Abstract
BACKGROUND
Liver metastases in patients with cancer are associated with poor survival. We conducted a phase I study of hepatic arterial infusion (HAI) oxaliplatin combination therapy in patients with advanced cancer and liver metastases.
PATIENTS AND METHODS
Treatment consisted of escalating doses of HAI oxaliplatin 60–175 mg/m2 and 3000 IU heparin intraarterially (day 1); leucovorin 200 mg/m2 intravenously (IV) and 5-fluorouracil 300 mg/m2 bolus + 600 mg/m2 IV (days 1–2); and bevacizumab 10 mg/kg IV (day 3). A conventional “3 + 3” design was used.
RESULTS
Fifty-seven patients were treated [median age, 57 years; 30 women, 27 men; median number of prior therapies, 3 (range, 1–7)]. The most common cancer was colorectal (n=29). Overall, 204 cycles were administered (median per patient, 2; range, 1–17). The maximum tolerated dose (MTD) of HAI oxaliplatin was 140 mg/m2. Dose-limiting toxicities were Grade 4 thrombocytopenia (n=1) and Grade 4 hypokalemia (n=1) at 150 mg/m2 (n=5). Thirty-three (58%) patients had no toxicity > Grade 1. The most common toxicities were thrombocytopenia (n=19), fatigue (n=15), nausea/vomiting (n=6), constipation (n=6), and diarrhea (n=4). Of 55 patients evaluable for response (RECIST), 4 (7%) had partial response (PR) and 32 (58%) had stable disease (SD), including 15 (48%) who had SD for ≥ 4 months. Of 28 patients with colorectal cancer, 3 (11%) had PR and 9 (32%) had SD for ≥ 4 months.
CONCLUSIONS
HAI oxaliplatin combined with systemic 5-fluorouracil, leucovorin, and bevacizumab has antitumor activity in patients with advanced cancer and liver metastases and warrants further study.
INTRODUCTION
The presence of liver metastases in patients with solid tumors is associated with a poor prognosis. Overall, 15% to 25% of patients with colorectal cancer present with liver metastases, and another 25% to 50% develop hepatic metastasis following resection of the primary tumor. 1–3 The use of liver resection alone in patients with liver metastases is limited by the number of patients with resectable disease at presentation because most patients develop recurrent disease in the liver and/or extrahepatic sites4.
Hepatic arterial infusion (HAI) has been used in the treatment of hepatic metastases from colorectal cancer since early 1970’s.5 The rationale is based on the concept that malignant lesions derive most of their blood supply from the hepatic artery, in contrast to normal hepatocytes, which are supplied through the portal venous circulation.6 Cytotoxic agents administered via the hepatic artery are thought to be extracted in their initial pass through the hepatic parenchyma, thus maximizing their antitumor activity in the liver metastases.6
In 1989, a controlled clinical trial of 5-fluoro-2′-deoxyuridine (FUDR) for hepatic metastases of colorectal carcinoma via continuous intraarterial/intravenous (IV) therapy prevented extrahepatic spread during therapy in most patients, resulting in prolonged survival.7
In subsequent clinical trials, regional adjuvant therapy with FUDR was shown to improve survival in patients with colorectal cancer and liver involvement.8–10 In a randomized trial (CALGB 9481) HAI therapy was associated with higher rates of response (p=.01) and survival (p = .003) compared to systemic therapy.11
In 2000, using the human tumor colony-forming assay, in liver tumors, significant concentration-dependent inhibition of colony formation occurred after a 2-hour exposure to oxaliplatin, suggesting that patients with colorectal or pancreatic liver metastases may benefit from HAI with oxaliplatin.12 In addition, studies in rabbits had shown that hepatic arterial infusion of oxaliplatin was associated with a 4.3 times higher concentration in liver tumors compared to normal liver tissue13. Pharmacokinetic studies in humans demonstrated a liver extraction ratio of 0.47 for oxaliplatin administered via the hepatic artery14.
Clinical studies of HAI oxaliplatin combined with IV 5-fluorouracil (5-FU) and leucovorin have also shown encouraging results in patients with colorectal cancer and liver metastases.15–18 Bevacizumab has antitumor activity in a broad spectrum of tumors, and it improves survival in metastatic colorectal cancer.
Therefore, we conducted a phase I study of HAI oxaliplatin combined with systemic 5-FU/leucovorin and bevacizumab in patients with advanced solid tumors metastatic to the liver. The primary objectives were to determine the dose-limiting toxicity (DLT) and maximum tolerated dose (MTD) of oxaliplatin and to assess toxicity. We also assessed response or clinical benefit, if any.
PATIENTS AND METHODS
From August 2006 to November 2008, 57 patients were treated on protocol. Eligibility criteria included patients seen in the Phase I Clinical Trials Program at The University of Texas M. D. Anderson Cancer Center with histologically confirmed advanced cancer (any type) and liver metastases who had failed at least one prior chemotherapy regimen. Other eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status 0–2; at least 3 weeks after completion of previous therapy; baseline toxicity ≤ Grade 1; and adequate renal (serum creatinine ≤ 2.0 mg/dL), hepatic (total bilirubin ≤ 3 mg/dL, aspartate transaminase or alanine transaminase ≤ 5 × upper normal reference value), and bone marrow function (absolute neutrophil counts ≥ 1.5 × 109/L; platelet counts ≥ 100 × 109/L).
Patients were excluded owing to pregnancy; peripheral neuropathy ≥ Grade 2; serious wound history of abdominal fistula, gastrointestinal perforation, or intraabdominal abscess; major surgical procedure within 28 days prior to therapy; bleeding diathesis; active gastric/duodenal ulcer; uncontrolled intercurrent illness or hypertension; 24-hour urine protein > 1 g; history of cerebrovascular accident within the past 6 months; myocardial infarction/unstable angina within the past 6 months, New York Heart Association ≥ Grade II congestive heart failure, serious cardiac arrhythmia and clinically significant peripheral vascular disease, or history of bleeding brain metastasis.
Prior to enrollment, all participants signed informed consent forms fully disclosing the investigational nature of the trial. Patients under 18 years of age (excluding those younger than 7 years) signed assent forms, and the parent/guardian signed consent. The study protocol was approved by the M. D. Anderson Cancer Center Institutional Review Board.
Treatment
Patients were admitted for treatment at M. D. Anderson. A hepatic intraarterial catheter was placed by an interventional radiologist using the femoral approach. A 5 French angiographic catheter was utilized to select the celiac and/or superior mesenteric artery, and a co-axial 3 French microcatheter was advanced into the desired hepatic artery. Hepatic artery flow evaluation was then performed in all patients following the injection of 5 mCi technetium-99m MAA particles through the HAI catheter, which was used to simulate the distribution of chemotherapeutic agents. Once extrahepatic flow was excluded and appropriate hepatic distribution was confirmed, patients were transferred to the inpatient unit for initiation of HAI therapy. The nuclear medicine flow study was also used to identify any evidence of extra-hepatic flow to reduce the risk of gastrointestinal complications. When issues were identified on the nuclear medicine flow study, patients were returned to Interventional Radiology for evaluation and catheter repositioning. Right common femoral arterial access was routinely used for each session. The catheter was removed at the end of the oxaliplatin infusion.
Patients were treated as follows: day 1—escalating doses of HAI oxaliplatin from 60 to 175 mg/m2 intraarterially over 2 hours and 3000 units of heparin intraarterially; days 1 and 2—leucovorin at 200 mg/m2 IV and a 300-mg/m2 bolus of 5-FU plus 600 mg/m2 5-fluorouracilin 250 mL dextrose 5% in water as a continuous IV infusion over 22 hours; and day 3—bevacizumab at 10 mg/kg IV over 60 minutes. Dexamethasone at 10 mg IV prior to the oxaliplatin dose and for 2 days afterwards was administered. Oxaliplatin dose escalation was as follows: 60, 80, 100, 120, 140, 150, 160, and 175 mg/m2. Cycles were repeated every 3 weeks.
Patient monitoring
Patients were monitored every 3 weeks by physical examination, hematology and chemistry laboratory studies, vital signs, electrocardiogram, and chest x-ray. Tumors were staged before treatment and after every two cycles of therapy.
Endpoints and statistical considerations
The study was originally designed using a conventional “3 + 3” design, followed by an expansion phase comprised of 31 patients. Toxicities were assessed using NCI CTC v 3.0.19 Dose-limiting toxicities (DLTs) were assessed during the first cycle. The use of growth factors was acceptable during the clinical study.
Response was assessed by an M. D. Anderson radiologist using CT imaging studies every two cycles of therapy (1 cycle = 3 weeks) using the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines.20 Waterfall plot analysis was used to illustrate antitumor activity, as previously described.21 Responses shown in the waterfall plot were grouped according to standard RECIST criteria.
Survival was measured from the start of treatment on protocol until death from any cause or last follow-up. Progression-free survival was measured from the start of treatment on protocol until progression or death, whichever occurred first. A p-value <0.05 was considered statistically significant. Statistical analyses were carried out using SAS 9.1 (SAS Institute, Cary, NC) and S-Plus, version 7.0 (Insightful Corp., Seattle, WA) software.
RESULTS
Demographics
Sixty-three patients were registered on protocol. Six patients were removed from the study owing to a decline in performance status (n=3) or brain metastases (n=1) at initial screening, withdrawal of consent because of adverse event concerns (n=1), or insurance/financial issues (n=1).
Fifty-seven patients were treated. Their median age was 57 years (range, 11–76). There were 30 women and 27 men. Diagnoses were colorectal cancer (n=29), melanoma (n=7), gastric cancer (n=4), hepatocellular carcinoma (n=3), ovarian cancer (n=3), cholangiocarcinoma (n=3), and other (n=8). The median number of prior therapies was 3 (range, 1–7). In the subset of patients with colorectal cancer, the median number of prior therapies was 4 (range, 1 to 8). Thirty-three patients had previously received oxaliplatin as salvage therapy. Prior therapies are listed in Table 1.
Table 1.
Prior therapies in patients with colorectal cancer who responded to treatment
| Prior therapies | No. of pts (n=57) | % |
|---|---|---|
| Cytotoxics | ||
| Oxaliplatin | 33 | 58 |
| Irinotecan | 29 | 51 |
| 5-fluorouracil | 29 | 51 |
| Taxanes | 17 | 30 |
| Capecitabine | 17 | 30 |
| Cisplatin | 11 | 19 |
| Gemcitabine | 9 | 16 |
| Anthracyclines | 9 | 16 |
| Carboplatin | 6 | 11 |
| Topotecan | 3 | 5 |
| Targeted agents | ||
| Bevacizumab | 34 | 60 |
| Cetuximab | 15 | 26 |
| Erlotinib | 3 | 5 |
| Hypomethylating agents | 3 | 5 |
Dose escalation and DLT
Table 2 summarizes the dose escalation schedule and outcomes. No DLTs were reported at the first five dose levels of HAI oxaliplatin (60 mg/m2 to 140 mg/m2).
Table 2.
Distribution of patients, treatment cycles, and dose-limiting toxicities across tested dose levels
| HAI oxaliplatin dose level, mg/m2 | No. of patients | No. of patients completing cycle 1 | No. of patients with DLTs | Description of DLTs |
|---|---|---|---|---|
| 60 | 3 | 3 | 0 | |
| 80 | 3 | 3 | 0 | |
| 100 | 3 | 3 | 0 | |
| 120 | 6 | 6 | 0 | |
| 140 | 6 | 6 | 0 | |
| 150 | 5 | 5 | 2 | Grade 4 thrombocytopenia Grade 4 hypokalemia |
| Expansion (140) | 31 | 31 | 0 |
DLT=dose-limiting toxicity
Note: Each line of symptoms represents 1 patient.
Three patients were treated at the first three dose levels of HAI oxaliplatin (60 mg/m2 to 100 mg/m2). Then, the protocol was amended to allow six patients per cohort in order to capture more safety data before escalation to the next dose level. Therefore, six patients each were treated at the 120 and 140 mg/m2 HAI oxaliplatin dose levels. Of the five patients treated at the 150 mg/m2 HAI oxaliplatin dose, the fourth patient developed Grade 4 thrombocytopenia and the fifth patient developed Grade 4 hypokalemia (Table 2). Owing to these DLTs, the next lower dose, i.e., 140 mg/m2 HAI oxaliplatin, was considered the maximum tolerated dose (MTD) in this treatment regimen. Subsequently, 31 patients were treated at the MTD during the expansion phase of the study, and none developed a DLT.
Toxicity
A total of 204 cycles were administered. The median number of cycles per patient was 2 (range, 1–17). The most common toxicities were thrombocytopenia (n=19), fatigue (n=15), nausea/vomiting (n=10), constipation (n=6), and diarrhea (n=4) (Table 3). Among the 57 patients who completed cycle 1, 33 (58%) patients had no toxicity > Grade 1.
Table 3.
Number of Adverse Events Reported by Grade
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Hyperlipidemia | 1 | |||
| Nausea | 7 | 2 | ||
| Vomiting | 3 | 1 | ||
| Constipation | 5 | 1 | ||
| Fatigue | 7 | 8 | ||
| Shortness of breath | 1 | |||
| Rash | 3 | |||
| Diarrhea | 4 | |||
| Neutropenia | 2 | |||
| Hyperglycemia | 1 | |||
| Leukopenia | 3 | 1 | ||
| AST | 4 | 2 | ||
| ALT | 3 | 1 | ||
| Thrombocytopenia | 7 | 10 | 1 | 1 |
| UTI | 1 | |||
| Anorexia | 1 | 2 | ||
| Neuropathy | 2 | |||
| Abdominal pain | 2 | 1 | ||
| Hypercalcemia | 1 | |||
| Hyperbilirubinemia | 1 | 1 | ||
| Myalgia | 1 | |||
| Drop in hemoglobin | 5 | |||
| Lymphopenia | 1 | |||
| Hypertension | 2 | 1 | ||
| Hypokalemia | 1 | |||
| Cold sensitivity | 1 | |||
| Mucositis | 1 |
Abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase; UTI = urinary tract infection
Toxicity profile at the recommended dose
Overall, 37 patients (dose escalation phase, 6; expansion phase, 31) were treated at the MTD of 140 mg/m2 for a total of 139 cycles (median, 2 cycles; range, 1 to 14). No serious treatment-related toxicities were noted in any of these 37 patients.
Response
Fifty-five patients were evaluable for response. Of these 55 patients, 4 (7%) (colorectal cancer, n=3; breast cancer, n=1) achieved a partial response (PR) (tumor reduction of 45%, 38%, 31%, and 30% for 17, 4, 9, and 3 months, respectively). Thirty-two (58%) patients had stable disease (SD), including 15 (48%) patients who had SD for ≥ 4 months. The remaining 19 (35%) patients had progressive disease (PD). Responses are shown by waterfall plot analysis in Figure 1a. The tumor types of the 32 patients with SD were as follows: colorectal cancer, n = 15; melanoma, n = 5; hepatocellular cancer, n = 3; ovarian cancer, n = 3; cholangiocarcinoma, n = 3, hemangiopericytoma, n = 1; cancer of the duodenum, n = 1; and cancer of the gastroesophageal junction, n = 1. The diagnoses of the 15 patients who had SD for ≥ 4 months were as follows: colorectal cancer, n = 9; hepatocellular cancer, n = 2; ovarian cancer, n = 2; and cholangiocarcinoma, n = 2.
Figure 1.
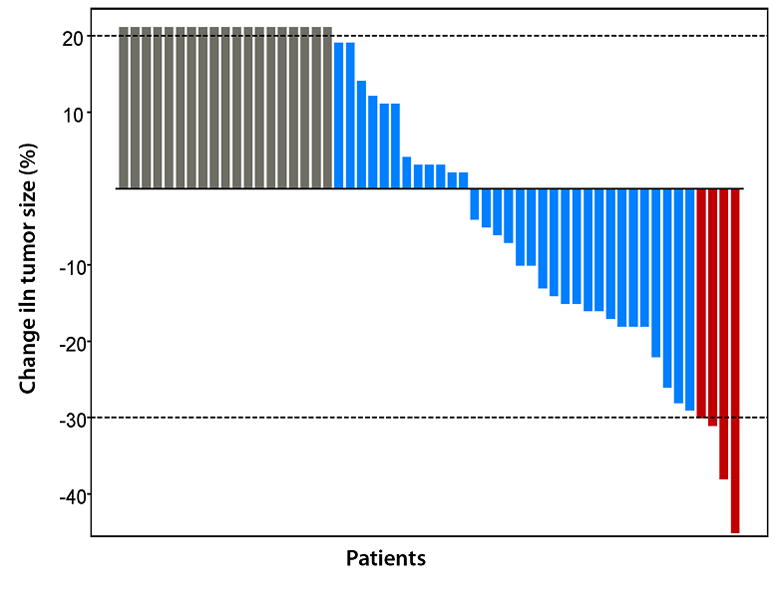
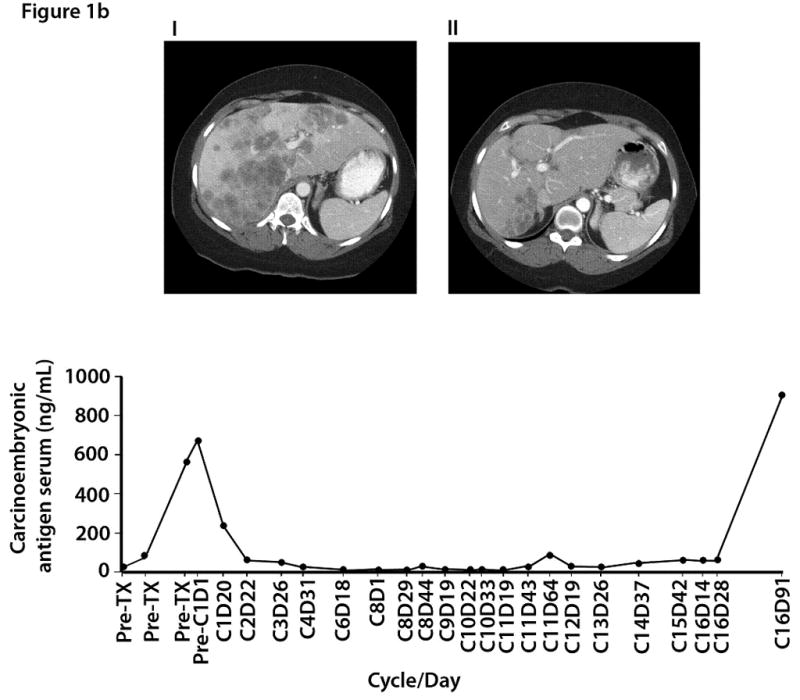
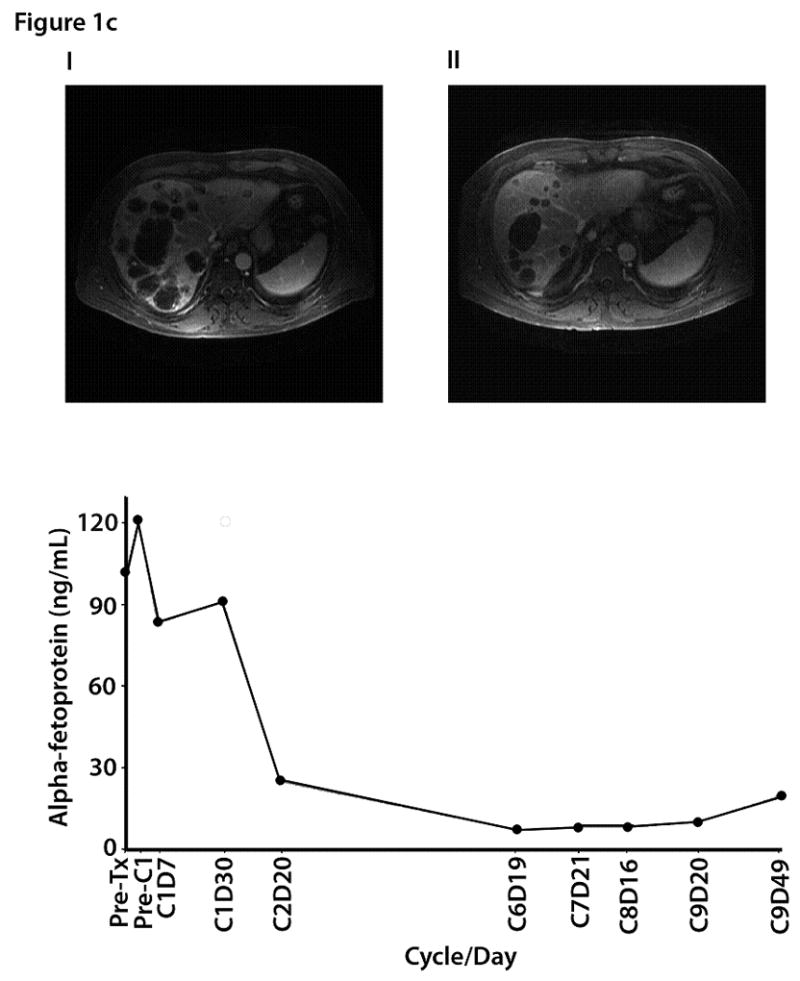
Figure 1a. Waterfall plot showing best response by RECIST to HAI oxaliplatin and systemic 5-FU, leucovorin, and bevacizumab: changes from baseline in tumor measurements. Each red box indicates a patient with maximum response, or a PR (> 30% reduction in tumor size) (n=4), each blue box indicates patients with SD (maximum response between 29% reduction and 19% increase in tumor size) (n = 32), and each grey box indicates a patient with clinically PD, or an increase in tumor > 20% (n=19).
Figure 1b. Computed tomography imaging from a 43-year-old female with colorectal carcinoma and liver metastases: (i) baseline (10/27/06) images demonstrate bilobar liver metastases and periportal adenopathy; (ii) after 8 months of treatment (06/07/07), improvement in the size and number of diffuse hepatic metastases was noted; (iii) carcinoembryonic antigen serum (CEA) levels by time of treatment.
Figure 1c. Magnetic resonance imaging (MRI) from a 51-year-old male with metastatic hepatocellular carcinoma: (i) baseline images demonstrated metastatic disease involving both lobes of the liver, right anterior diaphragmatic lymphadenopathy, and lower paraesophageal lymphadenopathy; (ii) 6 months later the size and number of multiple liver metastases had decreased; (iii) alpha fetoprotein trend levels by time of treatment.
Among 36 evaluable patients treated at the MTD, 3 (9%) patients had a PR and 20 (57%) patients had SD. Of 28 patients with colorectal cancer evaluable for response, 3 (11%) had a PR and 15 (54%) had SD, including 9 (32%) who had SD for ≥ 4 months. All patients with colorectal cancer who responded had prior oxaliplatin. The 3 patients with colorectal cancer who had a PR were previously treated as follows: 1 patient had prior FOLFOX (oxaliplatin, folinic acid and 5-FU) and bevacizumab; irinotecan and cetuximab; and the deoxycitidine analogue TAS-109; 1 patient was previously treated with FOLFOX and bevacizumab; FOLFIRI (irinotecan, leucovorin and 5-FU); capecitabine; cetuximab; and cetuximab, bevacizumab and capecitabine; and 1 patient was previously treated with FOLFOX and bevacizumab, FOLFIRI and bevacizumab, and 5-FU, leucovorin and bevacizumab.
Figures 1b and 1c illustrate improvements in the number and size of liver metastases and decreases in tumor markers in two patients (one with colorectal cancer and the other with metastatic hepatocellular carcinoma).
Two patients underwent resection of hepatic metastases after treatment on protocol. A 57-year-old woman with mixed cholangiocarcinoma and hepatocellular carcinoma had SD and underwent resection of hepatic tumors 5.5 months after initiation of HAI oxaliplatin in combination with systemic 5-FU, leucovorin, and bevacizumab. She died 11 months after the surgery. The second patient was an 11-year-old Asian male with hepatitis B-associated multifocal hepatocellular carcinoma for whom four prior therapies had failed. Two months after initiation of HAI oxaliplatin in combination with systemic 5-FU, leucovorin, and bevacizumab, he had shrinkage of his tumor by 23% compared to baseline and a significant tumor marker drop, and he underwent right and partial left hepatectomy. Eleven months later, his disease progressed, and he was treated with valproic acid and sunitinib, followed by gemcitabine and oxaliplatin. He remains alive 17 months after the hepatectomy.
Survival and failure-free survival
With a median follow-up of 14.7 months, 40 of 57 patients has died. The median overall survival duration was 8.7 months (95% CI: 5.4 – 11.6) (Figure 2a). All deaths were due to PD. Forty-four patients had PD. The median progression-free survival duration was 2.6 months (95% CI: 1.7 – 6.7) (Figure 2b).
Figure 2.
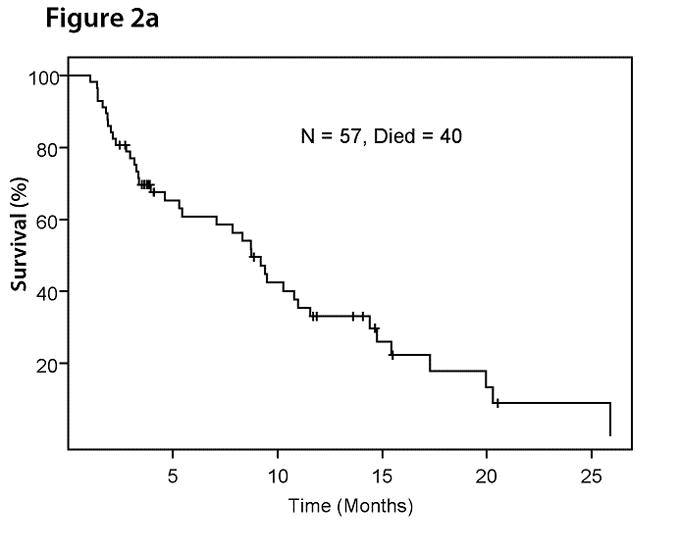
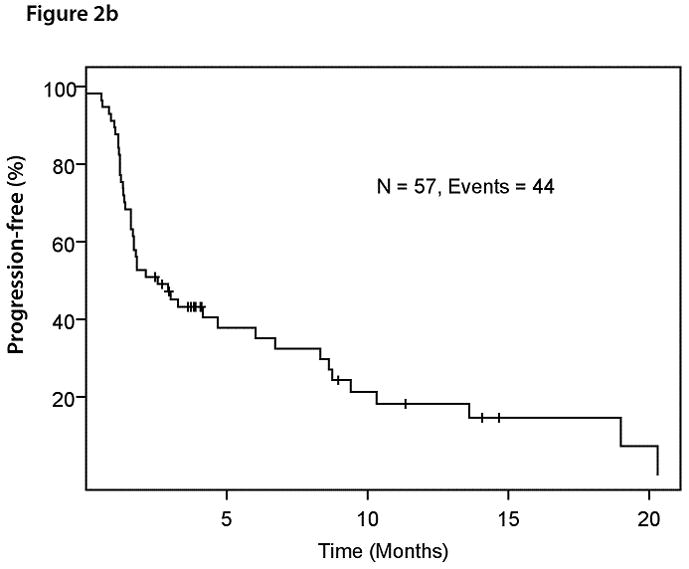
Figure 2a. Kaplan-Meier plot for overall survival in 57 patients with advanced solid tumors metastatic to the liver treated with HAI of oxaliplatin and systemic 5-FU, leucovorin, and bevacizumab.
Figure 2b. Kaplan-Meier plot for progression-free survival in 57 patients with advanced solid tumors metastatic to the liver treated with HAI of oxaliplatin and systemic 5-FU, leucovorin, and bevacizumab.
DISCUSSION
Our study demonstrates that the MTD of HAI oxaliplatin in combination with systemic 5-FU, leucovorin, and bevacizumab was 140 mg/m2. The most common toxicities were thrombocytopenia, fatigue, nausea/vomiting, constipation, and diarrhea. PRs were noted in 4 (7%) patients (colorectal carcinoma, n=3; breast cancer, n=1). SD for ≥ 4 months was noted in 15 patients (colorectal cancer, n = 9; hepatocellular cancer, n = 2; ovarian cancer, n = 2; and cholangiocarcinoma, n = 2). Among the 28 patients with colorectal cancer metastatic to the liver evaluable for response, 3 (11%) patients achieved a PR and 15 (54%) patients had disease stabilization, including 9 (32%) for ≥ 4 months.
The results of our study pertaining to MTD are consistent with those of another phase I study of HAI with oxaliplatin in combination with folinic acid and 5-FU in patients with hepatic metastases from colorectal cancer.15 Although no bevacizumab was administered in that study, oxaliplatin was given over a 4-hour period, and the DLT of oxaliplatin was 150 mg/m2. The investigators recommended the next lower dose level, i.e., 125 mg/m2, for phase II studies and reported a mean terminal half-life of administered ultrafiltrable platin of 18.8 +/− 9.3 hours.15
Our regimen was well tolerated, and 58% of patients did not experience toxicity greater than Grade 1. As expected, oxaliplatin-associated neurosensory symptoms were noted.22, 23 However, oxaliplatin infusion-associated abdominal pain was alleviated with prolongation of the infusion and concomitant administration of corticosteroids. Similarly, although hepatotoxicity, including the DLT of biliary sclerosis, was reported in earlier trials in 6% to 25% of patients treated with FUDR24, it was not observed in our study, probably because of premedication with corticosteroids to prevent oxaliplatin toxicity.
Keeping in mind that responders with colorectal cancers enrolled in the current trial were heavily pretreated and all were previously treated with oxaliplatin, the results noted in our study are encouraging. Higher rates of response and survival have been reported by the CALGB, but the baseline characteristics of patients between the two studies are not comparable, as only 3% of patients in that study had received prior adjuvant chemotherapy.11 Although HAI chemotherapy regimens are typically used to treat hepatic metastases in colorectal cancer and, therefore, the inclusion of any cancer type in the current study maybe be viewed as a limiting factor, an intriguing finding was that patients with other tumor types, such as hepatocellular carcinoma and breast cancer, responded to this treatment with SD.
Other investigators have reported encouraging results using HAI oxaliplatin-containing regimens for colorectal liver metastases.16, 17, 25 In a phase II study, HAI oxaliplatin at 100 mg/m2, IV leucovorin at 200 mg/m2, and 5-FU at a 400 mg/m2 IV bolus followed by a 5-FU 600 mg/m2 22-hour continuous infusion were administered on days 1 and 2 every 2 weeks. In 26 treated patients, the intent-to-treat objective response rate was 64% and the median overall and disease-free survival times were both 27 months.16 The same regimen showed an overall response rate of 62% in patients with unresectable liver metastases from colorectal cancer after systemic chemotherapy failure.17 In another study, in patients with colorectal liver metastases who underwent hepatectomy, some liver metastases vanished on imaging studies and remained undetected during hepatectomy so that posthepatectomy oxaliplatin HAI was associated with favorable outcomes (recurrence, survival, disease-free survival).26
In prior years, HAI regimens used 5-fluoro-2′-deoxyuridine (FUDR)-containing regimens, to treat patients with liver metastases from colorectal cancer because FUDR has a very high (94%–99%) first-pass extraction rate via the hepatic artery.6 27, 28 In a phase III clinical trial, HAI with FUDR combination therapy was associated with higher survival rates compared to similar systemic therapy alone at the time of resection.27
Ten phase III clinical trials have compared HAI FUDR or 5-FU and leucovorin to systemic FUDR or 5-FU and leucovorin.11, 28–37 In a randomized trial (CALGB 9481) HAI therapy was associated with higher rates of response (p=.01) and survival (p = .003) compared to systemic therapy.11 The HAI arms had superior response rates compared to the systemic arms (42% to 62% vs. 9% to 21%), but a survival benefit was shown in patients randomized to the HAI arms in only two of these phase III trials.28
Other combination regimens with HAI chemotherapy have also shown promising results38–40. A phase II study with HAI of 5-FU and IV oxaliplatin and folinic acid in patients with colorectal liver metastases was associated with an overall response rate of 41%, a median time to progression of 10 months, and a median overall survival duration of 21 months.18
In a phase I study of HAI and systemic oxaliplatin and irinotecan (Group A) or oxaliplatin, fluorouracil, and leucovorin (Group B), 36 patients (89% previously treated) with unresectable liver metastases were treated.41 Systemic chemotherapy was administered every 2 weeks, concurrent with 2 weeks of HAI FUDR and dexamethasone every 28 days. The overall response rate was 88%.41 In a retrospective analysis, HAI FUDR and dexamethasone plus IV irinotecan resulted in a 44% response rate in patients with unresectable liver metastases from colorectal cancer.42 In another phase I clinical trial with HAI FUDR and dexamethasone plus systemic oxaliplatin and irinotecan in patients with unresectable liver metastases form colorectal cancer, the rate of conversion to resection in the 49 chemotherapy-naïve and pretreated patients was 47% (chemotherapy-naïve patients, 57%).39
More recently, cetuximab, a chimeric IgG1 monoclonal antibody that targets epidermal growth factor receptor, has shown promising activity in combination with HAI chemotherapy.43
In conclusion, the MTD of HAI oxaliplatin was 140 mg/m2. HAI oxaliplatin combined with systemic 5-FU, leucovorin, and bevacizumab has antitumor activity in patients with advanced solid tumors metastatic to the liver and warrants further study.
Acknowledgments
Supported in part by Grant Number RR024148 from the National Center for Research Resources, a component of the NIH Roadmap for Medical Research (http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp)
Footnotes
The authors have no financial disclosures
References
- 1.Power DG, Healey-Bird BR, Kemeny NE. Regional chemotherapy for liver-limited metastatic colorectal cancer. Clin Colorectal Cancer. 2008;7:247–259. doi: 10.3816/CCC.2008.n.032. [DOI] [PubMed] [Google Scholar]
- 2.Khatri VP, Chee KG, Petrelli NJ. Modern multimodality approach to hepatic colorectal metastases: solutions and controversies. Surg Oncol. 2007;16:71–83. doi: 10.1016/j.suronc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007;11:1057–1077. doi: 10.1007/s11605-006-0061-3. [DOI] [PubMed] [Google Scholar]
- 4.Curley SA, Izzo F, Abdalla E, Vauthey JN. Surgical treatment of colorectal cancer metastasis. Cancer Metastasis Rev. 2004;23:165–182. doi: 10.1023/a:1025875332255. [DOI] [PubMed] [Google Scholar]
- 5.Fortner JG, Mulcare RJ, Solis A, Watson RC, Golbey RB. Treatment of primary and secondary liver cancer by hepatic artery ligation and infusion chemotherapy. Ann Surg. 1973;178:162–172. doi: 10.1097/00000658-197308000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ragnhammar P, Hafstrom L, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncol. 2001;40:282–308. doi: 10.1080/02841860151116367. [DOI] [PubMed] [Google Scholar]
- 7.Safi F, Bittner R, Roscher R, Schuhmacher K, Gaus W, Beger GH. Regional chemotherapy for hepatic metastases of colorectal carcinoma (continuous intraarterial versus continuous intraarterial/intravenous therapy). Results of a controlled clinical trial. Cancer. 1989;64:379–387. doi: 10.1002/1097-0142(19890715)64:2<379::aid-cncr2820640207>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Kelly H, Goldberg RM. Systemic therapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol. 2005;23:4553–4560. doi: 10.1200/JCO.2005.17.749. [DOI] [PubMed] [Google Scholar]
- 9.Kemeny N, Fata F. Hepatic-arterial chemotherapy. Lancet Oncol. 2001;2:418–428. doi: 10.1016/S1470-2045(00)00419-8. [DOI] [PubMed] [Google Scholar]
- 10.Barber FD, Mavligit G, Kurzrock R. Hepatic arterial infusion chemotherapy for metastatic colorectal cancer: a concise overview. Cancer Treat Rev. 2004;30:425–436. doi: 10.1016/j.ctrv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481) J Clin Oncol. 2006;24:1395–1403. doi: 10.1200/JCO.2005.03.8166. [DOI] [PubMed] [Google Scholar]
- 12.Kornmann M, Fakler H, Butzer U, Beger HG, Link KH. Oxaliplatin exerts potent in vitro cytotoxicity in colorectal and pancreatic cancer cell lines and liver metastases. Anticancer Res. 2000;20:3259–3264. [PubMed] [Google Scholar]
- 13.Dzodic R, Gomez-Abuin G, Rougier P, et al. Pharmacokinetic advantage of intra-arterial hepatic oxaliplatin administration: comparative results with cisplatin using a rabbit VX2 tumor model. Anticancer Drugs. 2004;15:647–650. doi: 10.1097/01.cad.0000131684.06390.fe. [DOI] [PubMed] [Google Scholar]
- 14.Guthoff I, Lotspeich E, Fester C, et al. Hepatic artery infusion using oxaliplatin in combination with 5-fluorouracil, folinic acid and mitomycin C: oxaliplatin pharmacokinetics and feasibility. Anticancer Res. 2003;23:5203–5208. [PubMed] [Google Scholar]
- 15.Kern W, Beckert B, Lang N, et al. Phase I and pharmacokinetic study of hepatic arterial infusion with oxaliplatin in combination with folinic acid and 5-fluorouracil in patients with hepatic metastases from colorectal cancer. Ann Oncol. 2001;12:599–603. doi: 10.1023/a:1011186708754. [DOI] [PubMed] [Google Scholar]
- 16.Ducreux M, Ychou M, Laplanche A, et al. Hepatic arterial oxaliplatin infusion plus intravenous chemotherapy in colorectal cancer with inoperable hepatic metastases: a trial of the gastrointestinal group of the Federation Nationale des Centres de Lutte Contre le Cancer. J Clin Oncol. 2005;23:4881–4887. doi: 10.1200/JCO.2005.05.120. [DOI] [PubMed] [Google Scholar]
- 17.Boige V, Malka D, Elias D, et al. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol. 2008;15:219–226. doi: 10.1245/s10434-007-9581-7. [DOI] [PubMed] [Google Scholar]
- 18.Carnaghi C, Santoro A, Rimassa L, et al. The efficacy of hybrid chemotherapy with intravenous oxaliplatin and folinic acid and intra-hepatic infusion of 5-fluorouracil in patients with colorectal liver metastases: a phase II study. Invest New Drugs. 2007;25:479–485. doi: 10.1007/s10637-007-9048-5. [DOI] [PubMed] [Google Scholar]
- 19.http://ctep.cancer.gov/
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 22.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Wilson RH, Lehky T, Thomas RR, Quinn MG, Floeter MK, Grem JL. Acute oxaliplatin-induced peripheral nerve hyperexcitability. J Clin Oncol. 2002;20:1767–1774. doi: 10.1200/JCO.2002.07.056. [DOI] [PubMed] [Google Scholar]
- 24.Kemeny NE, Ron IG. Hepatic arterial chemotherapy in metastatic colorectal patients. Semin Oncol. 1999;26:524–535. [PubMed] [Google Scholar]
- 25.Neyns B, Van Nieuwenhove Y, Aerts M, et al. Hepatic arterial infusion of oxaliplatin and L-folinic acid-modulated 5-fluorouracil for colorectal cancer liver metastases. Anticancer Res. 2006;26:611–619. [PubMed] [Google Scholar]
- 26.Elias D, Goere D, Boige V, et al. Outcome of posthepatectomy-missing colorectal liver metastases after complete response to chemotherapy: impact of adjuvant intra-arterial hepatic oxaliplatin. Ann Surg Oncol. 2007;14:3188–3194. doi: 10.1245/s10434-007-9482-9. [DOI] [PubMed] [Google Scholar]
- 27.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 28.Power DG, Kemeny NE. The role of floxuridine in metastatic liver disease. Mol Cancer Ther. 2009 doi: 10.1158/1535-7163.MCT-08-0709. [DOI] [PubMed] [Google Scholar]
- 29.Kemeny N, Daly J, Reichman B, Geller N, Botet J, Oderman P. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med. 1987;107:459–465. doi: 10.7326/0003-4819-107-4-459. [DOI] [PubMed] [Google Scholar]
- 30.Chang AE, Schneider PD, Sugarbaker PH, Simpson C, Culnane M, Steinberg SM. A prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg. 1987;206:685–693. doi: 10.1097/00000658-198712000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hohn DC, Stagg RJ, Friedman MA, et al. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: the Northern California Oncology Group trial. J Clin Oncol. 1989;7:1646–1654. doi: 10.1200/JCO.1989.7.11.1646. [DOI] [PubMed] [Google Scholar]
- 32.Wagman LD, Kemeny MM, Leong L, et al. A prospective, randomized evaluation of the treatment of colorectal cancer metastatic to the liver. J Clin Oncol. 1990;8:1885–1893. doi: 10.1200/JCO.1990.8.11.1885. [DOI] [PubMed] [Google Scholar]
- 33.Martin JK, Jr, O’Connell MJ, Wieand HS, et al. Intra-arterial floxuridine vs systemic fluorouracil for hepatic metastases from colorectal cancer. A randomized trial. Arch Surg. 1990;125:1022–1027. doi: 10.1001/archsurg.1990.01410200086013. [DOI] [PubMed] [Google Scholar]
- 34.Rougier P, Laplanche A, Huguier M, et al. Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: long-term results of a prospective randomized trial. J Clin Oncol. 1992;10:1112–1118. doi: 10.1200/JCO.1992.10.7.1112. [DOI] [PubMed] [Google Scholar]
- 35.Allen-Mersh TG, Earlam S, Fordy C, Abrams K, Houghton J. Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases. Lancet. 1994;344:1255–1260. doi: 10.1016/s0140-6736(94)90750-1. [DOI] [PubMed] [Google Scholar]
- 36.Lorenz M, Muller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2000;18:243–254. doi: 10.1200/JCO.2000.18.2.243. [DOI] [PubMed] [Google Scholar]
- 37.Kerr DJ, McArdle CS, Ledermann J, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet. 2003;361:368–373. doi: 10.1016/S0140-6736(03)12388-4. [DOI] [PubMed] [Google Scholar]
- 38.Kemeny N, Capanu M, D’Angelica M, et al. Phase I trial of adjuvant hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone plus systemic oxaliplatin, 5-fluorouracil and leucovorin in patients with resected liver metastases from colorectal cancer. Ann Oncol. 2009;20:1236–1241. doi: 10.1093/annonc/mdn769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kemeny NE, Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27:3465–3471. doi: 10.1200/JCO.2008.20.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White RR, Schwartz LH, Munoz JA, et al. Assessing the optimal duration of chemotherapy in patients with colorectal liver metastases. J Surg Oncol. 2008;97:601–604. doi: 10.1002/jso.21042. [DOI] [PubMed] [Google Scholar]
- 41.Kemeny N, Jarnagin W, Paty P, et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol. 2005;23:4888–4896. doi: 10.1200/JCO.2005.07.100. [DOI] [PubMed] [Google Scholar]
- 42.Gallagher DJ, Capanu M, Raggio G, Kemeny N. Hepatic arterial infusion plus systemic irinotecan in patients with unresectable hepatic metastases from colorectal cancer previously treated with systemic oxaliplatin: a retrospective analysis. Ann Oncol. 2007;18:1995–1999. doi: 10.1093/annonc/mdm405. [DOI] [PubMed] [Google Scholar]
- 43.Neyns B, Aerts M, Van Nieuwenhove Y, et al. Cetuximab with hepatic arterial infusion of chemotherapy for the treatment of colorectal cancer liver metastases. Anticancer Res. 2008;28:2459–2467. [PubMed] [Google Scholar]


