Abstract
Host prion protein (PrP) is most abundant in neurons where its functions are unclear. PrP mRNA transcripts accumulate at key developmental times linked to cell division arrest and terminal differentiation. We sought to find if proliferative arrest was sufficient to cause an increase in PrP in developing neurons. Rat neuronal precursor cells transduced with the temperature sensitive SV40 T antigen just before terminal differentiation (permissive at 33°C but not at 37.5°C) were analyzed. By 2 days, T antigen was decreased in all cells at 37.5°C, with few DNA synthesizing (BrdU+) cells. Proliferative arrest induced by 37.5°C yielded a 4-fold PrP increase. When combined with reduced serum, a 7-fold increase was found. Within 2 days additional neuritic processes with abundant plasma membrane PrP connected many cells. PrP also concentrated between apposed stationary cells, and on extending growth cones and their filopodia. Stationary cells were maintained for 30 days in their original plate, and they reverted to a proliferating low PrP state at 33°C. Ultrastructural studies confirmed increased nanotubes and adherent junctions between high PrP cells. Additionally, some cells shared cytoplasm and these apparently open regions are likely conduits for the exchange of organelles and viruses that have been observed in living cells. Thus PrP is associated with dynamic recognition and contact functions, and may be involved in the transient formation of neural syncytia at key times in embryogenesis. This system can be used to identify drugs that inhibit the transport and spread of infectious CJD particles through the nervous system.
Keywords: filopodia, terminal differentiation, neurons, adherent junctions, dynamic attachment, synapse, virus, CJD, scrapie
INTRODUCTION
One of the fundamental features of neurogenesis is its exquisitely ordered timing. In less complex regions, such as the embryonic spinal cord, neuron precursors progress through stages of proliferation, precise anatomical migration, and a final loss of replicative capacity. Replication arrest coincides with the appearance of spinal cord synaptic connections and subsequent glial differentiation at 14–18 days post-implantation in rodents [Manuelidis and Manuelidis, 1971]. In granule neurons of the cerebellum, DNA synthesis, mitosis, and migration occurs later, and synapses start to form only 9 days after birth [Manuelidis, 1974]. This developmental background led us to realize that accumulation of host prion protein (PrP), a small mammalian membrane protein of 34kd, coincides with an arrest in neuronal division and the formation of communicating synaptic junctions in the brain. PrP transcripts in dissected rat spinal cord are already apparent by embryonic day 20, after synapses formed, whereas in the later developing cerebellum, PrP mRNA increases only after 3 days postpartum [Lieberburg, 1987]. In-situ hybridization mRNA studies further support a cell division to PrP synaptic relationship. Spinal ganglia are heavily labeled in the mouse embryo at 16.5 days [Manson et al., 1992], the time when their abundant synaptic contacts actively form. It is not clear if specialized cell-to-cell contacts lead to proliferative arrest, or if arrest itself initiates these contacts [Manuelidis and Manuelidis, 1971].
PrP is best known for its pathological amyloid state in Transmissible Encephalopathies (TSE), a group of infectious diseases affecting many mammalian species. In this setting, host PrP has reputed protean properties [Prusiner, 1999], including its ability to be an infectious protein or “prion”, to have inherited and spontaneous prion forms, to cause neurodegeneration, to protect neurons, and most recently, to mutate and create new agent strains without any nucleic acid [Li et al., 2010]. Presumably strain-specific mutation occurs by some type of protein misfolding that was not demonstrable. This PrP infectious amyloid model bears a remarkable resemblance to the self-catalytic crystalline protein model of Tobacco Mosaic Virus proposed in 1936 [Kay, 1986]. This Nobel Prize work ignored the fact that infectious preparations were not pure, and contained nucleic acids, the fundamental genetic and mutable molecules of all organisms. Since all infectious TSE preparations contain nucleic acid, and also exhibit classical biological and structural properties of an ~25nm virus, we think it more likely that host PrP acts as an essential membrane receptor for the infectious particle [Manuelidis, 2007; Manuelidis et al., 2007]. The host also recognizes TSE agents as foreign rather than host encoded, and geographic agent isolates show very different patterns of virulence [Manuelidis et al., 2009a; Manuelidis et al., 2009b]. Furthermore, removal of the environmental source of infection results in a dramatic decrease in disease, as shown by reductions of epidemic bovine TSE in the UK. Understanding the normal role of PrP can yield new insights for retarding the spread of TSE agents.
Because knockout PrP mice are essentially normal, a “bewildering variety of subtle abnormalities” in circadian rhythm, olfaction, hypoxia sensitivity, oxidative capacity, copper metabolism and signal transduction have been proposed [Chiesa and Harris, 2009; Pantera et al., 2009]. Studies on TSE infections in culture led us back to their probable function in both cell communication and neuronal differentiation. In establishing new tissue culture models of many different TSE agents, we found that cell-to-cell contacts were of major importance in the natural spread of sheep scrapie and human Creutzfeldt-Jakob Disease (CJD). Most of the major different TSE agent strains, derived from human and animal sources, have now been propagated in neuronal GT1 cells [Manuelidis et al., 2009a; Manuelidis et al., 2009b], and co-culture experiments showed that infectious particles were effectively transmitted through cell contacts, rather than by particles secreted into the medium [Nishida et al., 2005]. Indeed, debris cleared medium from a variety of infected cells normally contains ≤1% to undetectable levels of the cellular infectivity [Nishida et al., 2005; Leblanc et al., 2006; Race et al., 1987]. We thus chose to investigate the relationship of nuclear DNA synthesis to both PrP production and the formation of new cell-to-cell contacts in a culture model of rat neurogenesis. We used neural progenitor septal (SEP) cultures that could be induced to differentiate both morphologically and molecularly by arresting cell proliferation [Eves et al., 1994; Vogt Weisenhorn et al., 2001]. Proliferative arrest in a neuroblastoma tumor line was previously reported to have no effect on PrP in uninfected cells [Ghaemmaghami et al., 2007]. However, neither DNA synthesis nor in-situ PrP changes were examined. No other studies have reported on PrP changes caused by the arrest of neural cell proliferation.
We demonstrate that when normal SEP cells become stationary they rapidly accumulate 7-fold elevations in PrP, and also elaborate new cell processes with PrP rich cell-to-cell junctions. Stationary cell cultures were achieved by a mild physiological temperature shift and/or a reduction of serum factors. Proliferative arrest, as monitored by BrdU uptake, was rapid and without apparent pathological consequences. Of particular interest was the formation of abundant thin cell-to-cell attachments, consistent with tunneling nanotubes that are known to be conduits for exchange of cytoplasmic contents and viral spread [Rustom et al., 2004; Gerdes et al., 2007; Sherer et al., 2007; Sowinski et al., 2008]. Our results suggest that membrane PrP probably participates in the normal formation of these conduits. Ultrastructural studies confirmed apparently open cytoplasmic regions between cells, as well as electron dense adherent junctions. A syncytial network of connections among many cells may be an essential part of programmed tissue differentiation, particularly in the nervous system during specific transitional times of development.
MATERIALS and METHODS
Low passage SEP AS583 cells (subclone e422), a generous gift of B. Wainer, were maintained in high glucose DMEM and various concentrations of FBS (see Results) with penicillin/streptomycin (50 units/ml) and G418 (0.2mg/ml). Proliferating cells were kept at 33°C and split 1:4 every 4 days. Stationary cells were refed every 2 days in their original seed plate. For Western blots, cells were lysed in detergent and analyzed with two different PrP antibodies as previously detailed [Arjona et al., 2004]. To detect SV-40 T antigen on blots it was necessary to separate a nuclear pellet after lysis by low speed centrifugation [Sun et al., 2008]; viscous DNA was sheared by bath sonication before loading. Chemiluminescent signals on blots [Arjona et al., 2004] were normalized with respect to lane protein loads and additionally confirmed by β-actin and tubulin signals on the same blot, as well as by Annexin II, a plasma membrane molecule.
In situ studies were done on cells plated in chamber slides. To assess DNA synthesis, cells were labeled with BrdU [Manuelidis and Borden, 1988] for 2hr, fixed in cold methanol, treated with 2N HCl for 20 min, and detected with a mouse anti-BrdU antibody as described (26), except that strepavidin alkaline phosphatase (KPL) was used as the reporter. The SV40 T antigen was also detected after methanol fixation, whereas PrP and nestin were evaluated on cells fixed in 2% paraformaldehyde [Manuelidis et al., 2009b]. For electron microscopy cells were fixed in situ with 2% glutaraldehyde in 0.1M Cacodylate buffer for 20 min, gently scraped from the flask in sheets, pelleted at 1500g prior to addition of 2% melted agarose and conventionally post-fixed in 1% tannic acid and then 1% osmium before embedding.
RESULTS
We first assessed the best conditions to arrest nuclear DNA synthesis in a clone of neural SEP cells with many features of neural differentiation [Eves et al., 1994; Vogt Weisenhorn et al., 2001]. Just before their terminal brain mitosis, SEP precursor neurons had been transduced with a defective retrovirus carrying an SV-40 temperature sensitive (ts) T antigen. They can be induced to elaborate neurites, neurofilaments, neuron-specific markers, and synaptic terminals [Eves et al., 1994; Vogt Weisenhorn et al., 2001]. We cultured SEP cells under several temperature and serum conditions, and exposed them to BrdU to monitor DNA synthesis in situ. Growth at 39°C resulted in some cell loss, whereas typical incubation at 37.5°C did not. We therefore evaluated the combined effect of temperature and reduced serum in cells at DNA synthetic (33°C) and arrest-inducing (37.5°C) temperatures.
Fig. 1A shows the percent of SEP cells that incorporated of BrdU during a short 2hr pulse under standard proliferative and arresting conditions. Under standard conditions, 39% of cells were BrdU labeled, consistent with a 24 hr doubling time found here and previously. DNA synthesis was significantly decreased with either elevated temperature alone, or in combination with reduced serum. Within 2 days of conditional change there was a marked decrease in nuclear DNA synthesis (Fig. 1A). Merely incubating cells at 37.5°C in standard 10% serum reduced DNA synthesizing cells by half (P<0.0001). Thus partial arrest was already induced in physiologic conditions. More dramatic decreases were seen when serum reduction was combined with a shift to 37.5°C. With 2% or 1% serum only 11% and 4% of cells, respectively, incorporated BrdU at 2 days. Some positive cells were expected at this time since cells with residual stores of serum factors still could initiate DNA synthesis. In accord with this, SEP neurons fed with 1–2% serum became completely stationary within 4 days, and maintained a stable plate density if kept under these conditions. They never reached complete confluence or attained the density of non-arrested controls. Piling up of arrested cells was not observed, unlike transformed and tumor lines. There was some cell loss in 1% serum flasks arrested for more that a week, likely due to insufficient serum maintenance factors. Nevertheless, these conditions still resulted in stationary viable cells for 30 days (see Fig 2B). Fig. 1A also shows a comparable T antigen reduction by Western blot analysis. Again, there is a large 5-fold decrease in T antigen (P<0.0001) when SEP neurons are induced to become stationary, and these low values persisted.
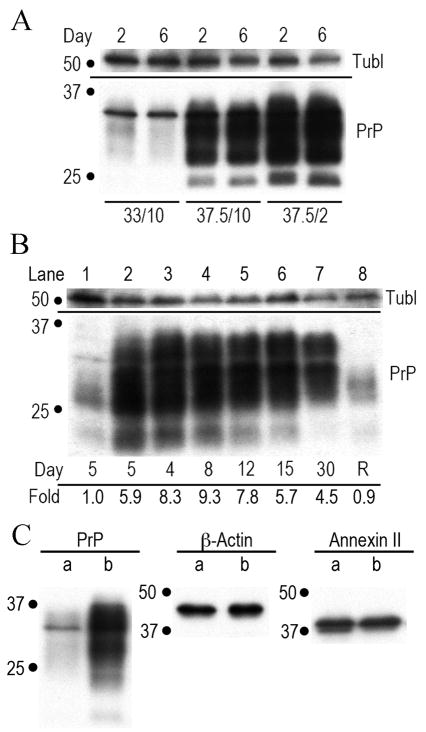
Fig 1.
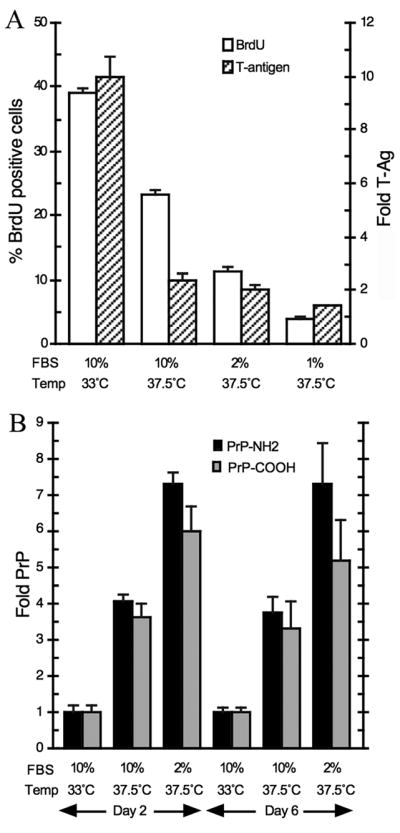
Temperature and serum conditions to induce stationary SEP neural progenitor cells. Fig. 1A graphs the % of BrdU positive cells (white bars), and nuclear T antigen levels (hatched bars). Fig. 1B shows the amount of PrP produced under these conditions at 2 and 6 days. In 5 independent experiments, PrP antibodies for both the NH2 (black bars) and central amyloid region (gray bars) show a significant elevation in PrP in stationary versus proliferating cells at 2 days (P<0.0001), as well as to temperature only arrest (P<0.05). The amyloid PrP antibody assay at 6 days was repeated only 3 times and a single low determination decreased the mean, although alternate processing of more carboxy PrP cannot be excluded.
Fig. 2.
shows representative Western blots of PrP and other normalization markers such as tubulin (tubl). All cells were equally loaded as determined by protein assay. Fig. 2A shows the PrP in control proliferating cells (first two lanes) with 1x PrP at 2 and 6 days. Lanes 3–4 and 5–6 show cells at 37.5°C with high serum and low serum respectively. Fig 2B shows that stationary cells continuously produce high amounts of PrP for 30 days (days indicated). Lane 8 shows stationary cells returned to standard proliferative conditions after 14 days (R) revert to their original low PrP state. PrP levels (fold) are shown under the lanes. Fig. 2C show proliferative (a) and 37.5°C-2% serum arrested cells at 5 days (b) stained for PrP, β-Actin and Annexin II. Only the PrP amount is changed (5.3 fold the proliferative control).
Fig. 1B graphs the change in cell PrP levels under these same conditions. Standard proliferative conditions were taken as 1x, and arrested cells clearly accumulated more PrP. In the graph (5 independent experiments), by day 2 it was apparent that cells at 37.5°C with high serum already accumulated a 4-fold increase in PrP by both the NH2 and central PrP antibodies [Arjona et al., 2004]. In the cells with a more complete proliferative arrest (2% serum at 37.5°C), there was a 7.3-fold increase (P<0.0001) in NH2-PrP, and the central PrP antibody was also significantly increased (6-fold, P<0.0001). After 6 days of stationary arrest PrP remained significantly higher than proliferating cells.
Fig. 2A shows a representative blot of PrP under different culture conditions. There is an obvious increase in PrP from controls (first 2 lanes) when cells are arrested for 2 or 6 days with only a shift to 37.5°C (middle two lanes). The PrP accumulation is even higher when serum is reduced to 2% (two last lanes). Moreover, SEP cells kept in a stringent stationary phase for up to 30 days without replating, maintained high PrP levels as shown at sequential time points in Fig. 2B. Cells maintained for 14 days in 1% serum at 37.5°C, and then switched to standard proliferative conditions, reverted to a proliferative state and their original low levels of PrP (lane 8). Thus high levels of normal PrP can be cleared by cell division.
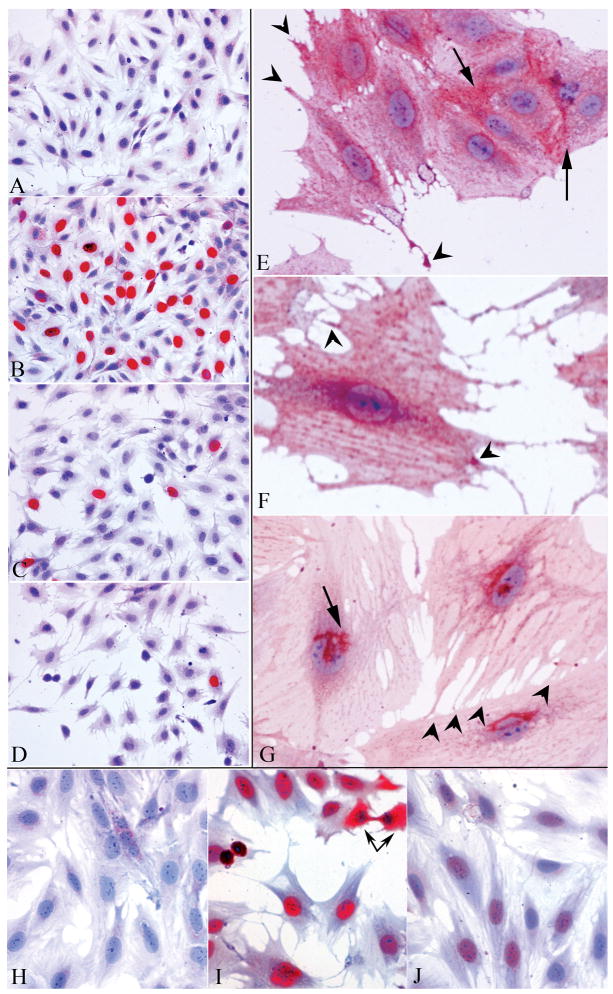
In situ studies of SEP cell proliferation and PrP localization at 2 days are shown in Fig. 3. Representative fields of a BrdU negative antigen control (A) show no staining in proliferating cells. In contrast, many labeled nuclei (red) are apparent (B) when exposed to the primary BrdU antibody in parallel proliferating cells at 33°C-10% serum. Very few positive cells (C) are seen with 2% serum at 37.5°C, and only very rare BrdU positive cells are present with 1% serum (D). The majority of cells also show the same cell size under proliferative and stationary conditions.
Fig. 3.
In situ antibody studies of proliferating and stationary cells at 2 days. Fig. 3A-D shows cells exposed to BrdU for 2 hr. No BrdU is detected in controls exposed only to the 2nd antibody (A) whereas many BrdU positive (red) nuclei are observed in proliferating SEP cells at 33°C (B). There is a marked reduction in BrdU positive cells fed with 2% serum (C) or 1% serum (D) at 37.5°C. Fewer cells are also apparent, and arrested cells show more extensive thin processes but no change in overall cell size. Fig. 3E-G show cells labeled with the NH2-PrP antibody arrested in 2% serum. Fig. 3E shows a growth cone and other extending process tips with intense PrP staining (arrowheads). Note intense staining between several closely adjacent cells at membrane contact regions (arrows). In F, red dots of PrP are aligned on the plasma membrane, horizontally orientated to cytoplasmic stress fibers. The lower arrowhead points to an intense positive signal where the process from another cell contacts the large cell body. The other arrowhead shows increased PrP in varicosities of an extending nanotube. The cell in G was extracted with 0.2% saponin prior to NH2-PrP detection. The plasma membrane signal is abolished and the characteristic Golgi region is prominently stained (arrow). Many tiny processes connect to the bottom cell (arrowheads). Figs. 3H-J show T antigen staining at 2 days. The control (H, 2nd antibody only) has low background whereas proliferating cells (I) show a strong T signal (red) in the nuclei of all cells, and in the cytoplasm of an anaphase cell (linked arrows). In contrast to BrdU, all cells (J) have only faint T signals at 2 days (2% serum, 37.5°C). Hematoxylin counterstained.
At 2 days in either 2% or 1% serum, arrested cells elaborated many new thin processes. Figs. 3E and F show early stages of process formation. The thin processes were consistent with neurites, filopodia and/or tunneling nanotubes. These processes were positive for plasma membrane NH2-PrP, most prominently at the ends of their growth cones (red), and at the beginning of new processes sprouts (arrowheads). PrP was also very concentrated between regions of nanotube attachment to cell bodies as well as between closely apposed cell bodies (Fig. 3E, arrows). Ruffled edges of some cells also showed intense plasma membrane PrP staining. Fig. 3F shows a strong PrP signal at a nanotube to cell junction, as well as at varicosities in a thin nanotube process (arrowheads). Interestingly, punctate PrP surface membrane staining on some cells lined up with a striation pattern common to actin stress filaments. In these cells, PrP positive red dots on the plasma membrane were aligned in horizontal red stripes on cells as shown in Fig. 3F.
The NH2 PrP antibody also labeled the internal membrane rich Golgi to rough endoplasmic reticulum (RER) membranes where PrP is synthesized and transported. Heavy Golgi labeling within cells was demonstrated unambiguously by removal of plasma membrane PrP with the detergent saponin (Fig 3G). Saponin substitutes for membrane cholesterol and releases the GPI anchored PrP molecules with the cholesterol in neural cell types such as GT1 hypothalamic cells. In the SEP cells here as well, only the characteristic connected channels of Golgi to RER adjacent to the nucleus remained positive for PrP after saponin extraction. In Fig. 3G, a typical Golgi profile is prominently labeled at arrow (red), and it partially overlies the PrP negative nucleus; PrP plasma membrane dots and labeled processes were abolished. Golgi networks in stationary cells were typically ~3× larger than in proliferating cells (data not shown). Cells that were slightly less extracted with lower saponin concentrations showed more extensive intracellular labeling of the RER, and these labeling results are in accord with ultrastructural labeling of the RER by PrP antibodies [Manuelidis et al., 2007]. Fig. 3G additionally shows multiple small processes that attach the top right cell to the one beneath it (arrowheads). These terminal small diameter attachments were numerous in arrested cells, and are consistent with intimate nanotube cell-to-cell connections. Nuclear PrP labeling was not seen in any of these or previous preparations, including sectioned cells, with our PrP antibodies [Manuelidis et al., 2009a; Manuelidis et al., 2009b; Arjona et al., 2004].
We also assessed T antigen suppression it in-situ. Fig. 3H shows the secondary antibody alone gives no positive signal. In proliferating cells (Fig. 3I), all the nuclei show strong T staining, indicating robust elaboration this antigen by every cell. Because the nuclear membrane disintegrates during mitosis, the T antigen is intense in the cytoplasm of a mitotic anaphase cell (at connected arrows). Only a faint amount of T antigen, however, is apparent in the nuclei of cells arrested for as little as 2 days. Fig. 3J shows all nuclei contain markedly diminished or undetectable nuclear T antigen, regardless of the few remaining nuclei that were still incorporating BrdU. This pan T antigen reduction additionally demonstrated that these neural stem cells were homogeneous and derived from a single cell clone. These in situ results were also in good accord with T antigen changes on Western blots (Fig. 1A). Additionally, many proliferating cells were strongly positive for nestin, a marker of early progenitor neurons, whereas this marker was reduced in arrested cells, consistent with their increased differentiation. Finally, because some arrested cells were larger and flatter than controls and displayed more numerous extending processes and cell connections by light microscopy, we sought to find if cells with high PrP had more extensive plasma membranes. In accord with no change in cytoplasmic tubulin and actin markers, Annexin II, a plasma membrane receptor also showed no change on Western blots. Fig. 2C shows control low PrP cells (a lanes) and arrested high PrP cells (b lanes) had equivalent amounts of Annexin II as well as β-Actin.
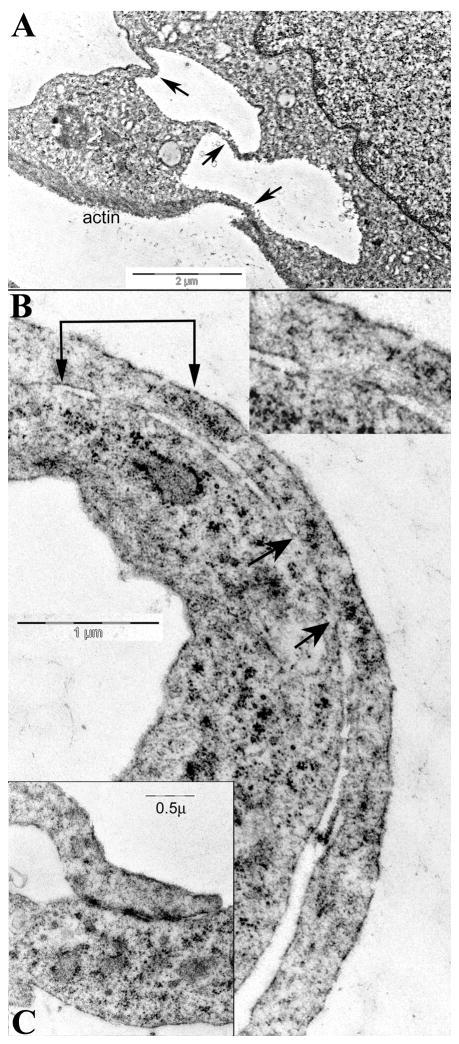
To understand more about the PrP rich cell-to cell attachments we evaluated the ultrastructure of cell processes after fixation in situ. There were many cell-to-to cell attachments between SEP cells that had features of nanotubes as well as growth cone filopodia. Typical processes did not contain packed aligned arrays of microtubules, a characteristic of more developed neurites. Rather, nanotubes and their communicating attachments to other cells were prominent in stationary cells with increased PrP. Fig. 4A shows two cells connected by 2 nanotubes, and by 1 filopodium distinguished by its substrate oriented underlying actin (arrows). Nanotube processes measured 200–500nm in diameter and thus could transport at least some organelles as well as viral particles. At nanotube to nanotube junctions it was sometimes impossible to find the limiting membranes separating the two cells. Cytoplasmic exchange regions also appeared between cell extensions and underlying cell body. These open regions may be transient, and part of a sliding membrane process where cells sample a small bit of each other. Fig. 4B shows two such intimate regions of communication, with the overlying process en passant on the right. The cytoplasm of both cells appears to be shared, as shown between the two sets of arrows. Thin nanotube to nanotube junctions distant from the cell body also showed only an interrupted and/or delicate single cell membrane similar to that seen in fenestrated epithelia. Such regions typically contained small membranous irregular vesicles that bridged both cells. Other types of specialized cell junctions, including gap junctions, as well as adherent junctions with densities on both contacting membranes (Fig. 4C), were also obvious. Communicating processes, as in light microscopic preparations, were more numerous in stationary cells. The dense adherent contact junctions were typically ~2× longer in these high PrP cells. In sum, the ultrastructural findings here strongly support the involvement of PrP in the elaboration of differentiating neural processes as well as several different types of cell-to-cell contact and communication.
Fig. 4.
Representative ultrastructural images of contacts and processes in arrested cells. Fig. 4A shows three thin processes connecting two cells (arrows), and one has actin at its bottom surface. In B, two regions appear to share cytoplasm (at 2 connected arrows, magnified 2x in inset). Another larger region with cytoplasmic connection is also between the unconnected arrows. In C, a characteristic nanotube forms an adherent junction with another cell process that contains small vesicles.
DISCUSSION
We have shown that both acute and chronic arrest of cell proliferation through mild physiological conditions is sufficient to both induce and maintain marked elevations of PrP in developing neurons. Concomitant with this PrP elevation, other features of neural differentiation, including nanotube and neural growth cone extensions, were observed. PrP was most concentrated at the connection sites between cells and processes, as well as at the tips of growth cones. This induction of processes with increased cell-to-cell attachments and neural molecular differentiation, including reduced nestin, is comparable to previous studies of these cells [Eves et al., 1994; Vogt Weisenhorn et al., 2001]. While PrP clearly accumulates in this morphological transition, its exact molecular role in process extension and membrane attachment remains unknown.
Since PrP knockout mice do not show major neurological deficits, the effects of PrP in mammals may be additive rather than essential. However, knock down of a PrP homolog in zebrafish led to an arrest in gastrulation that was due to deficient cell movements and E-cadherin adhesion [Málaga-Trillo et al., 2009]; adherent junctions were also stabilized by PrP. Data here similarly support a functional role for PrP as a dynamic plasma membrane receptor, one that interacts with other membrane components, including those in other cells, to both sort and define attachment sites. The elaboration of cell processes and their specific junctional connections, such as the adherent junctions associated with high PrP, are fundamental for the transport of cytoplasmic materials between cells, as well as for electrical and chemical coupling. Moreover, PrP also concentrates on the abundant thin extending processes of follicular dendritic spleen cells [Manuelidis et al., 2000], and these cells contact and exchange TSE agents with transiting white blood cells. Thus PrP receptor and contact functions are not limited to neural cells. Indeed, a study of intestinal enterocytes further substantiates a general role for PrP in the elaboration of adherent junctions as well as their villi [Morel et al., 2008]. Both were reduced in PrP knockout mice, a confirming complement to the experiments here. Additionally, the deletion of PrP in mice had no effect on BrdU uptake in intestinal enterocytes. This also strengthens the conclusion that proliferative arrest itself induces PrP synthesis and process formation. In contrast, one complex animal model of neurogenesis concluded the reverse: that PrP stimulates cell proliferation [Steele et al., 2006].
There have been few studies of tunneling nanotubes at the ultrastructural level, but comparable connecting nanotube and filopodial processes have been described elsewhere [Rustom et al., 2004; Gerdes et al., 2007; Sherer et al., 2007; Sowinski et al., 2008]. Our ultrastructural studies additionally showed regions of cytoplasmic communication that seemed structurally patent, with incomplete or fenestrated membranes between a nanotube and its target cells. Electron lucent membrane vesicles also crossed through these regions. These syncytial cytoplasmic regions have not been described elsewhere, and it is possible that staining of the plasma membrane in these regions was inadequate, despite the fact that tannic acid and osmium were used to intensify membrane detection. On the other hand, confocal time lapse and other studies have shown that large organelles, such as mitochondria, RER, lysosomes and other cytoplasmic components, are transported through nanotubes and delivered into connected cells [Gerdes et al., 2007]. This gives credence to the presence of dynamic cytoplasmic exchange pathways where a membrane barrier is either not present or not operational. Because many cells are linked by nanotube attachments, it is possible that during neural embryogenesis and differentiation there is a transient phase of syncytial collaboration among cells, one that may be enhanced by host PrP. The susceptibility of a neuronal progenitor cell line to many diverse geographic TSE agents [Manuelidis et al., 2009a; Manuelidis et al., 2009b] may likewise be facilitated by transient syncytial communications. Notably, most of these very distinct and different agent strains show the same misfolded tissue and cell type specific PrP-res pattern, a finding not in accord with the claim that PrP misfolding encodes agent strain characteristics. On the other hand a cell type specific PrP-res band pattern is consistent with the concept that host PrP is a necessary receptor for these diverse agents.
More extensive dense adherent junctions, similar to those seen in synaptic regions, were also consistent with a role for PrP in synaptic development, as well as for the transport of viruses across synaptic clefts. Trans-synaptic transport of large viruses between neurons is historically well established, and best documented in the spread of the neurotropic rabies virus. TSE agents can also spread transneuronally from the eye to the brain, as first shown in 1936, as well as by non-neural pathways [Manuelidis et al., 1977]. Furthermore, when only a single eye is inoculated with a scrapie agent, the neurodegenerative pattern follows the projections from that eye, including its path to the lateral geniculate body on opposite side of the brain [Scott and Fraser, 1989]. This trans-synaptic spread of scrapie has a defined intra-axonal rate that is slower than the spread of viruses through nanotubes and their viral “synapses” in culture [Sherer et al., 2007; Sowinski et al., 2008]. In scrapie infected brain, transport correlated with tubule and neurofilament transport mechanisms, whereas in HIV infected cultures, the viral particles rapidly spread on the surface of nanotubes and entered connected cells at junctional regions [Sherer et al., 2007]. Although the mechanical basis of spread may not be identical, it is clear that normal PrP can facilitate TSE agent transport and delivery both in vivo, and in vitro [Nishida et al., 2005]. The high concentration of CJD infectivity and viruslike particle arrays in synaptosomal fractions [Manuelidis, 2007] also suggests that PrP facilitates transport of TSE agents across neuronal synapses.
Most interesting is the recent demonstration that abnormal large internal PrP aggregates in an infected cell, as well as fluorescently labeled lysosomes, appeared to shuttle inside nanotubes to deliver their components to uninfected cells [Gousset et al., 2009]. It will be of interest to further examine the rapid transfer of infectivity as well as ultrastructural viruslike particles and fluorescently labeled cytoplasmic organelles in other TSE infected cultures. We suspect that high surface PrP will facilitate the natural cell-to-cell transfer of TSE agents, but only minimal PrP may be necessary once the agent is established inside the cell. We should be able to test this because we have been able to infect stationary rat SEP cells with a rat adapted CJD agent (manuscript in preparation). Moreover, this agent can also infect mouse neuronal GT1 cells used for rapid and reliable infectivity assays [Sun et al., 2008]. A co-culture of SEP and GT1 cells can also provide many new ways to evaluate different types of inhibitory molecules, and to screen therapeutic drugs that target specific membrane and filament functions that are most essential for communication, transport and delivery. Interfering with several of these transport molecules should delay TSE agent spread in both peripheral tissues and brain, and thus retard disease progression.
Acknowledgments
This work was supported by NINDS Grant RO1 012674 and NIAID Grant R21 A1076645. Plastic embedding and thin sectioning was done by the Yale EM Core.
Abbreviations
- PrP
prion protein
- TSE
transmissible spongiform encephalopathy
- SEP
septal neurons
- CJD
Creutzfeldt-Jakob Disease
References
- Arjona A, Simarro L, Islinger F, Nishida N, Manuelidis L. Two Creutzfeldt-Jakob disease agents reproduce prion protein-independent identities in cell cultures. Proc Natl Acad Sci USA. 2004;101:8768–8773. doi: 10.1073/pnas.0400158101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa R, Harris DA. Fishing for prion protein function. PloS Biology. 2009;7:0439–43. doi: 10.1371/journal.pbio.1000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eves E, Kwon J, Downen M, Tucker M, Wainer B, Rosner M. Conditional immortalization of neuronal cells from postmitotic cultures and adult CNS. Brain Res. 1994;656:396–404. doi: 10.1016/0006-8993(94)91484-2. [DOI] [PubMed] [Google Scholar]
- Gerdes H-H, Bukoreshtilev N, Barroso J. Tunneling nanotubes: A new route for the exchange of components between animal cells ++ good review with em & cell types and organelles. FEBS lett. 2007;581:2194–2201. doi: 10.1016/j.febslet.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami S, Phuan P, Perkins B, Ullman J, May B, Cohen F, Prusiner S. Cell division modulates prion accumulation in cultured cells. Proc Natl Acad Sci U S A. 2007;104:17971–6. doi: 10.1073/pnas.0708372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman D, Chenouard N, de Chaumont F, Martino A, Enninga J, Olivo-Marin J, Männel D, Zurzolo C. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009:11. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- Kay L. W. M. Stanley’s Crystallization of the Tobacco Mosaic Virus, 1930–1940. Isis. 1986;77:450–472. doi: 10.1086/354205. [DOI] [PubMed] [Google Scholar]
- Leblanc P, Alais S, Porto-Carreiro I, Lehmann S, Grassi J, Raposo G, Darlix J. Retrovirus infection strongly enhances scrapie infectivity release in cell culture. EMBO J. 2006;25:2674–85. doi: 10.1038/sj.emboj.7601162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Browning S, Mahal S, Oelschlegel A, Weissmann C. Darwinian Evolution of Prions in Cell Culture. Science. 2010;327:869–872. doi: 10.1126/science.1183218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberburg I. Developmental expression and regional distribution of the scrapie-associated protein mRNA in the rat central nervous system. Brain Research. 1987;417:363–366. doi: 10.1016/0006-8993(87)90465-3. [DOI] [PubMed] [Google Scholar]
- Mälaga-Trillo E, Solis G, Schrock Y, Geiss C, Luncz L, Thomanetz V, Stuermer C. Regulation of embryonic cell adhesion by the prion protein. Plos Biol. 2009;7:e55. doi: 10.1371/journal.pbio.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson J, West J, Thomson V, McBride P, Kaufman M, Hope J. The prion protein gene: a role in mouse embryogenesis? Development. 1992;115:117–22. doi: 10.1242/dev.115.1.117. [DOI] [PubMed] [Google Scholar]
- Manuelidis EE, Angelo JN, Gorgacz EJ, Kim JH, Manuelidis L. Experimental Creutzfeldt-Jakob disease transmitted via the eye with infected cornea. N Eng J Med. 1977;296:1334–1336. doi: 10.1056/NEJM197706092962308. [DOI] [PubMed] [Google Scholar]
- Manuelidis L. A 25 nm Virion Is the Likely Cause of Transmissible Spongiform Encephalopathies. J Cell Biochem. 2007;100:897–915. doi: 10.1002/jcb.21090. [DOI] [PubMed] [Google Scholar]
- Manuelidis L, Manuelidis EE. On the DNA content of cerebellar Purkinje cells in vivo and in vitro. Exp Neurol. 1974;43:192–206. doi: 10.1016/0014-4886(74)90140-x. [DOI] [PubMed] [Google Scholar]
- Manuelidis L, Borden J. Reproducible compartmentalization of individual chromosome domains in human CNS cells revealed by in situ hybridication and three-dimensional reconstruction. Chromosoma. 1988;96:397–410. doi: 10.1007/BF00303033. [DOI] [PubMed] [Google Scholar]
- Manuelidis L, Chakrabarty T, Miyazawa K, Nduom N-A, Emmerling K. The kuru infectious agent is a unique geographic isolate distinct from Creutzfeldt–Jakob disease and scrapie agents. Proc Natl Acad Sci U S A. 2009a;106:13529–13534. doi: 10.1073/pnas.0905825106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L, Liu Y, Mullins B. Strain-specific viral properties of variant Creutzfeldt–Jakob Disease (vCJD) are encoded by the agent and not by host prion protein. J Cell Biochem. 2009b;106:220–231. doi: 10.1002/jcb.21988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L, Manuelidis E. An autoradiographic study of the proliferation and differentiation of glial cells in vitro. Acta Neuropathol. 1971;18:193–213. doi: 10.1007/BF00685066. [DOI] [PubMed] [Google Scholar]
- Manuelidis L, Yu Z-X, Barquero N, Mullins B. Cells infected with scrapie and Creutzfeldt-Jakob disease agents produce intracellular 25-nm virus-like particles. Proc Natl Acad Sci USA. 2007;104:1965–1970. doi: 10.1073/pnas.0610999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L, Zaitsev I, Koni P, Lu Z-Y, Flavell R, Fritch W. Follicular Dendritic Cells and the dissemination of Creutzfeldt-Jakob Disease. J Virol. 2000;74:8614–8622. doi: 10.1128/jvi.74.18.8614-8622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel E, Fouquet S, Strup-Perrot C, Pichol Thievend C, Petit C, Loew D, Faussat A, LY, Pinçon-Raymond M, Chambaz J, Rousset M, Thenet S, Clair C. The cellular prion protein PrP(c) is involved in the proliferation of epithelial cells and in the distribution of junction-associated proteins. PLoS One. 2008;3:e3000. doi: 10.1371/journal.pone.0003000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida N, Katamine S, Manuelidis L. Reciprocal interference between specific CJD and scrapie agents in neural cell cultures. Science. 2005;310:493–6. doi: 10.1126/science.1118155. [DOI] [PubMed] [Google Scholar]
- Pantera B, Bini C, Cirri P, Paoli P, Camici G, Manao G, Caselli A. PrPc activation induces neurite outgrowth and differentiation in PC12 cells: role for caveolin-1 in the signal transduction pathway. J Neurochem. 2009;110:194–207. doi: 10.1111/j.1471-4159.2009.06123.x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. Development of the Prion Concept. In: Prusiner S, editor. Prion Biology and Diseases. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1999. pp. 67–112. (cf. p81) [Google Scholar]
- Race R, Fadness L, Chesebro B. Characterization of scrapie infection in mouse neuroblastoma cells. J Gen Virol. 1987;68:1391–9. doi: 10.1099/0022-1317-68-5-1391. [DOI] [PubMed] [Google Scholar]
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes H-H. Nanotubular Highways for Intercellular Organelle Transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- Scott J, Fraser H. Transport and targeting of scrapie infectivity and pathology in the optic nerve projections following intraocular infection. Prog Clin Biol Res. 1989;317:645–52. [PubMed] [Google Scholar]
- Sherer N, Lehmann M, Jimenez-Soto L, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nature Cell Biology. 2007:9. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowinski S, Jolly C, Berninghausen O, Purbhoo M, Chauveau A, Köhler K, Oddos S, Eissmann P, Brodsky F, Hopkins C, Onfelt B, Sattentau Q, Davis D. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–19. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- Steele A, Emsley J, Ozdinler P, Lindquist S, Macklis J. Prion protein (PrPc) positively regulates neural precursor proliferation during developmental and adult mammalian neurogenesis. Proc Natl Acad Sci U S A. 2006;103:3416–21. doi: 10.1073/pnas.0511290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Liu Y, Zhang H, Manuelidis L. Quantitative recovery of scrapie agent with minimal protein from highly infectious cultures. Viral Immunol. 2008;21:293–302. doi: 10.1089/vim.2008.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt Weisenhorn D, Roback L, Kwon J, Wainer B. Coupling of cAMP/PKA and MAPK signaling in neuronal cells is dependent on developmental stage. Exp Neurol. 2001;169:44–55. doi: 10.1006/exnr.2001.7651. [DOI] [PubMed] [Google Scholar]