Abstract
BACKGROUND
Dietary lycopene combined with other constituents from whole tomatoes was previously found to have greater chemopreventive effects against prostate cancer as compared to pure lycopene provided in a beadlet formulation. We hypothesized that tomato paste would have greater chemopreventive effects in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice relative to equivalent lycopene doses provided from lycopene beadlets.
METHODS
Fifty-nine TRAMP mice were randomized to a control diet or to diets providing 28 mg lycopene per kg diet from tomato paste (TP) or from lycopene beadlet (LB), and sacrificed at 20 weeks. Prostate histopathology, prostate weight and serum levels of IGF-I and IGF binding protein-3 were evaluated.
RESULTS
The incidence of prostate cancer was significantly decreased in the LB group relative to the control group (60% vs 95% respectively, p=0.0197) whereas the difference between the TP and control groups was not statistically significant (80% vs. 95%, p=0.34). There was no difference in prostate weights between the groups. Total lycopene levels in the serum and prostate tissue were similarly elevated in the LB and TP groups relative to the control group. The ratio of 5-cis-lycopene to trans-lycopene in the serum was significantly greater in the LB group relative to the TP group (p=0.0001). Oxidative DNA damage was significantly reduced in the livers of mice fed LB and TP diets relative to the control group.
CONCLUSIONS
This preclinical trial suggests significant chemopreventive activity with a lycopene beadlet-enriched diet. The chemopreventive effects of lycopene from beadlets versus whole tomato products requires further testing in preclinical and clinical models of prostate cancer.
Keywords: Lycopene, Prostatic Neoplasms, Carotenoids, Insulin-Like Growth Factor I, Transgenic
INTRODUCTION
Prostate cancer (CaP) continues to be one of the leading causes of cancer-related mortality in the United States. As such, a number of potentially chemopreventive agents are being actively investigated in CaP to delay the process of carcinogenesis. One agent currently under active investigation is lycopene, a carotenoid found in highest levels in processed tomato products such as tomato sauce, tomato paste, and tomato juice. Lycopene is a potent antioxidant and may exert chemopreventive effects through scavenging of free radicals, induction of apoptosis, and inhibition of cellular proliferative processes.[1] In natural sources, lycopene is present entirely in the all trans-isomer form. Upon exposure to light, heat, or chemical reactions, isomerization can occur producing various forms of mono- and poly-cis isomers.[2]
Lycopene has been found in a number of epidemiologic studies to be associated with a lower risk of prostate cancer.[3] Two studies, in particular, support the intake of lycopene and tomato products as a potential contributor to the reduction of prostate cancer risk.[4–5] Another epidemiologic study did not demonstrate an association between lycopene intake and decreased prostate cancer risk.[2] In a prospective, case-controlled clinical trial, Chen, et al. and Kim et al. demonstrated that daily intake of commercial spaghetti sauce (30mg of lycopene in 200g of sauce) in pasta dishes for three weeks prior to radical prostatectomy resulted in significantly decreased oxidative DNA damage in prostate tissue compared to controls and increased apoptosis of prostate cancer epithelial cells.[6,7]
In a preclinical study, Venkateswaran, et al., demonstrated that administration of antioxidants (including lycopene, selenium, and vitamin E) in the diet of Lady transgenic mice inhibited prostate cancer development and increased disease-free survival.[8] Limpens, et al. demonstrated that lycopene in combination with vitamin E inhibited prostate cancer progression and median survival in an orthotopic model of prostate cancer in nude mice.[9] Utilizing a Dunning R3327-H prostate adenocarcinoma model, Canene-Adams, et al. demonstrated that dietary supplementation with whole tomato powder resulted in significantly decreased tumor weights compared to a control diet, whereas there was no difference seen in rats fed a diet containing lycopene from beadlets.[10] Boileau, et al. utilized a rat model for prostate carcinogenesis (male rats treated with N-methyl-N-nitrosourea and testosterone) and found that animals consuming a semipurified diet containing a tomato powder product that includes the skin and seeds of the tomato had a reduced incidence of prostate cancer compared to rats consuming lycopene from a beadlet formulation. This suggested that tomato products contain components in addition to lycopene that may interfere with prostate carcinogenesis.[11]
To further evaluate the chemopreventive effects of lycopene with or without other constituents of the whole tomato, we fed transgenic adenocarcinoma of the prostate (TRAMP) mice equivalent doses of lycopene from a tomato paste product or from a lycopene beadlet and compared prostate histopathology at 20 weeks with mice on a control diet. We hypothesized that tomato paste would have greater chemopreventive effects in TRAMP mice relative to equivalent lycopene doses provided from lycopene beadlets.
MATERIALS AND METHODS
Animal husbandry
The experimental protocol was approved by the University of California at Los Angeles Chancellor's Animal Research Committee and the animals were cared for in accordance with institutional guidelines. Briefly, male and female heterozygous C57BL/TGN TRAMP mice were purchased as breeding pairs from The Jackson Laboratory (Bar Harbor, ME). The animals were bred on the same genetic background using standard procedures and maintained in a manner consistent with the NIH Guidelines for the Care and Use of Laboratory Animals. Transgenic males for these studies were routinely obtained as [TRAMP × C57BL/6] F1 or as [TRAMP × C57BL/6] F2 offspring. Identity of the mice was established by screening of DNA by PCR.
Study Design and Feeding Protocol
Male TRAMP (C57BL/6-TRAMP/+) mice were randomized to their respective diets at weaning age and were caged 3–4 mice per cage. Randomization occurred to one of three diets: 1) AIN 93G (control) 2) AIN 93G with tomato paste formulated with 100 mg lycopene/kg diet 3) AIN 93G with 10% lycopene beadlets providing 100 mg lycopene/kg diet. The tomato paste was provided by Campbell's Soup Company (Camden, NJ) and contained all components of the tomato excluding the seeds and skin. Note that the amount of lycopene provided by each diet was approximately 28 ppm as this was the measured lycopene levels in the feed after exposure to the environment (see second paragraph in the Results Section). The composition of macronutrients in the tomato paste was: 1) protein – 6.4 grams/100 grams paste, 2) fat – 0 grams, 3) carbohydrate 24.2 grams/100 grams paste. The lycopene beadlets were obtained from DSM (Parsippany, NJ) and contained 5% ascorbyl palmitate and 1.5% α-tocopherol as antioxidants. The diets were prepared and pelleted by Dyets, Inc. (Bethlehem, PA) and the composition of the three diets is listed in Table I. The vitamin mix added to each of the 3 diets provides 75 IU of α-tocopherol per kilogram of diet thus resulting in comparable amounts of total α-tocopherol in each diet. Mice were fed ad libitum twice weekly and weighed weekly. Uneaten food was weighed and measured twice weekly. The diets were stored in packaging that blocked exposure to light and were stored in dark refrigerators prior to use. The weekly caloric intake per mouse was estimated by dividing the amount of food consumed per week per cage by the number of mice per cage. Diets were continued until mice were 20 weeks of age. Mice were provided access to water ad libitum.
TABLE I.
Composition of Diets
| INGREDIENTS | AIN-93G DIET (gm/kg diet) | AIN-93G DIET & PASTE (gm/kg diet) | AIN-93G DIET & BEADLET (gm/kg diet) |
|---|---|---|---|
| Casein, High Nitrogen | 200 | 191 | 200 |
| L-Cystine | 3 | 2.8 | 3 |
| Sucrose | 90 | 90 | 90 |
| Cornstarch | 397.486 | 374.486 | 396.486 |
| Dyetrose | 132 | 124 | 132 |
| Soybean Oil | 70.016 | 70.016 | 70.014 |
| Cellulose | 50 | 50 | 50 |
| Mineral Mix #210025 | 35 | 35 | 35 |
| Vitamin Mix #310025λ | 10 | 10 | 10 |
| Supplement #410750* | 10 | 10 | 10 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 |
| Tomato Paste | 0 | 128 | 0 |
| 10% Lycopene Beadlet+ | 0 | 0 | 1 |
Supplement includes vitamin B12, vitamin K, L-methionine to meet 1995 NRC requirements for rats.
Lycopene beadlet purchased from DSM and contains 5% ascorbyl palmitate and 1.5% DL-α-tocopherol as antioxidants
Vitamin Mix contains 75 IU vitamin E per kilogram diet
Tissue Procurement and Pathologic Evaluation
At 20 weeks of age, mice were anesthetized with isoflurane, cardiac puncture was performed, and mice were euthanized as per institutional guidelines. The mice were not fasted prior to sacrifice. The prostate from each mouse was dissected, measured, weighed, and fixed in 10% neutral buffered formalin and embedded in paraffin blocks for subsequent histologic analysis.
Four micron sections were stained with hematoxylin and eosin to assess the presence or absence of prostatic intraepithelial neoplasia, and/or carcinoma. Grading of the carcinomas (well, moderately,or poorly differentiated) was determined using criteria previously described.[12] All stained slides were read by a single pathologist (JWS) who was blinded to the treatment groups.
Measurement of Serum IGF-I and IGFBP-3 Levels
The levels of murine IGF-I and IGFBP-3 were measured as described previously.[13–14] Briefly, in-house mouse-specific ELISAs were used, using mouse-specific monoclonal antibodies and recombinant mouse IGF and IGFBP standards from R&D Systems (Minneapolis, MN). The mouse IGF-I assay has a sensitivity of 0.1 ng/mL and no cross-reactivity with mouse IGF-II or human IGF-I. The intraassay and interassay coefficients of variation were <10%. The mouse IGFBP-3 assays have sensitivities of 0.2 ng/mL and no cross-reactivity with other IGFBPs or the human homologues. The intraassay and interassay coefficients of variations were <6% and <8%, respectively.
Determination of Lycopene and α-Tocopherol (Vitamin E) Levels and Oxidative DNA Damage
In a separate experiment, 22 C57BL/TGN (same background as TRAMP mice) wild-type mice were randomly assigned to the three experimental diets and fed ad lib twice weekly for three weeks. After an overnight fast of eight hours, mice were sacrificed as described above, serum obtained via cardiac puncture, livers and prostates dissected, and immediately frozen in liquid nitrogen. Serum and prostate tissue lycopene levels and serum α-tocopherol levels were determined by high performance liquid chromatography (HPLC). Prostate tissue was extracted following the method by Ferreira, et al.[15] Briefly, 200–300mg of tissue was homogenized in 5mL chloroform:methanol mixture (2:1) including echinenone as internal standard. The mixture was vortexed and 0.5 mL of saline (8.5 grams/L) was added. The mixture was centrifuged at 800 × g at 4°C. The lower phase was extracted with hexane containing 1% butylated hydroxytoluene. The hexane extract was combined with the upper layer evaporated and the residue was dissolved in methanol:ethyl acetate (2:3 vol/vol). After HPLC, lycopene and lycopene isomers were quantitated at 445 nanometers (nm) and α-tocopherol at 292 nm compared to a standard curve.[15,16] Carotenoids and tocopherols were separated using a C18 Bakerbond narrow-pore, 250 × 4.6 mm reverse phase column (J.T. Baker, Inc., Phillipsburg, NJ), a 1050 Agilent Technology quaternary pump (Wilmington, DE), and autosampler, and a multiple wavelength detector. Data was evaluated using the Agilent Technology Chemstation Software 9.01 (Wilmington, DE). Serum and feed lycopene and serum tocopherol were quantitated in the same manner, using HPLC. For the prostate tissue lycopene measurements, two to three prostate specimens from each diet group were pooled..Echinenone was purchased from CaroteNature (Lupsingen, Switzerland). Lycopene, α-tocopherol, butylated hydroxytoluene, deferoxamine mesylate, guanidine thiocyanate, N-lauroylsarcosinate were purchased from Sigma-Aldrich (St. Louis, MO). Chelex 100 resin was purchased from BioRad Laboratories (Hercules, CA). All HPLC-grade solvents were purchased from Fisher Scientific Co. (Tustin, CA).
DNA was isolated from liver tissue, as described by Hofer, et al.[17] Briefly, liver tissue was homogenized in ten volumes of extraction buffer (10 mM deferoxamine mesylate, 3M guanidine thiocyanate, 0.2% w/v N-laroylsacrosinate, 20 mM Tris, ph 7.5, chelex-treated with resin, pre-colled at 4°C). DNA was extracted from the homogenate with phenol/chloroform/isoamyl alcohol (25:24:1 vol:vol:vol). One hundred milligrams of DNA was digested using a modification of the method described by Shigenga, et al and Huang, et al.[18–19] 8-hydroxydeoxyguanosine (8-oxodG) and deoxyguanosine were detected by HPLC (Agilent Technology 1100) using an Agilent Technology variable wavelength detector to determine deoxyguanosine (dG) at 245 nm (Wilmington, DE) and ESA coulochem II electrochemical detector (ESA, Bedford, MA) to detect 8-oxodG. The concentration of 8-oxodG was expressed as a ratio of 8-oxodG to 106 dG. 8-oxo-dG was purchased from Calbiochem (EMD Biosciences, La Jolla, CA).
Statistical Analysis
Statistical analysis was performed using GraphPad InStat Version 3.06 Software (La Jolla, CA) and S-plus version 6.0 (Insightful Corp.). The Fisher's exact test was used to compare cancer incidence between the three diet groups and subsequently to perform pairwise testing of the groups. Cancer was defined as the presence of well-, moderately-, or poorly- differentiated CaP, whereas either mPIN or benign tissue was classified as “non-cancer.” Quantitative measures were compared across groups using one-way analysis of variance (ANOVA) and post-hoc analysis was perfomed using Tukey's test. A p value <0.05 was considered to be significant.
RESULTS
Caloric Intake and Mouse Weights
The mean weekly caloric intake was not significantly different between mice in the 3 diet groups. Mice in the control, TP, and LB groups consumed on average 721.6 +/− 24.9 kcal/gram mouse weight, 735.2 +/− 26.1 kcal/gram mouse weight, and 734.1 +/− 14.0 kcal/gram mouse weight weekly, respectively. Likewise, average mouse weights did not differ throughout the experiment. The mean mouse weight at sacrifice (20 weeks of age) was 24.0 +/− 3.4 grams, 24.3 +/− 2.7 grams, and 23.9 +/− 3.0 grams in the control, TP, and LB groups, respectively.
Lycopene Content and Deterioration Over Time
There was no significant difference in the lycopene content of the TP and LB feed pellets immediately after removal from storage at 4° (27.69 +/− 0.54 mg/kg vs. 28.37 +/− 1.8 mg/kg) whereas no lycopene was detectable in the control diet. The lycopene content in TP and LB diets deteriorated similarly over a period of seven days with a mean decrease in lycopene content of 9.3 mg/kg and 8.9 mg/kg diet in the TP and LB diets, respectively.
Histopathology, Prostate Weights, and Serum IGF-I and IGFBP-3 levels in TRAMP Mice
The histopathology results at 20 weeks of age are depicted in Figure I. The LB group had a reduced incidence of prostate cancer relative to the control group (60% vs 95% respectively, p=0.0197) whereas the difference in prostate cancer incidence between the TP and control group was not statistically significant (80% vs. 95%, p=0.34). Six of 20 mice (30%) in the LB group had a benign prostate phenotype (no evidence of mouse PIN or cancer) whereas the purely benign phenotype was not present in the control or TP group. The incidence of benign phenotype was significantly higher in the LB group relative to the control group (p=0.0202) and TP group (p=0.0202). There was no difference in mean prostate weight between the groups at 20 weeks of age. The mean weights were 0.16 +/− 0.04 grams, 0.13 +/− 0.04 grams, and 0.15 +/− 0.05 grams in the control, TP, and LB groups, respectively.
FIGURE I. Cancer Incidence and Histopathology.
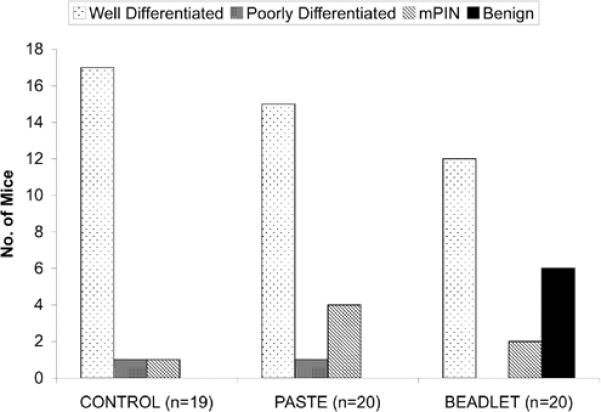
The LB group had a reduced incidence of prostate cancer relative to the control group (60% vs 95% respectively, p<0.00197) whereas the difference in prostate cancer incidence between the TP and control groups was not statistically significant (80% vs. 95%, p=0.34). The purely benign phenotype was only present in the LB group. mPIN = mouse prostatic intraepithelial neoplasia.
There was no significant difference in mean serum levels of IGF-I and IGFBP-3 between the groups at age 20 weeks. Mean IGF-I levels were 188.6 +/− 27.0 ng/mL, 198.0 +/− 27.0 ng/mL, and 199.8 +/− 19.9 ng/mL in the control, TP, and LB groups, respectively. Mean IGFBP-3 levels were 883.4 +/− 186.5 ng/mL, 901.2 +/− 284.6 ng/mL, and 983.2 +/− 192.1 ng/mL in the control, TP, and LB groups, respectively. Though mean serum IGFBP-3 level was higher in the LB group than the control group (983.2 +/− 192.1 ng/mL vs. 883.4 +/− 186.5 ng/mL), the difference was not statistically significant (p=0.3659).
Lycopene and Vitamin E Levels in Wild-Type Mice
There was no difference in mean serum lycopene levels in mice fed TP and LB diets (0.2 +/− 0.2 μmol/L and 0.2 +/− 0.04 μmol/L, respectively), and lycopene levels were undetectable in mice on the control diet (Figure IIA). Similarly, there was no difference in lycopene levels in pooled prostate tissue in the TP and LB groups (0.25 +/− 0.04 nmol/g, and 0.26 +/− 0.1 nmol/g, respectively) and lycopene levels were undetectable in mice on the control diet (Figure IIB). There was a significantly higher ratio of serum 5-cis- to trans- lycopene in the LB group relative to the TP group (LB 5-cis-:trans- 1.3 +/− 0.1, TP 5-cis-:trans- 0.79 +/− 0.1, p<0.0001). No difference was noted in the mean serum α-tocopherol levels in mice fed TP, LB, or control diets (10.8 +/− 1.7 μmol/L, 12.4 +/− 0.76 μmol/L, 11.2 +/− 2.1 μmol/L, respectively).
FIGURE IIA. Serum Lycopene Levels.
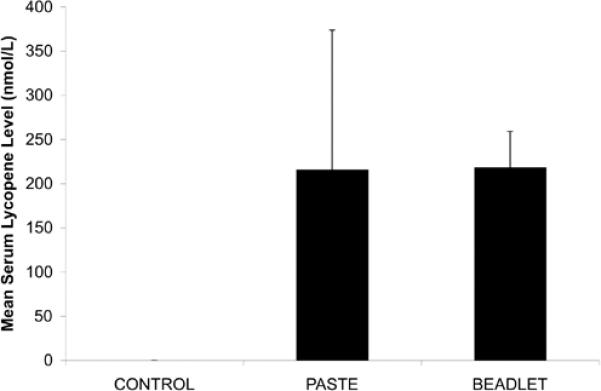
Fasting serum lycopene levels 6-week-old in wild-type C57BL/TGN male mice fed control, TP, and LB diets for three weeks (0 μmol/L, 0.2 +/− 0.2 μmol/L, 0.2 +/− 0.04 μmol/L, respectively). Lycopene levels were undetectable in mice on the control diet.
FIGURE IIB. Prostate Lycopene Levels.
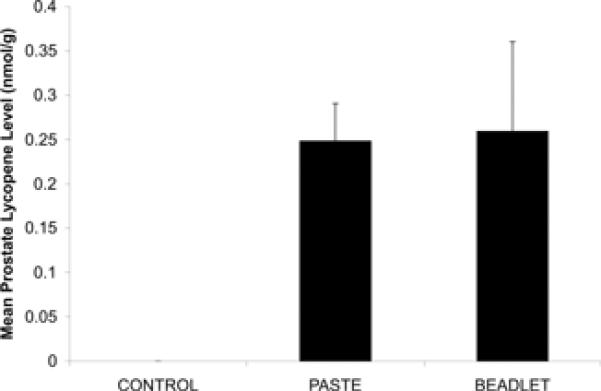
Prostate lycopene levels in 6-week-old wild-type C57BL/TGN male mice fed control, TP, and LB diets for three weeks (0 nmol/g, 0.25 +/− 0.04 nmol/g, and 0.26 +/− 0.1 nmol/g, respectively). Two to three prostate specimens were pooled for analysis in the control (n=6), LB (n=4), and TP (n=5) groups. Prostate lycopene levels were undetectable in mice on the control diet. Sample measurements were performed in quadruplicate.
Oxidative DNA Damage in Wild-Type Mice
The mean oxidative DNA damage (ratio of 8-oxodG:106 dG) in the liver was significantly reduced in the TP and LB groups relative to the control group (TP: 3.2 +/− 0.9, LB: 3.4 +/− 0.8, Control: 5.2 +/− 0.6, p=0.01, Figure III). There was no difference in oxidative DNA damage in the liver between the TP and LB beadlet groups.
FIGURE III. Liver Oxidative DNA Damage.
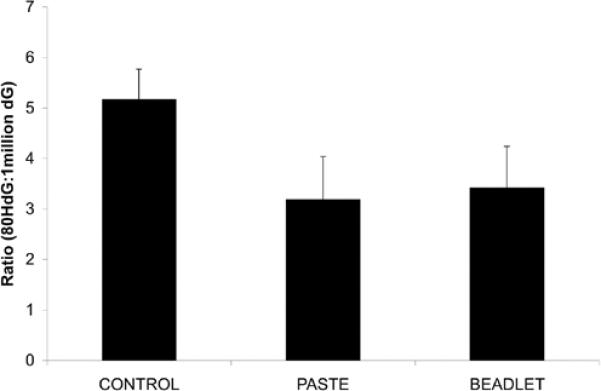
Oxidative DNA damage (ratio of 80 hydoxydeoxyguanosine (HdG):106 deoxyguanosine (dG)) in the liver of 6-week-old wild-type C57BL/TGN male mice fed control, TP, and LB diets for three weeks. HdG/dG levels were significantly reduced in the TP and LB groups relative to the control group. Values are expressed as mean +/− SEM.
DISCUSSION
Based on prior work demonstrating that dietary lycopene provided from a whole tomato product (tomato powder) inhibited carcinogenesis to a greater degree than lycopene beadlet in an NMU-testosterone treated rat model, we hypothesized that TP but not LB would inhibit carcinogenesis in TRAMP mice. On the contrary in the present study, TRAMP mice in the LB group had a reduced incidence of prostate cancer relative to the control group whereas the difference in prostate cancer incidence between the TP and control group was not significant. The reasons for these disparate findings are unclear. Analysis of serum levels of IGF-I, IGFBP-3, prostate tissue levels of lycopene, and oxidative DNA damage in the liver demonstrated no significant difference between mice fed TP and mice fed LB. In addition, there was no difference in lycopene levels in the TP and LB feed pellets and no difference in lycopene content deterioration in the pellets over time. Given the known deterioration of lycopene that can occur with exposure to air and light, feeding experiments that incorporate lycopene should document lycopene levels in the experimental diets and deterioration over time.
One difference between the tomato powder preparation used by Boileau, et al. and the paste used in the present trial was that the tomato powder contained the skin and seeds of the tomato, whereas the tomato paste used in the present trial did not contain these components. As part of the manufacturing process, the skin and seeds are removed when making tomato paste and tomato sauce. There may potentially be anticarcinogenic constituents in the skin and/or seeds of whole tomatoes. Another difference between the study by Boileau, et al. and the present study is a lower dose of dietary lycopene was provided to mice in the tomato powder group (13mg lycopene/kg diet) relative to the beadlet groups (161mg lycopene/kg diet) in the study by Boileau et al., whereas in the present study an equivalent dose of lycopene was provided in the TP and LB diets. It may be that the lower dose of lycopene used in the trial by Boileau, et al. had greater chemopreventive effects than the higher dose used in the group that received the lycopene beadlet supplemented diet. Further preclinical studies are required evaluating the dose response effects of lycopene and evaluating the different components of whole tomatoes for prostate cancer chemoprevention.
In the present trial the ratio of 5-cis to trans- lycopene in the serum was higher in the LB group relative to the TP group. Lycopene has a series of conjugated double bonds and is sensitive to cis-trans isomerization. Diet-derived lycopene is primarily in the trans- form, whereas cis-lycopene appears to be more bioavailable and may be a better substrate to carotene oxygenases than the trans- form.[20,21] The biologic activity of lycopene may be due, in part, to its metabolites.[21] Lycopene and its metabolites may play a role in chemoprevention through a variety of mechanisms including antioxidant effects, effects on gap junction communications, retinoid signalling, induction of phase-2 detoxification enzymes, and effects on proliferation and apoptosis.[20] Further studies are required to elucidate if the isomerization state of lycopene plays a significant role in chemoprevention of prostate cancer.
Whereas several trials demonstrated that dietary lycopene decreases serum IGF-I levels and increases serum IGFBP-3 levels, other studies did not confirm these findings.[22–23] Herzog, et al., demonstrated decreased local expression of IGF-I in the prostates of rats given lycopene supplementation.[24] Liu, et al., found that lycopene decreased cell IGF-I production and inhibited IGF-I mediated cell growth.[25] In the present trial, there was no difference in serum IGF-I and IGFBP-3 levels between the control, TP and LB groups. However, the IGFBP-3 results, while not significant, represent an increase with lycopene supplementation, consistent with previous findings by other groups.[22–23] TRAMP mice are known to have progressive increases in serum IGF-I levels and in prostate tissue IGF-I mRNA expression during androgen sensitive prostate cancer development.[26] However, reduction of liver-derived IGF-I and the resultant reduction in serum IGF-I levels in TRAMP with a targeted deletion of liver-derived IGF-I did not inhibit the development of prostate cancer at 9 and 19 weeks of age.[14] Interestingly, a recent study in patients at risk for cancer valuated the effects of 8-weeks lycopene intervention and detected increases in IGFBP-1 as the main effect on the IGF system. In this study IGFBP-1 levels were not measured, however, this represents an additional potential mechanism for the actions of lycopene on tumor progression and should be evaluated in future studies.[27]
Limitations of this study include a relatively small sample size of 59 mice. The TP group had a 15% lower incidence of prostate cancer relative to the control group but the p-value was 0.34. With a larger sample size we may have potentially seen significant chemopreventive effects with the TP diet. Another potential shortcoming is that serum and prostate tissue levels of lycopene and liver oxidative DNA damage was measured after a 3-week experiment in wild-type mice with the same genetic background as TRAMP mice. We performed this abbreviated experiment because we did not have sufficient serum from the TRAMP mice we studied, and we did not collect liver tissue from the TRAMP mice at sacrifice. In addition, these results may have been different at different time points of progression in TRAMP mice. The lycopene beadlet contained 5% ascorbyl palmitate as an antioxidant, which was not present in the vitamin mix added to the diets. This difference is unlikely to account for the results seen in the present trial given that the ascorbyl palmitate makes up an extremely small fraction of the total diet once the beadlets are mixed with the other components of the experimental diets
CONCLUSION
Epidemiologic and pre-clinical studies suggest a potential role for dietary lycopene products for prostate cancer chemoprevention. Whereas prior studies suggest bioactivity of whole tomato products such as tomato sauce and tomato powder, the present preclinical trial suggests greater chemopreventive effects from consuming lycopene beadlets. Further preclinical and clinical testing is required to elucidate the optimal form of dietary lycopene for future chemoprevention trials.
Acknowledgements
We thank Yantao Niu for assistance with diet content analysis and Michael Ittman for assistance with evaluation of the TRAMP pathology.
Grant Sponsor: National Institutes of Health 5R01CA100938-05, National Institutes of Health SPORE in Prostate Cancer 5P50CA092131-07, The Ruby Family Foundation
Footnotes
Work Performed at: Division of Clinical Nutrition Department of Medicine David Geffen School of Medicine at UCLA Los Angeles, CA
Author Disclosures: None
References
- 1.Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol Biomarkers Prev. 2007;16:2193–203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 2.Kirsh VA, Mayne ST, Peters U, Chatterjee N, Leitzmann MF, Dixon LB, Urban DA, Crawford ED, Hayes RB. A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:92–8. doi: 10.1158/1055-9965.EPI-05-0563. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM. Chemoprevention of prostate cancer: agents and study designs. J Urol. 2007;178:S9–S13. doi: 10.1016/j.juro.2007.03.138. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94:391–8. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 5.Gann PH, Ma J, Giovannucci E, Willett W, Sacks FM, Hennekens CH, Stampfer MJ. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res. 1999;59:1225–30. [PubMed] [Google Scholar]
- 6.Chen L, Stacewicz-Sapuntzakis M, Duncan C, Sharifi R, Ghosh L, van Breemen R, Ashton D, Bowen PE. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a wholefood intervention. J Natl Cancer Inst. 2001;93:1872–9. doi: 10.1093/jnci/93.24.1872. [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, Bowen P, Chen L, Duncan C, Ghosh L, Sharifi R, Christov K. Effects of tomato sauce consumption on apoptotic cell death in prostate benign hyperplasia and carcinoma. Nutr Cancer. 2003;47:40–7. doi: 10.1207/s15327914nc4701_5. [DOI] [PubMed] [Google Scholar]
- 8.Venkateswaran V, Fleshner NE, Sugar LM, Klotz LH. Antioxidants block prostate cancer in Lady transgenic mice. Cancer Res. 2004;64:5891–6. doi: 10.1158/0008-5472.CAN-04-0690. [DOI] [PubMed] [Google Scholar]
- 9.Limpens J, Schroder FH, de Ridder CMA, Bolder CA, Wildhagen MF, Obermuller-Jevic UC, Kramer K, van Weerden WM. Combined lycopene and vitamin E suppresses the growth of PC-346C human prostate cancer cells in nude mice. J Nutr. 2006;136:1287–1293. doi: 10.1093/jn/136.5.1287. [DOI] [PubMed] [Google Scholar]
- 10.Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman JW. Combinations of tomato and broccoli enhance antitumor activity in Dunning R3327-H prostate adenocarcinomas. Cancer Res. 2007;67:836–43. doi: 10.1158/0008-5472.CAN-06-3462. [DOI] [PubMed] [Google Scholar]
- 11.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95:1578–86. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi N, Barnard RJ, Said J, Hong-Gonzalez J, Corman DM, Ku M, Doan NB, Gui D, Elashoff D, Cohen P, Aronson WJ. Effect of low-fat diet on development of prostate cancer and Akt phosphorylation in the Hi-myc transgenic mouse model. Cancer Res. 2008;68:3066–73. doi: 10.1158/0008-5472.CAN-07-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang DL, Lee PD, Cohen P. Quantitative ontogeny of murine insulin-like growth factor (IGF)-I, IGF-binding protein-3 and the IGF-related acid-labile subunit. Growth Horm IGF Res. 2008;18:65–74. doi: 10.1016/j.ghir.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anzo M, Cobb LJ, Hwang DL, Mehta H, Said JW, Yakar S, LeRoith D, Cohen P. Targeted deletion of hepatic IGF-I in TRAMP mice leads to dramatic alterations in the circulating insulin-like growth factor axis but does not reduce tumor progression. Cancer Res. 2008;68:3342–9. doi: 10.1158/0008-5472.CAN-07-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira AL, Yeum KJ, Liu C, Smith D, Krinsky NI, Wang XD, Russell RM. Tissue distribution of lycopene in ferrets and rats after lycopene supplementation. J Nutr. 2000;130:1256–60. doi: 10.1093/jn/130.5.1256. [DOI] [PubMed] [Google Scholar]
- 16.Lu QY, Hung JC, Heber D, Go VL, Reuter VE, Cordon-Cardo C, Scher HI, Marshall JR, Zhang ZF. Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:749–56. [PubMed] [Google Scholar]
- 17.Hofer T, Seo AY, Prudencio M, Leeuwenburgh C. A method to determine RNA and DNA oxidation simultaneously by HPLC-ECD: greater RNA than DNA oxidation in rat liver after doxorubicin administration. Biol Chem. 2006;387:102–11. doi: 10.1515/BC.2006.014. [DOI] [PubMed] [Google Scholar]
- 18.Shigenga MK, Aboujaqoude EN, Chen Q, Ames BN. Assays of oxidative DNA damage biomarkers 8-oxo-2'-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1994;234:16–33. doi: 10.1016/0076-6879(94)34073-0. [DOI] [PubMed] [Google Scholar]
- 19.Huang X, Powell J, Mooney LA, Li C, Frenkel K. Importance of complete DNA digestion in minimizing variability of 8-oxo-dG analyses. Free Radic Biol Med. 2001;31:1341–51. doi: 10.1016/s0891-5849(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 20.Mein JR, Lian F, Wang XD. Biological activity of lycopene metabolites: implications for cancer prevention. Nutr Rev. 2008;66:667–83. doi: 10.1111/j.1753-4887.2008.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindshield BL, Canene-Adams K, Erdman JW. Lycopenoids: Are lycopene metabolites bioactive? Arch Biochem Biophys. 2007;458:136–40. doi: 10.1016/j.abb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Kanagaraj P, Vijayababu MR, Ravisankar B, Anbalagan J, Aruldhas MM, Arunakaran J. Effect of lycopene on insulin-like growth factor I, IGF binding protein-3 and IGF type-I receptor in prostate cancer cells. J Cancer Res Clin Oncol. 2007;133:351–9. doi: 10.1007/s00432-006-0177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graydon R, Gilchrist SE, Young IS, Obermuller-Jevic U, Hasselwander O, Woodside JV. Effect of lycopene supplementation on insulin-like growth factor-1 and insulin-like growth factor binding protein-3: a double-blind, placebo-controlled trial. Eur J Clin Nutr. 2007;61:1196–200. doi: 10.1038/sj.ejcn.1602632. [DOI] [PubMed] [Google Scholar]
- 24.Herzog A, Siler U, Spitzer V, Seifert N, Denelavas A, Hunziker PB, Hunziker W, Goralczyk R, Wertz K. Lycopene reduced gene expression of steroid targets and inflammatory markers in normal rat prostate. FASEB J. 2005;19:272–4. doi: 10.1096/fj.04-1905fje. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Allen JD, Arnold JT, Blackman MR. Lycopene inhibits IGF-I signal transduction and growth in normal prostate epithelial cells by decreasing DHT-modulated IGF-I production in co-cultured reactive stromal cells. Carcinogenesis. 2008;29:816–24. doi: 10.1093/carcin/bgn011. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan PJ, Mohan S, Cohen P, Foster BA, Greenberg NM. This insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Cancer Res. 1999;59:2203–9. [PubMed] [Google Scholar]
- 27.Vrieling A, Voskuil DW, Bonfrer JM, Korse CM, van Doorn J, Cats A, Depla AC, Timmer R, Witteman BJ, van Leeuwen FE, Van't Veer LJ, Rookus MA, Kampman E. Lycopene supplementation elevates circulating insulin-like growth factor binding protein-1 and -2 concentrations in persons at greater risk of colorectal cancer. Am J Clin Nutr. 2007;86:1456–62. doi: 10.1093/ajcn/86.5.1456. [DOI] [PubMed] [Google Scholar]


