Abstract
Purpose
Hispanics have a higher risk of early post-HCT treatment failure when compared to non-Hispanic whites. However, long-term morbidity among Hispanics has not been described.
Patients and Methods
Health-related outcomes were examined in 159 Hispanic and 825 non-Hispanic white patients who had undergone HCT between 1974 and 1998 and survived a mean of 8.7 years. Patients completed a detailed questionnaire about sociodemographic factors and the occurrence of chronic health conditions.
Results
Exposure to total body irradiation (TBI: OR=1.94, 95%CI, 1.06–3.56, p=0.03), presence of chronic graft vs. host disease (GvHD: OR=3.99, 95% CI, 1.94–8.24, p=0.002), and health insurance coverage (OR=3.46, 95% CI, 1.5–8.01, p=0.004), were significantly associated with severe/life-threatening conditions. Compared to non-Hispanic whites, Hispanics were 53% less likely to report severe/life-threatening conditions (OR=0.47, 95%CI, 0.27–0.83, p=0.009) after adjusting for relevant clinical variables. This effect size was mitigated (OR=0.56 [95%CI, 0.29–1.08], p=0.08) after adjusting for health insurance coverage.
Conclusions
Hispanics are less likely to report severe/life-threatening health conditions after HCT than non-Hispanic whites – a difference that decreases in magnitude and significance after taking health insurance into consideration. While confirming the role of TBI, and chronic GvHD, this study identifies the role of lack of health insurance coverage as a mediator of the lower prevalence of self-reported long-term morbidity in Hispanics.
INTRODUCTION
Hematopoietic cell transplantation (HCT) has now become the treatment of choice for several life-threatening disorders.1,2 Improvement in transplantation strategies has contributed to increments in survival rates of 10% per decade.3 Patients with hematologic malignancies who survive two years after HCT, have 10-year survival rates exceeding 70%.4–6 However, these improvements are not enjoyed equally by all. Data from the Center for International Blood and Marrow Transplant Research (CIBMTR) demonstrates Hispanics to be at a higher risk of early events such as treatment-related mortality (TRM) or relapse when compared to non-Hispanic whites undergoing allogeneic HCT.7,8 On the other hand, the Bone Marrow Transplant Survivor Study (BMTSS) does not identify ethnic/racial differences in late non-relapse mortality after autologous5 or allogeneic HCT6.
The Institute of Medicine (IOM) Report on Race and Unequal Treatment9 considers Hispanics to be a vulnerable population for adverse health-related outcomes due to socioeconomic and cultural issues, that could create barriers to access to the healthcare system. Despite being the largest minority group in the US (14.4% of the U.S. population) and the fastest growing (projected 29% of the U.S. population by 2050),10 the IOM Report found Hispanics to be disproportionately underrepresented in studies examining barriers to healthcare access and disparities in health-related outcome. Indeed, there exists a paucity of data regarding the long-term burden of morbidity that are unique to Hispanic HCT survivors.
In a previous report, we have shown that Hispanic HCT survivors are more likely to be uninsured, have lower family income and education, and are significantly less likely to establish contact with primary care providers after HCT.11 The purpose of the current study was to describe the burden of morbidity reported by long-term Hispanic HCT survivors in comparison to their non-Hispanic white counterparts, and to examine the role of sociodemographic factors, to explain the observed differences.
MATERIALS AND METHODS
Patients
BMTSS is a collaboration between the City of Hope National Medical Center (COH) and University of Minnesota (UMN) to examine the long-term outcomes of individuals who have survived two or more years after HCT. Eligible participants include individuals who received HCT at COH or UMN between 1974 and 1998 for a hematologic malignancy or severe aplastic anemia (SAA); survived at least two years post-transplantation; were alive and 18 years of age or older at study participation; and identified themselves as Hispanic or non-Hispanic white. The Human Subjects Protection committees at the participating institutions approved the study. Informed consent was obtained according to the Declaration of Helsinki.
Clinical Characteristics
Information regarding primary diagnosis, preparative regimens, source of stem cells (autologous or allogeneic), risk of relapse at HCT (standard vs. high risk), and management of chronic graft vs. host disease (GvHD), was obtained from institutional transplantation databases. Patients transplanted in first or second complete remission after acute leukemia (acute myeloid [AML] or lymphoid [ALL]) leukemia), and lymphoma (Hodgkin lymphoma [HL] or non-Hodgkin lymphoma [NHL]), and first chronic phase of chronic myeloid leukemia [CML], and all patients with SAA were considered being at standard risk for relapse; all others were considered at high-risk.
Outcome measure
A 255-item mailed questionnaire (available in English and Spanish) was used to collect information from all participants alive at the time of study enrollment, and covers the following general areas: sociodemographics (race/ethnicity, marital status, education, employment, annual household income, and health insurance); utilization of medical care over the past two years; presence of chronic GvHD; and diagnosis of physical health conditions with age at diagnosis (endocrinopathies; central nervous system compromise; cardiopulmonary dysfunction; gastrointestinal and hepatic sequelae; musculoskeletal abnormalities; and subsequent malignancies). An individual was considered to have active chronic GvHD if they reported active management of their chronic GvHD at the time of or within 12 months of study participation. The reliability and validity of the BMTSS questionnaire has been tested, and the results indicate moderate to high agreement between self-reported outcomes and medical records.12
Both Spanish and English versions of the BMTSS questionnaire were mailed to individuals with a Spanish surname, with bilingual instructions to complete the questionnaire in the language of their preference. A follow-up telephone call was made to all study participants (irrespective of race/ ethnicity) by a bilingual interviewer, confirming the language of preference and addressing any concerns or questions regarding the study, in the appropriate language. If the need for a Spanish version of the questionnaire was identified during the call (where only the English version had been mailed initially), the Spanish version was mailed.
Chronic Health Conditions
All chronic health conditions diagnosed after HCT were graded using the Common Terminology Criteria for Adverse Events version 3.0 (CTCAEv3.0), a scoring system developed through the National Cancer Institute by a multidisciplinary group13. The CTCAEv3.0 is used to grade acute and chronic conditions in patients with cancer and cancer survivors and distinguishes grades 1 through 5 with unique clinical descriptions of the severity for each event (grade 1: mild; grade 2: moderate; grade 3: severe; grade 4: life-threatening/disabling; grade 5: adverse event-related death). Gonadal dysfunction and psychosocial outcomes were not included in this analysis. This scoring system was adapted from that used by the Childhood Cancer survivor Study;14 with additional complications (such as avascular necrosis, dry eye, dry mouth, bleeding or swelling gums) unique to the HCT population.
Statistical Analyses
The prevalence of chronic health conditions was determined for participating HCT survivors. Chronic health conditions were reported as presence of any condition (grades 1–4); and dichotomized as mild-to-moderate (grades 1–2) or severe/life-threatening/disabling (grades 3–4). For participants with more than one condition, the maximum grade was used in the analysis. Descriptive statistics were calculated using standard parametric and non-parametric tests. Multivariate logistic regression was used to identify variables associated with the development of severe/life-threatening/disabling (grades 3 or 4) chronic health conditions after HCT (dependent variable). In addition to the primary independent variable of interest (ethnicity), other variables in Model 1 included those with a biological basis for association with health conditions (sex, age at study participation, primary diagnosis, year of transplantation, source of stem cells, exposure to total body irradiation (TBI), and presence of active chronic GvHD). This was followed by the introduction of education, and health insurance status into the model (Model 2) to help explain any association between ethnicity and health conditions identified in Model 1. The results were reported as odds ratios (ORs) with 95% confidence intervals (CIs).
Cumulative incidence of chronic health conditions was calculated for Hispanic and non-Hispanic white HCT recipients, using competing risk methods.15 This analysis included patients who had undergone HCT and survived at least two years (including 519 patients who died subsequently). Cause of death information was obtained from medical records and National Death Index.5,6 Deaths due to chronic health conditions (n=217) were given grade 5; deaths from primary disease/accident/suicide (n=302) were considered as competing risk. Non-participants were assumed to have no chronic health conditions, with follow-up censored at the date of last contact available from the institutional databases, providing a conservative estimate of the cumulative incidence. Data were analyzed using SAS 9.1 (SAS institute, Cary, NC). All statistical tests were two-sided.
RESULTS
Of the 1,571 eligible patients, 1,414 (90%) were successfully contacted, and of these, 984 (70%) agreed to participate, including 159 Hispanics (116 English-speaking; 43 Spanish-speaking), and 825 non-Hispanic whites. Participants were more likely to be female (44.6% vs. 38.7%, p=0.02); older at HCT (34.7 vs. 29.3 years, P<0.01) and at study participation (43.4 vs. 39.8 years, p<0.01); with shorter follow-up from HCT (mean length 8.7 vs. 10.5 years, p<0.01); more likely to have undergone autologous transplant (45% vs. 37%, p=0.002), and have received TBI (77.0% vs. 69.7, p<0.01). Hispanics were significantly less likely to participates (61%) compared with the non-Hispanic whites (71%, p<0.01). Patients who had undergone HCT for SAA were less likely to participate compared to survivors with other diagnoses. Participation rate did not differ by risk of relapse at HCT or by transplanting institution.
Characteristics of the study participants are presented in Table 1. Mean age at study participation was 41.5 years (range, 20.0–67.4) for Hispanics and 43.8 years (18.2–73.0) for non-Hispanic whites (p=0.02), and mean follow-up was 9.0 years (2.5–25.2) and 8.7 years (2.0–27.8, p=0.45) respectively. Hispanics were significantly less likely to have completed high school (54.1% vs. 96.3%, p<0.01), and to have health insurance coverage (75.5% vs. 93.7%, p<0.01). Hispanic study participants were significantly more likely to have undergone allogeneic HCT (67.9% vs. 52.5%, p<0.01). Hispanic survivors had a higher prevalence of acute leukemia (42.1% vs. 32.4%) but a lower prevalence of lymphoma (17.6% vs. 31.3%) compared to non-Hispanic whites. There were no differences with respect to gender, exposure to TBI, or presence of active chronic GvHD.
Table 1.
Demographic characteristics of the study cohort by ethnicity.
| Characteristics | Non-Hispanic White (N=825) | Hispanic (N=159) | P Value |
|---|---|---|---|
| Age (years), mean (SD) | |||
| Age at transplantation | 35.1 (14.1) | 32.5 (11.6) | 0.03 |
| Age at study participation | 43.8 (12.2) | 41.5 (11.0) | 0.03 |
| Gender, No. (%) | |||
| Male | 448 (54.3) | 97 (61.0) | 0.12 |
| Marital status, No. (%) | |||
| Married | 510 (61.8) | 89 (55.9) | 0.17 |
| Education, No. (%) | |||
| Less than high school | 28 (3.4) | 86 (54.0) | |
| Completed High school | 797 (96.6) | 73 (45.9) | <0.01 |
| Current health insurance, No. (%) | |||
| Insured | 773 (93.7) | 120 (75.5) | <0.01 |
| Primary diagnosis, No. (%) | |||
| Lymphoma | 258 (31.3) | 28 (17.6) | |
| Non-Hodgkin’s lymphoma | 181 (21.9) | 18 (11.3) | |
| Hodgkin’s lymphoma | 77 (9.3) | 10 (6.3) | |
| Acute Leukemia | 267 (32.4) | 67 (42.1) | |
| Acute lymphatic leukemia | 71 (8.6) | 24 (15.1) | |
| Acute myeloid leukemia | 196 (23.8) | 43 (27.0) | <0.01 |
| Chronic myeloid leukemia | 190 (23.0) | 39 (24.5) | |
| Other | 110 (13.3) | 25 (15.7) | |
| Severe aplastic anemia | 35 (4.2) | 13 (8.2) | |
| Multiple myeloma | 30 (3.6) | 10 (6.3) | |
| Other | 45 (5.5 | 2 (1.3) | |
| Donor Source, No. (%) | |||
| Allogeneic transplant | 433 (52.5) | 108 (67.9) | <0.01 |
| Conditioning regimen, No. (%) | |||
| Total body irradiation | 635 (77.0) | 121 (76.1) | 0.88 |
| Relapse risk at HCT, No. (%) | |||
| High risk | 295 (35.9) | 52 (32.7) | 0.44 |
| Body Mass Index at HCT, Kg/m2 | |||
| Mean, SD | 25.6 (5.1) | 26.3 (5.4) | 0.13 |
| Year of transplant, No. (%) | |||
| ≤1985 | 103 (12.5) | 21 (13.2) | |
| 1986 to 1990 | 134 (16.2) | 25 (15.7) | |
| 1991 to 1995 | 305 (37.0) | 65 (40.9) | |
| ≥1996 | 283 (34.3) | 48 (30.2) | 0.73 |
Abbreviations: HCT – hematopoietic cell transplantation; SD – standard deviation
Hispanics were significantly less likely to report a chronic health condition of any severity (57.2% vs. 69.1%, p<0.01), grade 3 or 4 severity (16.4% vs. 19.6%, p=0.04) or multiple (≥2) health conditions (43.4% vs. 51.9%, p=0.05) when compared to non-Hispanic whites (Table 2). Commonly occurring grade 3 or 4 conditions for the entire study population included gastrointestinal complications (10.7%) musculoskeletal complications (10.1%), audiovisual impairment (8.5%), and second malignant neoplasms (7.6%). The prevalence of the specific types of chronic health conditions reported by Hispanics and non-Hispanic whites did not differ.
Table 2.
Severity of chronic health conditions among non-Hispanic whites and Hispanics
| Chronic condition | Non-Hispanic White (N=825) | Hispanic (N=159) | P Value |
|---|---|---|---|
| No. (%) | |||
| No condition | 255 (30.9) | 68 (42.8) | |
| Grade 1 (mild) | 123 (14.9) | 21 (13.2) | |
| Grade 2 (moderate) | 285 (34.6) | 44 (27.7) | 0.08 |
| Grade 3 (severe) | 123 (14.9) | 20 (12.6) | |
| Grade 4 (life threatening/disabling) | 39 (4.7) | 6 (3.8) | |
| Any condition | |||
| Grades 1–4 | 570 (69.1) | 91 (57.2) | <0.01 |
| Grades 3 or 4 | 162 (19.6) | 26 (16.4) | 0.04 |
| Multiple health conditions | |||
| ≥2 health conditions | 428 (51.9) | 69 (43.4) | 0.05 |
| ≥3 health conditions | 293 (35.5) | 51 (32.1) | 0.40 |
Multivariate analysis (Table 3) revealed that Hispanics were less likely to report a grade 3 or 4 health condition, compared with non-Hispanic whites (OR=0.47, 95% 0.27–0.83, p=0.009) in Model 1 (did not contain education, and health insurance status). Introduction of these sociodemographic variables diminished both the magnitude and the significance of the association between ethnicity and health conditions (OR=0.56, 95% CI, 0.29–1.08, p=0.08).
Table 3.
Multivariate analysis of risk factors associated with having a severe (grade 3) or life-threatening (grade 4) health condition
| Risk Factors | Model 1 Odds ratio (95% CI) | P-Value | Model 2 Odds ratio (95% CI) | P-Value |
|---|---|---|---|---|
| Gender | ||||
| Male | 1.00 | 1.00 | ||
| Female | 1.18 (0.79–1.77) | 0.41 | 1.15 (0.76–1.73) | 0.51 |
| Age | ||||
| Age at HCT | 0.88 (0.81–0.96) | 0.004 | 0.88 (0.80–0.96) | 0.003 |
| Age at study participation | 1.17 (1.07–1.27) | 0.0007 | 1.17 (1.07–1.27) | 0.0008 |
| Ethnicity | ||||
| Non-Hispanic white | 1.00 | 1.00 | ||
| Hispanic | 0.47 (0.27–0.83) | 0.009 | 0.56 (0.29–1.08) | 0.08 |
| Diagnosis | ||||
| SAA/MM/Other | 1.00 | 1.00 | ||
| Chronic myeloid leukemia | 0.70 (0.30–1.65) | 0.41 | 0.65 (0.27–1.54) | 0.32 |
| Acute leukemia | 0.95 (0.44–2.08) | 0.90 | 0.93 (0.42–2.06) | 0.85 |
| Lymphoma | 1.02 (0.47–2.23) | 0.95 | 0.94 (0.43–2.07) | 0.88 |
| Type of transplant | ||||
| Autologous | 1.00 | 1.00 | ||
| Allogeneic, no active chronic GvHD | 1.13 (0.63–2.06) | 0.68 | 1.17 (0.64–2.15) | 0.61 |
| Allogeneic, active chronic GvHD | 4.01 (1.97–8.14) | 0.001 | 3.99 (1.94–8.24) | 0.002 |
| Conditioning regimen | ||||
| Chemotherapy | 1.00 | 1.00 | ||
| Chemotherapy + TBI | 1.81 (0.99–3.29) | 0.05 | 1.94 (1.06–3.56) | 0.03 |
| Year of transplant | ||||
| ≤1990 | 1.00 | 1.00 | ||
| 1991 to 1995 | 1.21 (0.54–2.67) | 0.64 | 1.23 (0.55–2.77) | 0.61 |
| ≥1996 | 0.91 (0.31–2.72) | 0.87 | 0.92 (0.30–2.81) | 0.89 |
| Insurance status | ||||
| Uninsured | 1.00 | |||
| Insured | 3.46 (1.50–8.01) | 0.004 | ||
| Education | ||||
| Less than high school | 1.00 | |||
| Completed high school | 0.98 (0.45–2.12) | 0.95 | ||
Abbreviations: HCT – hematopoietic cell transplantation; GvHD –graft versus host disease; TBI – total body irradiation; CI – confidence interval.
Other variables identified to be associated with an increased reporting of grade 3 or 4 chronic health conditions included presence of active chronic GvHD (OR=3.99, 95% CI, 1.94–8.24, p=0.002), health insurance coverage (OR=3.46, 95% CI, 1.5–8.01, p=0.004), and exposure to TBI (OR=1.94, 95%CI, 1.06–3.56, p=0.03). Individuals with active chronic GvHD were significantly more likely to report grade 3 or 4 audiovisual impairment (25% vs. 9.1%; p= 0.04), endocrinopathies (25% vs. 7.7%; p= 0.03) and gastrointestinal complications (28% vs. 9.1%; p= 0.02) when compared to other allogeneic HCT recipients without active chronic GvHD. Participants who received TBI were significantly more likely to report grade 3 or 4 musculoskeletal (12.8% vs. 3.1%; p=0.01) or gastrointestinal (10.9% vs. 3.1%; p=0.03) complications when compared to those who not exposed to TBI.
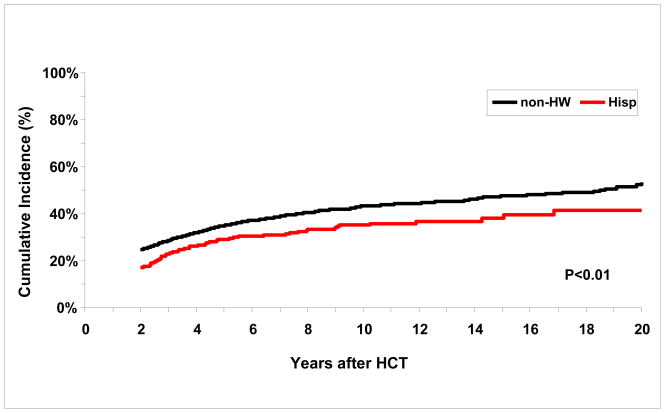
The cumulative incidence of chronic health conditions among Hispanics and non-Hispanic whites was 35% (95% CI, 30%–40%) and 43%% (95% CI, 41%–46%) at 10 years after HCT, respectively (Figure 1); p<0.01. Corresponding estimates for grades 3 or 4 chronic health conditions were 8% (5%–11%) and 10% (8%–11%), while those for grade 5 chronic health conditions were 11% (8%–15%) and 14% (12%–16%).
Figure 1.
Cumulative Incidence of Grades 1 through 5 chronic health conditions in non-Hispanic white (non-HW) and Hispanic (Hisp) survivors.
DISCUSSION
The overall goal of this study was to determine whether ethnic differences existed in the prevalence of chronic health conditions after HCT. While the CIBMTR report found that Hispanics had a 30% increased risk of early post-HCT events,7 others have failed to replicate these findings and have suggested that the differences in outcome may be less about ethnicity and more about socioeconomic status and barriers to healthcare access faced by these populations.16 Furthermore, in previous reports, BMTSS identified no ethnic/racial differences in late mortality due to non-relapse causes.5,6
There exists a paucity of data regarding the magnitude of long-term morbidity in Hispanic HCT survivors; whether there are differences in morbidity between non-Hispanic whites and Hispanics; and the possible causes of these differences. In the current study, we found that Hispanics are 53% less likely to report chronic severe/life-threatening/disabling conditions when compared with non-Hispanic whites after adjusting for relevant clinical variables an effect size that was mitigated after adjusting for known sociodemographic factors, most notably, health insurance coverage.
Availability of health insurance determines the timeliness and quality of healthcare received by survivors.9 Hispanics face greater barriers to health insurance access than all other racial/ethnic groups, making them less likely to have a regular source of care.9,17,18 The prevalence of being uninsured among Hispanics is 33% compared with 17% for the general population.9,19 The current study found Hispanics to be significantly more likely to be uninsured at study participation (24.5%) compared with non-Hispanic whites (6.3%). Those who had health insurance coverage were three-fold more likely to report a severe/life-threatening condition, independent of other modifying risk factors such as socioeconomic status, age, gender, and treatment-related conditions. In fact, having health insurance coverage was associated with a higher likelihood of diagnosis and reporting of severe/life-threatening/disabling health conditions following HCT in both Hispanics and non-Hispanic whites. There are several reasons as to why having health insurance would increase the likelihood of reporting chronic health conditions: first, as shown by others, long-term cancer survivors who have health insurance are more likely to undergo recommended health-related screenings such as echocardiograms, mammograms and pap smears, thus increasing the likelihood of detection of the condition being screened.20–22 In fact, we have demonstrated in a previous study that lack of health insurance is associated with a lower prevalence of healthcare utilization by this cohort of HCT survivors.11 Second, availability of health-related resources and access to healthcare would presumably lead to improvement in health-related-knowledge, enabling these survivors to more accurately characterize the nature and severity of their chronic medical conditions.23,24
The paucity of information regarding these barriers in previous studies is likely due to the relatively small numbers of Hispanics included in studies evaluating long-term outcomes following HCT.7,16 For example, it is increasingly recognized that language proficiency plays an important role in health-related knowledge and access to primary care.9,25,26 With nearly 30% of Hispanics in the U.S. currently living in a linguistically isolated household,10 language proficiency is likely to play an increasingly important role in the design of studies evaluating long-term outcomes in these at risk populations. As highlighted in the recent American Society of Clinical Oncology (ASCO) Policy Statement on Disparities in Cancer Care,27 these issues need to be addressed comprehensively to develop support systems and informed interventions for the vulnerable sub-populations.
We also demonstrate that presence of active chronic GvHD increases the risk of chronic severe/life-threatening/disabling conditions. Chronic GvHD is a leading cause of non-relapse mortality and is associated with chronic renal toxicity, musculoskeletal abnormalities, cardiopulmonary compromise, and gastrointestinal complications.4,6,28–31 The current study summarizes the burden attributable to chronic GvHD, by demonstrating a 4-fold increased risk of severe/life-threatening health conditions among those with active chronic GvHD. Chronic health conditions reported include those that are well known consequences of chronic GvHD and its management, i.e., visual impairment, gastrointestinal complications, and musculoskeletal disabilities. Of note, the prevalence of active chronic GvHD did not differ by ethnicity in the current study.
Complications associated with TBI include renal insufficiency,30,32 cataracts,33–35 cardiopulmonary dysfunction,29,36–38 endocrinopathies,39–41 and subsequent malignancies.42–44 Several of these conditions such as cataracts, chronic renal insufficiency, and pulmonary dysfunction are in turn exacerbated by chronic GvHD and its treatment. In the current study, individuals receiving TBI reported a nearly two-fold increased risk of severe or life-threatening chronic health conditions, independent of chronic GvHD status.
The results of this study must be interpreted in the context of potential limitations. There was an overrepresentation of Hispanics among non-participants, potentially contributing to an underestimation of chronic health conditions in this population of survivors, if the non-participants were to have had a higher prevalence of chronic health conditions than participants. However, non-participants were younger at the time of HCT and at the time of survey, and were less likely to have received TBI – characteristics associated with a lower risk of chronic health conditions – suggesting that the non-participants should have been at a lower risk of developing these complications than the participants, and would therefore not have resulted in an underestimation of the prevalence.
Ethnic differences in mortality due to chronic health conditions prior to study participation could potentially have introduced bias in the prevalence of chronic health conditions reported by the survivors. However, we did not find ethnic/racial difference in non-relapse-related late mortality after HCT.5,6 We therefore believe that ethnic differences in deaths due to chronic health conditions would not have contributed to bias in the current study.
This study is designed to capture self-reported chronic health conditions diagnosed as a consequence of delivery of “standard care” after HCT. Large studies of cancer cohorts have shown that self-report can be used effectively to describe post-treatment complications that were diagnosed as part of routine healthcare delivery.14,39,45–47 Furthermore, we have demonstrated that HCT survivors have the ability to report post-HCT complications accurately12 – as long as they have been diagnosed, which depends on access to care, which in turn is dependent on availability of health insurance coverage.
This study was not designed to conduct a comprehensive evaluation of all HCT survivors to identify pre-clinical or clinically overt long-term complications – an approach that is needed to describe the burden of long-term morbidity in all patient populations – but is logistically and financially prohibitive in a large and geographically diverse cohort such as ours. On the contrary, this study summarizes the prevalence of chronic health conditions diagnosed by our healthcare system and communicated to the HCT survivors, taking into account issues related to health insurance coverage, access to care, awareness among the healthcare providers of the risk of long-term complications, and communication of these outcomes to the HCT survivors. Keeping these issues in mind, our study finds Hispanics to be less likely to report severe/life-threatening health conditions after HCT – a difference that decreases in magnitude and significance after taking health insurance into consideration. While confirming the role of TBI, and chronic GvHD, overall, this study identifies the role of health insurance coverage as a mediator of the lower prevalence of self-reported long-term morbidity in Hispanics.
Acknowledgments
Funding/support: NIH R01 CA078938 (S. Bhatia), P01 CA30206 (S. Forman), Lymphoma/Leukemia Society Scholar Award for Clinical Research 2192 (S. Bhatia).
Financial support. All authors: no financial disclosures.
Footnotes
Financial disclosures. All authors: no financial disclosures.
Presented, in part, at the 50th annual meeting of the American Society of Hematology, San Francisco; December 8, 2008.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz MM, Rowlings PA. An update from the International Bone Marrow Transplant Registry and the Autologous Blood and Marrow Transplant Registry on current activity in hematopoietic stem cell transplantation. Curr Opin Hematol. 1997;4:395–400. doi: 10.1097/00062752-199704060-00006. [DOI] [PubMed] [Google Scholar]
- 3.Wingard JR, Vogelsang GB, Deeg HJ. Stem cell transplantation: supportive care and long-term complications. Hematology Am Soc Hematol Educ Program. 2002:422–44. doi: 10.1182/asheducation-2002.1.422. [DOI] [PubMed] [Google Scholar]
- 4.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105:4215–22. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–92. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker KS, Loberiza FR, Jr, Yu H, et al. Outcome of ethnic minorities with acute or chronic leukemia treated with hematopoietic stem-cell transplantation in the United States. J Clin Oncol. 2005;23:7032–42. doi: 10.1200/JCO.2005.01.7269. [DOI] [PubMed] [Google Scholar]
- 8.Serna DS, Lee SJ, Zhang MJ, et al. Trends in survival rates after allogeneic hematopoietic stem-cell transplantation for acute and chronic leukemia by ethnicity in the United States and Canada. J Clin Oncol. 2003;21:3754–60. doi: 10.1200/JCO.2003.03.133. [DOI] [PubMed] [Google Scholar]
- 9.Unequal Treatment. Confronting racial and Ethnic Disparities in Health Care. Washington, DC: national Academy Press; 2002. [Google Scholar]
- 10.Census 2005. American fact finder. Washington, D.C: U.S. Census bureau; 2005. [Google Scholar]
- 11.Prasad PK, Sun CL, Baker KS, et al. Health care utilization by adult Hispanic long-term survivors of hematopoietic stem cell transplantation: report from the Bone Marrow Transplant Survivor Study. Cancer. 2008 doi: 10.1002/cncr.23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louie AD, Robison LL, Bogue M, et al. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25:1191–6. doi: 10.1038/sj.bmt.1702419. [DOI] [PubMed] [Google Scholar]
- 13.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 14.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 15.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.Mielcarek M, Gooley T, Martin PJ, et al. Effects of race on survival after stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:231–9. doi: 10.1016/j.bbmt.2004.12.327. [DOI] [PubMed] [Google Scholar]
- 17.Betancourt JR. Eliminating racial and ethnic disparities in health care: what is the role of academic medicine? Acad Med. 2006;81:788–92. doi: 10.1097/00001888-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Andrulis DP. Access to care is the centerpiece in the elimination of socioeconomic disparities in health. Ann Intern Med. 1998;129:412–6. doi: 10.7326/0003-4819-129-5-199809010-00012. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. National Center for Health Statistics. [accessed, 2/17/09];Health Data Interactive. 2007 www.cdc.gov/nchs/hdi.htm.
- 20.Nathan PC, Jovcevska V, Ness KK, et al. The prevalence of overweight and obesity in pediatric survivors of cancer. J Pediatr. 2006;149:518–25. doi: 10.1016/j.jpeds.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 21.Sabatino SA, Coates RJ, Uhler RJ, et al. Health insurance coverage and cost barriers to needed medical care among U.S. adult cancer survivors age<65 years. Cancer. 2006;106:2466–75. doi: 10.1002/cncr.21879. [DOI] [PubMed] [Google Scholar]
- 22.Castellino SM, Casillas J, Hudson MM, et al. Minority adult survivors of childhood cancer: a comparison of long-term outcomes, health care utilization, and health-related behaviors from the childhood cancer survivor study. J Clin Oncol. 2005;23:6499–507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 23.Owen JE, Goldstein MS, Lee JH, et al. Use of health-related and cancer-specific support groups among adult cancer survivors. Cancer. 2007;109:2580–9. doi: 10.1002/cncr.22719. [DOI] [PubMed] [Google Scholar]
- 24.Shankar SM, Carter A, Sun CL, et al. Health care utilization by adult long-term survivors of hematopoietic cell transplant: report from the Bone Marrow Transplant Survivor Study. Cancer Epidemiol Biomarkers Prev. 2007;16:834–9. doi: 10.1158/1055-9965.EPI-06-0714. [DOI] [PubMed] [Google Scholar]
- 25.DuBard CA, Gizlice Z. Language spoken and differences in health status, access to care, and receipt of preventive services among US Hispanics. Am J Public Health. 2008;98:2021–8. doi: 10.2105/AJPH.2007.119008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson WS, Ahluwalia IB, Ford ES, et al. Language preference as a predictor of access to and use of healthcare services among Hispanics in the United States. Ethn Dis. 2008;18:93–7. [PubMed] [Google Scholar]
- 27.Goss E, Lopez AM, Brown CL, et al. American society of clinical oncology policy statement: disparities in cancer care. J Clin Oncol. 2009;27:2881–5. doi: 10.1200/JCO.2008.21.1680. [DOI] [PubMed] [Google Scholar]
- 28.Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108:2867–73. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uderzo C, Pillon M, Corti P, et al. Impact of cumulative anthracycline dose, preparative regimen and chronic graft-versus-host disease on pulmonary and cardiac function in children 5 years after allogeneic hematopoietic stem cell transplantation: a prospective evaluation on behalf of the EBMT Pediatric Diseases and Late Effects Working Parties. Bone Marrow Transplant. 2007;39:667–75. doi: 10.1038/sj.bmt.1705652. [DOI] [PubMed] [Google Scholar]
- 30.Choi M, Sun CL, Kurian S, et al. Incidence and predictors of delayed chronic kidney disease in long-term survivors of hematopoietic cell transplantation. Cancer. 2008;113:1580–7. doi: 10.1002/cncr.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell S, Sun CL, Kurian S, et al. Predictors of avascular necrosis of bone in long-term survivors of hematopoietic cell transplantation. Cancer. 2009 doi: 10.1002/cncr.24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kal HB, van Kempen-Harteveld ML. Renal dysfunction after total body irradiation: dose-effect relationship. Int J Radiat Oncol Biol Phys. 2006;65:1228–32. doi: 10.1016/j.ijrobp.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Gurney JG, Ness KK, Rosenthal J, et al. Visual, auditory, sensory, and motor impairments in long-term survivors of hematopoietic stem cell transplantation performed in childhood: results from the Bone Marrow Transplant Survivor study. Cancer. 2006;106:1402–8. doi: 10.1002/cncr.21752. [DOI] [PubMed] [Google Scholar]
- 34.Majhail NS, Ness KK, Burns LJ, et al. Late effects in survivors of Hodgkin and non-Hodgkin lymphoma treated with autologous hematopoietic cell transplantation: a report from the bone marrow transplant survivor study. Biol Blood Marrow Transplant. 2007;13:1153–9. doi: 10.1016/j.bbmt.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas O, Mahe M, Campion L, et al. Long-term complications of total body irradiation in adults. Int J Radiat Oncol Biol Phys. 2001;49:125–31. doi: 10.1016/s0360-3016(00)01373-0. [DOI] [PubMed] [Google Scholar]
- 36.Tichelli A, Bhatia S, Socie G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. Br J Haematol. 2008;142:11–26. doi: 10.1111/j.1365-2141.2008.07165.x. [DOI] [PubMed] [Google Scholar]
- 37.Ferry C, Gemayel G, Rocha V, et al. Long-term outcomes after allogeneic stem cell transplantation for children with hematological malignancies. Bone Marrow Transplant. 2007;40:219–24. doi: 10.1038/sj.bmt.1705710. [DOI] [PubMed] [Google Scholar]
- 38.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 39.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–72. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steffens M, Beauloye V, Brichard B, et al. Endocrine and metabolic disorders in young adult survivors of childhood acute lymphoblastic leukaemia (ALL) or non-Hodgkin lymphoma (NHL) Clin Endocrinol (Oxf) 2008;69:819–27. doi: 10.1111/j.1365-2265.2008.03283.x. [DOI] [PubMed] [Google Scholar]
- 41.Chemaitilly W, Sklar CA. Endocrine complications of hematopoietic stem cell transplantation. Endocrinol Metab Clin North Am. 2007;36:983–98. ix. doi: 10.1016/j.ecl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Krishnan A, Bhatia S, Slovak ML, et al. Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma: an assessment of risk factors. Blood. 2000;95:1588–93. [PubMed] [Google Scholar]
- 43.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–71. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 44.Brown JR, Yeckes H, Friedberg JW, et al. Increasing incidence of late second malignancies after conditioning with cyclophosphamide and total-body irradiation and autologous bone marrow transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:2208–14. doi: 10.1200/JCO.2005.05.158. [DOI] [PubMed] [Google Scholar]
- 45.Kadan-Lottick NS, Dinu I, Wasilewski-Masker K, et al. Osteonecrosis in adult survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26:3038–45. doi: 10.1200/JCO.2007.14.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood. 2008;111:5515–23. doi: 10.1182/blood-2007-10-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:5277–82. doi: 10.1200/JCO.2006.07.2884. [DOI] [PubMed] [Google Scholar]