1. Introduction
With over 190,000 new cases of breast cancer each year in the United States [1], there is tremendous interest in discovery of a modifiable risk factor for breast cancer. Observational studies have shown that physical activity is inversely associated with breast cancer etiology via alterations in mechanisms associated with breast cancer risk [2]. However, these mechanisms are not fully elucidated. Proposed mechanisms include increased oxidative stress, circulating estrogen levels, body weight and/or body fat, and fasting insulin, along with insulin resistance and alterations in circulating insulin growth factor (IGF) proteins [3-4]. Each of these variables has been observed to be associated with the development of breast cancer in vitro, in vivo, and/or in observational studies [5-8]. Breast cancer risk has been shown to increase with increasing levels of urinary F2-isoprostanes (a measure of oxidative stress) in recent case-control studies [6-7]. Women with higher levels of urinary F2-isoprostanes were found to have an increase of 80% in breast cancer risk compared with women with isoprostane levels below the median [7]. Likewise, prospective cohort studies have shown that risk of breast cancer was increased in postmenopausal women with levels of estrone, estradiol, and testosterone in the upper quintiles [8]. Epidemiological studies have also indicated that adiposity contributes to the incidence of breast cancer [8] and increased levels of serum IGF-1, which has been associated with increased risk of breast cancer, especially in premenopausal women [5].
The Women In Steady Exercise Research (WISER) study was a randomized controlled trial designed to test the hypothesis that regular aerobic exercise among young healthy women will result in positive physiologic changes associated with reduction of risk of breast cancer. Previous studies examining the effects of exercise on markers associated with breast cancer risk have been conducted in postmenopausal women [9-10] and breast cancer survivors [11]. These studies tested exercise interventions that lasted longer than the present study (12 months vs 16 weeks) but at lower intensity (40 to 75% vs 60-85% in the present study). Based on pilot data, our rationale was that 16 weeks would be the minimum length of weight bearing aerobic exercise to improve markers associated with breast cancer risk in a young population. To our knowledge, only one previous randomized controlled trial has examined the effects of aerobic exercise on markers of breast cancer risk (i.e., estrogen metabolites) in young women [12]. The innovation of the WISER study was to concurrently examine multiple biomarkers for risk of breast cancer, including oxidative stress, IGF-I axis proteins, insulin, estrogens, exercise related menstrual changes (such as shortened luteal phase and anovulation), and body composition in a study adequately powered to assess the relationships among these variables. Because the physiologic changes that initiate cancer may take place earlier in life, even if the disease does not become evident until after menopause, it is important to examine these changes among young women. This paper describes the design and methodology of the WISER study. The WISER study was one of three studies that formed the core of the University of Minnesota Transdisciplinary Research on Energetics and Cancer (TREC) Center, funded by the National Cancer Institute. Enrollment and participation in the WISER study are currently completed. Data analysis and publication of the results will take place in 2010.
2. Specific Aims
The overall aim of the WISER study was to explore the physiologic mechanisms by which exercise may alter breast cancer risk in young women. This was the first adequately powered clinical trial designed to concomitantly examine the effects of exercise on several factors related to breast cancer risk. The primary aim of the trial was to assess the changes in plasma levels of a stable marker of oxidative stress, F2-isoprostanes, resultant to approximately 16 weeks of exercise training compared to sedentary controls. We hypothesized that exercise would decrease the levels of F2-isoprostanes compared to levels in sedentary women. Additional adequately powered aims were:
To assess changes in other metabolic factors and explore potential relationships among factors that may explain the purported association between physical activity and breast cancer. We hypothesized that exercise would: increase the levels of sex-hormone binding globulin (SHBG), decrease the levels of blood hormones and urinary estrogen metabolites, improve insulin and glucose levels as well as insulin resistance, decrease IGF-1 levels, increase the levels of IGF binding proteins, increase lean body mass, and decrease fat mass.
To estimate incidence of, and to assess demographic and physiological predictors for, shortened luteal phase length and anovulatory menstrual cycles (markers for menstrual disturbances) among women aged 18-30 resultant to 16 weeks of exercise training compared to sedentary controls.
3. Research Design
Overview
The WISER study was a randomized controlled parallel-arm trial to compare an aerobic exercise intervention group and a sedentary control group in 319 healthy, sedentary, stable-weight, eumenorrheic women (18-30 years old). All outcome measurements were obtained between days 6 and 10 of the menstrual cycle at baseline and follow-up (4 menstrual cycles later). Participants were recruited over a period of three and a half years, with initial screening by telephone and at an orientation session. Eligible participants were randomly assigned to the exercise intervention (5 times a week for 4 menstrual cycles) or the sedentary control group.
3.1. Subject Recruitment
Recruitment for the study was conducted from May 2006 to April 2009 and aimed to reach young women living in the ten-county Minneapolis-St. Paul metropolitan area. The primary recruitment method was an email sent to female state university students and staff in the age range of 18-30 years. Recruitment expanded from year 2 forward to include an email sent to county employees from the two major cities, letters sent to childcare workers, fliers posted at area universities, colleges and community colleges, and ads in a local newspaper and a free weekly variety paper. Potential participants were directed to a website for online prescreening.
3.2. Subject Screening and Selection
Screening was done via a telephone interview to identify healthy women who were aged between 18 and 30 years, non-smokers, with a body mass index (BMI) ranging from 18-40 kg/m2 (inclusive). Additional inclusion criteria were designed to balance goals of feasibility, safety, and recruitment of a study sample who would be relatively homogeneous as to ovarian hormones: sedentary, defined as not currently participating in any habitual exercise training of moderate to vigorous intensity for two or less sessions weekly; self-reported menstrual cycle length of 24 to 35 days over the two months prior to entering the study; intact ovaries and uterus; no history of gynecologic problems such as fibroids, endometriosis, or polycystic ovary syndrome; no hormonal contraception use (for the past three months for oral, patch, and vaginal ring methods and past 12 months for IUDs with hormones and depo-provera); no medical conditions or medications that would prohibit participation in a vigorous program of weight bearing exercise or would negatively impact our ability to test our hypotheses; controlled hypertension; no history of cancer within the past five years, excepting non-melanoma skin cancers; not currently or recently (past six months) pregnant; not planning to become pregnant during the study period; alcohol consumption equal to or less than seven servings per week; not planning to move away from the Twin Cities area during the period of the study; stable weight (no changes greater than 10% over past year); and not currently trying to lose weight (by dieting, exercise or other means).
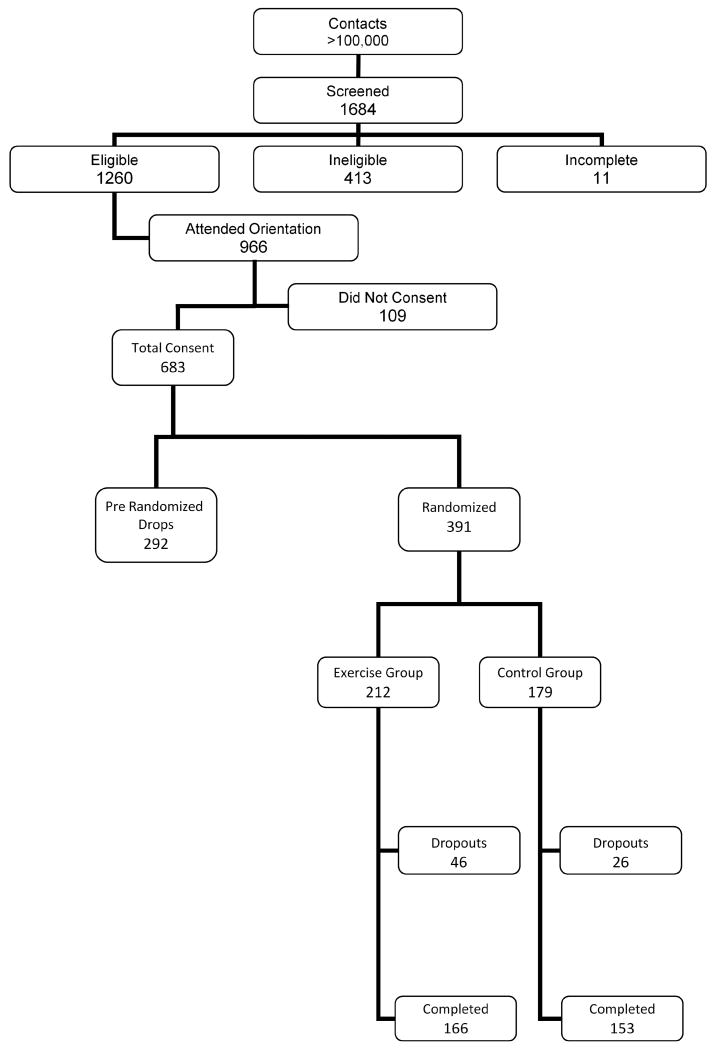
Full screening was completed with 1684 women, 1260 were found eligible, 683 were consented and 391 enrolled in WISER, out of which 319 successfully completed the study (Figure 1).
Figure 1.
Flowchart of recruitment numbers
The WISER study screened 1684 women over three and a half years, among which 1260 were eligible and 683 were consented. A total of 391 were randomized to the exercise or control group and 72 of these women dropped out of the study. Among the women who completed the study, 166 were in the exercise group and 153 in the control group.
Women who met the initial criteria via the telephone interview were invited to attend a two-hour orientation session in which the study staff explained the study procedures in detail. Interested participants had the opportunity to sign up for the study at the orientation sessions, which were held twice a month. Upon signing the consent form, women were given a home ovulation predictor kit, and the first clinic visit was scheduled upon a positive ovulation test. Failure to respond to the ovulation predictor kit constituted an exclusion criterion for the study, as our goal was to recruit young women with normal, ovulatory menstrual cycles. The test registered the luteinizing hormone (LH) surge, thereby predicting ovulation.
Table 1 shows selected characteristics of women who completed the trial compared to those who did not complete baseline measurements (were not randomized) or dropped out after randomization. The completers were statistically significantly (but not meaningfully) older than non-completers (less than 1 year age difference in means) and had a higher degree of education. We also compared randomized participants who dropped out of the study (n = 72) with completers (n = 319) and found a significant difference in level of education between the two groups (Table 2).
Table 1. Selected Characteristics of Participants who completed the study compared to those lost to follow upa.
| Non-completersb N = 364 |
Completers N = 319 |
p-valuec | |
|---|---|---|---|
| Age (years) | 24.1 ± 0.17 | 24.9 ± 0.19 | 0.003 |
| Race | |||
| Asian | 57 (15.7%) | 46 (14.5%) | 0.80 |
| Black or African American | 35 (9.6%) | 26 (8.1%) | |
| American Indian or Alaskan Native | 3 (0.8%) | 1 (0.3%) | |
| White | 252 (69.2%) | 232 (72.7%) | |
| Multi-race | 17 (4.7%) | 14 (4.4%) | |
| Ethnicity | |||
| Hispanic | 345 (94.8%) | 304 (95.3%) | 0.75 |
| Non-Hispanic | 19 (5.2%) | 15 (4.7%) | |
| Education | |||
| Less than high school | 1 (0.3%) | 1 (0.3%) | 0.002 |
| High school | 10 (2.7%) | 12 (3.8%) | |
| Vocational training | 7 (1.9%) | 5 (1.6%) | |
| Some college | 150 (41%) | 86 (27.1%) | |
| College degree | 129 (35.3%) | 118 (37.2%) | |
| Graduate or professional degree | 68 (18.6%) | 95 (30%) | |
| Marital Status | |||
| Never married | 301 (82.6%) | 262 (82.1%) | 0.92 |
| Married | 52 (14.3%) | 47 (14.7%) | |
| Separated | 2 (0.6) | 1 (0.3%) | |
| Divorced | 6 (1.7%) | 5 (1.6%) | |
| Domestic partnered | 3 (0.8%) | 4 (1.3%) | |
| Previous Use of Hormonal Contraceptives | |||
| No | 347 (95.3%) | 299 (93.7%) | 0.36 |
| Yes | 17 (4.7%) | 20 (6.3%) | |
| Parity | |||
| Nulliparous | 332 (91.2%) | 298 (93.4%) | 0.28 |
| Parous | 32 (8.8%) | 21 (6.6%) | |
Continuous variables are expressed as means ± SE and categorical variables are expressed as frequency (%).
Non-completers includes women who consented to the study but were not randomized because of failure to complete baseline measurements (n = 292) and women who were randomized but dropped out after randomization (n = 72).
p-values are based on Student t-test for continuous variables and χ2 test for categorical variables.
Table 2. Baseline Characteristics of Randomized Participants (n = 391) by Treatment Groupa.
| Completers | Dropouts | |||
|---|---|---|---|---|
| Controlb n = 153 |
Exercisec n = 166 |
Control n = 26 |
Exercise n = 46 |
|
| Age (years) | 25.2 ± 0.3 | 25.4 ± 0.3 | 25.0 ± 0.6 | 24.5 ± 0.5 |
| Height (cm) | 165.4 ± 0.6 | 164.9 ± 0.5 | 166.0 ± 1.1 | 164.4 ± 1.0 |
| Weight (kg) | 67.6 ± 1.2 | 67.4 ± 1.1 | 73.1 ± 3.4 | 67.5 ± 2.4 |
| BMI (kg/m2) | 24.7 ± 0.4 | 24.7 ± 0.4 | 26.4 ± 1.1 | 24.8 ± 0.7 |
| Race | ||||
| Asian | 26 (17%) | 20 (12.1%) | 1 (3.8%) | 9 (19.6%) |
| Black/African American | 12 (7.8%) | 13 (7.8%) | 4 (15.4%) | 6 (13%) |
| American Indian | 0 (0) | 1 (0.6%) | 0 (0) | 0 (0) |
| White | 107 (70%) | 124 (74.7%) | 20 (76.9%) | 28 (60.9%) |
| Multi-race | 8 (5.2%) | 8 (4.8%) | 1 (3.9%) | 3 (6.5%) |
| Ethnicity | ||||
| Non-Hispanic | 147 (96.1%) | 158 (95.2%) | 23 (88.5%) | 42 (91.3%) |
| Hispanic | 6 (3.9%) | 8 (4.8%) | 3 (11.5%) | 4 (8.7%) |
| Education | ||||
| Less than high school | 1 (0.6%) | 0 (0) | 0 (0) | 0 (0) |
| High school | 4 (2.6%) | 8 (4.8%) | 1 (3.8%) | 2 (4.3%) |
| Vocational training | 2 (1.3%) | 3 (1.8%) | 2 (7.7%) | 0 (0) |
| Some college | 44 (28.8%) | 43 (26%) | 9 (34.6%) | 21 (45.7%) |
| College degree | 63 (41.2%) | 56 (33.7%) | 9 (37%) | 16 (34.8%) |
| Grad/professional degree | 39 (25.5%) | 56 (33.7%) | 5 (19.2%) | 7 (15.2%) |
| Marital Status | ||||
| Never married | 124 (81%) | 138 (83.1%) | 22 (84.6%) | 36 (78.3%) |
| Married | 25 (16.3%) | 22 (13.2%) | 4 (15.4%) | 10 (21.7%) |
| Separated | 0 (0) | 1 (0.7%) | 0 (0%) | 0 (0%) |
| Divorced | 3 (2%) | 2 (1.2%) | 0 (0%) | 0 (0%) |
| Domestic partnered | 1 (0.6%) | 3 (1.8%) | 0 (0%) | 0 (0%) |
| Previous Use of Contraceptives | ||||
| No | 71 (46.4%) | 82 (49.4%) | 10 (38.5%) | 20 (43.5%) |
| Yes | 82 (53.6%) | 84 (50.6%) | 16 (61.5%) | 26 (56.5%) |
| Parity | ||||
| Nulliparous | 144 (94.1%) | 154 (92.8%) | 24 (92.3%) | 43 (93.5%) |
| Parous | 9 (5.9%) | 12 (7.2%) | 2 (7.7%) | 3 (6.5%) |
Continuous variables are expressed as means ± SE and categorical variables are expressed as frequencies (%).
p-values for comparisons between controls (completers × dropouts): 0.62 (height), 0.13 (weight), 0.78 (age), 0.14 (BMI), 0.24 (race), 0.39 (education), 0.10 (ethnicity), 0.72 (previous use of contraceptives) 0.87 (marital status), 0.77 (parity).
p-values for comparisons between exercisers (completers × dropouts): 0.70 (height), 0.99 (weight), 0.14 (age), 0.91 (BMI), 0.38 (race), 0.05 (education), 0.31 (ethnicity), 0.48 (previous use of contraceptives) 0.48 (marital status), 0.87 (parity).
This study was approved by the University of Minnesota Human Subjects Review Committee (Institutional Review Board; IRB ID# 0505M69867). Written informed consent was obtained from all participants at the orientation sessions prior to beginning any study activities.
3.3. Randomization
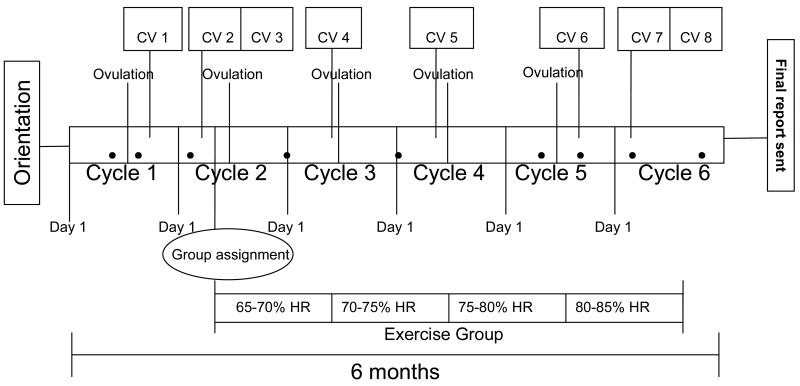
Randomization occurred after completion of all baseline measurements and was balanced by baseline BMI and age in order to assure that these potentially important confounding variables were balanced across treatment and control participants. BMI was divided by tertiles based on the 50th and 75th percentiles from NHANES I data (≤ 22.8, 22.8-26.3, ≥ 26.3) and age dichotomized (18-24 versus 25-30) to form six cells. Participants were randomized within cell group to treatment or control arm, thus ensuring a balance of BMI and age characteristics. Initial randomization was done at 1:1 ratio but was modified to 60:40 (exercise:control) in August 2007, due to the higher drop-out rate observed in the exercise group and concerns about ensuring adequate sample size in the treatment group to test study hypotheses (Table 2). Figure 2 shows the flow of activities for participants in the WISER study, including all clinic visits, ovulation tests, and exercise sessions for those randomized to the exercise treatment group.
Figure 2.
Flow Diagram of Participation.
Orientation: Participants are asked to attend a 2-hour orientation session to learn details about the study and to sign the consent forms. Upon signing of the consent form, they are given an ovulation kit to test at home.
Ovulation: Participants are provided with ovulation kits to use during five months of participation in the study.
CV: clinic visit. CV1 and CV6: These clinic visits occur during the luteal phase eight days after participants receive a positive ovulation test during menstrual cycles 1 and 5, respectively. CV2 and CV7: These clinic visits occur during the follicular phase on day eight of menstrual cycles 2 and 6, respectively. CV3 and CV8: Participants fill out food records and collect their urine on days 7, 8, and 9 of cycles 2 and 6. Clinic visits 3 and 8 refer to urine processing at the clinic.
Mentorship contact: The 9 filled circles shown in the diagram represent a point of contact between participants and staff with the purpose of monitoring participant progression in the study.
Group assignment: Randomization into groups occurs after all baseline measurements are taken during a baseline fitness assessment with trainers around day 11 of cycle 2. The follow-up fitness assessment occurs around day 11 of cycle 6.
Exercise group: Participants randomized into the exercise group are asked to exercise 5 times per week for about 16 weeks. The intensity of the activity increases every 4 weeks by 5% of the age predicted maximum heart rate.
Final report: Upon completing of the study, participants receive a packet in the mail with study results.
3.4. Exercise treatment
The exercise training sessions took place at various fitness facilities in the Twin Cities area based on the proximity to participants' homes. Each exercise session was approximately 45 minutes in length: 30 minutes of weight bearing aerobic exercise at a specified intensity based on age-predicted maximal heart rate (max HR), and five minutes each for the warm-up, cool-down, and stretching. Participants were asked to attend five exercise sessions per week, wear a Polar Heart Rate monitor (Polar Electro Inc., Lake Success, NY) and keep an exercise log. Exercise intensity was set at 65-70% of age predicted max HR for the first four weeks of exercise (max HR=220-age), 70-75% of max HR for weeks 5-8, 75-80% of max HR for weeks 9-12, and 80-85% of max HR for the final stage, which lasted until they completed the study (day 5 of the 6th menstrual cycle). The final stage ranged from two to four weeks depending on participants menstrual cycle length. Participants randomized into the exercise group worked with a certified personal trainer for the intervention as well as for ongoing support and monitoring of their adherence to the protocol. At the first several exercise sessions, the trainer instructed participants in the proper use of the heart rate monitors, as well as warm-up, cool-down, stretches, and aerobic exercise equipment safety, and once a week thereafter to provide ongoing support and monitoring. Further, instruction was provided regarding completing the exercise logs, which involved recording exercise date, time of day, and duration, average heart rate from the polar heart rate monitor, equipment used for each exercise session, and any additional comments. If participants traveled for work or vacation, they worked with the trainer to create an exercise plan for during their trip. If illness or injury prevented exercise for over a week, participation in the study was extended to meet protocol requirements of at least 2 weeks of exercise at each heart rate range. Exercise logs were reviewed by study staff weekly, and participants who missed exercise sessions were contacted to determine reason for missed sessions and to encourage compliance with the study protocol. Because the aim of the study was to explore effects of exercise and not to show feasibility of an exercise program, participants were informed that if more than 15 sessions were missed over the study period, they would be excluded from the study. In addition, approximately 50% of the participants in the exercise group were provided heart rate monitors that recorded a single exercise session. Halfway through the study, the research team switched to using an upgraded heart rate monitor that recorded up to 12 workouts to facilitate monitoring, and allow trainers to assess adherence more rigorously. On weeks when exercise intensity increased, trainers provided guidance regarding the increased heart rate range and monitored for any adverse events with the increased exercise intensity.
3.5. Non-exercise control treatment
Participants randomized into the control group were asked to maintain their usual level of physical activity and to not engage in any new exercise program during study participation.
4. Data Collection
4.1. Biological Samples
All women provided blood samples four times during the study: twice at baseline and twice at follow-up. The blood draws occurred in the morning between 6:45–11:00 am and at specific days of the women's menstrual cycles, once during the luteal phase (6-10 days after ovulation) and once during the follicular phase (6-10 days after start of flow) of cycles 1 and 2 (baseline), as well as cycles 5 and 6 (follow-up). Serum and plasma were obtained from whole blood by centrifugation for 15 minutes (4°C at 1000 × g) and stored in aliquots of 1-1.5 ml at -70°C.
Forty-eight hours prior to the start of urine collections, participants were asked to not engage in any moderate or higher intensity exercise, or to drink alcohol. Participants collected all of their urine for three consecutive 24-hour periods on days 7, 8, and 9 of menstrual cycles 2 (baseline) and 6 (follow-up). Participants were given an insulated bag with ice packs to carry a one-liter urine collection container with them throughout the day and keep the container cold. Once they returned home, they were instructed to transfer the day's urine into a three-liter bottle with ascorbic acid preservative, wash and dry the one liter bottle, and repeat the process for the remaining 48 hours. Three-liter bottles were kept in home refrigerators or coolers provided by the study until completion of the urine collection, at which point a staff person picked up the bottles and brought them to the clinic. The participant's collection bottles were kept cold and 0.1% sodium azide was added before separating into aliquots. Urine samples for each participant were pooled and the total volume was recorded followed by analysis of urinary creatinine. Aliquots of untreated and sodium azide-treated urine were then stored at -20°C.
4.2. Anthropometric Measurements
Body weight was assessed four times during the study to the nearest 0.1 kg, using an electronic scale (Scale Tronix, White Plains, NY); in addition to the baseline and follow-up measurements, body weight was assessed at two interim time points (during menstrual cycles 3 and 4) to ensure there were no large energy balance changes that went unnoticed in either group (see Figure 3). Height was measured at baseline without shoes to the nearest 0.1 cm (Scale Tronix, White Plains, NY). Body mass index (BMI) was calculated as the weight in kg divided by height in meters squared (kg/m2).
4.3. Menstrual Logs
Participants were asked to keep a menstrual log to record characteristics of all menstrual cycles during study participation. The menstrual characteristics log asked participants to record the date and approximate time (morning, afternoon, evening, or while sleeping) when menstrual flow began and ended, any adverse symptoms, and a qualitative statement regarding whether the flow was the same as usual. To assess the timing and occurrence of the luteal surge, participants were given four 9-day ovulation kits (Assure LH™, Conception Technologies, San Diego, CA) for each menstrual cycle during study participation. The Assure LH™ kit measures the luteal surge by Enzyme-Linked Immunosorbent Assays (ELISA) with 96% accuracy with home use. Luteal surge has been observed to coincide within one day of ovulation [13]. Participants recorded on the menstrual cycle log the date on which the ovulation test kit indicated the luteal surge. For menstrual cycles 1 and 5, the luteal surge indicated by the ovulation test kit was the basis for scheduling blood draws during the luteal phase. The luteal phase length was determined by subtracting the date the next menstrual cycle started from the date after which the luteal surge was detected by the ovulation kit.
4.4. Physical Activity Assessment and Fitness Assessment
Physical activity prior and during study participation was assessed via a modified version of the Modifiable Activity Questionnaire described elsewhere [14]. The choice to use this instrument was based, in part, on the desire to have a physical activity outcome in common with another TREC project that was also examining effects of an exercise intervention on similar cancer biomarkers, but in post-menopausal women (the NEW study at the Fred Hutchinson Cancer Research Center, C. Ulrich and A. McTiernan, Co-Project Leaders). The interviewer-administered questionnaire asked about physical activity over the past year at baseline and over five months at follow-up, in order to capture changes that may have occurred across seasons. Physical activity was measured in three domains: leisure-time, occupational, and sedentary. All the information collected in this questionnaire was transformed into metabolic equivalents per hour per week (METS-h/week) using commonly accepted MET values for each activity [15]. This questionnaire is reliable with rank order correlations ranging from 0.62 to 0.92 for leisure and occupational activity. Validity of the leisure activity section of the questionnaire was demonstrated through comparisons with the Caltrac activity monitor counts per hour (rho = 0.62, p< 0.05) [14].
Participants' fitness level was assessed using a sub-maximal treadmill test at baseline (between days 8-12 of menstrual cycle 2) and after the intervention period (between days 8-12 of menstrual cycle 6). The reasons why a sub-maximal test was used to assess fitness in this study were: the exercise protocol used was not designed to increase VO2 max, fitness was not a primary outcome but rather a measurement of adherence, participant burden, and cost. The modified Bruce protocol sub-maximal fitness assessment [16] was performed in the first sixty-nine participants. However, it was noticed that participants were reaching their age-predicted maximum heart rate (max HR), defined as 220 – age, very quickly, which led to a modification of the protocol. The protocol used for the remainder of the study involved a five minute warm-up, followed by a treadmill walk at a steady speed (3.5 miles per hour), and the grade on the treadmill was increased by 2-percent every two minutes until the participants reached 80 percent of max HR. If a participant was within ten heart beats of her predicted max HR, treadmill grade was increased by 1-percent every two minutes until 80 percent max HR was reached. Heart rate during this test was measured using Polar Heart Rate monitors (Polar Electro Inc., Woodbury, NY). This workload was then converted into Metabolic Equivalents (MET) using a standard conversion formula [17]. Because the decision to change the fitness assessment protocol during the study may cause potential problems when interpreting results, a subset of the participants (n=19) was assessed using both protocols for comparison. No statistically significant or meaningful differences were found between the two (data not shown). All participants were tested with the same protocol pre- and post-intervention.
4.5. Body Composition Assessment
Body composition was measured by dual energy x-ray absorptiometry (DXA) in the total body scanning mode with a Lunar Prodigy DXA apparatus (Lunar Radiation Corp., Madison, WI) at the General Clinical Research Center. Jensen et al found that duplicate scans showed a difference of 0.6% ± 0.5% body fat using DXA [18]. Body fat measurement with DXA has been shown to correlate with underwater weighing, total body potassium counting, and total body water methods of measuring body composition with correlations of 0.7 to 0.8 [19].
4.6. Dietary Intake Assessment
Dietary intake was measured in this study in order to be sure that any observed intervention effects on the primary outcomes of interest were not the result of concomitant dietary intake changes. As a result, we chose to assess food and nutrient intake through 3-day dietary records completed concomitantly with the urine collections described previously, i.e. days 7, 8 and 9 of menstrual cycles 2 (baseline) and 6 (follow-up). The purpose of obtaining 3-day dietary records was to allow us to accomplish two goals: first, to have the best possible dietary data for controlling for any confounding effect of dietary changes, and additionally to enable us to help participants with advice on diet adjustments if weight changes at interim measurement time points indicated that the participant had altered her diet.
In addition to the dietary records, participants completed a diet history questionnaire (DHQ) once during the study (baseline). Validation studies indicate that the DHQ out-performs the Block and the Willett questionnaires in women [20]. Participants were able to complete the DHQ at their convenience, provided they had Internet access, otherwise study staff provided access to the DHQ at a scheduled time. All the results were stored in a server and were retrieved by logging into a designated website.
4.7. Safety Monitoring
This randomized controlled trial aimed to test the hypothesis that regular aerobic exercise among young healthy women will result in positive physiologic changes associated with reduction in risk of hormonally related cancers. The intervention and measurement protocols posed minimal risk to participants. In fact, the exercise prescription for the study was taken specifically from public health recommendations regarding the amount of physical activity associated with improving and maintaining health and preventing chronic disease [21]. There were also few risks associated with the measurements. Because of this low risk status, the data safety monitoring (DSM) plan for this trial focused on close monitoring by the principal investigator (PI), along with prompt reporting of excessive adverse events and any serious adverse events to the National Institutes of Health and to the Institutional Review Board at the University of Minnesota. No serious adverse events associated with the exercise intervention were reported by participants during the study.
4.8. Adherence
All participants randomized to the treatment group were asked to keep a workout log to record the date, time and location of workout, type of equipment used, average heart rate obtained from a heart rate monitor, duration of workout and stretching, and any comments regarding the workout. Participants were instructed to leave their workout sheets at the gym for weekly checks by the research staff.
Adherence was measured in four different ways, including: 1) frequency of workouts (5 times per week), 2) session duration (30 minutes per exercise session), 3) mode of exercise (always weight-bearing, but could be stair-steppers, elliptical machines, treadmills, or other weight-bearing exercise) and 4) % of session duration within prescribed exercise intensity (% of age predicted heart rate, which varied by month of participation in the study).
4.9. Participant Tracking System
The study protocol was relatively complex for participants to follow. To make sure participants scheduled the clinic visits at the right moment and were compliant with respect to the data collection as well as to offer support throughout the study, study staff created the “buddy system” to track participants' across all participating menstrual cycles. Through this system, each participant was assigned a staff member who was in charge of contacting participants regarding upcoming clinic visits, and to remind them to use the ovulation kit or to turn in a survey. The buddy system was a key component of the study as it contributed to participant compliance to intervention as well as measurement of study endpoints and to minimizing deviations to the protocol. In addition, weekly staff meetings occurred in which the status of each participant was reviewed.
5. Laboratory Analyses
5.1. F2-isoprostanes
Free F2-isoprostanes were measured in plasma by a gas chromatography-mass spectrometry (GC-MS)-based method [22] at the Molecular Epidemiology and Biomarker Research Laboratory (MEBRL), University of Minnesota, Minneapolis, Minnesota. The method utilized an internal standard, [2H4]8-iso-PGF2alpha (>98% pure from Cayman Chemical), wherein the deuterium atoms were located at the non-exchangeable positions 3 and 4 of the molecule. The internal standard was added directly to the plasma samples before the initiation of analysis and therefore accounted for the recovery of F2-isoprostanes. The assay provides a quantitative measurement of plasma F2-isoprostanes; specificity of the assay for F2-isoprostanes has been demonstrated previously [22]. Overall analytical variation of the method was 10% for each of three control pools which had a range of concentrations. Long-term studies at the MEBRL have shown that levels of plasma F2-isoprostanes are stable for >5 years with storage at −70°C.
5.2. Blood hormones and estrogen metabolites
Blood testosterone, progesterone and estrogens were measured in serum using commercial radioimmunoassay kits (DSL, Inc. Webster, TX). Sex-hormone binding globulin (SHBG) was measured by enzyme-linked immunosorbent assay (IBL, Minneapolis, MN). Urinary estrogen metabolites were analyzed by LC/MS-MS performed on a Thermo Electron Quantum Discovery Max Triple Quadrupole LC-MS/MS Instrument [23-24]. Quantitative analysis was performed using Thermo Electron Xcalibur proprietary software.
The 12 urinary estrogens analyzed included the primary estrogens and their metabolites: E1, E2, E3, 2-OHE1, 2-OHE2, 4-OHE1, 4-OHE2, 2-MeE1, 2-MeE2, 4-MeE1, 4-MeE2, and 16OHE1. Each participant's baseline and follow-up samples were run in the same batch. A quality control urine sample was included in duplicate in each assay.
5.3. IGF axis variables, Insulin, Glucose, HOMA
Fasting blood glucose and plasma insulin levels were measured at the Fairview University Diagnostic Laboratories (Minneapolis, MN). The glucose levels were assessed using colorimetric reflectance spectrophotometry. Insulin levels were assessed by chemiluminescent immunoassay (Immulite, Diagnostic Products Corporation, Los Angeles, CA). The measure of insulin resistance was determined by the homeostatic model assessment (HOMA) index. The HOMA index was calculated by multiplying fasting plasma insulin (mmols/L) by fasting glucose (mmols/L) and then dividing by 22.5. HOMA correlates well (r = -0.83) with insulin sensitivity as measured by the gold standard euglycemic clamp [25].
For assessment of IGF-axis variables, plasma samples were stored at −70°C, and then sent in batches to the laboratory of Dr. Michael Pollack at McGill University in Montreal, Canada. Random samples (5% of total participant pool) were sent in duplicate to test for variation. The lab was blinded to which samples were sent in duplicate. Enzyme-Linked Immunosorbent Assays (ELISA) of IGF-I and IGFBP-3 were performed. Samples were run with two standard controls included in the kit for each analyte. Reagents were purchased from Diagnostic Systems Laboratory (Webster, TX).
6. Power Calculations and Statistical Analyses
The primary outcome of the WISER study is change in plasma F2-isoprostanes from baseline to post-treatment follow-up. For power calculations, preliminary estimates of variances and correlations came from pilot studies conducted by the WISER Study investigators.
Table 3 presents estimates of minimum differences that the study had 80% power to detect with a two-sided two-sample t-test at the 0.05 level. Participants' baseline characteristics and demographic data will be tested using Student t-tests for continuous variables and χ2 test for categorical variables. Statistical analysis will use adjustment for baseline covariates and a longitudinal model with random subject effects. Further, as the study is built on a theoretical model of interplay between various biochemical pathways as modified by the exercise regimen, Structural Equation Modeling (SEM) with repeated measures in these data will provide a test of the overall theoretical framework, and identify significant direct and indirect pathways between posited activator/suppressor antagonists, as well as possible feedback mechanisms. SAS (V9.2, proc CALIS) will be used for fitting the SEM. For the primary endpoint, change in plasma F2-isoprostane, we will include in the analysis all participants with both baseline and final measurement of F2-isoprostane (intent to treat analysis). We will also perform an analysis of those who complied with the weight and exercise restrictions in their assigned group.
Table 3.
List of study endpoints with minimum detectable differences at 80% power, based on estimates of variability in outcome changes from pilot studies.
| Endpoint | Standard deviation of changes from pilot study | Minimum detectable difference (80% power) |
|---|---|---|
| Primary Outcome | ||
| F2-isoprostanes (pg/ml) | 40 | 12.6 |
| Secondary Outcomes Estrogensa: | ||
| Estradiol | 6 | 1.8 |
| Estrone | 15 | 4.6 |
| Estriol | 10 | 3.2 |
| 16-hydroxyestrone | 11 | 3.5 |
| 2-hydroxyestrone | 5 | 1.6 |
| 2-hydroxyestradiol | 1.004291 | 0.315347 |
| 2-hydroxyestrogens combined | 6.416084 | 2.01465 |
| 4-Hydroxyestrone | 0.462954 | 0.145367 |
| 4-hydroxyestradiol | 5.205566 | 1.634548 |
| 4-hydroxyestrogens combined | 0.898837 | 0.282235 |
| 2-Methyhydroxyestrone | 2.712885 | 0.851846 |
| 2-methyhydroxyestradiol | 18.06073 | 5.671068 |
| 2/16 hydroxyestrone ratio | 0.511265 | 0.160537 |
| 4/2 hydroxyestrogens ratio | 0.108517 | 0.034074 |
| Body composition variables: | ||
| BMI (kg/m2) | 1.593873 | 0.500476 |
| %Body fat (by DXA scan) | 2.296699 | 0.721164 |
| Insulin and Glucose: | ||
| Insulin (mU/L) | 1.800009 | 0.565203 |
| Glucose (mg/dL) | 6.849493 | 2.150741 |
| Insulin resistance index (HOMA) | 0.5432 | 0.170565 |
| Insulin resistance ln (HOMA) | 0.384855 | 0.120845 |
| IGF proteins: | ||
| IGF-I (ng/ml) | 43.10523 | 13.53504 |
| IGFBP-1 (ng/ml) | 22.44738 | 7.048477 |
| IGFBP-2 (ng/ml) (from WTBS) | 99.70928 | 31.30871 |
| IGFBP-3 (ng/ml) | 367.35 | 115.3479 |
7. Discussion
The WISER study was a randomized controlled trial examining the effects of exercise on several biomarkers associated with breast cancer risk. WISER study data collection was completed in October 2009. Figure 1 shows a diagram of the study. The main recruitment strategy in this study was mass emails sent to University of Minnesota students and staff. Around 80% of study participants were recruited via mass emails. The most common reason for ineligibility for the study was irregularity of menstrual cycles followed by being too active. Some reasons for not consenting to the study were lack of time, unwillingness to collect urine for a 3-day period, aversion to blood draws, and unwilling to not use birth control over the period of the study. Among the consented women, 292 were lost to follow up before completion of baseline measurements, 391 were randomized into the exercise or control group and 319 women successfully completed the trial whereas 72 dropped out. We found a statistically significant difference in education between participants who dropped out of the study either prior to or after randomization (n = 366) and completers. It is possible that those who dropped out realized that their work and academic obligations could not accommodate the study requirements. Their lives were also possibly more in transition than higher educated participants. Among randomized participants the overall dropout rate was 18.4%. However, there were almost twice as many dropouts in the exercise group (n = 46) than in the control group (n = 26). This finding was not surprising given that women randomized into the exercise group were asked to devote significantly more time to the study (approximately 5 hours more per week) than those randomized into the control group. As mentioned earlier, the randomization procedure was adjusted earlier in the study to account for this difference in dropout rates between the two groups. Reasons for dropping out of the study were time constraints, pregnancy, moving, and illness. In the exercise group, participants completed an average 92% of assigned minutes of exercise, and 140 women (86%) exercised for at least 14 weeks. Mean adherence was 97% in the first stage of exercise and 85% in the final stage.
Findings from this trial will be useful in understanding the physiologic mechanisms by which exercise possibly contributes to decreased markers of breast cancer risk. One limitation of the WISER study is its generalizability. Most of the participants in the study were college women not taking any hormonal contraceptives, recruited from the University of Minnesota and a few other colleges in the Twin Cities area; therefore, the findings are only applicable to this specific population. It should be noted, however, that according to the National Center for Education Statistics, 42% of women aged between 18-24 years were enrolled in degree granting institutions in 2007 [26]. In addition, it has been estimated that more than 50% of women aged 20-29 do not use hormonal contraceptives [27]. As the first adequately powered clinical trial designed to concomitantly examine the effects of exercise on F2-isoprostanes and several other factors related to cancer risk, the WISER study has several strengths that promise to contribute to the literature on the effects of exercise on biomarkers associated with risk of breast cancer.
Acknowledgments
The authors wish to acknowledge the General Clinical Research Center at the University of Minnesota, the study administrative staff, the Minneapolis YWCAs, the study participants, and the National Institutes of Health/National Cancer Institute for grant 1U54CA116849-010003.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer facts and figures 2009. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 2.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med. 2008;42:636–47. doi: 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- 3.Vanio H, Bianchini F. Weight control and physical activity. Lyon: IARC Press; 2002. [DOI] [PubMed] [Google Scholar]
- 4.Friedenreich CM. Physical activity and cancer prevention: from observational to intervention research. Cancer Epidemiol Biomarkers Prev. 2001;10:287–301. [PubMed] [Google Scholar]
- 5.Kaaks R, Lundin E, Rinaldi S, Manjer J, Biessy C, Soderberg S, et al. Prospective study of IGF-I, IGF-binding proteins, and breast cancer risk, in northern and southern Sweden. Cancer Causes Control. 2002;13:307–16. doi: 10.1023/a:1015270324325. [DOI] [PubMed] [Google Scholar]
- 6.Rossner P, Jr, Gammon MD, Terry MB, Agrawal M, Zhang FF, Teitelbaum SL, et al. Relationship between urinary 15-F2t-isoprostane and 8-oxodeoxyguanosine levels and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:639–44. doi: 10.1158/1055-9965.EPI-05-0554. [DOI] [PubMed] [Google Scholar]
- 7.Rossner P, Jr, Terry MB, Gammon MD, Agrawal M, Zhang FF, Ferris JS, et al. Plasma protein carbonyl levels and breast cancer risk. J Cell Mol Med. 2007;11:1138–48. doi: 10.1111/j.1582-4934.2007.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 9.Monninkhof EM, Velthuis MJ, Peeters PH, Twisk JW, Schuit AJ. Effect of exercise on postmenopausal sex hormone levels and role of body fat: a randomized controlled trial. J Clin Oncol. 2009;27:4492–9. doi: 10.1200/JCO.2008.19.7459. [DOI] [PubMed] [Google Scholar]
- 10.McTiernan A, Tworoger SS, Ulrich CM, Yasui Y, Irwin ML, Rajan KB, et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res. 2004;64:2923–8. doi: 10.1158/0008-5472.can-03-3393. [DOI] [PubMed] [Google Scholar]
- 11.Irwin ML, Varma K, Alvarez-Reeves M, Cadmus L, Wiley A, Chung GG, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18:306–13. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell KL, Westerlind KC, Harber VJ, Bell GJ, Mackey JR, Courneya KS. Effects of aerobic exercise training on estrogen metabolism in premenopausal women: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2007;16:731–9. doi: 10.1158/1055-9965.EPI-06-0784. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Chen J, Overstreet JW, Nakajima ST, Lasley BL. Urinary follicle-stimulating hormone peak as a biomarker for estimating the day of ovulation. Fertil Steril. 2002;77:961–6. doi: 10.1016/s0015-0282(02)02998-9. [DOI] [PubMed] [Google Scholar]
- 14.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–11. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 15.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 16.Bruce RA. Multi-stage treadmill test of maximal and sub maximal exercise. In: AHA, editor. Exercise testing and training of apparently health individuals: a handbook for physicians. New York: AHA; 1972. [Google Scholar]
- 17.Guidelines for Exercise Testing and Prescription. 6th. Philadelpha, PA: Lippincott, Williams & Wilkins; 2000. American College of Sports Medicine. [Google Scholar]
- 18.Jensen MD, Kanaley JA, Roust LR, O'Brien PC, Braun JS, Dunn WL, et al. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc. 1993;68:867–73. doi: 10.1016/s0025-6196(12)60695-8. [DOI] [PubMed] [Google Scholar]
- 19.Wang JP, Russell M, Mazariegos M, Burastero S, Thornton J, Lichtman S, et al. Body fat by dual photon absorptiometry: comparisons with traditional methods in Asians, blacks and whites. Am J Hum Biol. 1992;4:501–10. doi: 10.1002/ajhb.1310040409. [DOI] [PubMed] [Google Scholar]
- 20.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett and National Cancer Institute food frequency questionnaires. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 21.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. Jama. 1995;273:402–7. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 22.Morrow JD, Roberts LJ., 2nd Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, et al. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77:6646–54. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 24.Nelson RE, Grebe SK, OK DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–84. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Education Statistics. Digest of Education Statistics. Washington, DC: National Center for Education Statistics; 2008. Table 204. Enrollment orates of 18- to 24-year-olds in degree-granting institutions, by type of institution and sex and race/ethnicity of student: 1967 through 2007. [Google Scholar]
- 27.Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004:1–36. [PubMed] [Google Scholar]