Abstract
Mesenchymal stromal cells (MSCs) are multipotent cells that have high expansion yields, and fibrin is a native extracellular matrix (ECM) material widely used for cell delivery and surgeries. MSCs and fibrin have tremendous potential for tissue engineering applications, but the effect of fibrin on MSCs is not well characterized. The purpose of this study was to analyze the role of fibrin in modulating MSC phenotype by gene expression analysis. The results demonstrate that fibrin upregulated MSC gene expression of vasculogenic (FLK1, ACTA2, VECAD, SM22, CNN1), myogenic (MYF5, MYH13), neurogenic (TH, GFAP), and chondrogenic (COL2A1) markers after 5 days of incubation. These gene expression results were supported by induction of expression on the protein level for early lineage-specific markers such as ACTA2, FLK1, and MYF5. The ability of fibrin to modulate MSC gene expression was not affected by matrix pore size (80–110μm diameter) or Young’s Modulus (5–25kPa), and the differential expression of some phenotypic markers could be partially mimicked by other ECMs like fibronectin and collagen I. In some cases, the inductive effect of fibrin on gene expression could be further augmented by the treatment with growth factors such as nerve growth factor (NGF). However, fibrin’s effect appeared to be limited, as MSCs did not differentiate into fully mature cells based on immunofluorescence staining after 12 days. This body of work provides a rationale approach for studying the interactions of MSC with fibrin, which has important therapeutic implications for the delivery of stem cells.
Keywords: extracellular matrix, fibrin, bone marrow mesenchymal cell, collagen, vascular
INTRODUCTION
Mesenchymal stromal cells (MSCs) are a promising cell type for tissue engineering and cell transplantation applications. MSCs are multipotent in their ability to differentiate into numerous cell types, including those that comprise bone, skeletal muscle, fat, tendon, heart, and blood vessels [1–3]. They can be isolated from the bone marrow as well as the spleen, liver, and adipose tissue [4–6]. Other advantages of MSCs include their ease of isolation, high expansion ratio, and limited immunogenicity [3, 7]. Currently, MSCs are under investigation in over 50 clinical trials in the US for treatment of a wide range of diseases, including heart disease, diabetes, liver failure, graft versus host disease, and Crohn’s disease [8].
Besides the delivery of cells alone, transplantation of bioengineered constructs also holds tremendous potential for replacing diseased or dysfunctional tissues. Extracellular matrix (ECM) proteins provide a physical structure to support cell growth, proliferation, and migration. Recent studies also demonstrate an important role of matrix proteins in modulating the self-renewal or differentiation of stem cells [9–11]. Among the various types of ECMs, fibrin is an FDA-approved biocompatible matrix material that has been shown in numerous studies to promote cell survival and proliferation both in vitro and in vivo [12–16]. In our previous studies, we have shown that delivery of fibrin alone or with therapeutic cells could enhance neovasculature formation and attenuate dilatation of the left ventricle after myocardial infarction [4, 15, 17, 18]. Consequently, fibrin may be a favorable matrix material for delivering MSCs to ischemic tissues.
However, the interaction of fibrin with MSCs is not well understood. A limited number of recent studies report that fibrin can stimulate MSC differentiation towards osteogenic and chondrogenic lineages in vitro [19–22], but it is unclear whether fibrin can modulate specificity towards other cell types, including those of vascular, neural, and muscular lineages. Since the interactions between fibrin and MSCs may influence their therapeutic efficacy and cell fate when delivered together to the site of the ischemic tissues, we sought to determine the role of fibrin in modulating MSC phenotype in vitro by gene expression analysis. We also examined combined matrix and growth factor treatments for further regulating MSC phenotype.
MATERIALS AND METHODS
Fibrin Preparation
Fibrin (Tisseel, Baxter Healthcare Corp., Glendale, CA) was prepared by mixing the fibrinogen (75–115 mg/ml in aprotinin solution) and thrombin (400–600 I.U.ml) components, according to our previous studies [17, 23]. The standard formulation of fibrin was prepared by mixing fibrinogen and thrombin components at a 1:1 ratio and then applying the mixture onto 6 well tissue culture-treated dishes.
MSC Culture on Fibrin-Coated Substrates
Human bone marrow-derived MSCs (Cambrex, Walkersville, MD) were cultured in Mesenchymal Stem Cell Growth Medium (MSCGM, Cambrex) with 1% penicillin/streptomycin. The cells were previously purified by the manufacturer for positive expression of bone marrow MSC phenotypic markers that include CD105, CD166, and CD44. For characterization of MSC phenotype on the standard formulation of fibrin-coated substrates, 1.5 × 105 cells were grown on either fibrin-coated or non-coated tissue culture dishes in MSCGM (n ≥ 3).
MSC Proliferation on Fibrin-Coated Substrates
For characterization of MSC proliferation on the standard formulation of fibrin-coated substrates, cells were grown on either fibrin-coated or non-coated tissue culture dishes in MSCGM (n ≥ 3). At various time points, the cells in the fibrin-coated samples were dissociated from fibrin using bovine pancreatic trypsin (Sigma, St. Louis, MO) and counted using a brightline hemacytometer. Cells on non-coated tissue culture dishes were dissociated with TrypLE Express (Invitrogen, Carlsbad, CA) stable trypsin replacement enzyme and counted using a brightline hemacytometer.
RNA Isolation and Quantitative Polymerase Chain Reaction (qPCR)
At specified time points, the cells on either fibrin-coated or non-coated substrates were lysed with Trizol (Invitrogen). RNA isolation and qPCR was carried out according to previous literature [24]. The primers of interest included those specific to cardiovascular, neural muscular, osteogenic, chondrogenic, and adipogenic phenotypes. Primers for qPCR were designed by ABI Prism Primer Express software (Applied Biosystems, Foster City, CA), as listed in Supplemental Table 1. The mRNA expression for all treatment groups was normalized by the level of 18S ribosomal RNA in respective sample, and expressed as normalized mRNA quantities. The data is shown as mean ± standard deviation (n≥3). Statistical analysis between two groups was performed by the Student’s t-test, whereas comparisons for greater than two groups were assessed by ANOVA with Holm’s adjustment. Statistical significance was accepted at P<0.05.
Immunoblotting
Immunoblotting was performed according to our previous studies [25]. Briefly, samples were lysed in RIPA buffer (Pierce, Rockford, IL) at specified incubation periods, resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred onto nitrocellulose membranes (Invitrogen). Primary antibodies consisted of FLK1 (Cell Signaling Technology, Danvers, MA), CNN1 (Millipore, Billerica, MA), MYF5 (Novus Biologicals, Littleton, CO), skeletal muscle myosin (Santa Cruz Biotechnology, Santa Cruz, CA) GFAP (Novus Biologicals), ACTA2 (Sigma), and total actin (Sigma). After application of primary antibodies, the membranes were incubated with horse radish peroxidase (HRP)-conjugated secondary antibodies. The proteins were visualized by an ECL Detection Kit (Amersham, Piscataway, NJ) and then quantified by densitometry. Protein levels are normalized to total actin abundance and expressed in the form of relative fold change (n=3). Statistical analysis was performed by the unpaired t test, and significance was accepted at P<0.05.
Fibrin Porosity
Besides the standard fibrin formulation which consisted of a 1:1 ratio of fibrinogen:thrombin, other formulations were prepared by diluting the thrombin component 10-fold or 50-fold before mixing at equal ratios with fibrinogen, and those formulations were denoted T10 and T50, respectively. Additionally, the formulation consisting of 10-fold dilutions of both thrombin and fibrinogen before mixing at equal ratios was denoted as T10F10. To assess for porosity, 100μl fibrin of each formulation was allowed to solidify at 37°C for 30 minutes before embedding into OCT (Sakura Finetek, Torrance, CA) and cryosectioning into 10-μm sections. For visualization of the fibrin pore sizes, the frozen sections were processed by routine hematoxylin and eosin (H&E) staining according to previous reports [26]. The sections were imaged using a Zeiss Axiophot microscope (Carl Zeiss, Oberkochen, Germany). For quantification of pore size, Image J software was used to measure the perimeter (P) of each pore, and the equivalent diameter was calculated by P/π. To eliminate artifacts due to sample processing, the pore size range for analysis was restricted to 20–600 μm. The data is shown as mean ± standard deviation (n≥3). Statistical analysis was performed by analysis of variance (ANOVA) with Holm’s adjustment, and significance was accepted at P<0.05.
Fibrin Bulk Tensile Properties
The bulk tensile properties of fibrin were assessed according to previous reports [27, 28]. Briefly, fibrin constructs containing various concentrations of fibrinogen and thrombin were casted into molds forming 2cm × 0.2cm × 0.3cm constructs. After allowing the matrix to solidify for 2 hours, the constructs were hung vertically and known loads were applied to the construct. The final construct dimensions were measured upon reaching equilibrium after approximately 1 minute. The Young’s Modulus was calculated according to the equation (m × g)/(t × w)/[(l−lo)/lo] where m is the mass, g is the gravitational constant (9.806 m/s2), t is thickness, w is width, l is final length, and lo is original length. The data is shown as average ± standard deviation (n≥3). Statistical analysis was performed by ANOVA with Holm’s adjustment, and significance was accepted at P<0.05.
MSC Studies on Various ECMs
To compare the effect of fibrin with other ECMs on MSC phenotype, MSCs were cultured on substrates coated with murine laminin (1μg/ml, BD Biosciences, Bedford, MA), bovine fibronectin (1μg/ml, Sigma), rat tail collagen I monomer (800 μg/ml, BD Biosciences), rat tail collagen I polymer (800μg mg/ml, BD Biosciences), or the bare tissue culture substrate (control). Collagen I polymer was prepared by neutralizing collagen I monomer in acetic acid with 0.1N NaOH until reaching pH 7. The ECM substrates were incubated for 2 hours at 37°C before rinsing with PBS and proceeding with cell seeding.
MSC Culture on Fibrin-Coated Substrates with Growth Factors
To further examine the combined effect of growth factor and fibrin modulation, the media was enriched by the addition of one of the following recombinant human growth factors, namely vascular endothelial growth factor-A (VEGF, 50 ng/ml), transforming growth factor β1, TGFβ, 5 ng/ml) insulin-like growth factor-1 (IGF-1, 10 ng/ml), platelet-derived growth factor-BB (PDGF, 15 ng/ml), or nerve growth factor (NGF, 50 ng/ml) (all obtained from Peprotech, Rocky Hill, NJ) (n ≥ 3). The control substrate for these growth factor experiments were non-coated tissue culture dishes in the absence of growth factors. The cells were used within 8 passages and the media was replaced every 2–3 days.
Immunofluorescence Staining
At specified time points, samples were fixed in 4% paraformaldehyde. Immunofluorescence staining was performed as previously described [29]. The antibodies consisted of those against vascular endothelial cadherin (VECAD), smooth muscle calponin1 (CNN1), and skeletal muscle myosin, and tyrosine hydroxylase (TH) (all from Sigma, St. Louis, MO).
RESULTS
Effect of Fibrin on MSC Proliferation and Gene Expression of Phenotypic Markers
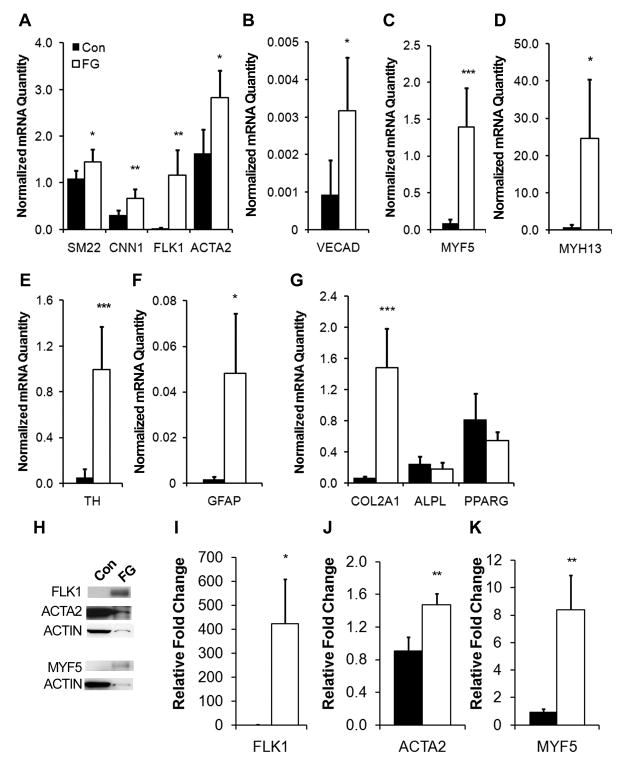
We first examined whether fibrin supports MSC proliferation by quantifying cell numbers at various time points. In agreement with published literature, MSCs cultured on fibrin-coated dishes increased in number with time (Suppl. Fig. 1) [12, 19]. We next determined whether fibrin-coated substrates could modulate MSC phenotype across a range of cell lineages, including vascul ogenic, myogenic, neurogenic, chondrogenic, osteogenic, and adipogenic lineages. Based on qPCR analysis after 5 days of culture on fibrin-coated substrates, we showed that fibrin differentially regulated phenotypic markers of multiple lineages (Fig. 1 and Table 1). Gene expression was markedly induced on the fibrin-coated substrate for smooth muscle markers. In particular, smooth muscle calponin (CNN1) demonstrated a 2-fold increase (0.67±0.20 fibrin vs 0.30±0.10 control), smooth muscle α-actin (ACTA2) increased by 1.5-fold (2.47±0.94 fibrin vs 1.62±0.52 control), and transgelin (SM22) showed a modest increase by 1.3-fold (1.45±0.28 fibrin vs 1.09±0.18 control) (Fig. 1A). Endothelial marker vascular endothelial growth factor receptor 2 (VEGFR2/FLK1) was upregulated by 40-fold (1.17±0.53 fibrin vs 0.02± 0.01 control) and vascular endothelial cadherin (VECAD) increased by 3-fold (0.0032±0.0014 fibrin vs 0.00092± 0.0056 control) (Fig. 1A–B). In stark contrast, cardiac markers troponin-I and GATA-4 were not detectable in any treatment groups (data not shown).
Fig 1. Mesenchymal stem cell (MSC) phenotype on fibrin-coated surfaces.
Real-time PCR data shows the differential gene expression after 5 days, as quantified as normalized mRNA quantity, for phenotypic markers comprising vasculogenic (A–B), myogenic (C–D), neurogenic (E–F), chondrogenic (G), osteogenic (G), and adipogenic (G) lineages. Immunoblots depicting FLK1, ACTA2, MYF5, and total actin protein expression (H) are shown and quantified by densitometry (I–K). Error bars indicate standard deviation. Con represents control tissue culture substrate and FG represents fibrin-coated substrates. * P<0.05, **P<0.01, ***P<0.001 (n≥3).
Table 1.
qPCR Primers
| Lineage | Abbreviation | Primer Name |
|---|---|---|
| Vasculogenic | SM22 | Transgelin |
| CNN1 | Calponin 1 | |
| FLK1 | Vascular endothelial growth factor receptor 2 | |
| ACTA2 | Smooth muscle α-actin | |
| VECAD | Vascular endothelial-cadherin | |
| Myogenic | MYF5 | Myogenic factor 5 |
| MYH13 | Skeletal muscle myosin, heavy polypeptide 13 | |
| Neurogenic | TH | Tyrosine hydoxylase |
| GFAP | Glial fibrillary acidic protein | |
| Chondrogenic | COL2A1 | Collagen II α1 |
| Osteogenic | ALPL | Alkaline phosphatase |
| BMP2 | Bone morphogenetic protein 2 | |
| Adipogenic | PPARG | Peroxisome proliferator-activated receptor γ |
| Cardiogenic | GATA-4 | GATA-4 |
| Troponin-I | Troponin-I |
Besides vascular lineages, we also surveyed phenotypic markers representing myogenic, chondrogenic, osteogenic, neurogenic, and adipogenic lineages (Fig. 1C–G). Myogenic factor 5 (MYF5), an early muscular transcriptional factor, was significantly upregulated by 16-fold (1.39±0.53 fibrin vs 0.08±0.05 control) on fibrin-coated substrates (Fig. 1C). Similarly, skeletal myosin heavy chain (MYH13), a mature skeletal muscle marker, was significantly induced by 34-fold (24.68±15.69 fibrin vs 0.72±0.87 control) (Fig. 1D). Among neurogenic markers, the dopaminergic neuronal marker TH was upregulated by 14-fold (0.99±0.38 fibrin vs 0.05±0.07 control) (Fig. 1E), and astrocyte phenotypic marker glial fibrillary acidic protein (GFAP) was upregulated by 29-fold (0.048±0.026 fibrin vs 0.0016±0.0012 control) (Fig. 1F). In addition, chondrogenic extracellular matrix collagen IIα1 (COL2A1) was also significantly induced by the fibrin-coated substrate by 24-fold (1.48±0.51 fibrin vs 0.06±0.03 control) (Fig. 1G). On the other hand, osteogenic lineage marker alkaline phosphatase (ALPL) and adipogenic marker peroxisome proliferator-activated receptor γ (PPARG) did not show significant differential gene expression on fibrin-coated substrates (Fig. 1G). These results suggested that fibrin could modulate phenotypic expression of MSCs towards numerous lineages, including vascular (SM22, CNN1, FLK1, VECAD), neural (TH, GFAP), muscular (MYF5, MYH13), and chondrogenic (COL2A1) lineages after 5 days of incubation.
To confirm the differential gene expression results, we carried out immunoblotting assay after 5 days of incubation for a subset of markers. In agreement with the gene expression patterns, the relative expression of early lineage-specific markers such as FLK1, ACTA2, and MYF5 were significantly induced on the fibrin substrate, when normalized to total actin abundance (Fig. 1H). Fibrin-coated substrates promoted an increased expression of FLK1 by over 400-fold, ACTA2 by 0.5-fold, and MYF5 by 8-fold (Fig. 1I–K). On the other hand, we could not detect the expression of mature markers such as skeletal muscle myosin and GFAP (data not shown). These results suggest that fibrin could mediate phenotypic changes in MSCs at the gene and, to some degree, the protein levels.
Effect of Physical and Mechanical Properties of Fibrin on MSC Gene Expression
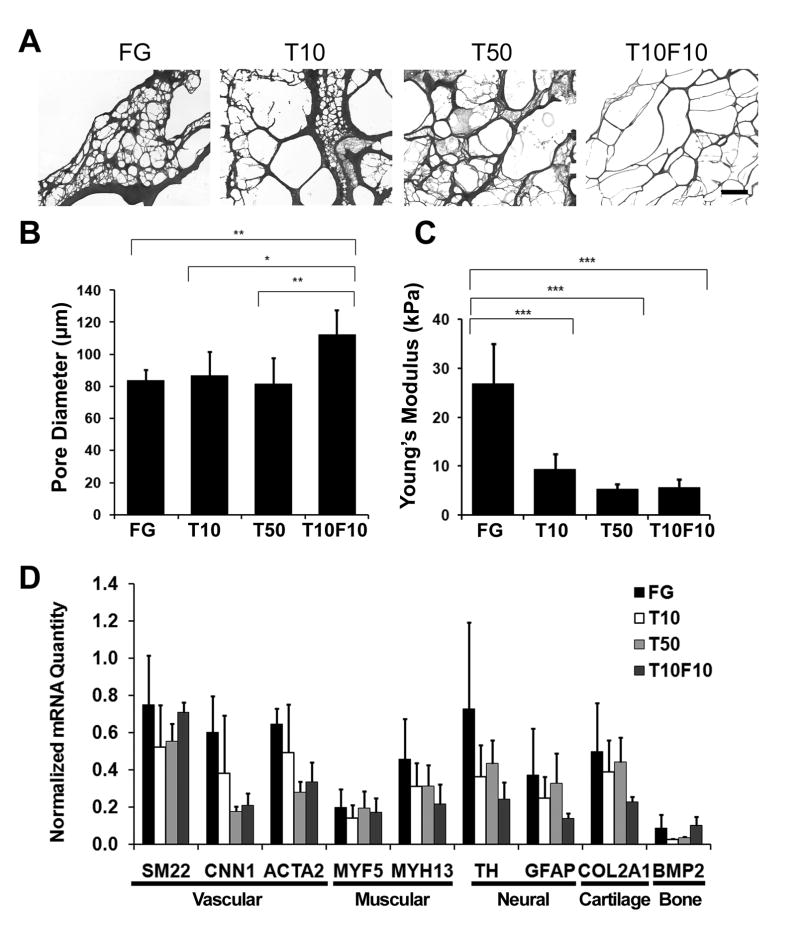
To understand the mechanism of fibrin-induced phenotypic expression, we questioned whether the differential gene expression was related to the physical or mechanical properties of fibrin, namely its pore size and elastic modulus. To address this, we modulated the pore size and elastic modulus by changing the composition of the fibrinogen and thrombin components. We prepared several formulations of fibrin by diluting fibrinogen 10-fold (F10) and/or thrombin by 10-fold (T10) or 50-fold (T50) and found that the pore size of fibrin could be significantly altered. As shown by the H&E-stained cross sections (Fig. 2A–B), the equivalent pore diameter was significantly higher in the T10F10 formulation (112.1±15.41 μm), when compared to that of the standard formulation (83.53±6.81 μm), T10 (86.89±14.70 μm), and T50 (81.29±16.48 μm) formulations (P<0.05). In addition to significantly modulating the fibrin pore size, diluting the thrombin and fibrinogen components also altered its mechanical properties. As shown in Fig. 2C, the Young’s modulus for the standard FG formulation was 26.96 ± 8.14 kPa, which was significantly higher than the T10 (9.45±3.10 kPa), T50 (5.29±1.08 kPa), and T10F10 (5.74±1.64 kPa) formulations (P<0.05).
Fig. 2. Effects of physical and mechanical properties of fibrin on gene expression changes in MSCs.
A. H&E-stained cross sections of fibrin in the standard formulation (FG), or when thrombin was further diluted 10-fold (T10) or 50-fold (T50), respectively. T10F10 represents fibrin with 10-fold dilution of both thrombin and fibrinogen. Quantification of (B) the average equivalent pore diameter and (C) Young’s modulus for fibrin compositions. D. Real-time PCR data shows the differential gene expression after 2 days of incubation on various fibrin compositions. The PCR data is expressed as normalized mRNA quantities, and error bars indicate standard deviation (n≥3). * P<0.05, **P<0.01, ***P<0.001 (n≥3). Scale bar: 500 μm.
After characterizing the physical and mechanical properties of different fibrin formulations, we next studied gene expression of MSCs on the 4 formulations of fibrin. To minimize the possible changes in physical and mechanical properties during in vitro studies due to protease digestion or other factors, we chose an early time point of 2 days for the assessment of gene expression changes. Within the range of mechanical properties and pore sizes examined, we did not detect statistically significant differences in gene expression of phenotypic markers after 2 days of culture (Fig. 2D). This data suggests that these biomechanical and biophysical factors did not play a significant role in modulating MSC behavior after 2 days of culture.
Comparison of MSC Gene Expression on Various ECMs
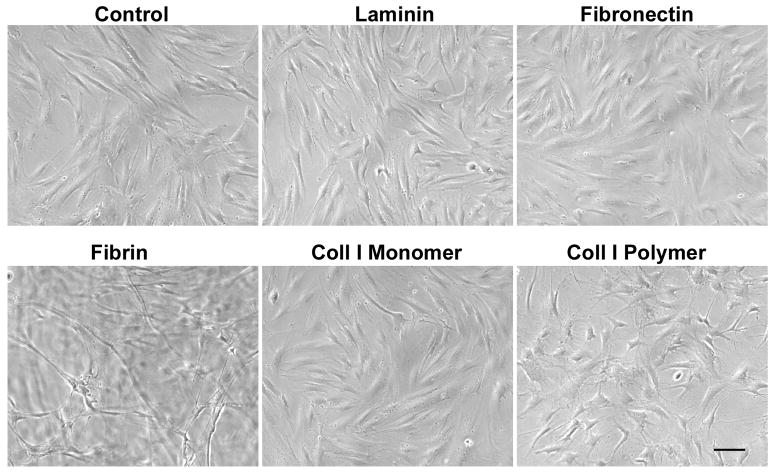
In addition to assessing the effect of the physical and mechanical properties of fibrin on MSC gene expression, we sought to determine whether the modulation of gene expression changes was specific to the chemical composition of fibrin. To address this, we compared MSC gene expression on fibrin or on other ECMs, namely laminin, fibronectin, and collagen I. To further elucidate the structural effect of substrates on gene expression, collagen I was prepared as either a monomeric coating or polymerized thin gel. A 2-day time point was selected to minimize the possibility of biochemical and biophysical changes in ECM caused by cells during in vitro studies. The MSCs on cultured on laminin, fibronectin, and collagen I matrices appeared to have an elongated morphology as on the non-coated control substrate (Fig. 3). On the other hand, the cells on fibrin-coated substrates appeared to form tube-like structures that were consistent with early phenotypic changes towards vascular cell phenotype.
Fig. 3. MSC morphology on substrates coated with laminin, fibronectin, fibrin, collagen I (coll I) monomer, or coll I polymer matrices after 2 days of culture.
Scale bar: 100 μm.
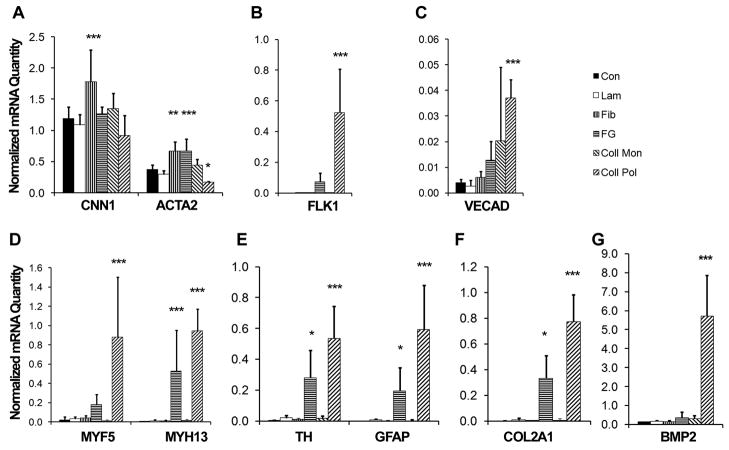
Comparative gene expression analysis of MSCs on the various ECM substrates revealed a number of differentially expressed phenotypic markers. The mature smooth muscle marker, CNN1 was shown to be significantly upregulated only on the fibronectin substrate by 1.5-fold (1.7 ±0.51 fibronectin vs 1.20±0.18 control) (Fig. 4A). Another smooth muscle marker, ACTA2, was induced on both fibronectin- and fibrin-coated substrates by 1.5-fold and 1.1-fold, when compared to the control sample, whereas collagen I polymer induced a 1-fold downregulation in gene expression respectively (1.78±0.51 fibronectin vs 1.27±0.11 fibrin vs 0.17±0.02 collagen polymer vs 1.20±0.18 control) (Fig. 4A). In addition to smooth muscle phenotypic markers, endothelial precursor marker FLK1 was upregulated on both fibrin and collagen I polymer substrates by 55-fold and 395-fold, respectively (0.07±0.06 fibrin vs 0.53±0.28 collagen polymer vs 0.0013±0.0019 control) (Fig. 4B), although the mature endothelial marker, VECAD, was differentially expressed only on collagen I polymer (0.037±0.007 collagen polymer vs 0.004±0.001 control) substrate (Fig. 4C). The gene expression of CNN1 and VECAD was not significantly different on fibrin and control surfaces at day 2, suggesting that these two gene were induced at later time points (e.g., day 5; Fig. 1).
Fig. 4. Effect of ECM on MSC differentiation markers after 2 days.
Real-time PCR data shows the differential gene expression for phenotypic markers when MSCs were cultured on laminin (Lam), fibronectin (Fib), fibrin (FG), collagen monomer (Coll Mon), or collagen polymer (Coll Pol). The phenotypic markers consisted of those comprising vasculogenic (A–C), myogenic (D), neurogenic (E), chondrogenic (F), and osteogenic (G) lineages. The PCR data is expressed as a normalized abundance to the control tissue culture substrate (Con) for markers comprising multiple lineages. Error bars represent standard deviation.). * P<0.05, **P<0.01, ***P<0.001 when compared to Con group (n≥3).
In regards to myogenic phenotypic markers, MYF5 was significantly induced on the collagen I polymer substrate by 40-fold, when compared to the control (0.88±0.62 collagen polymer vs 0.02±0.03), and MYH13 was induced on both the fibrin and collagen I polymer (70-fold and 120-fold, respectively) substrates (0.53±0.43 fibrin vs 0.94±0.23 collagen polymer vs 0.01±0.01 control) (Fig. 4D). In regards to neural markers, fibrin induced a 67-fold upregulation of TH and 173-fold increase in GFAP, whereas collagen polymer I induced these same genes by 173-fold and 526-fold, respectively (0.28±0.18 fibrin vs 0.53±0.21 collagen polymer vs 0.0041±0.0029 control for TH; 0.20±0.15 fibrin vs 0.59±0.28 collagen polymer vs 0.0011±0.0013 control for GFAP) (Fig. 4E). In addition, chondrogenic marker COL2A1 was upregulated on both fibrin and collagen I polymer by 98-fold and 226-fold, respectively (0.33±0.36 fibrin vs 0.77±0.30 collagen polymer vs 0.0034± 0.0033 control) (Fig. 4F), and osteogenic marker BMP2 was 40-fold higher on the collagen I polymer substrate when compared to the control substrate (5.72±2.14 collagen polymer vs 0.14±0.01 control) (Fig. 4G).
Interestingly, the effects on collagen I thin gels were not reproduced on collagen I monomeric coating, suggesting that the structural aspect of the substrate may also be an important factor in matrix regulation of cell phenotype. Together, these findings reveal that some phenotypic markers could be differentially expressed on both fibrin-coated substrates as well as others such as fibronectin and collagen I polymer, suggesting that more than one ECM composition may be capable of modulating MSC phenotype.
Combined Effects of Fibrin with Growth Factors on MSC Expression
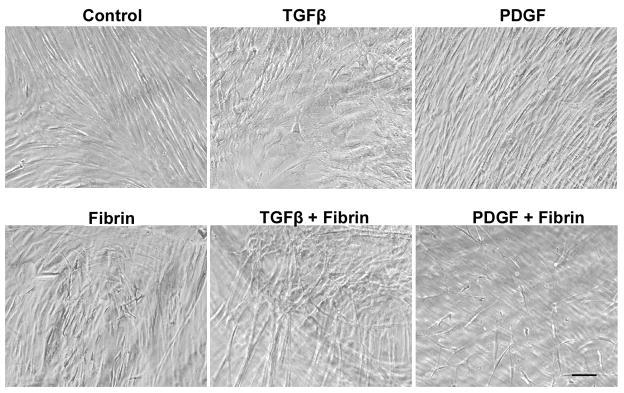
In addition to assessing the effect of fibrin on MSC phenotype, the combined effect of fibrin and growth factors was examined by culturing MSCs on fibrin-coated substrates for 12 days in the presence of growth factors known to stimulate muscular, vascular, or neural lineages, namely VEGF, TGFβ, IGF-1, PDGF, and NGF. Morphologically, the cells in the presence of TGFβ on the non-coated substrate appeared to have a flattened cellular shape and pronounced formation of stress fibers, whereas no obvious differences were observed in the remaining growth factor-treated groups (Fig. 5). In the presence of fibrin, the cells had an elongated morphology for all growth factor-treated groups. After 12 days of incubation, fibrin was macroscopically visible on the dish.
Fig. 5. MSC morphology when cultured on fibrin and/or growth factors for 12 days.
Scale bar: 100 μm.
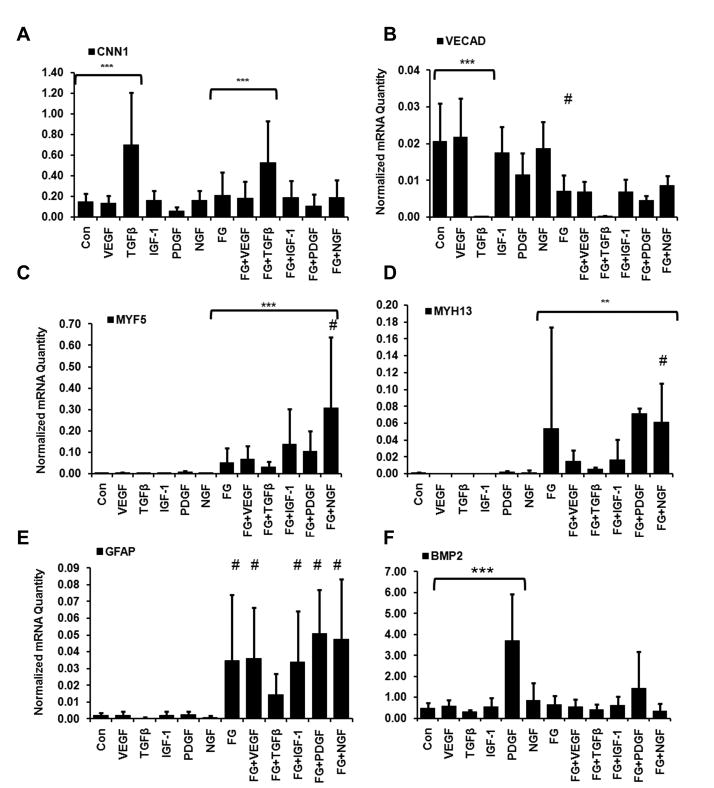
At day 12, in the absence of growth factors, only GFAP gene was still induced by fibrin matrix, suggesting that the effects of fibrin are time-dependent and the further differentiation of MSCs needs other biochemical factors (Fig. 6E). In some cases, the addition of growth factors to fibrin could further modulate differential gene expression changes after 12 days. Whereas fibrin appeared to have no significant effect in modulating TGFβ-induced upregulation of CNN1 (Fig. 6A) or TGFβ-mediated inhibition of VECAD expression (Fig. 6B), the early myogenic transcriptional factor, MYF5, was significantly induced in the fibrin+NGF (0.31±0.33) group when compared to fibrin (0.05±0.07) or NGF (0.0013±0.0010) alone (Fig. 6C). Similarly, MYH13 resulted with a significantly higher mRNA abundance in the fibrin+NGF (0.06±0.05) group when compared to fibrin (0.05±0.12) or to NGF (0.0015±0.0026) alone (Fig. 6D). In the area of neural phenotype, the expression of GFAP was significantly upregulated in the presence of fibrin by VEGF, PDGF, IGF-1, or NGF, when compared to growth factor treatment alone (Fig. 6E). Since VEGF, PDGF, and IGF-1 are not commonly associated with neural phenotype, this data suggests that fibrin may be involved in growth factor-stimulated pathways that enhance neural lineage phenotype. Finally, BMP2 gene expression was not modulated by the presence of fibrin, but could be significantly induced in the presence of PDGF alone, when compared to the control group (Fig. 6F). These results suggest that the combined delivery of fibrin with growth factors play diverse roles in upregulating, downregulation, or having no effect on MSC gene expression.
Fig. 6. Combined effects of fibrin and growth factors on MSC phenotypic markers after 12 days.
Real-time PCR data shows the differential gene expression for vasculogenic markers CNN1 (A) and VECAD (B); myogenic markers MHF5 (C) and MYH13 (D); neurogenic marker GFAP (E), and osteogenic marker BMP2 (F). The data is expressed as normalized raw abundance. Con represents control tissue culture substrate and FG represents fibrin-coated substrates. # indicates statistically significant compared to growth factor treatment without fibrin. Statistically significant relationships are denoted by *P<0.05, **P<0.01, and ***P<0.001 (n≥3).
To determine whether fibrin and/or growth factors could induce the post-transcriptional expression of mature lineage-specific markers, we carried out immunofluorescence staining on samples cultured for 12 days using antibodies against VECAD, smooth muscle calponin, and skeletal muscle myosin. Contrary to transcriptional analysis, no treatment group could produce detectable immunofluorescence staining of the mature phenotypic markers, suggesting that fibrin could modulate cell phenotype on the transcriptional level but could not induce MSCs to become maturely differentiated cells (data not shown).
DISCUSSION
MSCs and fibrin are both promising candidates for therapeutic cell transplantation or tissue engineering applications, but the effect of fibrin on MSCs is not well characterized. Therefore, we assessed the gene expression effects of fibrin on MSC phenotype and found that fibrin could regulate MSC gene expression for phenotypic markers across multiple lineages. The effect of fibrin on MSC gene expression was assessed at multiple incubation periods ranging from 2 to 12 days. In particular, fibrin alone induced the expression of vascular (SM22, CNN1, FLK1, VECAD), neural (TH, GFAP), muscular (MYF5, MYH13), and chondrogenic (COL2A1) lineages after 5 days of incubation (Fig. 1). Furthermore, the combination of fibrin and growth factors for 12 days could enhance the differential expression of certain lineage-specific phenotypic markers, including MYF5, MYH13, and GFAP (Fig. 6). On the other hand, fibrin did not appear to affect the TGFβ-mediated inhibition of VECAD or stimulation of CNN1. These findings demonstrate that fibrin and soluble factors interact with MSCs in regulating cell phenotype. However, the growth factor studies are limited by the use of serum-containing media that contains trace amounts of growth factors, which may contribute to gene expression changes.
Although NGF is commonly associated with neural differentiation, the finding that NGF with fibrin induces myogenic gene expression of MYF5 and MYH13 in the presence of fibrin reveals a novel role of NGF for regulating myogenic phenotype. Previous studies have described a role of NGF in regulating myogenic differentiation and proliferation of skeletal myoblasts. In particular, exogenously added NGF increased the rate of myoblast proliferation as well as fusion into myotubes, whereas neutralizing antibodies that block the action of NGF decreased the incidence of myotube formation [30]. However, this is the first report the role of fibrin+NGF for modulating myogenic genes in MSCs.
To elucidate the mechanism of fibrin’s involvement in modulating MSC phenotype, we developed multiple formulations of fibrin by varying the concentration of fibrinogen and/or thrombin and found that the stiffness and pore size of fibrin could be significantly modified in this manner. In particular, the T10F10 formulation had a 30% higher pore diameter than the standard formulation, and all three modified formulations (T10, T50, and T10F10) could lower the Young’s modulus by 60–80% (Fig. 2). However, the modified formulations of fibrin, which span a range of mechanical properties and porosities, did not significantly alter gene expression.
We also assessed the role of various ECM compositions on MSC phenotype for a subset of genes. Interestingly, fibronectin and collagen I polymer shared some similar effects as fibrin in modulating MSC gene expression, whereas laminin and collagen I monomer showed no significant induction of gene expression (Fig. 4). These results may, in part, be associated with some similar cell binding domains among the ECMs [31]. However, the differences in MSC gene expression when seeded on collagen I monomer or polymer suggests that integrin binding and chemical composition alone may not account for the results observed. Rather, these results implicate a role of other factors such as matrix nano- or microtopography on cell phenotype [32, 33] and warrant further investigation. Future studies would also be required to distinguish the roles of fibrin chemical composition, mechanical properties, and pore size on MSC phenotype. It will also be interesting to elucidate the effects of fibrin matrix on MSC gene expression in three-dimensional culture.
The present study demonstrates that fibrin significantly modulates MSC phenotypic markers on the transcriptional level, but distinctive immunofluorescence expression of mature lineage-specific markers could not be detected after 12 days of incubation. One possible explanation for these results is that fibrin may have transient effects on the regulation of lineage-specific markers. For example, CNN1, VECAD, and MYF5 were upregulated on fibrin-coated substrates after 5 days, but not after 12 days (Figs 1 and 6). Another explanation is that fibrin alone in the absence of additional biochemical or mechanical cues is insufficient for mature differentiation of MSCs. This is supported by other studies showing the requirement of specific induction media to promote the differentiation of MSCs into osteoblasts and chondrocytes, in addition to fibrin [19, 20]. To fully realize cell differentiation, it is likely that multiple soluble factors and appropriate ECM are needed. How fibrin affects MSC differentiation into specific lineage in the presence of a cocktail of soluble differentiation factors awaits further investigations. A third explanation is that incubation periods longer than 12 days may be necessary to observe post-transcriptional effects of fibrin on cell phenotype. Our results highlight the complex cell-matrix interactions that modulate the MSC phenotype.
Whereas the role of growth factors on MSC phenotype has been widely assessed [34–36], few studies have examined the diverse lineages of MSC differentiation in the presence of fibrin. One study showed that higher fibrinogen concentrations supported higher ALPL activity and osteogenic differentiation, but proliferation was higher in formulations containing a lower fibrinogen concentration [19]. In another study that examined the chondrogenic differentiation of MSCs embedded in collagen I and fibrin gels, the authors concluded that both matrices could support chondrogenesis [20]. Fibrin encapsulation of MSCs has been shown to promote differentiation into chondrocytes that secrete aggrecan and collagen II [22]. In addition to chondrogenic differentiation media and fibrin, hypoxic (3% O2) environments also appear to induce chondrocyte-like cell types and a chondrogenic phenotype in MSCs based on collagen II expression and Alcian blue staining [21]. These studies provide evidence that fibrin is a potent modulator of MSCs towards osteogenic and chondrogenic differentiation and prompted the investigation of the effects of fibrin in modulating MSC differentiation towards other lineages.
Future studies by using DNA microarray will provide more information on the global changes in MSC gene expression. In addition, how other cell types (e.g., vascular cells, osteoblasts and chondrocytes) respond to fibrin matrix can be investigated t the effects of fibrin matrix are cell-type dependent.
CONCLUSION
In summary, this study demonstrates the role of fibrin in inducing MSC gene expression of vasculogenic, myogenic, neurogenic, chondrogenic, and osteogenic phenotypic markers. The modification of fibrin concentration by varying thrombin and fibrinogen ratios affected the pore size of the structure and Young’s Modulus, but these changes to physical and mechanical properties did not significantly alter gene expression. However, comparison of various ECMs demonstrated similar trends in fibrin-induced gene expression by fibronectin or collagen I polymer. In some cases, the inductive effect of fibrin on gene expression could be further augmented by the treatment with growth factors. In particular, the seeding of MSCs on fibrin-coated substrates in the presence of NGF resulted in significant induction of myogenic markers MYF5 and MYH13, whereas neural marker GFAP could be upregulated in the presence of fibrin by VEGF, PDGF, IGF-1, or NGF, when compared to growth factor treatment alone. Fibrin-mediated gene expression changes were verified at the protein level for early markers of vasculogenic and myogenic lineages, namely ACTA2, MYF5, and FLK1. However, fibrin and growth factors alone did not induce MSC differentiation into fully mature cells based on immunofluorescence staining of lineage-specific markers. Our results suggest that understanding the interaction between MSCs and fibrin can be beneficial for directing stem cell fate for therapeutic applications. This study provides a rationale approach for studying MSC and fibrin interactions, which can influence the therapeutic efficacy and cell phenotype when delivered together to the site of the ischemic tissues.
Supplementary Material
Supplementary Figure 1. Cell Proliferation on Fibrin
Supplementary Table 1. Primer sequences for qPCR
Acknowledgments
This work was supported by a research grant to S.L. from the National Institute of Health (HL078534). N.H. was supported by a graduate fellowship from the National Science Foundation.
Footnotes
The authors report no financial interests or conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Huang NF, Li S. Mesenchymal stem cells for vascular regeneration. Regen Med. 2008;3:877–892. doi: 10.2217/17460751.3.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 6.Bernacki SH, Wall ME, Loboa EG. Isolation of human mesenchymal stem cells from bone and adipose tissue. Methods Cell Biol. 2008;86:257–278. doi: 10.1016/S0091-679X(08)00011-3. [DOI] [PubMed] [Google Scholar]
- 7.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 8.
- 9.Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat Methods. 2005;2:119–125. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 10.Li YJ, Chung EH, Rodriguez RT, Firpo MT, Healy KE. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A. 2006;79:1–5. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 11.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci U S A. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho W, Tawil B, Dunn JC, Wu BM. The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006;12:1587–1595. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 13.Zhang G, Wang X, Wang Z, Zhang J, Suggs L. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Eng. 2006;12:9–19. doi: 10.1089/ten.2006.12.9. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G, Hu Q, Braunlin EA, Suggs LJ, Zhang J. Enhancing Efficacy of Stem Cell Transplantation to the Heart with a PEGylated Fibrin Biomatrix. Tissue Eng Part A. 2008;14:1025–1036. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]
- 15.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 16.Syedain ZH, Bjork J, Sando L, Tranquillo RT. Controlled compaction with ruthenium-catalyzed photochemical cross-linking of fibrin-based engineered connective tissue. Biomaterials. 2009;30:6695–6701. doi: 10.1016/j.biomaterials.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 18.Huang NF, Lam A, Fang Q, Sievers RE, Li S, Lee RJ. Bone marrow-derived mesenchymal stem cells in fibrin augment angiogenesis in the chronically infarcted myocardium. Regen Med. 2009;4:527–538. doi: 10.2217/rme.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catelas I, Sese N, Wu BM, Dunn JC, Helgerson S, Tawil B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006;12:2385–2396. doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- 20.Dickhut A, Gottwald E, Steck E, Heisel C, Richter W. Chondrogenesis of mesenchymal stem cells in gel-like biomaterials in vitro and in vivo. Front Biosci. 2008;13:4517–4528. doi: 10.2741/3020. [DOI] [PubMed] [Google Scholar]
- 21.Baumgartner L, Arnhold S, Brixius K, Addicks K, Bloch W. Human mesenchymal stem cells: Influence of oxygen pressure on proliferation and chondrogenic differentiation in fibrin glue in vitro. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32577. [DOI] [PubMed] [Google Scholar]
- 22.Ho ST, Cool SM, Hui JH, Hutmacher DW. The influence of fibrin based hydrogels on the chondrogenic differentiation of human bone marrow stromal cells. Biomaterials. 2010;31:38–47. doi: 10.1016/j.biomaterials.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Huang NF, Yu J, Sievers R, Li S, Lee RJ. Injectable Biopolymers Enhance Angiogenesis after Myocardial Infarction. Tissue Eng. 2005;11:1860–1866. doi: 10.1089/ten.2005.11.1860. [DOI] [PubMed] [Google Scholar]
- 24.Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88:359–368. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Huang NF, Wilson KD, Velotta JB, Huang M, Li Z, Lee A, Robbins RC, Cooke JP, Wu JC. nAChRs mediate human embryonic stem cell-derived endothelial cells: proliferation, apoptosis, and angiogenesis. PLoS One. 2009;4:e7040. doi: 10.1371/journal.pone.0007040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang NF, Sievers RE, Park JS, Fang Q, Li S, Lee RJ. A rodent model of myocardial infarction for testing the efficacy of cells and polymers for myocardial reconstruction. Nat Protoc. 2006;1:1596–1609. doi: 10.1038/nprot.2006.188. [DOI] [PubMed] [Google Scholar]
- 27.Jacot JG, Dianis S, Schnall J, Wong JY. A simple microindentation technique for mapping the microscale compliance of soft hydrated materials and tissues. J Biomed Mater Res A. 2006;79:485–494. doi: 10.1002/jbm.a.30812. [DOI] [PubMed] [Google Scholar]
- 28.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang NF, Patel S, Thakar RG, Wu J, Hsiao BS, Chu B, Lee RJ, Li S. Myotube Assembly on Nanofibrous and Micropatterned Polymers. Nano Lett. 2006;6:537–542. doi: 10.1021/nl060060o. [DOI] [PubMed] [Google Scholar]
- 30.Rende M, Brizi E, Conner J, Treves S, Censier K, Provenzano C, Taglialatela G, Sanna PP, Donato R. Nerve growth factor (NGF) influences differentiation and proliferation of myogenic cells in vitro via TrKA. Int J Dev Neurosci. 2000;18:869–885. doi: 10.1016/s0736-5748(00)00041-1. [DOI] [PubMed] [Google Scholar]
- 31.Martino MM, Mochizuki M, Rothenfluh DA, Rempel SA, Hubbell JA, Barker TH. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30:1089–1097. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26:5158–5166. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Binulal NS, Deepthy M, Selvamurugan N, Shalumon KT, Suja S, Mony U, Jayakumar R, Nair SV. Role of Nanofibrous Poly(Caprolactone) Scaffolds in Human Mesenchymal Stem Cell Attachment and Spreading for In Vitro Bone Tissue Engineering-Response to Osteogenic Regulators. Tissue Eng Part A. 2010 doi: 10.1089/ten.TEA.2009.0242. epub. [DOI] [PubMed] [Google Scholar]
- 34.Tao H, Rao R, Ma DD. Cytokine-induced stable neuronal differentiation of human bone marrow mesenchymal stem cells in a serum/feeder cell-free condition. Dev Growth Differ. 2005;47:423–433. doi: 10.1111/j.1440-169X.2005.00810.x. [DOI] [PubMed] [Google Scholar]
- 35.Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS, Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 36.Narita Y, Yamawaki A, Kagami H, Ueda M, Ueda Y. Effects of transforming growth factor-beta 1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Cell Tissue Res. 2008;333:449–459. doi: 10.1007/s00441-008-0654-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Cell Proliferation on Fibrin
Supplementary Table 1. Primer sequences for qPCR