Abstract
The molecules and environment to direct pluripotent stem cell differentiation into cardiomyocytes are largely unknown. Here, we determined a critical role of receptor tyrosine kinase, EphB4, in regulating cardiomyocyte generation from embryonic stem (ES) cells through endothelial cells. The number of spontaneous contracting cardiomyocytes, and the expression of cardiac-specific genes, including α-MHC and MLC-2V, was significantly decreased in EphB4-null ES cells. The peptide blocking study indicated that EphB4 signaling is critical for cardiomyocyte development in the early phase of ES cell differentiation. EphB4 was expressed in endothelial cells underneath contracting cardiomyocytes, but not in cardiomyocytes. Angiogenic inhibitors, including endostatin and angiostatin, inhibited endothelial cell differentiation and diminished cardiomyogenesis in ES cells. Generation of functional cardiomyocytes and the expression of cardiac-specific genes were significantly enhanced by co-culture of ES cells with human endothelial cells. Furthermore, the defects of cardiomyocyte differentiation in EphB4-deficient ES cells were rescued by human endothelial cells. For the first time, our study demonstrated that endothelial cells play an essential role in facilitating cardiomyocyte differentiation from pluripotent stem cells. EphB4 signaling is a critical component of the endothelial niche to regulate regeneration of cardiomyocytes.
Keywords: embryonic stem (ES) cells, cardiomyocytes, endothelial cells, EphB4, ephrin
Introduction
Functional cardiomyocytes in the adult heart are terminally differentiated and unable to reenter the cell cycle for regeneration. The damage to cardiomyocytes resulting from ischemic injury is irreversible and leads to the development of progressive heart failure. Recently, therapies based on cardiac progenitor cells have emerged as potential cardiac therapeutics. Cardiac stem or progenitor cells can be isolated from early embryos or pluripotent stem cells, and cultured to differentiate into functional cardiomyocytes. A small amount of cardiac stem or progenitor cells are identified in the adult myocardium [Beltrami et al., 2003; Laugwitz et al., 2005; Matsuura et al., 2004; Oh et al., 2003; Oyama et al., 2007; Pfister et al., 2005; Wang et al., 2006]. However, the quantity of cardiac progenitor cells in the adult heart may not be sufficient for regeneration after myocardial injury. Therefore, different non-cardiac cells, including skeletal myoblasts [Menasche et al., 2003], bone marrow cells [Jackson et al., 2001], endothelial progenitor cells [Kocher et al., 2001], and mesenchymal stem cells [Makino et al., 1999], were examined as potential cell sources to repair the injured heart after cardiac damage [Davani et al., 2005; Laflamme and Murry, 2005]. One of the major obstacles for using these cells in transplantation study is the low efficiency of myogenic differentiation in vivo and the modest effect on cardiac function.
Pluripotent embryonic stem (ES) cells are capable to differentiate into cardiomyocytes [Laflamme and Murry, 2005]. Transplantation of cardiomyocytes derived from human or mouse ES cells improves cardiac function in animal models [Caspi et al., 2007; Klug et al., 1996; Xue et al., 2005]. Although ES cells have the potential to be renewable sources of cell-based therapy, efficiently generating cardiomyocytes from ES cells is a major challenge because the condition and factors to direct ES cells differentiation into cardiomyocytes are largely unknown. Several studies demonstrate that endothelial progenitor cells harvested from the bone marrow [Kocher et al., 2001; Schachinger et al., 2004; Takahashi et al., 1999], circulating peripheral blood [Asahara et al., 1999; Urbich and Dimmeler, 2004], and ES cells [Li et al., 2007] can contribute to angiogenesis and improve the function of ischemic myocardium. The developmental relationship of endothelial cells and cardiomyocytes, and mechanisms by which endothelial cells improve cardiac function after ischemic injury are not well understood.
During embryonic heart development, endothelial cells and cardiomyocytes arise from the same cardiac mesodermal precursors [Narumiya et al., 2007][Linask and Lash, 1993]. There are two types of cardiac endothelial cells in the heart: i) endocardial endothelial cells and ii) myocardial capillary endothelial cells (MyoCapE) [Brutsaert, 2003]. Cardiac endothelial cells release some factors, such as nitric oxide, endothelin-1, prostanoids, adenylpurines, natriuretic peptides [Shah, 1996]. Interaction between endothelial cells and cardiomyocytes in the heart regulates normal heart functions, such as cardiac growth, contractile performance, and rhythmicity [Brutsaert, 2003; Hsieh et al., 2006; Kuruvilla and Kartha, 2003]. The receptor tyrosine kinase, EphB4, and its ligand, ephrinB2, are key regulators of vascular development [Conway et al., 2001; Gale and Yancopoulos, 1999]. Signaling between Eph receptors and their ephrin ligands is restricted to sites of direct cell-cell contact, and can induce reciprocal bidirectional events between interacting cells [Bruckner and Klein, 1998; Davis et al., 1994; Gale and Yancopoulos, 1999]. The bidirectional signaling of EphB4 and ephrinB2 regulates arteriovenous asymmetry in the developing vasculature [Conway et al., 2001; Gale and Yancopoulos, 1999]. During embryonic development, EphB4 is predominantly expressed in the venous endothelium, whereas ephrinB2 is primarily expressed in arterial endothelial cells [Gerety et al., 1999; Wang et al., 1998]. Null mutations in either EphB4 or ephrinB2 genes result in embryonic lethality, and display identical defects in forming capillary connections between arterial and venous networks of the head and yolk sac [Gerety et al., 1999; Wang et al., 1998]. Arrested heart development at E9.5 to E10, such as failure to increase in size and incomplete cardiac looping, occurs in EphB4-null and ephrinB2-null mice, which corresponds to early defects of vascular development [Gerety et al., 1999].
We previously demonstrated that EphB4 signaling regulates hematopoietic and endothelial development. The differentiation of ES cells into cardiomyocytes is also impaired in EphB4−/− ES cells [Wang et al., 2004]. In this study, we investigated effects of EphB4 on ES cell differentiation into cardiomyocytes. Although differentiated cell masses from ES cells, called embryoid bodies (EBs), are far less organized than the actual embryo, they can partially mimic the spatial organization of the embryo. Our data demonstrate that generation of functional cardiomyocytes is dependent on endothelial cells, which may provide a “niche” in dishes to direct ES cell differentiation. EphB4, which is expressed in endothelial cells at regions containing functional cardiomyocytes, is a critical component for endothelial cells promoting ES cell differentiation into cardiomyocytes. The cardiomyocyte differentiation defect in EphB4−/− ES cells is rescued by human endothelial cells. Our studies, for the first time, demonstrate that interaction of endothelial cells and ES cells facilitates cardiomyocyte differentiation, in which EphB4 signaling is essential.
Materials and Methods
Cell culture
CGR8-GFP mouse ES cell line was generously provided by Dr. Richard T. Lee (Harvard Medical School, Boston, MA). The EGFP expression in CGR8-GFP cells is under the control of cardiac muscle specific α-myosin heavy chain (MHC) promoter [Takahashi et al., 2003]. EphB4+/− and EphB4−/− ES cell lines were generously provided by Dr. David Anderson (California Institute of Technology, CA). Mouse ES cells were cultured as we described previously [Wang et al., 2004]. Briefly, mouse ES cells were maintained on a mouse feeder cell line (SNL) in Dulbecco’s modified Eagle medium (DMEM) containing 15% defined-fetal bovine serum (FBS, HyClone, Logan, UT), 2 mM sodium pyruvate, 2 mM L-glutamine, 0.1 mM nonessential amino acid, 150 μM monothioglycerol (MTG, Sigma-Aldrich, St Louis, MO), 50 U/ml penicillin, 50 μg/ml streptomycin, and 10 ng/ml leukemia inhibitory factor (LIF) (Chemicon International). The ES cells were cultured on gelatin-coated plates for two passages to remove feeder cells before EB differentiation.
Human umbilical vein endothelial cells (HUVEC) were purchased from Clonetics (Cambrex, Walkersville, MD). HUVEC and human bone marrow microvascular endothelial cells (BMEC) [Candal et al., 1996; Rafii et al., 1994] were cultured on gelatin-coated dishes in EGM-2 media (Cambrex, Walkersville, MD). NIH3T3 cells and mouse embryonic fibroblast (MEF) cells were cultured in DMEM with 10% FBS, 2 mM L-glutamine, 0.1 mM nonessential amino acid, 50 U/ml penicillin, and 50 μg/ml streptomycin. To generate conditioned medium (CM), BMEC were cultured in ES cell differentiation medium for 48 hours, and then filtered through a 0.22 μm Steriflip filter (Millpore, Billerica, MA).
Cardiomyocyte differentiation of ES cells
ES cell differentiation into EBs was performed as described previously [Maltsev et al., 1994]. Briefly, 400 ES cells were suspended in hanging-drop cultures in 30 μl differentiation medium containing Iscove modified Dulbecco’s medium (IMDM), 15% defined-FBS, 2 mM L-glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin. After 5 days of differentiation in the presence or the absence of 100μM ascorbic acid (Vitamin C), the individual EBs were transferred to a gelatin-coated well in 48-well plates. Differentiation medium was changed every 3 days. After 24 hours, the EBs were attached to the surface of the wells. After 7 to 8 days of differentiation, GFP+ cardiomyocytes from CGR8-GFP ES cells were observed under a fluorescent microscope. Spontaneously contracting areas in beating EBs were observed after 8 to 9 days of differentiation. In general, the GFP+ cardiomyocytes formed cell clusters that generated spontaneously beating areas after 24 hours.
ES cells were cocultured with endothelial cells, NIH3T3 cells, and MEFs. Undifferentiated ES cells (400 cells) and cocultured cells (100 to 400 of HUVEC, BMEC, NIH3T3, or MEF cells) were formed into EBs in 30 μl of ES cell differentiation medium using the hanging-drop method. ES cell differentiation was carried out with or without endostatin (2 μg/ml), angiostatin (1μg/ml) or TNYL-RAW (5 μM). The TNYL-RAW peptide (TNYLFSPNGPIARAW) is an antagonist of ephrinB2 binding to EphB4 [Koolpe et al., 2005]. The control peptide (WYPSNTRPGILNAFA) contains a scramble sequence of TNYL-RAW. Both TNYL-RAW and control peptide are synthesized by EZBiolab (www.ezbiolab.com, Westfield, IN). Endostatin, angiostatin, TNYL-RAW, or control peptide was added into the differentiation medium at different times, depending on experiment design.
RT-PCR and Quantitative PCR (Q-PCR)
Undifferentiated ES cells and differentiated EBs were harvested at different time points. Total RNA was extracted with TRIzol reagent (Invitrogen Life Technologies). One μg RNA was used to generate cDNA in 20 μl by reverse transcription (RT) using SuperScript III reverse transcriptase (Invitrogen). Specific primers used for PCR are listed in supplemental table 1.
For quantitative PCR (real-time PCR), 0.5μl of cDNA was added to 19.5μl of master mix containing 1× SYBR Green (Bio-Rad Laboratories, Hercules, CA) and 25μM sense and antisense primers. Triplicates of the cDNA were amplified for 40 cycles with the iCycler iQ Real-Time thermal cycler (Bio-Rad Laboratories). The PCR product level was expressed as Ct, the amplification cycle at which the emission intensity of the product rises above an arbitrary threshold level. Specific primers used for Q-PCR are listed in supplemental table 2.
Flow cytometric analysis and immunostaining for confocal microscope analysis
Differentiated EBs at different time points were trypsinized for 2 minutes, and passed through a 30μm pre-separation filter (Miltenyi Biotec Inc., Auburn, CA) to produce a single cell suspension. Cells (1×106) were then stained with primary antibodies as follows: VE-Cadherin (VE-Cad) at 1:100 dilution, Flk-1 (VEGFR-2) at 1:100 dilution, rat IgG2a (control antibodies) at 1:100 dilution. PE-conjugated or APC-conjugated secondary antibodies were used for flow cytometric analysis. Dead cells were gated out by a DNA dye (7AAD staining). All antibodies were purchased from BD Bioscience (Franklin Lakes, NJ).
Differentiated EBs cultured in chamber slides were fixed in 4% paraformaldehyde for confocal microscopy (Leica microscope LTCS-SP, Heidelberg, Germany). The following primary antibodies were used: CD31 (PE-CAM) (Serotec, Oxon, United Kingdom) at 1:100 dilution, VE-Cad at 1:100 dilution (BD, Franklin lakes, NJ), EphB4 at 1:100 dilution (R&D System, Minneapolis, MN), and ephrin B2 at 1:100 dilution (R&D System). Nuclei were labeled with TO-Pro-3 iodide (Invitrogen). Expression of human EphB4 and ephrinB2 was analyzed using a rabbit polyclonal IgG anti-human EphB4 at 1:100 and a rabbit polyclonal IgG anti-human ephrinB2 at 1:100 (both from Santa Cruz Biotechnology, CA). Nuclei were labeled with DAPI (Invitrogen).
Statistical Analysis
The results were subjected to statistical analysis by the Student’s t-test. For all analyses, values of p<0.05 were considered statistically significant.
Results
Differentiation into cardiomyocytes is impaired by inhibition of EphB4 signaling
Our previous study indicated that beating cardiomyocytes were significantly decreased in EphB4-null (EphB4−/−) ES cells [Wang et al., 2004]. To further characterize the role of EphB4 signaling in cardiomyocyte differentiation, we used the hanging-drop method to generate embryoid bodies (EBs) in a controlled manner during ES cell differentiation. After 5 days of initial aggregation of ES cells in hanging-drops, individual EBs were transferred into gelatin coated wells for further cardiomyocyte differentiation. Ascorbic acid (vitamin C) has been shown to enhance cardiomyocyte differentiation, which isnot enhanced by the other antioxidants [Takahashi et al., 2003]. To test whether ascorbic acid promotes cardiogenesis in EphB4−/− ES cells, we measured the kinetic of beating EB at different times with or without ascorbic acid. As shown in Figure 1A, no beating EBs were observed from EphB4−/− ES cells up to day 20 of differentiation without ascorbic acid, whereas addition of ascorbic acid increased the number of beating EBs in both EphB4+/− ES cells and EphB4−/− ES cells. By RT-PCR analysis of EBs at day 9 and day 15, the expression of the muscle specific genes, α-MHC and MLC-2V, were negative at day 9, but detected at day 15 in EphB4−/− EBs (Figure 1B, lane 5 and 6). The addition of ascorbic acid increased cardiac gene expression (Figure 1B, lane 5 and 7) in EphB4−/− ES cells. These data demonstrated that EphB4 signaling can be compensated for by ascorbic acid. The mechanisms of ascorbic acid enhancing cardiogenesis in ES cells have not been defined. Whether EphB4 and ascorbic acid share downstream signaling pathway in cardiac differentiation is under investigation.
Figure 1. Defect of cardiomyocyte differentiation in EphB4-null ES cells.
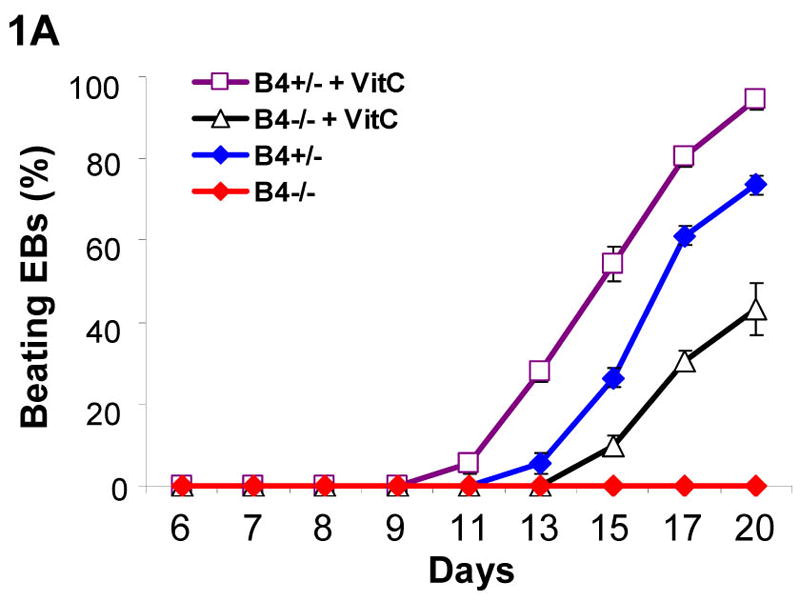
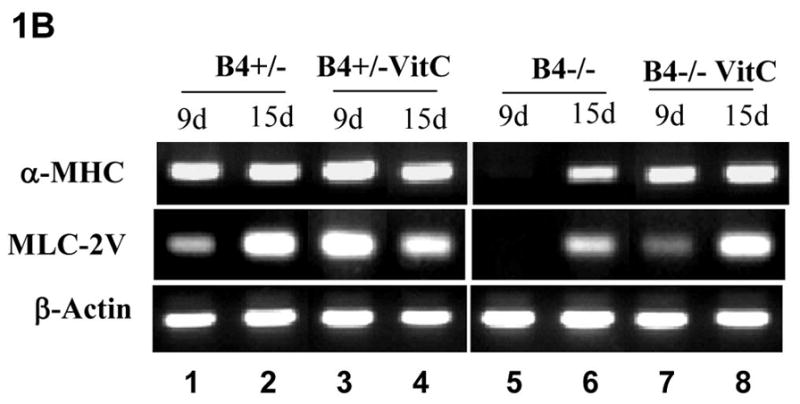
Four hundreds of undifferentiated ES cells were differentiated in hanging-drops. Individual EBs were transferred to gelatin-coated wells at day 5. Spontaneous contracting EBs (beating EBs) were observed after EB attachment. (A) Undifferentiated EphB4+/− ES cells (B4+/−) or EphB4−/− ES cells (B4−/−) formed EBs in hanging-drop with or without ascorbic acid (VitC). After 5 days, individual EBs were transferred to 48-well plates. Beating EBs were counted at different time points. For each example, percentile of beating EB was calculated from total of 24 wells. Data are representative from 3 independent experiments. (B) RT-PCR analysis of cardiac genes, α-MHC and MLC-2V, in EphB4+/− or EphB4−/− ES cells. ES cells were differentiated in the presence or absence of ascorbic acid for 5 days. RNA samples were harvested at day 9 or day 15.
EphB4 and ephrinB2 are expressed in beating EBs
To determine whether EphB4 signaling is directly involved in regulating cardiomyocyte beating function, we analyzed EphB4 expression in CGR8-GFP mouse ES cells, in which GFP expression is controlled by the α-cardiac MHC promoter [Takahashi et al., 2003]. During ES cell differentiation, GFP is exclusively expressed in cardiomyocytes in CGR8-GFP ES cells. High expression of EphB4 in the beating EBs was observed by RT-PCR analysis (Figure 2A). To further determine whether EphB4 is expressed in functional cardiomyocytes, beating and non-beating EBs were analyzed by confocal microscopy with PE-conjugated EphB4 antibodies. Within a beating EB, GFP+ cardiomyocytes were clustered together in sheets, and were located on the upper layer of beating EBs (Figure 2B). EphB4 was expressed in cell aggregates underneath the cardiomyocytes (Figure 2B-3 and 4), but not in GFP+ cardiomyocytes (Figure 2B-1 and 2). EphB4 was rarely expressed in non-beating EBs (Figure 2B, right panel). These results indicate that EphB4+ cells are colocalized in regions of beating cardiomyocytes within a beating EB.
Figure 2. EphB4 expression in beating EBs.
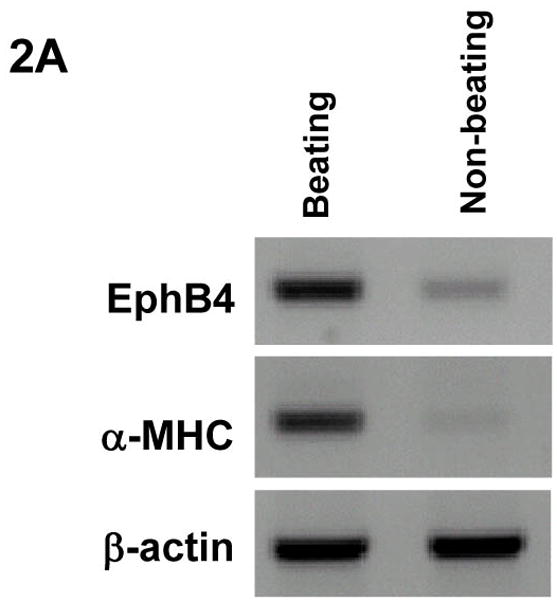
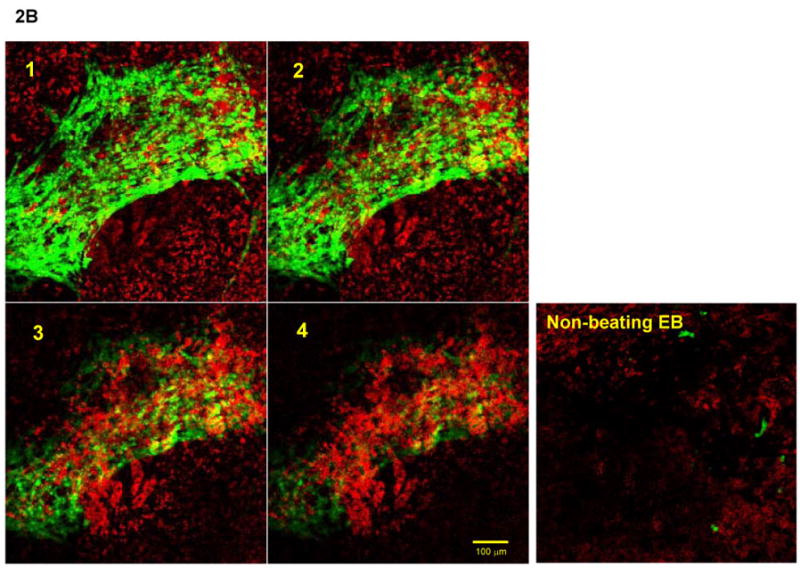
α-MHC-GFP ES cells were differentiated into EBs as in Figure 1. (A) RNA samples were isolated from beating EBs and non-beating EBs. RT-PCR was performed using primers listed in supplemental table 1. (B) EphB4 localization was detected by immunohistochemistry with PE-conjugated EphB4 antibodies. A beating EB was analyzed by confocal microscopy at 10 μm intervals (from 1 to 4). GFP+ cells were abundant on the upper layer (1 and 2), and a high density of EphB4+ cells was observed underlying the GFP+ layer (3 and 4). A non-beating EB is showed for comparison in the right panel. The scale bar = 100 μm.
EphB4+ cells underneath cardiomyocytes expressed the endothelial marker, CD31
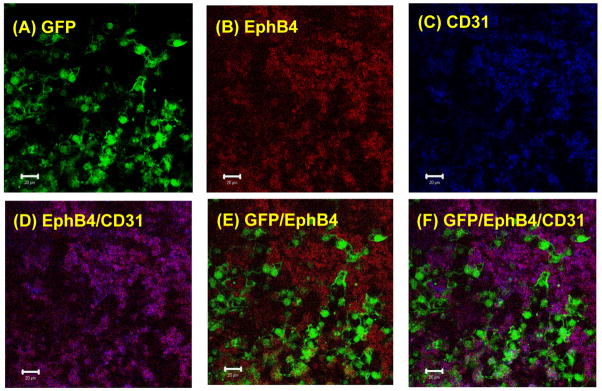
EphB4 plays an essential role in angiogenesis during embryonic development [Wang et al., 1998]. Our previous study demonstrated that endothelial development is impaired in EphB4−/− ES cells [Wang et al., 2004]. To determine whether EphB4-expressing cells underneath GFP+ clusters were endothelial cells, we analyzed individual beating EBs by a confocal microscopy with APC-conjugated CD31 antibodies and PE-conjugated EphB4 antibodies. As shown in Figure 3, GFP (A), EphB4 (B) and CD31 (C) were expressed in the beating EB under a single fluorescence wave length. Analyses of the beating EB with multi-wave lengths indicated that EphB4+ cells also expressed the endothelial cells marker, CD31 (D). However, neither EphB4 (E) nor CD31 (F) was expressed in GFP+ cardiomyocytes.
Figure 3. EphB4 expression in endothelial cells.
α-MHC-GFP ES cells was differentiated into EBs. At day 11 of differentiation, EBs were immunostained with PE-conjugated EphB4 (B) and APC-conjugated CD31 (C), and analyzed by confocal microscopy at different filters (wave lengths). GFP (A), EphB4 (B), and CD31 (C) were analyzed by single wave length in the same beating EB. The expression of EphB4 and CD31 (D), GFP and EphB4 (E), and GFP, EphB4 and CD31 (F) in the same EB were analyzed by multi-wave lengths. The scale bar = 20 μm. The data represent one of five EBs analyzed.
Endothelial cells enhance cardiomyocyte development in ES cells
Communication of endothelial cells and cardiomyocytes regulates cardiomyocyte function, including the contractile state of the adult heart [Brutsaert, 2003]. It is unclear whether endothelium provides signals to facilitate stem cells to differentiate into functional cardiomyocytes. Ascorbic acid promotes collagen synthesis during cardiomyocyte differentiation of ES cells [Sato et al., 2006]. In addition, ascorbic acid is essential in collagen synthesis by endothelial cells during angiogenesis [Telang et al., 2007]. To test the hypothesis that endothelial cells play an essential role in ES cell differentiation into cardiomyocytes, we cocultured endothelial cells with CGR8-GFP mouse ES cells in hanging-drop EB formation. Human endothelial cells (BMEC or HUVEC) or non-endothelial cells (NIH3T3 or MEF), were mixed and aggregated with 400 ES cells. By day 13 of ES cell differentiation, the EBs containing either BMEC or HUVEC endothelial cells dramatically increased the number of beating EBs (Figure 4A), as compared to ES cells alone (control). In contrast, the addition of MEF cells moderately decreased the number of spontaneous beating EBs, whereas no spontaneous beating EB was observed with the addition of NIH3T3 cells (Figure 4A). As showed in Figure 4B, for each EB containing 400 of ES cells, the addition of 200 or more of BMEC cells dramatically accelerated beating EB formation, indicating that the capacity of endothelial cells to promote cardiomyocyte generation is dependent on cell number.
Figure 4. Effects of endothelial cells on cardiomyocyte differentiation.
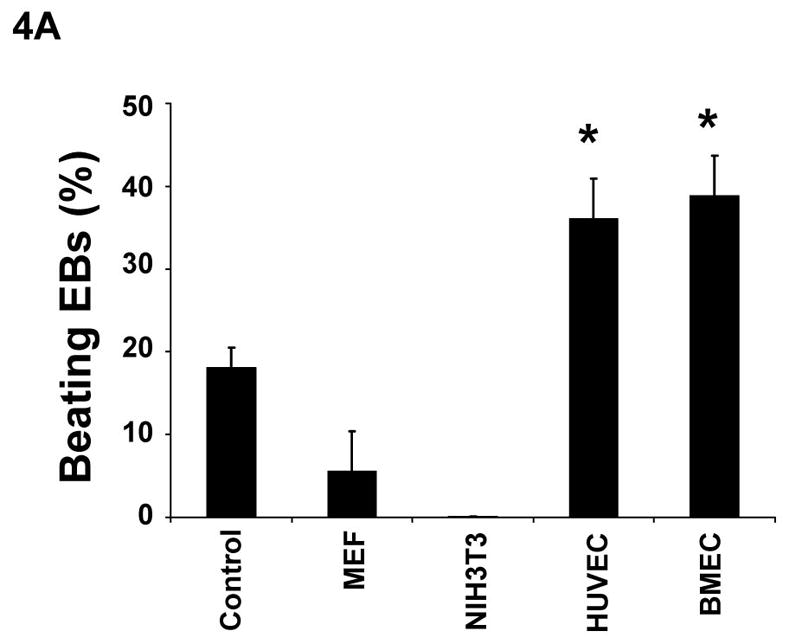
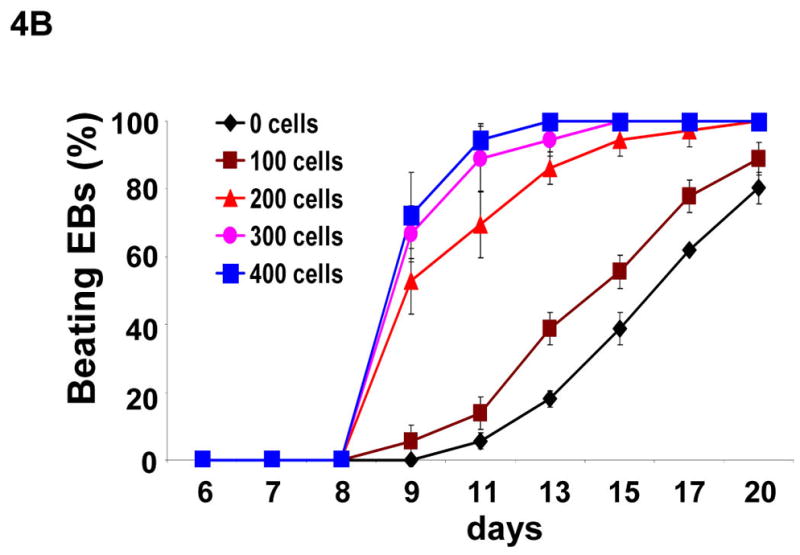
(A) Undifferentiated α-MHC-GFP ES cells formed EBs in hanging drops (400 ES cells/drop) without 200 exogenous cells (control) or with MEF, NIH3T3, HUVEC, or BMEC cells. After 5 days, individual EBs were transferred to individual wells in 24 wells coated with gelatin. The wells containing beating EBs were counted at day 13 of differentiation. (B) Undifferentiated ES cells formed EBs in hanging-drops (400 ES cells/drop) containing 0, 100, 200, 300, or 400 BMEC cells. The wells containing beating EBs were counted on different days. Data are representative from 3 independent experiments. Error bars represent standard deviation (* indicates p<0.05 vs. control).
Cardiomyocyte differentiation is impaired by angiogenic inhibitors
Our data indicated that the presence of exogenous endothelial cells promotes cardiomyogenesis during mouse ES cell differentiation. To investigate whether endogenous endothelial cells are essential for cardiomyogenesis during ES cell differentiation, we used angiogenic inhibitors to inhibit endothelial cells during ES cell differentiation. Endostatin and angiostatin predominantly interfere with the molecular pathways involved in endothelial cell migration, proliferation, and survival [O’Reilly et al., 1997; Tabruyn and Griffioen, 2007]. As showed in Figure 5A, the addition of either endostatin or angiostatin delayed formation of spontaneous beating EBs, and dramatically decreased the number of beating EBs. Furthermore, addition of endostatin partially abolished BMEC-promoted cardiomyocyte differentiation (Figure 5B).
Figure 5. Inhibition of cardiomyogenesis by angiogenic inhibitors.
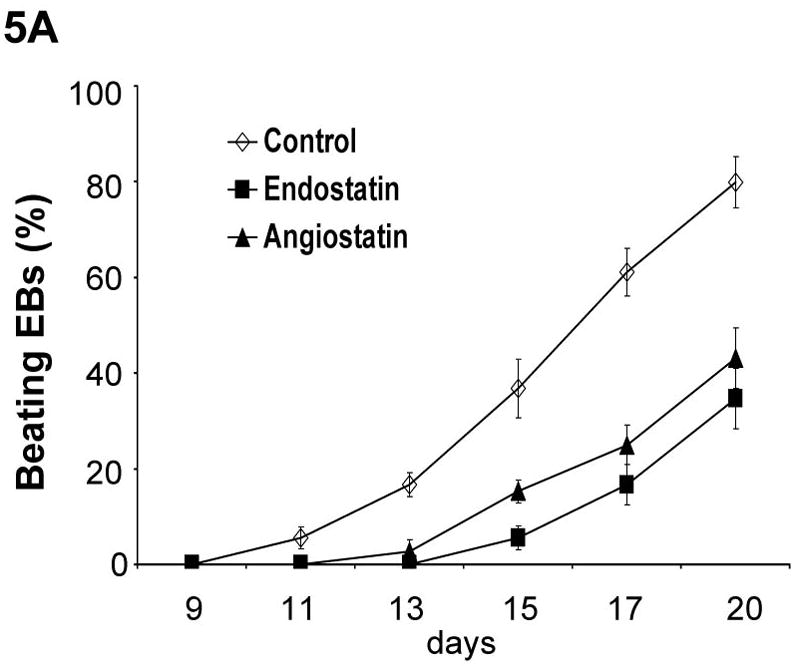
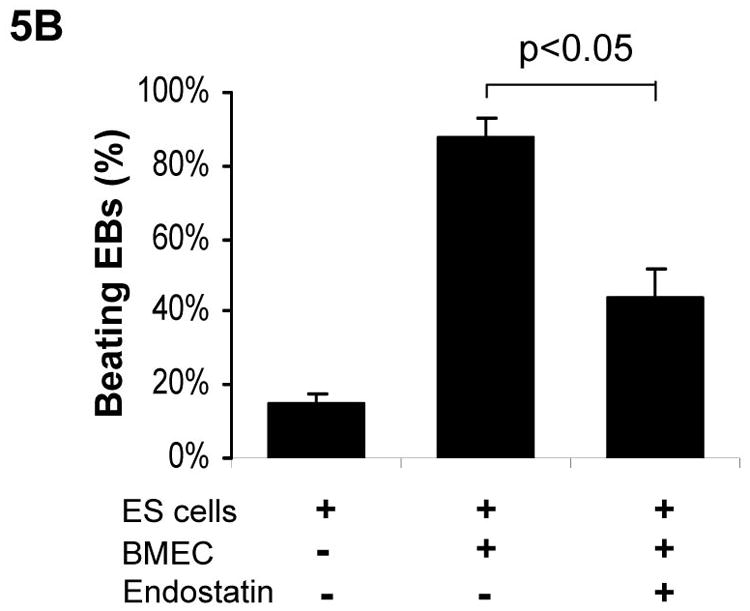
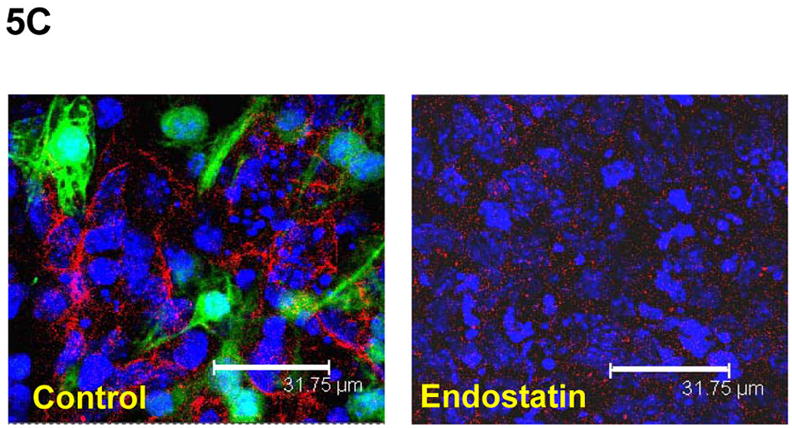
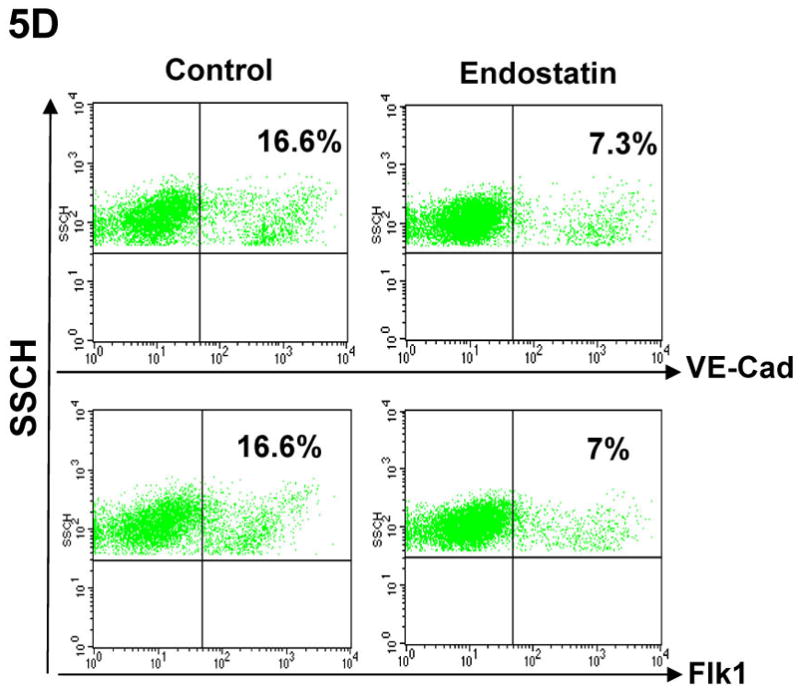
(A) Differentiation of αMHC-GFP ES cells was induced without (control) or with the angiogenic inhibitors, endostatin (2 μg/ml) or angiostatin (1 μg/ml). Percentages of beating EBs were quantified over time as in Figure 1. Data are representative from 3 independent experiments. Error bars represent standard deviation. (B) Cardiomyogenesis of αMHC-GFP ES cells was induced without (control) or with 200 of BMEC cells in the absence (+BMEC) or in the presence of endostatin (+BMEC/+Endostatin). Beating EBs were quantified at day 13 of differentiation. Data are representative from 3 independent experiments. Error bars represent standard deviation (p<0.05). (C) Cardiomyogenesis of αMHC-GFP ES cells was carried out in the absence (control) or presence of endostatin. At day 11 of differentiation, EBs were immunostained for VE-Cad expression with PE-conjugated antibodies (red). The VE-Cad+ endothelial cells and GFP+ cardiomyocytes were analyzed using confocal microscopy. Cell nuclei were stained with Topro-3 (blue). The scale bar = 31.75 μm. (D) Cardiomyogenesis of αMHC-GFP ES cells was induced without (control) or with endostatin. At day 11 of differentiation, VE-Cad+ cells and Flk1+ cells were analyzed by flow cytometry with PE-conjugated antibodies recognizing each antigen.
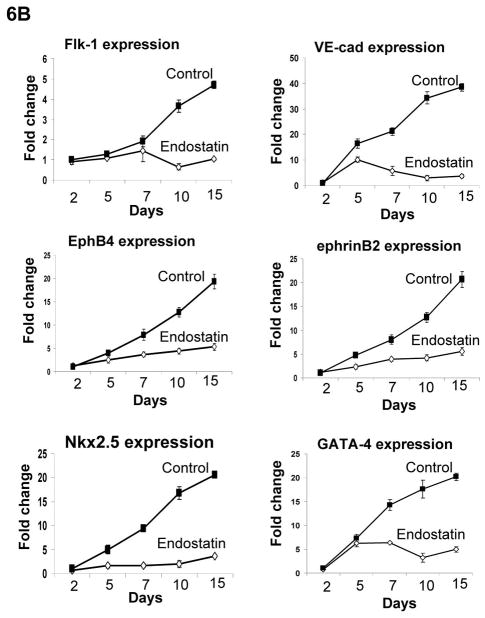
To examine whether angiogenic inhibitors inhibit endothelial cell-mediated differentiation of ES cells, we analyzed cardiomyocytes and endothelial cells by confocal microscopy during differentiation. As showed in Figure 5C, GFP+ cardiomyocytes and VE-Cad+ endothelial cells were decreased in the presence of endostatin during ES cell differentiation. The decreased VE-Cad+ and Flk1+ endothelial cells were confirmed by flow cytometric analysis (Figure 5D). RT-PCR analysis of differentiated ES cells indicated that expression of endothelial cell specific genes (CD31, Flk1, VE-Cad, EphB4, and ephrinB2) and cardiac specific genes (GATA4, Nkx2.5, β-MHC, and α-MHC) in ES cells were decreased by endostatin (Figure 6A). The expression of an endodermal gene (FoxA2) and ectodermal genes (MAP-2, FGF-5, and Nestin) were not affected by endostatin treatment of ES cells (Figure 6A). The inhibition of endothelial and cardiac gene expression by endostatin treatment was confirmed by real-time PCR at different time points of ES cell differentiation (Figure 6B). Expression of Flk1, VE-Cad, EphB4, ephrinB2, Nkx2.5, and GATA4 was initiated as early as day 2, and gradually increased during ES cell differentiation in control ES cells, whereas endostatin treatment blocked the expression of these genes during ES cell differentiation. These data suggest that endostatin inhibits endothelial cell development in ES cells, resulting in restraining cardiomyocyte differentiation.
Figure 6. Expression of endothelial and cardiomyocyte genes during ES cell differentiation.
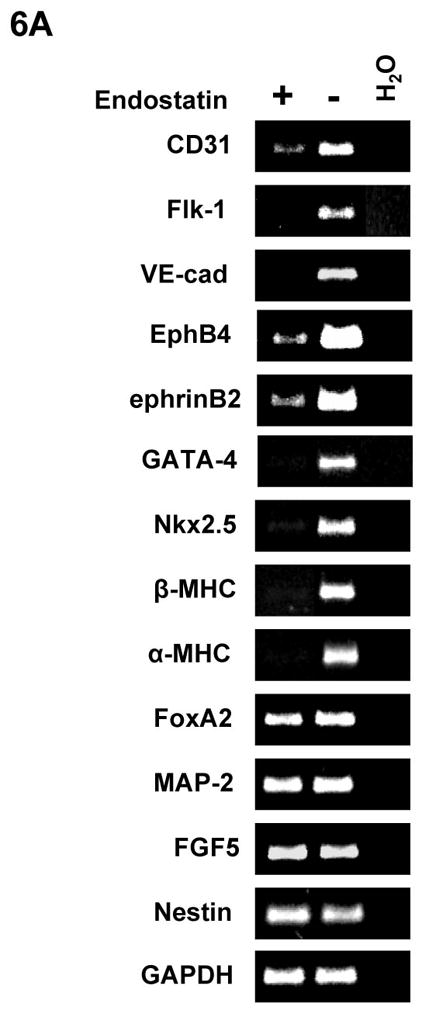
Differentiation of αMHC-GFP ES cells was carried out without (control) or with 2 μg/ml endostatin. (A) At day 10 of ES cell differentiation, RNA samples were harvested from EBs in the presence (+) or absence (−) of endostatin. RT-PCR was used to analyze gene expression. (B) RNA samples were harvested from EBs in the presence (Endostatin) or absence (Control) of endostatin at day 2, 5, 7, 10, and 15 of differentiation. Real-time PCR was performed with the primers listed in supplemental table 2. GAPDH was used as a standard.
EphB4-expressing endothelial cells rescues cardiomyocyte-defect in EphB4−/− ES cells
To further evaluate whether endothelial cells compensate EphB4 signaling to rescue cardiomyocyte differentiation-defects in EphB4−/− ES cells, we cocultured BMEC cells with EphB4−/− ES cells, and induced cardiomyocyte differentiation in hanging-drop EB formation. Immunohistochemistry and RT-PCR analysis confirmed EphB4 and ephrinB2 expression in BMEC and HUVEC (Figure 7A and B). EphB4−/− ES cells lost beating activity without BMEC cells. The addition of BMEC fully restored beating activity (Figure 7C), suggesting that EphB4-expressing endothelial cells provide a microenvironment that directs ES cell differentiation into cardiomyocytes in vitro.
Figure 7. Rescue of cardiomyocyte-defect in EphB4−/− ES cells by human endothelial cells.
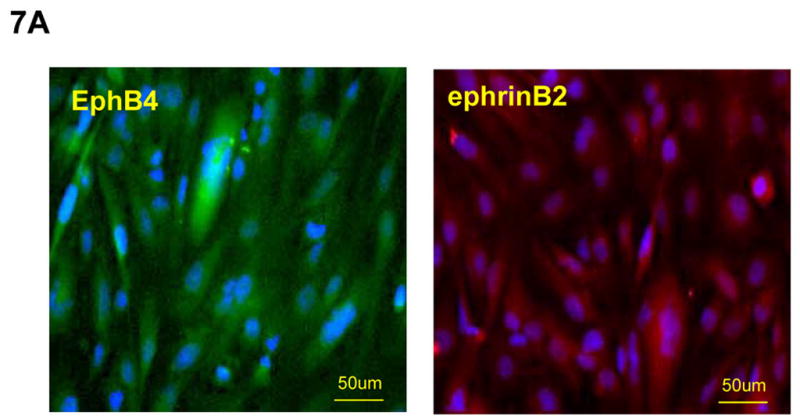
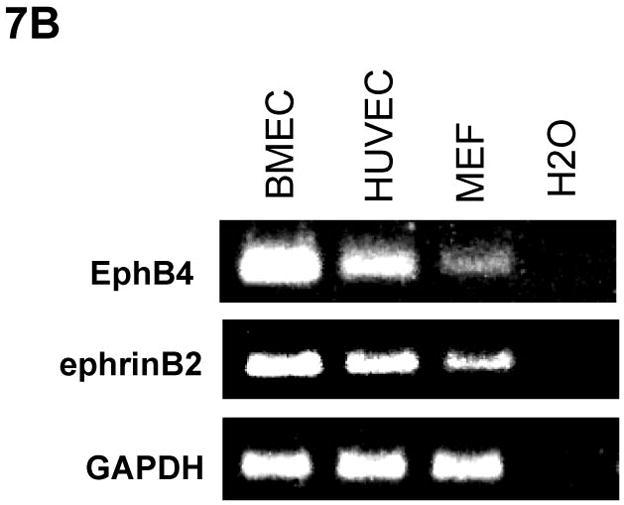
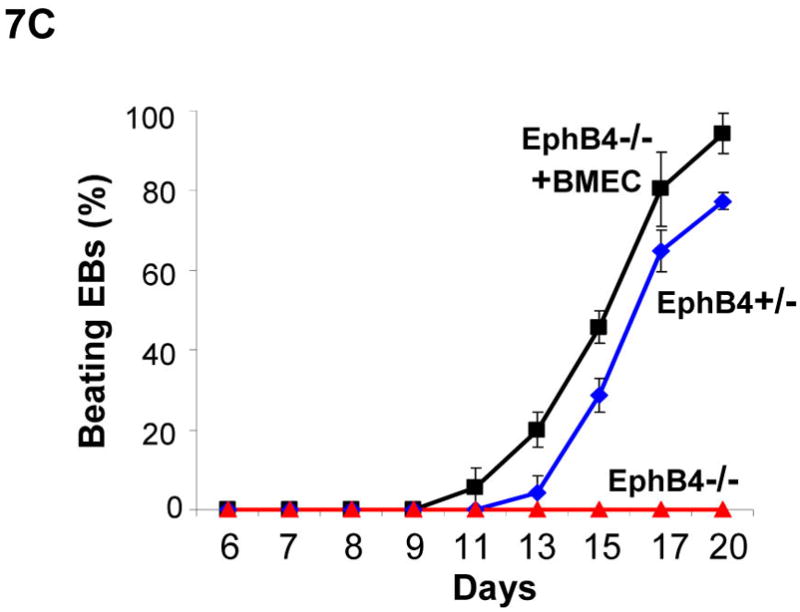
(A) Immunostaining was performed on BMEC cells to localize human EphB4 and ephrinB2 (rabbit polyclonal anti-human antibodies), using FITC-conjugated and PE-conjugated secondary antibodies, respectively. (B) RT-PCR analysis of EphB4 and ephrinB2 expression in endothelial cells compared to MEF. (C) EphB4−/− cells (400 cells) were induced to differentiate with (EphB4−/− and BMEC) or without (EphB4−/−) BMEC cells (200 cells) in hanging-drops. EphB4+/− ES cells were used as a control. The percentiles of beating EBs were quantified over time. Data are representative from 3 independent experiments. Error bars represent standard deviation.
Discussion
Eph receptor tyrosine kinases and their ligands, ephrins, play important roles in a variety of processes during embryonic development, including the targeting behavior of migratory neurons, vascular cell assembly and angiogenesis [Gale and Yancopoulos, 1999]. Fourteen different Eph receptors have been catalogued into EphA or EphB subclasses based on their affinity for ligands. Eight different ephrins have been identified to date. They are membrane proteins of either glycerophosphatidylinsital (GPI)-linked (ephrinA) or transmembrane (ephrinB) [Committee, 1997]. Rather than long range communication, signaling from Eph receptors and their ligands is restricted to sites of direct cell-cell contact and is capable of inducing reciprocal bidirectional events between interacting cells [Bruckner and Klein, 1998]. Essential roles for Eph receptor tyrosine kinases and their ephrin ligands emerged from studies of embryonic development [Himanen et al., 2007], including cardiovascular development [Adams and Klein, 2000; Kuijper et al., 2007]. EphB4 is specifically expressed at the venous endothelium, while the cognate ligand of EphB4, ephrinB2, is specifically and reciprocally expressed on arterial endothelial cells at the earliest stages of vascular development [Wang et al., 1998]. Mice lacking either EphB4 or ephrinB2 display identical defects in angiogenesis by both arteries and veins in the capillary networks of the head and yolk sac [Gerety et al., 1999; Wang et al., 1998]. EphB4-null or ephrinB2-null mice are also evident for heart defects [Gerety et al., 1999]. It is unclear whether the heart defects are due to direct impairment of cardiogenesis or from indirect impairment of angiogenesis. We previously demonstrated that differentiation of ES cells into endothelial cells and cardiomyocytes is impaired in EphB4−/− ES cells [Wang et al., 2004]. In this study, we provided evidences that EphB4 signaling on endothelial cells regulates ES cell differentiation into cardiomyocytes.
Ascorbic acid promotes collagen synthesis of endothelial cells, and enhances cardiomyocyte differentiation of ES cells [Sato et al., 2006; Takahashi et al., 2003; Telang et al., 2007]. We found that ascorbic acid partially restored cardiomyocyte differentiation in EphB4−/− ES cells, suggesting the overlapping function of ascorbic acid and EphB4 signaling pathways. Interestingly, EphB4 is expressed in endothelial cells at regions containing functional cardiomyocytes in beating EBs, but not in cardiomyocytes. Whether ephrinB2 ligand is involved in regulating cardiomyocyte differentiation in ES cells is under investigation.
Vascular endothelial niches play important roles in regulating self-renewal and differentiation of neural stem cells and hematopoietic stem cells in vivo [Li and Li, 2006; Shen et al., 2004; Shen et al., 2008]. Although, cardiac stem or progenitor cells are identified in vivo and in vitro [Beltrami et al., 2003; Laugwitz et al., 2005; Matsuura et al., 2004; Oh et al., 2003; Oyama et al., 2007; Pfister et al., 2005; Wang et al., 2006], it is unclear whether endothelial cells mediate cardiac regeneration from stem cells in the adult heart. In the adult heart, every cardiomyocyte in myocardium is surrounded by the capillary network of endocardial endothelial cells that provides not only oxygen and nutrients, but also local communication between endothelial cells and cardiomyocytes [Hsieh et al., 2006]. Interaction between endothelial cells and cardiomyocytes regulates normal function of the heart, such as cardiac growth, contractile performance, and rhythmicity [Brutsaert, 2003]. Recent studies demonstrate that implantation of endothelial cells from different sources to an infarcted heart improves cardiac function [Badorff et al., 2003; Kocher et al., 2001; Li et al., 2007]. Several mechanisms by which endothelial cells improve cardiac function have been proposed after transplantation: endothelial cells may transdifferentiate into functional cardiomyocytes in vivo [Badorff et al., 2003]; neovascularization by endothelial cells may promote cardiomyocyte survival and cardiac reorganization by direct interaction with endothelial cells and cardiomyocytes [Narmoneva et al., 2004]; and implanted cells may secret angiogenic cytokines to activate endogenous cells that accelerate repair of infarcted myocardium [Yoshioka et al., 2005]. Our study explored the hypothesis that endothelial cells provide signals to direct cardiomyocyte differentiation of ES cells. Directing differentiation of adult cardiac stem cells in vivo may be particularly significant for the treatment of patients with various heart diseases including chronic heart failure. It will be interesting to further investigate whether co-transplantation of ES cell-derived endothelial cells and cardiomyocytes into infarcted hearts can enhance cardiac regeneration in vivo.
Endocardial endothelial cells and cardiomyocytes are both derived from lateral plate mesoderm at about the same time during development, suggesting that myocardial and endocardial lineages have a common precursor [Linask and Lash, 1993]. Reciprocal signaling is evident between the endocardium and myocardium [Dor et al., 2001; Ramsdell and Markwald, 1997]. The interdependence of endothelial and myocardial cells during embryonic heart development is still largely unknown. ES cells not only have enormous potential as a source of therapeutic tissues, but also provide a unique system for studying embryonic development, including cardiovascular lineage commitment. Recent studies in ES cells demonstrate that common cardiovascular progenitors have the potential to differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells in the heart [Kattman et al., 2006; Moretti et al., 2006; Wu et al., 2006]. We found that exogenous human endothelial cells promoted the differentiation of spontaneously contracting cardiomyocytes from ES cells. Enhancement of cardiomyocyte differentiation by endothelial cells occurs in a dose-dependent manner, suggesting that signaling from endothelial cells specifically regulates the kinetics of cardiomyogenesis. Cardiac endothelial cells may regulate myocardial function through paracrine pathways, such as nitric oxide, endothelin-1, prostanoids, adenylpurines, and natriuretic peptides [Shah, 1996]. A recent study showed that paracrine factors of bovine aortic endothelial cells facilitate ES cell differentiation into Nkx2.5+ cells [Kado et al., 2008]. We will investigate whether conditioned medium from human bone marrow endothelial cells enhances generation of beating EBs, or a cell autonomous endothelial cell mechanism facilitates cardiomyocyte differentiation. Nevertheless, our study demonstrated that inhibition of endogenous endothelial cells with endostatin or angiostatin resulted in retardation of cardiogenesis, indicating that cardiogenesis does not occur to its full extent in the absence of endothelial cells.
To understand the mechanism of endothelial cell regulation of cardiomyocyte differentiation from pluripotent stem cells, we investigate the role of EphB4 in regulating cardiomyocyte differentiation. Our study demonstrated that EphB4 was highly expressed in endothelial cells that were underneath beating EBs. Human bone marrow endothelial cells rescued the defect of cardiomyocyte differentiation from pluripotent stem cells in EphB4-null ES cells. These data suggest that EphB4-expressing endothelial cells provide a niche for cardiomyogenesis from ES cells. It is unclear whether endothelial cells promote cardiac differentiation at the level of the pluripotent stem cells, at level of mesodermal development, or at the level of the cardiomyocyte progenitor cells. Further dissecting the role of endothelial cells in cardiomyocyte differentiation will be significant for cardiac regeneration.
Supplementary Material
Acknowledgments
The authors appreciate Dr. Richard T. Lee to generously provide CGR8-GFP mouse ES cell line, and thank Drs. David T. Scadden and Lucy Liaw for the valuable discussions. This study was partially supported by NIH grant K01DK064696 from NIDDK, and P20RR018789 from NCRR. Additional technical core support was provided by MMCRI’s Center of Excellence in Stem and Progenitor Cell Biology core facilities in ES cell and FACS (2P20RR18789) and Center of Excellence in Vascular Biology core facilities in confocal microscopy (2P20RR15555).
Footnotes
Disclosures
None.
Author contribution(s)
KC: Collection and assembly of data, Manuscript writing; HB: Collection and assembly of data; MA: Collection and assembly of data; YG: Collection and assembly of data; TA: Collection and assembly of data; WSW: Data analysis and interpretation; JKY: Data analysis and interpretation; LW: Conception and design; ZZW: Conception and design, Manuscript writing.
References
- Adams RH, Klein R. Eph receptors and ephrin ligands. essential mediators of vascular development. Trends Cardiovasc Med. 2000;10:183–8. doi: 10.1016/s1050-1738(00)00046-3. [DOI] [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–32. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Klein R. Signaling by Eph receptors and their ephrin ligands. Curr Opin Neurobiol. 1998;8:375–82. doi: 10.1016/s0959-4388(98)80064-0. [DOI] [PubMed] [Google Scholar]
- Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- Candal FJ, Rafii S, Parker JT, Ades EW, Ferris B, Nachman RL, Kellar KL. BMEC-1: a human bone marrow microvascular endothelial cell line with primary cell characteristics. Microvasc Res. 1996;52:221–34. doi: 10.1006/mvre.1996.0060. [DOI] [PubMed] [Google Scholar]
- Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–93. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Committee E. Unified Nomenclature for Eph Family Receptors and Their Ligands, the Ephrins. Cell. 1997;90:403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–21. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- Davani S, Deschaseaux F, Chalmers D, Tiberghien P, Kantelip JP. Can stem cells mend a broken heart? Cardiovasc Res. 2005;65:305–16. doi: 10.1016/j.cardiores.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–9. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Dor Y, Camenisch TD, Itin A, Fishman GI, McDonald JA, Carmeliet P, Keshet E. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development. 2001;128:1531–8. doi: 10.1242/dev.128.9.1531. [DOI] [PubMed] [Google Scholar]
- Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–66. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4:403–14. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Saha N, Nikolov DB. Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell Biol. 2007;19:534–42. doi: 10.1016/j.ceb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh PC, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol. 2006;68:51–66. doi: 10.1146/annurev.physiol.68.040104.124629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado M, Lee JK, Hidaka K, Miwa K, Murohara T, Kasai K, Saga S, Morisaki T, Ueda Y, Kodama I. Paracrine factors of vascular endothelial cells facilitate cardiomyocyte differentiation of mouse embryonic stem cells. Biochem Biophys Res Commun. 2008;377:413–8. doi: 10.1016/j.bbrc.2008.09.160. [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–32. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98:216–24. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- Koolpe M, Burgess R, Dail M, Pasquale EB. EphB receptor-binding peptides identified by phage display enable design of an antagonist with ephrin-like affinity. J Biol Chem. 2005;280:17301–11. doi: 10.1074/jbc.M500363200. [DOI] [PubMed] [Google Scholar]
- Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med. 2007;17:145–51. doi: 10.1016/j.tcm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Kuruvilla L, Kartha CC. Molecular mechanisms in endothelial regulation of cardiac function. Mol Cell Biochem. 2003;253:113–23. doi: 10.1023/a:1026061507004. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–56. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Li L. Understanding hematopoietic stem-cell microenvironments. Trends Biochem Sci. 2006;31:589–95. doi: 10.1016/j.tibs.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP, Wu JC. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linask KK, Lash JW. Early heart development: dynamics of endocardial cell sorting suggests a common origin with cardiomyocytes. Dev Dyn. 1993;196:62–9. doi: 10.1002/aja.1001960108. [DOI] [PubMed] [Google Scholar]
- Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev VA, Wobus AM, Rohwedel J, Bader M, Hescheler J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ Res. 1994;75:233–44. doi: 10.1161/01.res.75.2.233. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult Cardiac Sca-1-positive Cells Differentiate into Beating Cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, Bel A, Sarateanu S, Scorsin M, Schwartz K, Bruneval P, Benbunan M, Marolleau JP, Duboc D. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–83. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962–8. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya H, Hidaka K, Shirai M, Terami H, Aburatani H, Morisaki T. Endocardiogenesis in embryoid bodies: novel markers identified by gene expression profiling. Biochem Biophys Res Commun. 2007;357:896–902. doi: 10.1016/j.bbrc.2007.04.030. [DOI] [PubMed] [Google Scholar]
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N, Akazawa H, Uezumi A, Takeda S, Komuro I. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–41. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31−but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- Rafii S, Shapiro F, Rimarachin J, Nachman RL, Ferris B, Weksler B, Moore MA, Asch AS. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood. 1994;84:10–9. [PubMed] [Google Scholar]
- Ramsdell AF, Markwald RR. Induction of endocardial cushion tissue in the avian heart is regulated, in part, by TGFbeta-3-mediated autocrine signaling. Dev Biol. 1997;188:64–74. doi: 10.1006/dbio.1997.8637. [DOI] [PubMed] [Google Scholar]
- Sato H, Takahashi M, Ise H, Yamada A, Hirose S, Tagawa Y, Morimoto H, Izawa A, Ikeda U. Collagen synthesis is required for ascorbic acid-enhanced differentiation of mouse embryonic stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2006;342:107–12. doi: 10.1016/j.bbrc.2006.01.116. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–9. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Shah AM. Paracrine modulation of heart cell function by endothelial cells. Cardiovasc Res. 1996;31:847–67. [PubMed] [Google Scholar]
- Shen Q, Goderie S, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial Cells Stimulate Self-Renewal and Expand Neurogenesis of Neural Stem Cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabruyn SP, Griffioen AW. Molecular pathways of angiogenesis inhibition. Biochem Biophys Res Commun. 2007;355:1–5. doi: 10.1016/j.bbrc.2007.01.123. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, Lee RT. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107:1912–6. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
- Telang S, Clem AL, Eaton JW, Chesney J. Depletion of ascorbic acid restricts angiogenesis and retards tumor growth in a mouse model. Neoplasia. 2007;9:47–56. doi: 10.1593/neo.06664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4 [see comments] Cell. 1998;93:741–53. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J. The role of the sca-1+/CD31− cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–88. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cohen K, Shao Y, Mole P, Dombkowski D, Scadden DT. Ephrin receptor, EphB4, regulates ES cell differentiation of primitive mammalian hemangioblasts, blood, cardiomyocytes, and blood vessels. Blood. 2004;103:100–9. doi: 10.1182/blood-2003-04-1063. [DOI] [PubMed] [Google Scholar]
- Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–50. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Xue T, Cho HC, Akar FG, Tsang S-Y, Jones SP, Marban E, Tomaselli GF, Li RA. Functional Integration of Electrically Active Cardiac Derivatives From Genetically Engineered Human Embryonic Stem Cells With Quiescent Recipient Ventricular Cardiomyocytes: Insights Into the Development of Cell-Based Pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Ageyama N, Shibata H, Yasu T, Misawa Y, Takeuchi K, Matsui K, Yamamoto K, Terao K, Shimada K, Ikeda U, Ozawa K, Hanazono Y. Repair of infarcted myocardium mediated by transplanted bone marrow-derived CD34+ stem cells in a nonhuman primate model. Stem Cells. 2005;23:355–64. doi: 10.1634/stemcells.2004-0200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.