google Scholar]
- 27.Andrae B, Kemetli L, Sparen P, Silfverdal L, Strander B, Ryd W, Dillner J, Tornberg S. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst. 2008 May 7;100(9):622–629. doi: 10.1093/jnci/djn099. [DOI] [PubMed] [Google Scholar]