Abstract
We examined sugar-induced obesity in mouse strains polymorphic for Tas1r3, a gene that codes for the T1R3 sugar taste receptor. The T1R3 receptor in the FVB and B6 strains has a higher affinity for sugars than that in the AKR and 129P3 strains. In Experiment 1, mice had 40 days of access to lab chow plus water, sucrose (10 or 34%), or fructose (10 or 34%) solutions. The strains consumed more of the sucrose than isocaloric fructose solutions. The pattern of strain differences in caloric intake from the 10% sugar solutions was FVB > 129P3 = B6 > AKR; and that from the 34% sugar solutions was FVB > 129P3 > B6 ≥ AKR. Despite consuming more sugar calories, the FVB mice resisted obesity altogether. The AKR and 129P3 mice became obese exclusively on the 34% sucrose diet, while the B6 mice did so on the 34% sucrose and 34% fructose diets. In Experiment 2, we compared total caloric intake from diets containing chow versus chow plus 34% sucrose. All strains consumed 15-29% more calories from the sucrose-supplemented diet. In Experiment 3, we compared the oral acceptability of the sucrose and fructose solutions, using lick tests. All strains licked more avidly for the 10% sucrose solutions. The results indicate that in mice (a) Tas1r3 genotype does not predict sugar-induced hyperphagia or obesity; (b) sucrose solutions stimulate higher daily intakes than isocaloric fructose solutions; and (c) susceptibility to sugar-induced obesity varies with strain, sugar concentration and sugar type.
Keywords: sugar-induced obesity, hyperphagia, mice, sweet taste, sucrose, fructose
1. Introduction
The average weight of Americans has increased steadily over the last 30 years, leading many to infer the existence of an obesity epidemic [27,60]. Because the weight gain has occurred in a genetically stable population, it has been attributed primarily to environmental factors such as increased intake of calorically rich foods [10,61] and reduced expenditure of energy [47,75]. There is speculation that the overconsumption of sugar-sweetened beverages is contributing to the obesity epidemic [12,14], and that differences in desire for sweet tasting foods [22,68] may explain why some subpopulations are more prone to obesity [27,28]. At this point, however, the relationship between sugar preference, sugar-induced hyperphagia and obesity is unresolved [3,22,67]. To help fill this void, we examined this tripartite relationship in an animal model.
It is well-established that rats [42,49,50,70] and mice [18,41,51,78] become obese when offered free access to concentrated solutions of sugars. Although little is known about whether rodent strains differ in susceptibility to sugar-induced obesity, we expected large differences in mice. This is because mouse strains differ greatly in daily intake of sugar solutions [48,62] and susceptibility to diet-induced obesity on high-fat diets [7,82]. The strain differences in daily sugar intake have been related to polymorphisms in the Tas1r3 sweet taste receptor gene. Tas1r3 codes for a protein (T1R3) that is part of the heterodimeric sweet taste receptor of mammals (T1R2+T1R3) [9]. Mice with the Sacb allele of Tas1r3 are more sensitive to perithreshold concentrations of sugars, have stronger taste nerve responses to suprathreshold responses to sugars, and show higher daily intake of and preference for sugars [25,40,58]. It is not known, however, whether Tas1r3 genotype is associated with differences in long-term sugar intake or weight gain.
Another unresolved issue is whether specific types of sugars are more likely to cause diet-induced obesity. For instance, there is considerable speculation that the increased intake of free fructose (as part of soft drinks sweetened with high-fructose corn syrup) is an important contributor to the “obesity epidemic” [15,61,79]. Compatible with this view is a report of weight gain in mice offered a fructose solution plus lab chow [41], but other studies failed to observe fructose-induced weight gain in mice [11,56]. These discrepant findings are difficult to interpret, however, because they were derived from different strains of mice and different concentrations of fructose.
Below we describe three experiments to investigate these issues. In Experiment 1, we compared sugar intake and weight gain across four strains of mice offered chow supplemented with ad lib access to one of four sugar solutions (10% sucrose, 10% fructose, 34% sucrose and 34% fructose) over 40 days. In Experiment 2, we determined whether the strains that gained the most weight in the prior experiment exhibited the least accurate energy intake compensation when offered chow and a 34% sucrose solution (i.e., failed to reduce chow intake to compensate for the extra calories obtained from the sugar). In Experiment 3, we compared the palatability of the sucrose and fructose solutions in short-term lick tests.
2. Are there strain differences in sucrose or fructose intake and sugar-induced obesity? (Experiment 1)
This experiment addressed three questions. Are certain strains of mice more susceptible to weight gain on the sugar-supplemented diets? Does prolonged access to isocaloric sucrose versus fructose solutions have different effects on sugar intake, weight gain or adiposity? Does intake of 10% versus 34% sugar solutions have different effects on intake, weight gain and adiposity? We used the 10% concentration because it stimulates high daily intake in mice [8,48,62], and because it approximates the concentration present in many sugar-sweetened beverages. We used the 34% concentration because it is similar to that used in studies of sugar-induced obesity in rats [42,70].
We focused on four strains of mice: FVB/N (FVB), C57BL/6J (B6), 129P3/J (129P3), and AKR/J (AKR). The FVB and B6 strain express the Sacb allele of Tas1r3 [54], and thus have a more sensitive sweet taste receptor and show higher daily intake of 8-12% sucrose than the 129P3 and AKR strains, which express the Sacd allele [8,48,62,64,73]. In addition, these strains differ in their initial licking responses to caloric and non-caloric sweeteners [33]. Although there are reports of diet-induced obesity in all four strains [2,4,7,52,57,82], 129P3 and FVB mice appear less obesity-prone than B6 and AKR mice [4,7,38,44]. It is notable, however, that none of these prior studies of diet-induced obesity examined the impact of protracted consumption of sugar solutions on weight gain.
2.1. Methods
2.1.1. Animals and housing conditions
All mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). Approximately equal numbers of adult males and females were tested, beginning at 9 weeks of age (see Table 1 for sample sizes). Adults were used so as to minimize any potentially confounding effects of diet composition (e.g., relative amounts of lab chow versus sugar solution ingested) on growth and development. All mice were housed and tested individually in standard polycarbonate shoebox cages (27.5 × 17 × 12.5 cm) with Bed-O'Cobs™ bedding (Andersons; Maumee, OH) and Nestlets™ cotton pads (Ancare; Bellmore, NY). The housing facility had automatically controlled temperature, humidity and lighting (12 h:12 h light:dark cycle).
Table 1.
Initial body weights (mean ± S.E.) of the male and female mice from each strain in Experiment 1.
| Strain |
||||
|---|---|---|---|---|
| Gender | AKR | 129P3 | B6 | FVB |
| Male | 29.3 ± 2.3 | 22.8 ± 1.5 | 24.6 ± 1.6 | 25.9 ± 1.9 |
| Female | 26.0 ± 2.0 * | 17.7 ± 1.4 * | 18.8 ± 1.4 * | 19.7 ± 1.3 * |
N= 20 males and 19-20 females from each strain; these mice were divided evenly across the five diet treatments. We compare body weights across gender within each strain with unpaired t-tests.
P ≤ 0.05
Following one week of acclimation, testing began. The initial weights of the mice (broken down by gender) are provided in Table 1. All mice were offered laboratory chow (Rat Diet 5012, PMI Nutrition, Brentwood, MO) and tap water ad libitum throughout the experiments. According to the manufacturer, the chow has a physiological fuel value of 3.43 kcal/g and contains 13, 27, and 60% calories from fat, protein, carbohydrate, respectively. All testing procedures were approved by the Institutional Care and Use Committee of Columbia University.
2.1.2. Test solutions
The sugar solutions were prepared by dissolving food-grade sucrose (Domino Foods, Inc., Yonkers, NY) or fructose (ZooScape.com, Buffalo, NY) in deionized water at concentrations of 10% and 34%. The 10% fructose (0.57 M) and 10% sucrose (0.30 M) solutions each contained 0.52 kcal/g; and the 34% fructose (1.9 M) and 34% sucrose (1.0 M) solutions each contained 1.37 kcal/g.
All solutions were presented at room temperature, and were replaced with fresh solutions every two days. The mice in the sugar groups were given daily access to their designated sugar and water in sipper tubes, which were positioned approximately 5 cm apart. To control for side preferences, the left-right position of the sugar and water sipper tubes was alternated each day. The mice in the water groups were given two sipper tubes containing water.
Daily fluid intakes were measured (to the nearest 0.01 g) by recording the change in weight of the drinking bottles, using an electronic balance interfaced to a computer. Daily fluid spillage was estimated by recording the change in weight of bottles representing each fluid treatment in an empty cage. To correct for fluid spillage, we subtracted the estimated spill from the quantity consumed over the 24 hr test. We initially monitored daily chow intake by weighing the cage top with food hopper. However, we ceased once we realized that the daily intake measurements were confounded by two factors: weight of the hygroscopic lab chow fluctuated with room humidity, and the mouse strains differed in the amount of chow that they spilled into the cage each day (see Experiment 2).
2.1.3. Experimental procedures
Mice from each strain were assigned randomly to the five fluid treatment groups (i.e., water only, 10% sucrose, 10% fructose, 34% sucrose, 34% fructose). Because of space limitations, we could not test all mice concurrently. As a result, we tested the mice in four sequential cohorts (~ 40 mice per cohort). To avoid temporal confounds, we interspersed mice from different gender, strain and fluid treatments across the cohorts.
Daily intake of the water and sugar solutions was measured over a period of 40 days. In addition, body weight was measured on alternate days (to the nearest 0.01 g). At the end of the experiment, we euthanized each mouse, measured its weight and body length (nose-anus; to the nearest 1 mm), excised three intraperitoneal fat depots (retroperitoneal, gonadal and mesenteric) and measured the wet weight of each (to the nearest 0.001 g). We focused on these fat depots because they together constitute a large proportion of total body fat, can be removed easily, and respond differently to high-calorie diets in mice [82]. The retroperitoneal depot (in males and females) consisted of fat associated with the perirenal capsule and the dorsal body wall near the kidneys. In cases where an adrenal gland was embedded in the fat tissue, it was removed. The gonadal depot consisted of fat associated with the epididymis and vesicular gland in males, and the ovary, fallopian tubes and uterus in females. The mesenteric depot consisted of fat associated with the omental membrane and the mesentery along the duodenum, jejunum, and ileum. The mesentery was carefully stripped off the small intestine as a continuous piece; no attempt was made to dissociate fat from the mesenteric tissue. We standardized the fat depot extractions across mice by having the same person remove the fat depots from all mice (under a blind protocol).
2.1.4. Data Analyses
Because fluid intake tends to increase with body weight (e.g., see [6]), we tested for an effect of strain and gender on initial body weights, using a two-way ANOVA. There were significant main effects of strain (F3,151 = 168.5; P ≤ 0.05) and gender (F1,151 = 350.7; P ≤ 0.05), and a significant interaction of strain x gender (F3,151 = 9.8; P ≤ 0.05); the interaction reflected the fact that males were larger than females for all strains, though to varying degrees (Table 1). Owing to these body weight differences, we implemented two standardization procedures. First, in our strain comparisons of sugar intake, we calculated total sugar calories ingested per 30 g body weight (the approximate adult weight of many mouse strains) over the 40-day experiment, based on Bachmanov et al. [8]. To make these calculations for each mouse, we used the average of its initial and final weight. Second, we represented weight gain as the change in weight. To this end, we set the initial weight of each mouse as zero, and then calculated the change in weight during each successive 2-day period. Because the males and females within the same strain did not differ significantly in total weight gain over the 40-day experiment (in all unpaired t-test comparisons, P > 0.05), we combined data from both genders.
We examined the intake data in several ways. First, we analyzed changes in intake over time with mixed-model ANOVAs, treating time as a within factor and fluid intake as a between factor. Because we analyzed each strain separately and treated fluid intake as a repeated measure, we did not need to standardize intake. For the analysis of each sugar, we used three treatment levels (i.e., water, 10% sugar and 34% sugar); note that we used the same water intake data for the analyses involving sucrose and fructose. Second, because the ANOVAs revealed a significant interaction of time x solution intake, we compared initial and final daily intakes of each sugar solution. This involved determining mean daily intakes and preference scores (i.e., sugar solution intake / total intake × 100) across days 1-2 and days 39-40 of the experiment, separately for each sugar solution. We used two-way ANOVAs to examine the main effects of time (i.e., initial versus final) and sugar solution on the daily intakes and preference scores. Third, we used two-way ANOVAs to determine how total sugar calories ingested per 30 g body weight varied as a function of mouse strain, sugar type, and sugar concentration.
Next, we compared weight gain across mouse strains with a mixed-model ANOVA. Fluid treatment (i.e., water, 10% sugar, and 34% sugar) was the between factor and time was the within factor. We examined the results from each strain and sugar separately. Because the interactions were significant, we also ran one-way ANOVA and Newman-Keuls multiple comparison tests across the three fluid treatments levels on day 40 weights.
We ran two analyses on the fat depot data. First, we compared fat depot wet weights across fluid treatments with a one-way ANOVA and Newman-Keuls multiple comparison test, separately for each strain. Second, we used a two-way ANOVA to test for an effect of mouse strain and sugar type on total fat (i.e., combined weight of the gonadal, mesenteric and retroperitoneal depots); we limited this test to the 34% sugar solutions. To control for strain differences in constitutive fat weight, we calculated a standardized measure of fat depot weight, separately for each strain and sugar. This involved subtracting mean fat accumulated in mice offered water alone from that of each mouse offered 34% sucrose or 34% fructose.
Finally, we asked whether the experimental treatments altered two other measures of body size: lean body weight (i.e., final body weight minus total weight of gonadal, retroperitoneal and mesenteric fat pads) and body length. For each measure, we ran a one-way ANOVA (and Tukey post hoc test) across the fluids, separately for each strain.
2.2. Results
2.2.1. Intake patterns
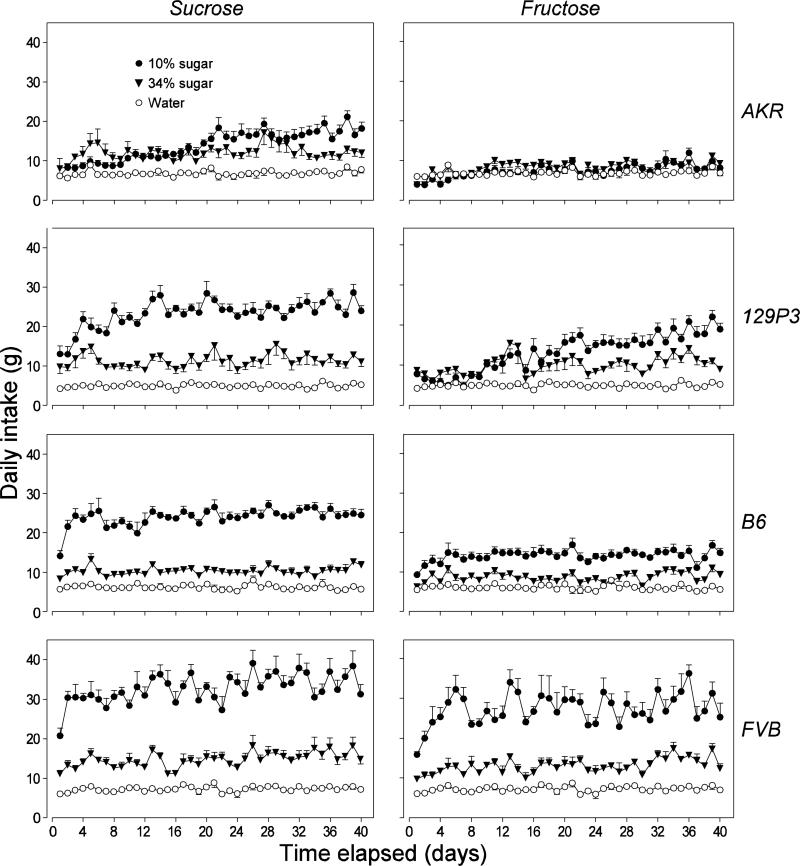
In Figure 1 (left column of panels), we illustrate how intake of water, 10% sucrose and 34% sucrose changed over the 40-day experiment, separately for each strain. See Table 2 for results of the two-way ANOVAs performed on these data. There was a significant main effect of fluid in each mouse strain. This result reflects the fact that for all strains, the relative daily intake was as follows: 10% sucrose > 34% sucrose > water. There was also a significant main effect of time, revealing an overall increase in fluid intake over the course of the experiment. The significant interaction of time x fluid demonstrates that the extent to which intake increased over time varied across groups. For instance, the time-dependent increase in intake of 10% sucrose was much more marked than that of the other fluids.
Figure 1.
Daily intakes from one of three diet treatments (10% sugar, 34% sugar, or water alone) over the 40-day experiment. The results from each strain are plotted in separate rows: FVB (first row), B6 (second row), 129P3 (third row) and AKR (fourth row). Further, the results from each type of sugar are plotted in separate columns: sucrose (left column) and fructose (right column). Because only one group of mice per strain received water alone, we presented the same water intake data in both the left and right panel for each strain. Further, even though the mice offered the sugar solutions also received water, we did not present their water intake data. These data are analyzed in Table 2. Each symbol represents mean ± S.E.; N = 8 mice per strain and solution treatment.
Table 2.
Analysis of the daily sugar solution intakes in Fig. 1. We show F-ratios (df) from two-way ANOVAs, performed separately on each sugar type and strain.
| Source of variation |
||||
|---|---|---|---|---|
| Sugar type | Strain | Sugar solution | Time | Interaction |
| Sucrose | AKR | 27.0 (2,819) * | 9.5 (39,819) * | 5.1 (78,819) * |
| 129P3 | 231.4 (2,819) * | 4.9 (39,819) * | 2.9 (78,819) * | |
| B6 | 530.0 (2,819) * | 4.9 (39,819) * | 2.9 (78,819) * | |
| FVB | 532.7 (2,819) * | 3.6 (39,819) * | 1.8 (78,819) * | |
| Fructose | AKR | 3.5 (2,819) * | 7.2 (39,819) * | 1.7 (78,819) * |
| 129P3 | 42.9 (2,780) * | 14.9 (39,780) * | 6.9 (78,780) * | |
| B6 | 52.9 (2,819) * | 5.3 (39,819) * | 1.8 (78,819) * | |
| FVB | 156.8 (2,819) * | 3.1 (39,819) * | 1.1 (78,819) | |
P ≤ 0.05
In Figure 1 (right column of panels), we show how intake of water, 10% fructose and 34% fructose changed over the 40-day experiment, separately for each strain. See Table 2 for results of the two-way ANOVAs performed on these data. As with sucrose, there was a significant main effect of fluid in each mouse strain. For all strains except AKR, the relative daily intake was as follows: 10% fructose > 34% fructose > water. For the AKR strain, the relative daily intake was 10% fructose = 34% fructose > water. The significant main effect of time reflects the fact that intake generally increased with time. However, the significant interactions of time x fluid shows that the extent to which intake increased was not uniform across groups.
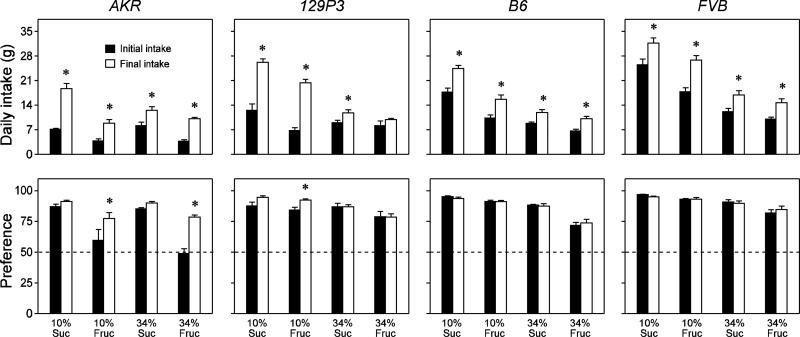
To better understand the time-dependent changes in sugar intake, we compared the daily intake between the initial and final two days of the experiment (Figure 2, top row of panels). See Table 3 for results of the two-way ANOVAs performed on these data. In virtually all cases, the main effect of time was significant, demonstrating that the mice increased daily intake of the four sugar solutions over the 40-day experiment; the only exception was that the 129P3 mice did not increase intake of the 34% fructose solution. The AKR and 129P3 mice exhibited the largest changes. For instance, the AKR mice increased intake of the 10% sucrose solution nearly three-fold, and the 129P3 mice increased intake of the 10% sucrose and fructose solutions two- to three-fold.
Figure 2.
Illustration of how daily intake of and preference for the four sugar solutions changed over the 40-day experiment in each mouse strain (mean ± S.E). Upper panels: comparison of initial (i.e., mean daily intake during days 1-2) versus final (i.e., mean daily intake during days 39-40) intake of each sugar solution. Lower panels: comparison of initial versus final preference for each sugar solution over water. In each lower panel, we indicate indifference (i.e., 50% preference) with a dashed line. For each solution and strain, we compare the initial and final intakes and preferences with paired t-tests (* P ≤ 0.05). Additional analyses are performed in Table 3. N = 7-8 mice per strain and solution treatment.
Table 3.
Analysis of the results in Fig. 2. We show F-ratios (df) from two-way ANOVAs, performed separately on each ingestive measure and strain.
| Ingestive measure | Source of variation |
|||
|---|---|---|---|---|
| Strain | Time | Sugar solution | Interaction | |
| Intake | AKR | 120.4 (1,28) * | 27.3 (3,28) * | 6.9 (3,28) * |
| 129P3 | 109.9 (1,27) * | 41.8 (3,27) * | 18.7 (3,27) * | |
| B6 | 117.2 (1,28) * | 74.4 (3,28) * | 3.9 (3,28) * | |
| FVB | 71.4 (1,28) * | 65.3 (3,28) * | 1.9 (3,28) | |
| Preference | AKR | 20.8 (1,28) * | 31.6 (3,28) * | 3.8 (3,28) * |
| 129P3 | 3.4 (1,27) * | 13.0 (3,27) * | 1.2 (3,27) | |
| B6 | < 0.1 (1,28) | 42.5 (3,28) * | 0.4 (3,28) | |
| FVB | < 0.1 (1,28) | 37.4 (3,28) * | 0.5 (3,28) | |
P ≤ 0.05
We also compared the initial and final preference scores (i.e., percent sugar intakes) for the different sugar solutions (Figure 2, bottom row of panels). See Table 3 for results of the two-way ANOVAs performed on these data. The 129P3, B6 and FVB mice displayed strong initial preferences for all of the sugar solutions over water, although those for 34% sucrose were slightly but consistently weaker. In nearly all cases, the main effect of time was not significant, indicating that the preferences were stable over the 40-day test—the one exception involved the 129P3, which displayed a small but significant increase in preference for 10% fructose. Whereas the AKR mice showed strong initial and final preferences for the sucrose solutions, their preference for the fructose solutions required time to develop. Initially, the AKR mice were largely indifferent to the 10% and 34% fructose solutions (i.e., preference ratios near 0.5), but eventually developed moderate (and significantly higher) preferences for both solutions. It is notable that the initial preference for 10% sucrose was significantly greater in the FVB and B6 strains than in the AKR and 129P3 strains (P ≤ 0.05, according to a Newman-Keuls multiple comparison test). A similar preference profile was observed for 10% fructose, although in this case only the AKR mice differed significantly from the FVB and B6 mice.
In Figure 3, we show total calories ingested per 30 g body weight from the sugar solutions over the 40-day experiment. To examine the influence of sugar concentration and strain on this dependent measure, we ran a two way ANOVA. For both sugars, there were significant main effects of concentration (sucrose: F1,56 = 33.6, P ≤ 0.05; fructose: F1,55 = 77.7, P ≤ 0.05) and mouse strain (sucrose: F3,56 = 40.8, P ≤ 0.05; fructose: F3,56 = 57.7, P ≤ 0.05); the interaction of concentration × strain was significant for sucrose (F3,56 = 4.4, P ≤ 0.05) but not fructose (F3,56 = 2.1, P > 0.05). These findings reveal that (a) the mice generally acquired more calories per 30 g body weight from the 34% than the 10% concentrations of both sugars; (b) the FVB strain obtained more calories per 30 g body weight than the other strains from the 10 and 34% concentrations of each sugar; and (c) the AKR mice obtained fewer calories per 30 g body weight than the other strains from all sugar solution except 34% sucrose.
Figure 3.
Total sugar intake (kcal/ 30 g body weight) over the 40-day test from the 10% and 34% solutions of sucrose and fructose by the AKR, 129P3, FVB and B6 mice (mean ± S.E.). Within each panel, we compare total caloric intake per 30 g body weight across strains with the Newman-Keuls multiple comparison test; different letters above the bars (a-d) indicate means that differ significantly from one another (P ≤ 0.05).
In a separate analysis of the data in Figure 3, we used two-way ANOVA to determine whether the mice obtained more calories from the sucrose or fructose solutions, separately at the 10 and 34% concentrations. The most important result was that at each sugar concentration, there was a significant main effect of sugar type on calories consumed (at 10%, F1,55 = 78.1, P ≤ 0.05; at 34%, F1,56 = 8.8, P ≤ 0.05). This establishes that the mice generally obtained more calories from sucrose than fructose.
It is notable that even though the 129P3 strain initially consumed less 10% sucrose and 10% fructose than the B6 strain (Figure 2), this difference disappeared after approximately 12 days, owing to a latent surge in intake by the 129P3 strain (Figure 1). As a result, the 129P3 strain ultimately obtained as many calories per 30 g body weight from the 10% sugar solutions as did the B6 strain (Figure 3).
2.2.2. Body weight and fat changes
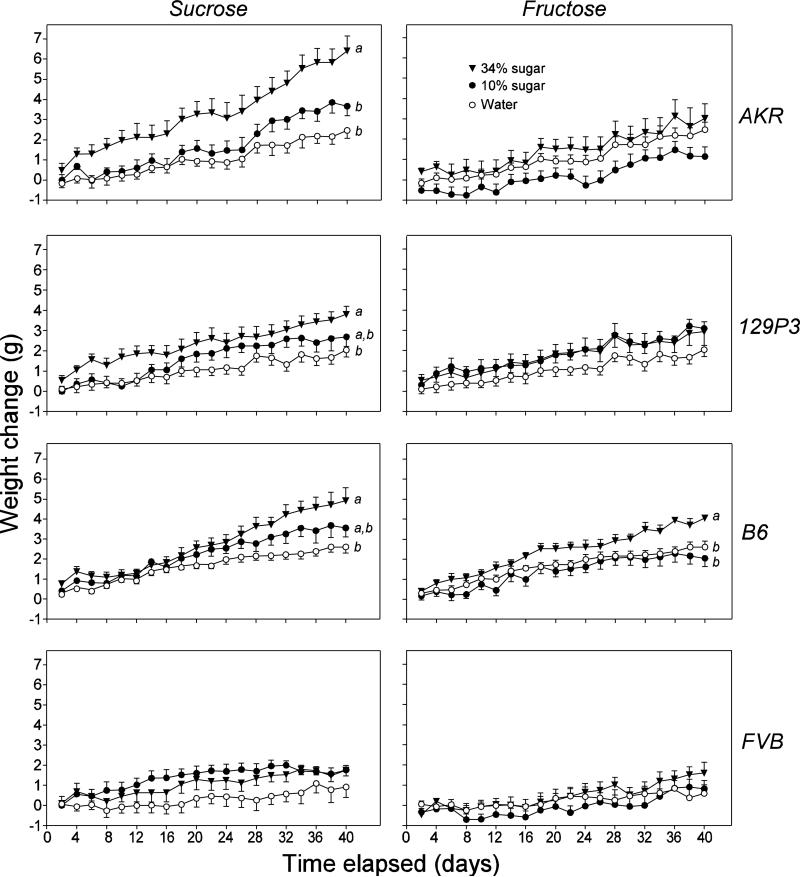
In Figure 4 (left column of panels), we show weight changes in mice offered 34% sucrose, 10% sucrose or water alone. We present the results of the two-way ANOVAs performed on these data in Table 4. For the FVB mice, there was a significant main effect of time, but no significant main effect of fluid or interaction of time x fluid. This shows that the FVB mice did not gain disproportionately more weight on the sucrose solutions. In contrast, for the AKR, 129P3 and B6 mice, there were significant main effects of fluid and time, and a significant interaction of fluid x time. To explore the nature of these interactions, we compared final weight gains (i.e., on day 40) across fluid treatments, separately for each strain and fluid treatment. These analyses revealed that the AKR, 129P3 and B6 mice in the 34% sucrose group (but not the 10% sucrose group) gained significantly more weight than did mice from the same strains in the water group (see Fig. 4).
Figure 4.
Weight changes in mice offered one of three diet treatments (10% sugar, 34% sugar, or water alone) over the 40-day experiment. The results from each strain are plotted in separate rows: FVB (first row), B6 (second row), 129P3 (third row) and AKR (fourth row). Further, the results from each type of sugar are plotted in separate columns: sucrose (left column) and fructose (right column). Within each panel, we compare the mean weights on day 40 across diet treatment with a Newman-Keuls multiple comparison test; treatment levels that differ significantly from one another (P ≤ 0.05) are labeled with distinct letters (i.e., a and b). Additional analyses on these data are performed in Table 4. Each symbol represents mean ± S.E.; N = 8 mice per strain and solution treatment.
Table 4.
Analysis of the weight changes in Fig. 4. We show F-ratios (df) from two-way ANOVAs, performed separately on each sugar type and strain.
| Source of variation |
||||
|---|---|---|---|---|
| Sugar type | Strain | Sugar solution | Time | Interaction |
| Sucrose | AKR | 9.9 (2,399) * | 57.2 (19,399) * | 2.6 (38,399) * |
| 129P3 | 3.9 (2,399) * | 53.3 (19,399) * | 1.9 (38,399) * | |
| B6 | 4.1 (2,399) * | 84.2 (19,399) * | 3.7 (38,399) * | |
| FVB | 3.0 (2,399) | 14.0 (19,399) * | 1.3 (38,399) | |
| Fructose | AKR | 3.1 (2,399) | 24.4 (19,399) * | 0.4 (38,399) |
| 129P3 | 1.6 (2,380) | 42.5 (19,380) * | 0.7 (38,380) | |
| B6 | 4.7 (2,399) * | 88.6 (19,399) * | 2.7 (38,399) * | |
| FVB | 0.7 (2,399) | 14.4 (19,399) * | 1.4 (38,399) | |
P ≤ 0.05
In Figure 4 (right column of panels), we illustrate weight changes in mice offered 34% fructose, 10% fructose or water alone. We present results of the two-way ANOVAs performed on these data in Table 4. For the B6 strain, there were significant main effects of fluid and time, and a significant interaction of fluid x time. These findings reflect the fact that the mice offered 34% fructose (but not 10% fructose) gained significantly more weight than those offered water alone. In contrast, for the AKR, 129P3 and FVB strains, there was a significant main effect of time, but no significant main effect of fluid or interaction of time x fluid. Accordingly, consumption of neither 10 nor 34% fructose caused significant weight increase in these strains (relative to mice on the control diet). In fact, the 10% fructose solution tended to reduce weight gain in the AKR, B6, and FVB during at least portions of the 40-day test period.
In Figure 5, we compare weights of the fat depots (retroperitoneal, mesenteric and gonadal) across the fluid treatments, separately for each mouse strain with Newman-Keuls multiple comparison tests. For the FVB strain, there was no effect of fluid treatment on any fat depot. For the AKR strain, the mesenteric and gonadal fat depots were heavier in mice offered 34% sucrose and 34% fructose than in control mice. For the B6 strain, the mesenteric and gonadal fat depots were heavier in mice offered 34% and 10% sucrose than in control mice; further, the gonadal fat depot was also heavier in mice offered 34% fructose. For the 129P3 strain, the retroperitoneal and gonadal fat depots were heavier in mice offered 34% sucrose than in control mice.
Figure 5.
Wet weight of retroperitoneal (left column of panels), mesenteric (middle column of panels) and gonadal (right column of panels) fat depots in FVB, B6, 129P3, and AKR mice at the end of the 40-day experiment. We present fat depot weights of mice that were offered water alone (Con), a sucrose solution (10 or 34%), or a fructose solution (10 or 34%). Within each panel, we compare fat depot weights across diet treatments with a Newman-Keuls multiple comparison test; treatment levels that differ significantly from one another (P ≤ 0.05) are labeled with distinct letters (i.e., a, b, c). Each bar represents mean ± S.E.; N = 8 mice per strain and solution treatment.
In Figure 6, we illustrate standardized fat depot weight in mice offered 34% sucrose or 34% fructose. A two factor ANOVA revealed significant main effects of sugar type (F1,56 = 13.5; P ≤ 0.05) and strain (F3,56 = 9.1; P ≤ 0.05), but no significant interaction of sugar type x strain (F = 2.1; P > 0.05). This result establishes that the mice on the 34% sucrose had significantly more fat than those on the 34% fructose diet on day 40 of the experiments. Further, among the mice offered 34% sucrose, standardized fat depot weight was higher in the AKR strain than in all other strains (P ≤ 0.05; Newman-Keuls multiple comparison test). Indeed, among the mice offered the 34% sucrose solution, those in the AKR strain had a standardized fat depot weight that was almost 3 times larger than that in the 129P3 and FVB strains, and more than 2 times larger than that in the B6 strain. In contrast, while there was a trend for the AKR and B6 mice on the 34% fructose diet to have greater fat accumulation than the B6 and AKR mice, the strain differences were not significant (P > 0.05; Newman-Keuls multiple comparison test).
Figure 6.
Strain comparison of standardized fat gain in mice offered the 34% sucrose or 34% fructose solution. The standardized fat gain measure includes the gonadal, retroperitoneal and mesenteric fat depots. A two-way ANOVA revealed a significant main effect of both sugar type and strain, but a non-significant interaction of sugar type x strain (see text for details). Each bar represents mean ± S.E.; N = 8 mice per strain and solution.
Finally, we examined two alternate explanations for the observed strained differences in weight gain. First, they could have stemmed from changes in body length. However, there were no significant main effects of the sucrose or fructose solutions on body length in any of the strains (in all one-way ANOVAs, P > 0.05; data not shown). Second, because we did not measure total body fat, it is possible that we overlooked fat accumulation in the depots that were not examined (e.g., subcutaneous and interscapular). If this was the case, then the mice on the sugar diets should have had a higher lean weight than mice on the control diet (note that we defined lean mass as final body weight minus total weight of gonadal, retroperitoneal and mesenteric fat pads). However, there were no significant main effects of the sucrose or fructose solutions on lean mass in any of the strains (in all one-way ANOVAs, P > 0.05; data not shown).
3. Are there strain differences in the energy compensatory response to sucrose intake? (Experiment 2)
In Experiment 1, there were large strain differences in weight gain and sugar intake on the 34% sucrose diet. Unexpectedly, however, the highest sugar calorie intakes were not associated with the highest weight gain and fat accumulation. In particular, when offered the 34% sucrose, the FVB strain ingested the most sugar calories per 30 g body weight, but gained the least weight. Also, the AKR mice gained the most body fat but did not consume more sucrose calories per 30 g body weight than any of the other strains. These findings were unexpected because weight gain usually increases with daily sugar energy intake in mice [18,41,51,78,81].
Because we did not record intake of laboratory chow in Experiment 1, we could not determine how many total calories the mice ingested each day. It is possible that even though the FVB mice consumed more sugar calories than the other strains, they may have reduced caloric intake from the laboratory chow, thereby obtaining the same number of calories per 30 g body weight as the other strains. When mice from the NMRI strain were offered a lab chow diet supplemented with a 10% sucrose or 15% fructose solution, they compensated for the sugar calories by reducing chow intake; in so doing, the mice obtained the same total number of calories as mice offered chow alone [41]. In contrast, there are other reports in which mice [11,51,78,81] and rats [42,49,50,70] failed to show accurate energy intake compensation when offered free access to a sugar solution.
Here, we examined energy intake compensation in the AKR, 129P3, B6 and FVB strains. We hypothesized that the strain that gained the most weight in Experiment 1 (i.e., AKR) would show the poorest energy intake compensation. To this end, we effectively repeated Experiment 1, but quantified intake of calories from both sugar and chow. We focused on the diet consisting of 34% sucrose solution and lab chow because it stimulated the greatest intake and weight gain in the previous experiment. Further, we limited the study to 10 days because our goal was to examine compensatory feeding responses, not long-term patterns of weight gain.
3.1. Methods
3.1.1. Animals, housing conditions, and intake measurements
Ten males and ten females from each strain (AKR, 129P3, B6, and FVB) were tested, beginning at 9 weeks of age. All animal housing and test procedures were identical to those in the prior experiment, except that the mice did not have Nestlets in their cages.
3.1.2. Experimental procedure
Mice from each strain and gender were assigned randomly to two diet treatment groups. The experimental groups received a 34% sucrose solution (1.37 kcal/g), water and laboratory chow (Rat Diet 5012; physiological fuel value = 3.43 kcal/g) ad libitum; the control groups received water and laboratory chow ad libitum. All mice were tested concurrently over a 10-day period.
Daily fluid intakes were measured as described in Experiment 1. Daily laboratory chow intakes were quantified as follows. Each mouse was given approximately 7 g of laboratory chow pellets per day in the cage lid hopper, and the change in weight of the pellets was measured daily. To correct for the effects of humidity on estimates of daily chow intake, we measured weight changes in ~7 g chow rations placed on four empty cages and adjusted the food intake measures accordingly. To recover any diet that fell into the cage as the mice ate, we sifted the cage bedding each day by passing it through two sieves; the first had a grid size of 2.3 × 2.3 mm and the second a grid size of 1.5 × 1.5 mm. The diet recovered from the sifting was subtracted from the daily intake for each mouse (range = 0.1 to 1.6 g/day). The absence of cotton bedding (i.e., Nestlets) in the bedding improved the yield of spilled diet.
To explore whether the strains differed in diet spillage, we calculated the percentage of diet that was removed from the lab chow in the cage lid hopper, but not eaten (i.e., fell to the bottom of the cage). Among mice offered the control diet, this percentage was 8.6% for the 129P3 and B6 strains; 6.1% for the FVB strain; and 4.1% for the AKR strain. The spillage by the 129P3 and B6 strains was significantly greater than that by the AKR strain (P ≤ 0.05, according to a Newman-Keuls multiple comparison test). Thus, failing to control for diet spillage would have confounded our strain comparisons of daily caloric intake.
3.1.3. Data Analyses
First, we compared weight gain across the two diets with unpaired t-tests, separately for each strain. Second, we tested for energy intake compensation. To this end, we estimated daily caloric intake from each diet component by multiplying daily intake (in g) of laboratory chow × 3.43 kcal/g, and daily intake (in g) of 34% sucrose solution × 1.37 kcal/g. Then, we compared daily kcal intake from each diet with an unpaired t-test, separately for each strain. Third, to standardize kcal intake across strains, we calculated total caloric intake per 30 g body weight over the 10-day experiment. We ran a two-way ANOVA on these standardized caloric intake values, using strain and diet as between factors; we also ran one-way ANOVAs (and Newman-Keuls multiple comparison tests) across strains, separately for each diet.
3.2. Results
As in Experiment 1, the AKR, 129P3, and B6 (but not FVB) strains gained significantly more weight on the experimental (chow + 34% sucrose) than on the control (chow only) diet. For all strains except FVB, unpaired t values were > 3.37, df = 18, P ≤ 0.05; for the FVB strain, t = 0.2, df = 18, P > 0.05. The weight gains for mice on the experimental versus control diets were, in respective order: 2.25 vs. 1.32 g for AKR mice; 1.95 vs. 0.51 g for 129P3 mice; 1.57 vs. 0.72 g for B6 mice; and 0.88 vs. 0.97 g for FVB mice.
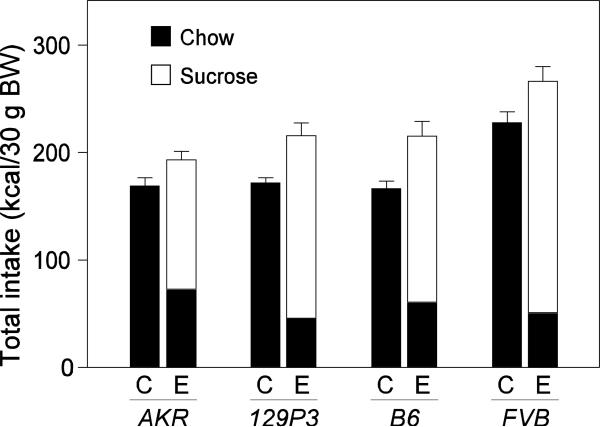
In Figure 7, we show total intakes (kcal/30 g body weight) over the 10-day experiment from the control and experimental diets, separately for each strain. A two-way ANOVA on these data revealed significant main effects of mouse strain (F3,72 = 18.4, P ≤ 0.05) and diet (F3172 = 31.4, P ≤ 0.05), but no significant interaction of strain x diet (F3,72 = 0.6, P ≤ 0.05). These findings establish that even though mice on the experimental diet consumed less chow than mice on the control diet, they did not accurately compensate for the increased calories obtained from the 34% sucrose solution. Indeed, the presence of the 34% sucrose solution caused the following percent increases in caloric intake per 30 g body weight (relative to the chow-only condition): 15% for AKR mice, 26% for 129P3 mice, 29% for B6 mice, and 17% for FVB mice.
Figure 7.
Total intake (kcal/30 g body weight) from the control (C; chow only) and experimental (E; chow + 34% sucrose solution) diets over the 10-day experiment by AKR, 129P3, B6 and FVB mice. For the experimental diet, we distinguish calories obtained from chow (black) versus sucrose solution (white). A two-way ANOVA revealed a significant main effect of both diet and strain on total kcal intake per 30 g body weight, but a non-significant interaction of diet x strain (see text for details). Each bar represents mean ± S.E.; N = 10 mice per strain and diet.
We compared total intake (kcal/30 g body weight) across strains, separately for each diet. There was a significant effect of strain for both the control (F3,39 = 15.4; P ≤ 0.05) and experimental (F3,39 = 7.0; P ≤ 0.05) diets. A Newman-Keuls multiple comparison test determined that both diets produced the same pattern of strain differences in total intake: FVB > AKR = B6 = 129P3.
We also compared the mouse strains in terms of the percentage of total caloric intake derived from the 34% sucrose solution. A one-way ANOVA revealed significant strain difference (F3,39 = 22.0; P < 0.05) with the pattern of differences being AKR (63%) < B6 (72%) < 129P3 (79%) = FVB (84%) according to a Tukey post hoc test. One implication of this finding is that the diet of the AKR mice contained a higher percentage of fat and protein than did that of the other strains, owing to their greater reliance on chow as a source of calories.
Taken together, these data provide three important insights. First, none of the strains showed precise energy intake compensation, despite reducing chow intake when offered the sucrose solution (see Fig. 7). Second, there was a disconnect between the pattern of strain differences in weight gain and fat accumulation (in both the present and previous experiments) and the pattern of strain difference in caloric intake per 30 g body weight (in the present experiment). For instance, even though the FVB mice accumulated less weight and fat than the AKR mice on the diet containing 34% sucrose, the FVB mice nevertheless consumed significantly more calories per 30 g body weight from this diet. Third, there was no relationship between the accuracy of energy intake compensation and weight gain. This is illustrated most clearly by the fact that the strain that accumulated the most weight and fat (i.e., AKR) exhibited the most accurate energy intake compensation (15%).
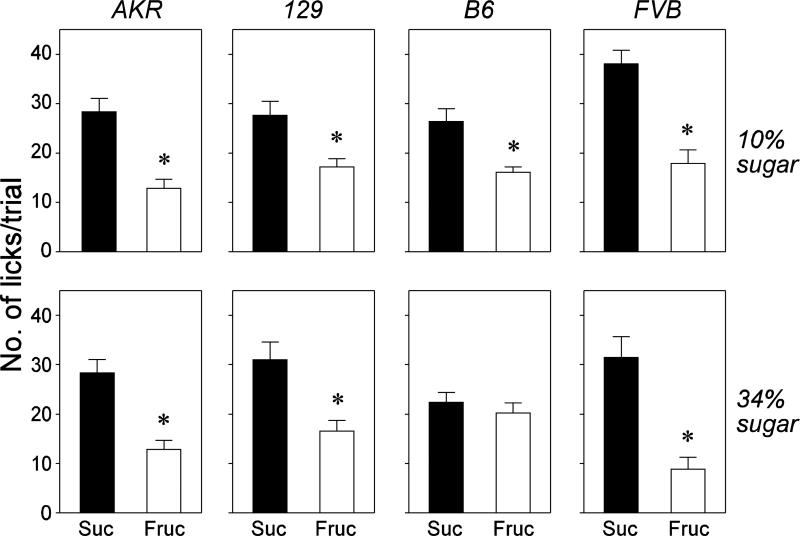
4. Does sucrose have a more acceptable taste than fructose at isocaloric concentrations? (Experiment 3)
In Experiment 1, all strains of mice consumed more of the sucrose than fructose solutions. Here, we asked whether the higher intake of sucrose solution could have stemmed, at least in part, from it having a more acceptable taste to the mice. In humans, the relative sweet intensity of isocaloric concentrations of fructose and sucrose is controversial—some studies report that fructose is sweeter [23,36,39], while others report that fructose is less sweet than (or isosweet to) sucrose [29,37] concentrations. Despite this lack of consensus, it is often assumed that fructose has a more acceptable taste than isocaloric concentrations of sucrose for many species of mammal. For instance, several investigators have inferred (without any empirical substantiation) that rats drank less fructose than sucrose solution in their studies because (a) the fructose solutions had a more intense sweet taste, and (b) the rats needed to drink less of the more intense fructose solution to satisfy their sweet tooth [49,50].
Given that the 10% and 34% fructose solutions have nearly twice the molar concentration as the corresponding sucrose solutions, one might assume that they would have a more acceptable taste. However, unpublished behavioral studies and chorda tympani nerve recordings in our laboratory indicate that the opposite is the case in mice. In this experiment, therefore, we compared the oral acceptability of two isocaloric concentrations of fructose and sucrose: 10% and 34%. We used a short-term lick test to minimize any potential post-ingestive effects on intake [31].
4.1. Methods
4.1.1. Experimental approach
A total of 7 mice from each strain (sex-ratio roughly balanced) were obtained from Jackson Labs, and were tested at approximately 9 weeks of age. To assess the relative taste acceptability of two isocaloric concentrations of sucrose and fructose (i.e., 10% or 34%), we recorded the number of licks for each solution during successive 5-s trials, and inferred that one solution was more acceptable if the mice took significantly more licks from it. All mice were naïve to the sugar solutions prior to testing.
4.1.2. Lick tests
Each test was conducted in a commercial gustometer (Davis MS160-Mouse; DiLog Instruments, Tallahassee, FL). We used a two-bottle no-choice testing procedure, during which the mice were alternately presented with each isocaloric concentration during 5-s trials across a 30-min test session. This testing procedure is described in detail elsewhere [32].
4.1.2.1. Training procedure
Before taste testing was conducted, the mice were given three days of training with water. This served to familiarize the mice with the gustometer and train them to lick from the sipper tube to obtain fluid. Because the mice were placed on a water-restriction schedule (see below) throughout training, they were highly motivated to lick from the sipper tube. Each training session began when the mouse took its first lick, and lasted 30 min. On Training Day 1, the mouse could drink freely from a single sipper tube throughout the session as the shutter was permanently open. On Training Days 2 and 3, the mouse could only drink from a sipper tube during successive 5 s trials, which were separated by 7.5-s inter-trial intervals.
To encourage sampling from the sipper tube, we water-deprived the mice for 22.5 h prior each training session. Following training sessions 1 and 2, we gave the mice water ad libitum for 1 h, and then began the next 22.5 hr period of water-deprivation.
4.1.2.2. Testing
Testing began once training was completed. Two sugar solutions were presented during successive 5-s trials during each 30-min test session. To control for potential order effects across trials within the test session, we treated the two sugar solutions as a block, and randomized (without replacement) the presentation sequence of each solution within each block. The mouse could initiate up to 288 trials throughout the test session.
Each mouse was run through two test sessions, each on different days. In one session, the mouse received 10% sucrose and 10% fructose, and in the other, it received 34% sucrose and 34% fructose. To control for testing order effects, the order of the two sessions was counter-balanced across mice within a strain.
To encourage sampling from the sugar solutions, we food- and water-restricted each mouse for 23.5 h prior to each test session. This involved limiting each mouse to 1 g of laboratory chow (dustless precision 1 g food-pellets; BioServ) and 2 ml of water. We interjected a recovery day between the test sessions, during which food and water were available ad libitum. Parenthetically, we have determined previously that this food- and water-restriction procedure does not alter licking responses of B6 mice for sugars [32].
4.1.3. Data analysis
We quantified the oral acceptability of each sugar solution by calculating mean number of licks per 5-s trial, separately for each sugar concentration and mouse. As each strain initiated a mean of at least 25 trials per test session, we considered our licking measures robust. We used paired t-tests to compare licks/trial for each isocaloric concentration of sucrose and fructose, separately for each mouse strain.
4.2. Results
All strains exhibited more licks/trial for sucrose than fructose at both 10% and 34% concentrations (Fig. 8). These differences were significant in all cases except one: the B6 mice licked only slightly more for 34% sucrose than 34% fructose. Thus, despite their lower molar concentrations, the sucrose solutions were more orally acceptable than the fructose solutions. The potential contribution of this finding to the long-term intakes of the sugar solutions in Experiment 1 is discussed below.
Figure 8.
Relative oral acceptability of the isocaloric concentrations of sucrose (Suc) and fructose (Fruc) for each of the mouse strains. Top row of panels: number of licks per 5 s trial for the 10% sucrose and fructose solutions. Bottom row of panels: number of licks for the 34% sucrose and fructose solutions. In each panel, we compare lick rates for the sucrose versus fructose solutions with a paired t-test (* P ≤ 0.05). Each bar indicates mean ± S.E.; N = 7 mice per strain and concentration.
5. General Discussion
Our results revealed significant strain, sugar and concentration differences in sugar-induced overeating and obesity in mice. Consistent with prior results [11,51], B6 mice gained excess weight and body fat when their chow diet was supplemented with a solution containing 34% sucrose. We observed a similar pattern of sucrose-induced obesity in the AKR and 129P3 mice, but not the FVB mice. Fructose was less effective than sucrose in promoting obesity. Only B6 mice gained excess weight with the 34% solution, although both AKR and B6 mice had slightly increased body fat. Overall, the 10% sugar solutions promoted more overdrinking than the 34% solutions, but did not significantly increase body weight or adiposity. That inbred mice differ in their obesity response to sugar solutions is not surprising given the many reports of strain differences in high-fat induced obesity [7,45,59,82,83]. Nevertheless, the strain differences observed here are notable in several respects.
The FVB mice consumed significantly more of the 10% and 34% sugar solutions than did the B6, 129P3 and AKR strains across the 40-day period in Experiment 1. Despite this high intake, the FVB was the only strain to resist sugar-induced obesity. We demonstrated in Experiment 2 that the FVB mice did not resist obesity by reducing chow intake to compensate for the elevated sucrose intake. In fact, the sucrose-fed FVB mice actually increased their total energy intake by 32% (relative to chow controls), which is more than the 25% increase observed in the AKR mice and only slightly less than the 34-36% increase of the 129P3 and B6 mice. It is possible that the sucrose-fed FVB mice expended their excess sugar calories by increasing energetic expenditure. This could have been accomplished by increasing activity levels [17,43], thermogenesis [20,63,76], expression of mitochondrial leak channels (e.g., uncoupling protein 1) in interscapular brown adipose tissue [26], and/or overall sympathetic nervous outflow [13,74,84]. In support of this possibility, there is evidence that FVB mice exhibit higher levels of spontaneous activity than many other mouse strains [80]. Increased energy expenditure may also account for the ability of FVB mice to resist weight gain when offered the 34% fructose solution given that their fructose intake was substantially higher than that of the other three strains.
The question of whether FVB mice are also resistant to fat-induced obesity is unclear. As with sugar, the FVB mice exhibit high lick rates for and daily intakes of oil emulsions compared with other mouse strains [34]. However, while some investigators reported that intake of high-fat diets promotes obesity in FVB mice [30,52,57], others reported that it does not [35,44]. There is no obvious explanation for these contradictory findings given that all of the studies used similar procedures—e.g., initiated testing with juvenile mice (i.e., 3-5 weeks of age), maintained the mice on the test diets for 8-10 weeks, and used high-fat diets with 41-58% of the calories derived from fat.
The overeating and obesity response of the AKR and 129P3 mice to the 34% sucrose solution is noteworthy given that they express the Sacd allele of Tas1r3 (i.e., the T1R3 receptor with a lower binding affinity for sweeteners; [58,64]) and show relatively low preference for and intake of 10% sugar solutions during 24-hr preference tests [48]. Here, we replicated these findings—at the start of Experiment 1, both the AKR and 129P3 exhibited relatively low preferences for (i.e., 87-88%) and intake of the 10% sucrose solutions, and the AKR mice initially showed weak-to-nonexistent preferences for the 10 and the 34% fructose solutions (60 and 49%, respectively). Yet, over the 40-day experiment, the AKR and 129P3 mice consumed as many calories per 30 g body weight from the 34% sucrose solution and gained as much (129P3) or more (AKR) body weight and fat as the B6 mice. Given that the AKR and 129P3 mice in the 10% sucrose groups increased their sucrose preference to the level of the B6 and FVB mice over the 40-day test period, it is possible that prolonged sucrose ingestion overrode the effect of Tas1r3 genotype on sugar intake and preference. Other findings indicate, however, that the sweet taste deficit of 129P3 mice persists—e.g., their relatively low preference for saccharin persists following extensive experience with sucrose [73]. Instead, the enhanced sucrose preference displayed by experienced 129P3 mice may represent an increased central evaluation of sucrose flavor (i.e., taste, smell, texture) conditioned by the post-ingestive nutritive actions of the sugar [72,71]. Accordingly, a genetically-determined sweet taste deficit does not protect mice from sugar-induced obesity because taste, while important, is not the only determinant of sugar intake.
It is notable that the AKR strain developed the highest sugar-induced obesity in this study, and fat-induced obesity in other studies [2,82]. While this observation indicates that the AKR strain is particularly susceptible to obesity on both fat- and sugar-rich diets, this may not be the case for all strains. For instance, the 129P3 strain developed sucrose-induced obesity in this study, but did not consistently display fat-induced obesity in prior studies [4,7]. These observations highlight the large amount of phenotypic diversity that exists among mouse strains in sugar and fat processing. Up to this point, most studies of strain differences in susceptibility to diet-induced obesity have focused on high-fat diets (e.g., see [5,24,66,77]). It might be useful to expand these studies to high-sugar diets, particularly given current concerns about the overconsumption of contribution of sugar-sweetened beverages [12,14,69].
In contrast to the 34% sucrose solution, the 10% solution did not significantly increase body weight gain or adiposity in any of the four strains. Even though 10% sucrose stimulated the greatest fluid intake, the mice obtained more calories from the 34% sucrose solution. The higher caloric intake from the 34% sucrose solution presumably accounts for its greater ability to promote weight gain and adiposity. Nevertheless, the AKR, B6, and 129P3 mice that were offered the 10% sucrose diets weighed slightly more than their respective controls. It is possible that more robust weight gains would have emerged if the animals had been tested for a longer period. In support of this possibility, significant weight gains have been reported when mouse strains (different from those tested herein) were maintained for > 40 days on a chow diet supplemented with a 10% sucrose solution [16,18].
5.1. Effect of sugar type on obesity and hyperphagia
Overall, fructose produced less weight gain and adiposity than did sucrose. The AKR and B6 mice on the 34% fructose solution accumulated more fat than controls, although less so than did the AKR and B6 mice fed 34% sucrose. In contrast, weight gain and adiposity of the 129P3 and FVB mice were not altered by fructose. Note that the differential weight gain response of B6 and AKR mice to fructose is opposite to that observed with sucrose. Further, note that the 10% fructose solution tended to decrease weight gain (relative to controls) in the AKR, B6 and FVB strains. Thus, we not only observed strain differences in sugar-induced obesity, but the pattern of the differences varied as a function of sugar type.
Two previous studies of B6 mice [11,56] reported that 30% fructose produced weight gains intermediate to that of the control, 30% sucrose and 30% glucose groups, whereas a 15% fructose solution had no effect on body weight. In another study [41], outbred mice (NMRI strain) gained excess weight on a 15% fructose solution, but not on a 10% sucrose solution. In rats (Sprague Dawley strain), iscaloric fructose and sucrose solutions (13 – 32%) were found to produce similar weight gains [42,49,50]. Accordingly, the present findings together with results reported elsewhere, indicate that fructose is no more obesogenic than sucrose in some rat and inbred mouse strains, and is less so in other mouse strains.
One consistent finding obtained in the present and previous studies is that mice and rats consume less fructose than sucrose at isocaloric concentrations [11,42,49,50]. Although some investigators attributed this to the higher oral acceptability of fructose [49,50], our data suggest just the opposite. That is, in the brief access one-bottle tests, the mice licked more for sucrose than fructose at 10% and 34% concentrations. Accordingly, higher oral acceptability could have stimulated higher daily intakes of sucrose than fructose. In addition, post-oral factors most likely contributed to the differential intakes of the two sugars. Rat studies indicate that intragastric (IG) infusions of sucrose (and glucose) condition much stronger flavor preferences than do fructose infusions [1]. This is also the case in B6 mice—e.g., matched IG infusions of 16% sucrose or glucose stimulated daily intake of a saccharin solution whereas 16% fructose infusions suppressed intake ([71]; Sclafani, unpublished findings).
5.2. Strain differences in body fat distribution
Several investigators have reported that fat depots of mice do not respond uniformly to high fat diets [7,78,82]. Indeed, each strain tends to distribute ingested fat across the depots in unique ways. Our results with high sugar diets corroborate this prior work. In the AKR and B6 mice, there were significant effects of 34% sucrose on the weights of the gonadal and mesenteric (but not the retroperitoneal) fat depots. Whereas fat accumulation occurred principally in the gonadal fat depot of the AKR mice, it was distributed more evenly across the gonadal and mesenteric fat depots of the B6 mice. In the 129P3 mice, there were significant effects of 34% sucrose on the weights of the gonadal and retroperitoneal (but not the mesenteric) fat depots. The vast majority of fat accumulation in the 129P3 mice occurred in the gonadal depot. These strain differences in body fat distribution are of general interest because higher mesenteric (i.e., abdominal) fat levels are co-morbid with cardiovascular disease in humans [21,53]. Several investigators have already begun to explore the genetic architecture of such strain differences in body fat distribution in mice (e.g., see [55,65]).
5.3. Conclusion
Our results serve as a cautionary tale. First, even though polymorphisms of the Tas1r3 gene are associated with strain differences in intake of and preference for sugars in sugar-naïve mice, we found that these genetically based differences are modified by dietary experience with sugars. Second, many investigators assume that a positive relationship exists between sugar intake and fat accumulation. We observed this positive relationship in the AKR, 129P3 and B6 mice, but not in the FVB mice. In fact, the FVB mice consumed significantly more sugar calories than the other mouse strains, but avoided weight gain. It may be that energy expenditure is a more important determinant of fat accumulation than energy intake in the FVB mice. Several studies provide support for this hypothesis in humans [19,46]. Finally, it is generally assumed that fructose is more obesogenic than sucrose. Among the strains we tested, we found the opposite result. We propose that because the sucrose solutions were more orally acceptable and provided stronger post-oral reinforcement of feeding, the mice consumed more of them. The elevated intake of the 34% sucrose solution in particular increased caloric intake, resulting in obesity in three of the four mouse strains.
Acknowledgements
This project was supported in part by grants from the Howard Hughes Medical Institute to Barnard College, and the National Institute of Diabetes and Digestive and Kidney Diseases to A. Sclafani (DK-031135).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackroff K, Yiin Y-M, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav. 2010;99:402–411. doi: 10.1016/j.physbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander J, Chang GQ, Dourmashkin JT, Leibowitz SF. Distinct phenotypes of obesity-prone AKR/J, DBA/2J and C57BL/6J mice compared to control strains. Int J Obesity. 2006;30:50–59. doi: 10.1038/sj.ijo.0803110. [DOI] [PubMed] [Google Scholar]
- 3.Allison DB, Mattes RD. Nutritively sweetened beverage consumption and obesity: the need for solid evidence on a fluid issue. JAMA. 2009;301:318–320. doi: 10.1001/jama.2008.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004;53:3274–3285. doi: 10.2337/diabetes.53.12.3274. [DOI] [PubMed] [Google Scholar]
- 5.Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. PNAS. 2007;104:2366–2371. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachmanov AA, Tordoff MG, Beauchamp GK. Voluntary sodium chloride consumption by mice: differences among five inbred strains. Behav Genet. 1998;28:117–124. doi: 10.1023/a:1021471924143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiol Behav. 2001;72:603–613. doi: 10.1016/s0031-9384(01)00412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–913. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmanov AA, Beauchamp GK. Taste receptor genes. Ann Rev Nutr. 2007;27:389–414. doi: 10.1146/annurev.nutr.26.061505.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg C, Lappas G, Wolk A, Strandhagen E, Torén K, Rosengren A, Thelle D, Lissner L. Eating patterns and portion size associated with obesity in a Swedish population. Appetite. 2008;52:21–26. doi: 10.1016/j.appet.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Bergheim I, Weber S, Vos M, Krämer S, Volynets V, Kaserouni S, McClain CJ, Bischof SC. Anitbiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983–992. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 12.Berkey CS, Rockett HRH, Field AE, Gillman MW, Colditz GA. Sugar-added beverages and adolescent weight change. Obesity Res. 2004;12:778–788. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- 13.Berne C, Fagius J, Niklasson F. Sympathetic response to oral carbohydrate administration evidence from microelectrode nerve recordings. J Clin Invest. 1989;84:1403–1409. doi: 10.1172/JCI114313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bray GA, Nielson SJ, Opokin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 15.Bray GA. Soft drink consumption and obesity: it is all about fructose. 2010:21. doi: 10.1097/MOL.0b013e3283346ca2. In press. [DOI] [PubMed] [Google Scholar]
- 16.Brito VB, Folmer V, Soares JCM, Silveira ID, Rocha JBT. Long-term sucrose and glucose consumption decreases the delta-aminolevulinate dehydratase activity in mice. Nutrition. 2007;23:818–826. doi: 10.1016/j.nut.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Brownlow BS, Petro A, Feinglos MN, Surwit RS. The role of motor activity in diet-induced obesity in C57BL/6J mice. Physiol Behav. 1996;60:37–41. doi: 10.1016/0031-9384(95)02210-4. [DOI] [PubMed] [Google Scholar]
- 18.Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- 19.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng Y-H, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeRuisseau LR, Parsons AD, Overton JM. Adaptive thermogenesis is intact in B6 and A/J mice studied at thermoneutrality. Metabolism. 2004;53:1417–1423. doi: 10.1016/j.metabol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Déspres J-P, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arterioscl Thromb Vasc Biol. 1990;10:497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- 22.Donaldson LF, Bennett L, Baic S, Melichar JK. Taste and weight: is there a link? Am J Clin Nutr. 2009;90(suppl):800–803. doi: 10.3945/ajcn.2009.27462Q. [DOI] [PubMed] [Google Scholar]
- 23.DuBois GE, Walters ET, Schiffman SS, Warwick ZE, Booth BJ, Pecore SD, Gibes K, Carr BT, Brands LM. Concentration-response relationships of sweeteners: a systematic study. In: Walters DE, Orthoefer FT, DuBois GE, editors. Sweeteners: discovery, molecular design, and chemoreception. American Chemical Society; 1991. pp. 261–276. (ACS Symposium Series 450). [Google Scholar]
- 24.Eberhart GP, West DB, Boozer CN, Atkinson RL. Insulin sensitivity of adipocytes from inbred mouse strains resistant or sensitive to diet-induced obesity. Am J Physiol. 1994;266:R1423–R1428. doi: 10.1152/ajpregu.1994.266.5.R1423. [DOI] [PubMed] [Google Scholar]
- 25.Eylam S, Spector AC. Stimulus processing of glycine is dissociable from that of sucrose and glucose based on behaviorally measured taste signal detection in Sac ‘taster’ and ‘non-taster’ mice. Chem Senses. 2004;29:639–649. doi: 10.1093/chemse/bjh068. [DOI] [PubMed] [Google Scholar]
- 26.Fink BD, Herlein JA, Almind K, Cinti S, Kahn CR, Sivitz WI. Mitochondrial proton leak in obesity-resistant and obesity-prone mice. Am J Physiol. 2007;293:R1773–R1780. doi: 10.1152/ajpregu.00478.2007. [DOI] [PubMed] [Google Scholar]
- 27.Flegal KM, Carrol MD, Ogden CL, Johnson CL. Overweight and obesity in the United States: prevalence and trends (1960-1994). Int J Obes. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 28.Flegal KM, Troiano RP. Changes in the distribution of body mass index of adults and children in the US population. Int J Obesity. 2000;24:807–818. doi: 10.1038/sj.ijo.0801232. [DOI] [PubMed] [Google Scholar]
- 29.Fontvielle AM, Faurion A, Helal I, Rizkalla SW, Falgon S, Letanoux M, Tchobroutsky G, Slama G. Relative sweetness of fructose compared with sucrose in healthy and diabetic subjects. Diabetes Care. 1989;12:481–486. doi: 10.2337/diacare.12.7.481. [DOI] [PubMed] [Google Scholar]
- 30.Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nature Med. 1995;1:1311–1414. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 31.Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 2002;27:461–474. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- 32.Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses. 2005;30:299–316. doi: 10.1093/chemse/bji025. [DOI] [PubMed] [Google Scholar]
- 33.Glendinning JI, Chyou S, Lin I, Onishi M, Patel P, Zheng KH. Initial licking responses of mice to sweeteners: effects of Tas1r3 polymorphisms. Chem Senses. 2005;30:601–614. doi: 10.1093/chemse/bji054. [DOI] [PubMed] [Google Scholar]
- 34.Glendinning JI, Feld N, Goodman L, Bayor R. Contribution of orosensory stimulation to strain differences in oil intake by mice. Physiol Behav. 2008;95:476–483. doi: 10.1016/j.physbeh.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Gnudit L, Tozzo E, Shepherds PR, Bliss JL, Kahn BB. High level overexpression of glucose transporter-4 driven by an adipose-specific promoter Is maintained in transgenic mice on a high fat diet, but does not prevent impaired glucose tolerance. Endocrinol. 1995;136:995–1002. doi: 10.1210/endo.136.3.7867610. [DOI] [PubMed] [Google Scholar]
- 36.Hanover LM, White JS. Manufacturing, composition, and applications of fructose. Am J Clin Nutr. 1993;58(suppl):724S–732S. doi: 10.1093/ajcn/58.5.724S. [DOI] [PubMed] [Google Scholar]
- 37.Hardy SL, Brennard CP, Wyse BW. Fructose: comparison with sucrose as sweetener in four products. J Am Dietetic Assoc. 1979;74:41–46. [PubMed] [Google Scholar]
- 38.Hu CC, Qing K, Chen Y. Diet-induced changes in stearoyl-CoA desaturase 1 expression in obesity-prone and -resistant mice. Obesity Res. 2004;12:1264–1270. doi: 10.1038/oby.2004.160. [DOI] [PubMed] [Google Scholar]
- 39.Hyvönen L, Kurkela R, Koivistoinen P, Merimaa P. Effects of temperature and concentration on the relative sweetness of fructose, glucose and xylitol. Lebensmittelwiss u -technolog. 1977;10:316–320. [Google Scholar]
- 40.Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiol Genomics. 2007;32:82–94. doi: 10.1152/physiolgenomics.00161.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jürgens H, Haass W, Castaneda TR, Schürmann A, Koebnick C, Dombrowski F, Otto B, Nawrocki AR, Scherer PE, Spranger J, Ristow M, Joost HG, Havel PJ, Tschöp MH. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res. 2005;13:1146–1156. doi: 10.1038/oby.2005.136. [DOI] [PubMed] [Google Scholar]
- 42.Kanarek RB, Orthen-Gambill N. Differential effects of sucrose, fructose and glucose on carbohydrate-induced obesity in rats. J Nutr. 1982;112:1546–1554. doi: 10.1093/jn/112.8.1546. [DOI] [PubMed] [Google Scholar]
- 43.Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol. 2008;294:R699–R710. doi: 10.1152/ajpregu.00095.2007. [DOI] [PubMed] [Google Scholar]
- 44.Le Lay S, Boucher J, Rey A, Castan-Laurell I, Krief S, Ferre P, Valet P, Dugail I. Decreased resistin expression in mice with different sensitivities to a high-fat diet. Biochem Biophys Res Comm. 2001;289:564–567. doi: 10.1006/bbrc.2001.6015. [DOI] [PubMed] [Google Scholar]
- 45.Leibowitz SF, Alexander J, Dourmashkin JT, Hill JO, Gayles EC, Chang G-Q. Phenotypic profile of SWR/J and A/J mice compared to control strains: possible mechanisms underlying resistance to obesity on a high-fat diet. Brain Res. 2005;1047:137–147. doi: 10.1016/j.brainres.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 46.Levine JA, Kotz CM. NEAT–non-exercise activity thermogenesis–egocentric and geocentric environmental factors vs. biological regulation. Acta Physiol Scand. 2005;184:309–318. doi: 10.1111/j.1365-201X.2005.01467.x. [DOI] [PubMed] [Google Scholar]
- 47.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 48.Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ. Inbred mouse strain survey of sucrose intake. Physiol Behav. 2005;85:546–556. doi: 10.1016/j.physbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Light HR, Tsanzi E, Gigliotti J, Morgan K, Tou JC. The type of caloric sweetener added to water influences weight gain, fat mass, and reproduction in growing Sprague-Dawley female rats. Exp Biol Med. 2009;234:651–661. doi: 10.3181/0812-RM-368. [DOI] [PubMed] [Google Scholar]
- 50.Lindqvist A, Baelemans A, Erlanson-Albertsson C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul Peptides. 2008;150:26–32. doi: 10.1016/j.regpep.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Marks-Kaufman R, Hamm MW, Barbato GF. The effects of dietary sucrose on opiate receptor binding in genetically obese (ob/ob) and lean mice. J Am College Nutr. 1989;8:9–14. doi: 10.1080/07315724.1989.10720272. [DOI] [PubMed] [Google Scholar]
- 52.Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem. 2006;281:18933–18941. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- 53.Matsuzawa Y, Shimomura I, Nakamura T, Keon Y, Kotani K, Tokunaga K. Pathophysiology and pathogenesis of visceral fat obesity. Obes Res. 1995;3(suppl.)):187S–194S. doi: 10.1002/j.1550-8528.1995.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 54.Max M, Shankar YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nature Gen. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- 55.McDaniel AH, Li X, Tordoff MG, Bachmanov AA, Reed DR. A locus on mouse Chromosome 9 (Adip5) affects the relative weight of the gonadal but not retroperitoneal adipose depot. Mamm Genome. 2006;17:1078–1092. doi: 10.1007/s00335-006-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Messier C, Whately K, Liang J, Du L, David Puissant D. The effects of a high-fat, high-fructose, and combination diet on learning, weight, and glucose regulation in C57BL/6 mice. Behav Brain Res. 2007;178:139–145. doi: 10.1016/j.bbr.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Metlakunta AS, Sahu M, Sahu A. Hypothalamic phosphatidylinositol 3-kinase pathway of leptin signaling is impaired during the development of diet-induced obesity in FVB/N mice. Endocrinol. 2008;149:1121–1128. doi: 10.1210/en.2007-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biology. 2005;15:1948–1952. doi: 10.1016/j.cub.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 59.Nishikawa S, Yasoshima A, Doi K, Nakayama H, Uetsuka K. Involvement of sex, strain and age factors in high fat diet-induced obesity in C57BL/6J and BALB/cA mice. Exp Anim. 2007;56:263–272. doi: 10.1538/expanim.56.263. [DOI] [PubMed] [Google Scholar]
- 60.Ogden C, Carrol MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;13:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 61.Pereira MA, Jacobs J, D.R. Sugar-sweetened beverages, weight gain and nutritional epidemiological study design. Brit J Nutr. 2008;99:1169–1170. doi: 10.1017/S0007114507868498. [DOI] [PubMed] [Google Scholar]
- 62.Pothion S, Bizot J-C, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res. 2004;155:135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Prpic V, Watson PM, Frampton IC, Sabol MA, Jezek GE, Gettys TW. Adaptive changes in adipocyte gene expression differ in AKR/J and SWR/J mice during diet-induced obesity. J Nutr. 2002;132:3325–3332. doi: 10.1093/jn/132.11.3325. [DOI] [PubMed] [Google Scholar]
- 64.Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24:938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reed DR, McDaniel AH, Li X, Tordoff MG, Bachmanov AA. Quantitative trait loci for individual adipose depot weights in C57BL/6ByJ x 129P3/J F2 mice. Mamm Genome. 2006;17:1065–1077. doi: 10.1007/s00335-006-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes-related traits in mouse strains susceptible to diet-Induced obesity. Diabetes. 2003;52:1958–1966. doi: 10.2337/diabetes.52.8.1958. [DOI] [PubMed] [Google Scholar]
- 67.Ruxton CHS, Garnder EJ, McNulty HM. Is sugar consumption detrimental to health? A review of the evidence 1995–2006. Crit Rev Food Sci Nutr. 2010;50:1–19. doi: 10.1080/10408390802248569. [DOI] [PubMed] [Google Scholar]
- 68.Schiffman SS, Brevick BG, Sattely-Miller EA, Peterson-Dancy M. Elevated and sustained desire for sweet taste in African-Americans: a potential factor in the development of obesity. Nutrition. 2000;16:886–893. doi: 10.1016/s0899-9007(00)00403-2. [DOI] [PubMed] [Google Scholar]
- 69.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of Type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 70.Sclafani A. Carbohydrate-induced hyperphagia and obesity in the rat: effects of saccharide type, form, and taste. Neurosci Biobehav Rev. 1987;11:155–162. doi: 10.1016/s0149-7634(87)80020-9. [DOI] [PubMed] [Google Scholar]
- 71.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol. 2005;289:R712–R720. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 72.Sclafani A. Sucrose motivation in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice measured by progressive ratio licking. Physio Behav. 2006;87:734–744. doi: 10.1016/j.physbeh.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 73.Sclafani A. Enhanced sucrose and Polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice after experience with these saccharides. Physiol Behav. 2006;87:745–756. doi: 10.1016/j.physbeh.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 74.Spraul M, Ravussin E, Fontvieille AM, Rising R, Larson DE, Anderson EA. Reduced sympathetic nervous activity. A potential mechanism predisposing to body weight gain. J Clin Invest. 1993;92:1730–1735. doi: 10.1172/JCI116760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stallmann-Jorgensen IS, Gutin B, Hatfield-Laube JL, Humphries MC, Johnson MH, Barbeau P. General and visceral adiposity in black and white adolescents and their relation with reported physical activity and diet. Int J Obesity. 2007;31:622–629. doi: 10.1038/sj.ijo.0803587. [DOI] [PubMed] [Google Scholar]
- 76.Surwit RS, Wang S, Petro AE, Sanchis D, Raimbault S, Ricquier S, Collins S. Diet-induced changes in uncoupling proteins in obesity-prone and obesity-resistant strains of mice. Proc Natl Acad Sci USA. 1998;95:4061–4065. doi: 10.1073/pnas.95.7.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takahashi N, Patel HR, Qi Y, Dushay J, Ahima RS. Divergent effects of leptin in mice susceptible or resistant to obesity. Horm Metab Res. 2002;34:691–697. doi: 10.1055/s-2002-38251. [DOI] [PubMed] [Google Scholar]
- 78.Takeda M, Imaizumi M, Sawano S, Manabe Y, Fushiki T. Long-term optional ingestion of corn oil induces excessive caloric intake and obesity in mice. Nutrition. 2001;17:117–120. doi: 10.1016/s0899-9007(00)00513-x. [DOI] [PubMed] [Google Scholar]
- 79.Tappy L, Lê K-A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 80.Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- 81.Weber S, Volynets V, Kanuri G, Bergheim I, Bischoff SC. Treatment with the 5-HT3 antagonist tropisetron modulates glucose-induced obesity in mice. Int J Obes. 2009;33:1339–1347. doi: 10.1038/ijo.2009.191. [DOI] [PubMed] [Google Scholar]
- 82.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262:R1025–R1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 83.West DB, Waguespack J, McCollister S. Dietary obesity in the mouse: interaction of strain with diet composition. Am J Physiol. 1995;268:R658–R665. doi: 10.1152/ajpregu.1995.268.3.R658. [DOI] [PubMed] [Google Scholar]
- 84.Young JB, Landsberg L. Diminished sympathetic nervous system activity in genetically obese (ob/ob) mouse. Am J Physiol. 1983;245:E148–E154. doi: 10.1152/ajpendo.1983.245.2.E148. [DOI] [PubMed] [Google Scholar]