Abstract
Background & Aims
Outcomes of undiagnosed celiac disease (CD) are unclear. We evaluated morbidity and mortality of undiagnosed CD in a population-based sample of individuals ≥50 years of age.
Methods
Stored sera from a population-based sample of 16,886 Olmsted County, Minnesota residents ≥50 years of age were tested for CD based on analysis of tissue transglutaminase (tTGA) and endomysial antibodies (EMA). A nested case-control study compared serologically defined subjects with CD to age- and sex-matched, sero-negative controls. Medical records were reviewed for comorbid conditions.
Results
We identified 129 (0.8%) subjects with undiagnosed CD in a cohort of 16,847 older adults. A total of 127 undiagnosed cases (49% male, median age 63.0 years) and 254 matched controls were included in a systematic evaluation for more than 100 potentially coexisting conditions. Subjects with undiagnosed CD had increased rates of osteoporosis and hypothyroidism, as well as lower body mass index and levels of cholesterol and ferritin. Overall survival was not associated with CD status. During a median follow-up period of 10.3 years after serum samples were collected, 20 cases but no controls were diagnosed with CD (15.2% Kaplan-Meier estimate at 10 years).
Conclusions
With the exception of reduced bone health, older adults with undiagnosed CD had limited comorbidity and no increase in mortality compared to controls. Some subjects were diagnosed with CD within a decade of serum collection, indicating that although most cases of undiagnosed CD are clinically silent, some result in symptoms. Undiagnosed CD can confer benefits and liabilities to older individuals.
Keywords: prevalence, epidemiology, autoantibodies, outcomes of undiagnosed celiac disease
Introduction
Celiac disease (CD) is one of the most common chronic inflammatory conditions of the digestive system. Once thought to be rare, CD affects approximately 1% of the population1–3 and appears to be associated with increased mortality4–6 along with substantial morbidity,7, 8 much of which is preventable or reversible with the gluten free diet.9, 10 Well recognized are the gastrointestinal consequences of severe malabsorption with weight loss or growth failure, macro- and micronutrient deficiencies and a host of extra-gastrointestinal manifestations varying from autoimmune disorders to arthralgia to neurologic problems.11–15 Historically considered a childhood disease, it has now become apparent that the diagnosis of CD may be delayed for many years and the condition often remains unrecognized.16–19 While there is no doubt that symptomatic CD can be a devastating illness, it is not clear if this outcome applies to all patients or just the small proportion that becomes clinically obvious.
Newer serological tests20–27 including tissue transglutaminase and endomysial antibodies now make CD readily detectable, but most screen-found patients tend to have few or no gastrointestinal symptoms at the time of detection.19, 28 Prior investigation has shown that the submerged part of the CD “iceberg” may be associated with certain comorbid conditions including metabolic bone disorders,29 type 1 diabetes mellitus,15 and iron deficiency anemia.28, 30 A recent study that includes young adults (median age = 20.5 years old) showed that undiagnosed CD was associated with a nearly 4-fold increased risk of death during 45-years of follow-up.31, 32 However, a recent study from Finland in adults with a mean age of 50 suggested the prognosis of adults with unrecognized CD appeared to be good, except for a significantly increased risk for lymphoma and esophageal carcinoma.33 Consequently, it is of crucial importance to know the impact that undetected, and hence untreated, CD has in older adults. This information could have profound implications for public health decisions and could help answer questions regarding the prognosis for patients in whom CD is detected in the absence of substantial gastrointestinal symptoms or other consequences of the disease. Thus, the aim of this study is to evaluate morbidity and mortality of undiagnosed CD in a population-based sample of subjects ≥50 years of age.
Materials and Methods
Setting
Population-based epidemiologic research can be conducted in Olmsted County (2000 population ~124,000) because medical care is virtually self-contained within the community and there are relatively few providers.34 The two major medical care providers (Mayo Clinic and Olmsted Medical Center) each use a dossier (or unit record) system whereby all medical information for each individual is accumulated in a single lifelong record. These clinical data are accessible because Mayo Clinic has maintained the original records as well as an extensive index of clinical and histologic diagnoses and surgical procedures since 1910. The medical records linkage system was further developed by the Rochester Epidemiology Project (REP) by indexing the records of the other providers into the same system used at Mayo.34
Participants
As part of a prior study of monoclonal gammopathy of undetermined significance (MGUS), serum samples of 24,727 Olmsted County residents age 50 years and over were obtained between the years of 1995 to 2001 and stored.35 During that time, study consent was granted for research of these specimens by 18,774 (75.9%) individuals. Thirty-four (0.2%) patients with known CD diagnosed prior to serum draw were excluded from the present study, leaving 18,738 subjects whose disease status was unknown at that time. Among these, 16,886 (90.1%) specimens still had sufficient volume for testing and were hence screened for CD.
Laboratory Testing
Serum was screened for CD using a sequential testing paradigm with tissue transglutaminase (tTGA) IgA ELISA as the initial screen. The ELISA procedure was performed on the ThermoLab DSX ELISA automated system (INOVA Diagnostics, Inc, San Diego, CA), and automated pipetting techniques were used to preserve sample volume. Each run had both positive and negative controls and calibrators. Those with positive screens were further tested with an endomysial antibody (EMA) immunofluorescence assay (Beckman Coulter, Inc, Brea, CA) for confirmation. The sensitivity and specificity of these tests have been described previously.20, 24, 27 Undiagnosed CD was defined by the presence of a tTGA test >2.0 U/ml with a positive EMA test. A tTGA <2.0 U/ml was considered negative and no EMA test was performed. Samples were also considered negative if the tTGA level was between 2.0–4.0 U/ml and the EMA test was negative. Tests were considered indeterminate if the tTGA level was >4.0 U/ml and the EMA test was negative. The technologist reading the EMA assay was unaware of the tTGA status and nature of the research study.
Data Collection
Upon completion of the serology testing, a nested, matched case-control design was proposed to compare serologically-defined undiagnosed CD subjects to sero-negative controls based on 2:1 matching of age and gender. Patients without general research authorization for use of their medical records were excluded from IRB approved review, including 2 seropositives and 278 potential controls. Complete medical records before and after the date of serum draw were reviewed by individuals unaware of serum status. These records included inpatient, outpatient, and emergency room documentation. Diagnosis lists, clinical notes, hospital notes, and laboratory results were used to obtain information pertaining to known comorbid conditions related to CD along with information regarding mortality. Comorbid conditions present before serum draw date were included in the association analysis. For review of laboratory testing, values obtained closest to the date of serum draw were utilized.
Statistical Analysis
Descriptive statistics summarizing the data included percentages for categorical data and medians and ranges for continuous data. To ensure adequate follow-up for each of the two controls within a matched set, the follow-up time of each subject was stopped at the latest recorded medical follow-up within that set. We assessed the risk of having various comorbidities before and after serum draw date in undiagnosed CD cases relative to controls using conditional logistic regression, or unconditional logistic regression if data were extensively missing (e.g., certain laboratory parameters). Odds ratios (with 95% confidence intervals) were used to measure the strength of association between comorbidity and serology status. In addition, the Kaplan Meier (KM) method was used to estimate overall survival and survival free of subsequent clinically diagnosed CD. Cox proportional hazards regression, stratified on matched set, was used to test for an association between positive serology and overall survival. In all models, the matching variables (age and gender) were included as covariates to control for any residual confounding not prevented by the matching itself. Given that more than 100 potential conditions, diseases, and laboratory findings were evaluated, these analyses are considered hypothesis-generating and results considered significant at the level of α = 0.05 should be interpreted cautiously. All analyses were carried out using the SAS statistical software package (version 9.1, SAS Institute, Cary, NC).
Results
Among subjects whose disease status was unknown, 16,886 Olmsted County, Minnesota residents ≥ 50 years of age who had consented to use of their serum for research were screened for CD. In total, 163 (1.0%) individuals tested positive for tTGA and underwent confirmatory EMA testing, while 143 had borderline tTGA levels (2.0–4.0 U/mL) and also were EMA tested. Based on a combined serology status of both the tTGA and EMA result, 39 subjects were considered equivocal and were excluded from subsequent analyses (final denominator, 16847). None of the 39 subjects were subsequently diagnosed with CD. A total of 129 subjects demonstrated a combined seropositive result for CD. Thus the seroprevalence of undiagnosed CD in our study population is 0.8 (95% CI, 0.6%–0.9%).
Patients without general research authorization for use of their medical records were excluded from subsequent analyses, including 2 seropositives and 278 potential controls. For the remaining 127 undiagnosed CD cases (51% female; median age=63.0 years; range, 51.7–87.7), 254 matched controls were selected for comparison (Table 1). Upon review, 20 seropositive patients were subsequently diagnosed (clinically) with CD (10-year KM rate, 15.2%; 95% CI, 8.2%–22.1%) after a median (range) of 10.3 (0.0–12.9) years of follow-up. Of note, no controls had yet to be subsequently diagnosed with CD.
Table 1.
Demographics of study participants
| Serology Negative (n=254)‡ | Serology Positive (n=127)‡ | Odds Ratio (95% CI)† | |
|---|---|---|---|
| Age (years) at serum draw | 62.9 (51.9, 87.7) | 63.0 (51.7, 87.7) | 1.19 (0.80, 1.78) |
| Female | 132 (52.0%) | 65 (51.2%) | 0.64 (0.13, 3.14) |
| Weight (kg) * | 78.0 (38.9, 142.0) (n=247) | 74.8 (44.0, 120.6) (n=125) | 0.98 (0.97, 1.00) |
| Height (cm) * | 166.4 (144.3, 189.7) (n=242) | 167.6 (124.0, 203.2) (n=123) | 1.01 (0.98, 1.04) |
| BMI | 27.4 (17.5, 55.5) (n=242) | 26.4 (17.2, 42.9) (n=123) | 0.94 (0.90, 0.99) |
Obtained from recorded weight and height closest to date of serum draw
descriptive statistics based on marginal distributions of sero-negatives and sero-positives and do not take into account the matching
Odds Ratio (95% CI) from conditional logistic regression which retains the matching
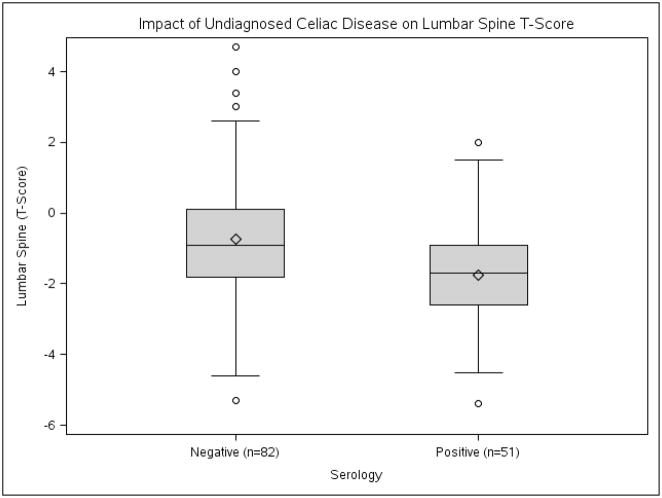
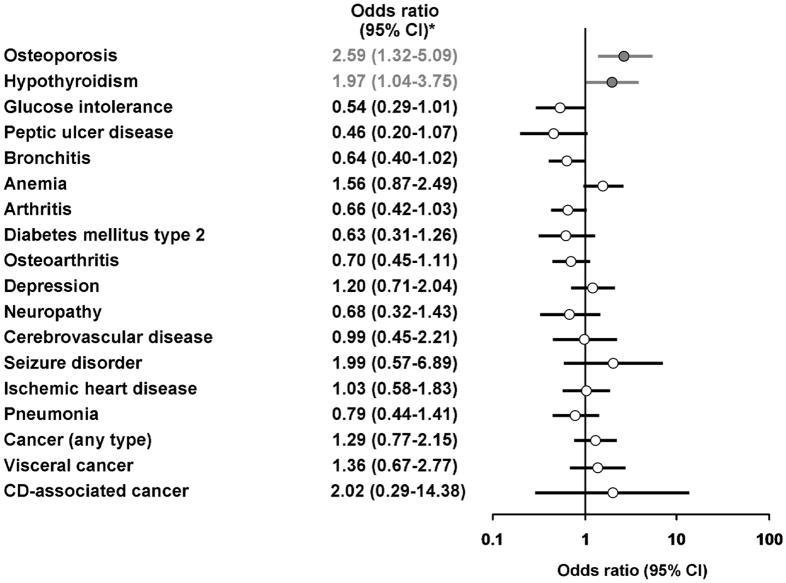
Undiagnosed CD was associated with decreased lumbar spine T-scores when compared to controls −1.7 vs. −0.9; OR=0.64; 95% CI, 0.48–0.85) (Figure 1) and an increased risk of osteoporosis (Figure 2) These patients also demonstrated higher rates of hypothyroidism. Conversely, undiagnosed CD patients had lower weight and BMI values (median value, 26.4 vs. 27.4) and while not statistically significant, a reduced rate of glucose intolerance. Laboratory evaluation showed undiagnosed CD was associated with reduced levels of cholesterol (median value, 200.0 vs. 213.0) and ferritin (25.0 vs. 78.5) (Table 2).
Figure 1. Impact of undiagnosed celiac disease on lumbar spine T-score.
Serology negative n=82, Serology positive n=51
Age, serology negative vs. serology positive = 62.2 vs. 63.7 (p=0.22)
Female gender, serology negative vs. serology positive = 85.4% vs. 82.4% (p=0.64)
Figure 2.
Summary of outcomes of undiagnosed celiac disease cases compared to serology-negative controls
†Odds Ratio (95% CI) from conditional logistic regression which retains the matching
Table 2.
Laboratory evaluation of patients with undiagnosed celiac disease cases compared to serology negative controls
| Parameter | Serology Negative (n=254)‡ | Serology Positive (n=127)‡ | Odds Ratio (95% CI) |
|---|---|---|---|
| Cholesterol (mg/dL) | 213.0 (99.0, 461.0) (n=242) | 200.0 (122.0, 314.0) (n= 120) | 0.91 (0.85, 0.98)^† |
| Ferritin (ug/L) | 78.5 (5.0, 1688.0) (n=86) | 25.0 (4.1, 443.0) (n=52) | 0.48 (0.32, 0.71)*Δ |
| Iron (ug/dL) | 74.0 (16.0, 433.0) (n=65) | 64.0 (4.0, 173.0) (n=39) | 0.65 (0.43, 1.00)*Δ |
| Hemoglobin (g/dL) | 14.0 (6.9, 17.0) (n=252) | 13.6 (8.8, 17.1) (n=125) | 0.87 (0.73, 1.05)† |
| B12 (ng/L) | 387.5 (84.9, 1318.0) (n=94) | 405.5 (33.0, 2000.0) (n=50) | 0.83 (0.58, 1.18)*Δ |
| Folate (ug/L) | 16.1 (3.7, 586.0) (n=74) | 13.8 (4.0, 24.0) (n=44) | 0.71 (0.45, 1.10)*Δ |
| Albumin (g/dL) | 4.2 (1.3, 9.2) (n=213) | 4.1 (2.3, 4.9) (n=106) | 0.80 (0.45, 1.40)† |
descriptive statistics based on marginal distributions of sero-negatives and sero-positives and do not take into account the matching
Odds Ratio (95% CI) from conditional logistic regression which retains the matching
Odds Ratio (95% CI) from regular (unmatched) logistic regression due to extensive missing values
Odds Ratio (95% CI) based on log-transformation of data, expressed as a 1-standard deviation change in the log scale
Odds Ratio (95% CI) expressed per 10-mg/dL change in cholesterol
Diagnosed and symptomatic celiac disease is known to be associated with an increased risk of cancer.5, 7 Upon review of the medical records, there was not a significantly increased risk of cancer detected in the undiagnosed CD patients compared to controls. The total number of cases identified as having cancer was 31 (24.4%) in the undiagnosed group compared to 51 (20.1%) in the controls (OR=1.29; 95% CI, 0.77–2.15). Two patients in the seropositive group were found to have a CD-associated malignancy (both small bowel lymphoma) as did two in the seronegative group (both esophageal cancer). Of the two undiagnosed CD patients with small bowel lymphoma, one was found to have a T-cell lymphoma.
Patient status (undiagnosed CD vs. controls) was not found to be associated with potential CD symptoms (Table 3). In particular, there was no difference in the proportion reported as having irritable bowel syndrome (10.4% vs. 12.6%) or experiencing weight loss (11.2% vs. 7.8%) around the time of serum draw. Furthermore, diarrhea was actually less prevalent, albeit not significantly, in undiagnosed cases than controls (21.4% vs. 26.2%). Overall, five seropositive subjects and none of the controls had a prior diagnosis of dermatitis herpetiformis.
Table 3.
Classic celiac disease symptoms in undiagnosed celiac disease cases compared to serology negative controls
| Symptom | Serology Negative (n=254)‡ | Serology Positive (n=127)‡ | Odds Ratio (95% CI)† |
|---|---|---|---|
| Diarrhea (%) | 65 (26.2%) | 27 (21.4%) | 0.77 (0.46, 1.31) |
| Weight Loss (%) | 19 (7.8%) | 14 (11.2%) | 1.67 (0.79, 3.51) |
| Abdominal Pain (%) | 92 (37.2%) | 46 (36.2%) | 0.96 (0.61, 1.51) |
| Dermatitis Herpetiformis (%) | 0 (0.0%) | 5 (4.0%) | - |
| Irritable Bowel Syndrome (%) | 31 (12.6%) | 13 (10.4%) | 0.79 (0.40, 1.54) |
| Deficient Hemoglobin (%) | 33 (13.1%) | 23 (18.4%) | 1.63 (0.86, 3.08) |
descriptive statistics based on marginal distributions of sero-negatives and sero-positives and do not take into account the matching
Odds Ratio (95% CI) from conditional logistic regression which retains the matching
In addition, undiagnosed CD was not found to be associated with an increased rate of all-cause mortality (HR=0.80; 95% CI, 0.45–1.41) or cancer –related mortality (Table 4). In particular, undiagnosed CD cases did not demonstrate a higher rate of mortality that was caused by any cancer, visceral types of cancer, or CD-associated types of cancer.
Table 4.
Association between undiagnosed celiac disease and mortality
| Type of Mortality | Hazard Ratio | (95% CI) | P-Value |
|---|---|---|---|
| All-cause mortality | 0.80 | (0.45, 1.41) | 0.44 |
| Cancer-related mortality | 0.63 | (0.16, 2.48) | 0.51 |
| Visceral cancer-related mortality | 0.79 | (0.25, 2.50) | 0.68 |
| CD-associated cancer mortality | 1.01 | (0.14–7.00) | 0.99 |
Hazard Ratio (95% CI) corresponds to risk in undiagnosed celiac disease cases compared to serology negative controls
Results obtained from Cox PH regression stratified on matched set
Of the 20 seropositive patients who were subsequently diagnosed with CD, iron deficiency (n=9, 45%) was the most common presenting symptom (Table 5). Three patients were diagnosed with CD after having been diagnosed with dermatitis herpetiformis first. Interestingly, only 3 of the 20 (15%) CD patients had presented with classic symptoms of diarrhea, malabsorption, and weight loss at the time of diagnosis. Other presenting symptoms included family history (n=3), nausea (n=1), and small bowel lymphoma (n=1). Gender was significantly associated with subsequent CD diagnosis, with 15 (75%) of these 20 being female in contrast to the nearly equally-divided gender distribution (47% female) in seropositives without a subsequent CD diagnosis (p=0.02 from a Chi-square test).
Table 5.
Presenting diagnosis of the 20 seropositive patients subsequently diagnosed clinically with celiac disease
| Symptom | n (%) |
|---|---|
| Iron deficiency | 9 (45%) |
| Dermatitis herpetiformis | 3 (15%) |
| Diarrhea, weight loss | 3 (15%) |
| Screened because of family history | 3 (15%) |
| Small bowel lymphoma | 1 (5%) |
| Nausea | 1 (5%) |
Discussion
Among the principal findings of this study, undiagnosed CD was found to be 1) associated with impaired bone health including increased rate of osteoporosis and lower bone density scores but was 2) not associated with increased mortality 3) nor the majority of comorbidities and symptoms commonly linked to diagnosed CD.
As found in our study, undiagnosed CD in older adults was not associated with an increased risk of mortality, data consistent with a recent study from Europe.33 This is in contrast to a recent study by Rubio-Tapia et al31 who found a 4-fold increased risk of death for undiagnosed CD among a younger cohort (age 18–24 years at the time of blood draw) with follow-up over 45 years. Possible explanations for this difference exist. Accumulated excess mortality in the Rubio-Tapia et al cohort did not occur until 25 years after the serum sample collection date, suggesting that if one got CD later in life, longer follow-up may be required to determine if excess mortality exists. Additionally, in our study, 15% of the initial undiagnosed CD were clinically detected and treated with a gluten-free diet while it is unlikely that any of those in the younger cohort were diagnosed with CD and treated. It is also possible that some subjects in the older group with undiagnosed CD died before age of sampling and are not represented in this cohort. Finally, a recent study suggests that loss of tolerance in CD may occur late in life.36 Thus, because of the cross-sectional design of the serologic testing in our study, we cannot exclude that late onset CD may explain the lack of morbidity and mortality in some of our patients with undiagnosed CD.
Although there is no difference in mortality among older patients with undiagnosed CD, it is evident these patients do have some excess morbidity related to CD. Significant results include a lower bone mineral density score and lower ferritin levels, findings consistent with what is found in clinically detected CD.37, 38 This suggests that although without an increase in symptoms from a gastrointestinal standpoint, undiagnosed CD is not entirely without nutritional consequences. On the other hand, undiagnosed CD patients did appear to have some theoretically protective characteristics that could be considered beneficial in a population where excess weight is the norm, such as a lower BMI and lower average cholesterol levels. Undiagnosed CD patients in our study also demonstrated a trend towards less arthritis and less glucose intolerance, which could be directly related to a lower BMI.
Of the 129 undiagnosed patients originally found to have a positive IgA tTGA and EMA tests, a significant minority were subsequently diagnosed with CD. Patients were most likely to be diagnosed with CD following a work-up of iron deficiency anemia and only 6 (30%) patients in this subgroup suffered from gastrointestinal symptoms. One patient was diagnosed with celiac disease only after being diagnosed first with small bowel lymphoma. What is interesting when comparing this group to the undiagnosed group is that 75% were female compared to 47% in the undiagnosed group suggesting females are more likely to be clinically diagnosed. Prior studies looking at CD in Olmsted County residents found a similar female predominance of diagnosed CD.39
The utility of mass screening of the general population for CD has been the topic of much discussion in the recent past.40–42 Those in favor of screening point out that CD is associated with an increase in mortality and morbidity, including certain cancers such as small bowel lymphoma and gastrointestinal cancers, many of which are diagnosed prior to or at the time of the diagnosis of CD. Also, treatment by virtue of a gluten-free diet is readily available. This, along with the development of improved screening tests for CD, including IgA tTGA and EMA immunofluorescence, make screening for the disease a possibility. The question still remains, however, whether the general population should be screened. As found in our study of middle-aged subjects, undiagnosed CD is not associated with an increased risk of mortality. Moreover, it has been suggested that those with undiagnosed CD who remain asymptomatic may be less likely to comply with the gluten-free diet, so benefits may be limited.43 Screening older populations for CD will find many individuals with undiagnosed or asymptomatic disease. Furthermore, a substantial minority of these patients will be clinically diagnosed with CD. It is possible that early identification of these patients may affect the ultimate outcome, but whether this will have a significant impact on quality of life and prevention of morbidity and mortality is not known.
There are several limitations to this study. Because the diagnosis of CD was not verified by small bowel biopsy, we are relying on the accuracy of serologic testing to make the diagnosis. We used IgA tTGA as an initial screening test. This test has been found to have a sensitivity approaching 91–98%20, 24, 25, 27. Those positive were tested for EMA antibodies with immunofluorescence, which has a specificity approaching 98–100%.24, 27 Obtaining small bowel biopsy from the surviving undiagnosed CD group could be the focus of a future study. We did not test for IgA deficiency. However, as about 1/400 of the general population are IgA deficient, one could anticipate from this that about 42/16,886 would then be deficient for IgA. If at most 10% of IgA deficient patients suffer from CD, then we would theoretically miss about 4 cases of undiagnosed CD.
In conclusion, our study finds a prevalence of undiagnosed CD of 0.8% in an adult population ≥ 50 years of age compared to prior studies that have found approximately 1% of the general population may suffer from CD, or more commonly have undiagnosed CD.1–3 Undiagnosed CD in older adults is not associated with an increase in mortality but is associated with impaired bone density and lower ferritin levels. Furthermore, a minority of subjects with undiagnosed CD, especially women, will eventually be clinically diagnosed with CD. As advances are made in testing for celiac disease, based on the results of this study it is not clear that a net benefit for detection of undiagnosed CD or at least CD that remains truly asymptomatic has been proven. Longer follow-up and studies in other populations would be necessary. Detection of the majority of patients with undiagnosed CD, even in this medically well-served population is unlikely to be achieved, even using an augmented case-finding approach. If undiagnosed CD has a net negative effect on morbidity or mortality, this strategy will likely leave the vast majority of patients undiagnosed.
Acknowledgments
This work was supported by research grants R01-DK57892, P01 CA62242, R01-AR30582, T-32 AI07047 (to A.R-T) from the National Institutes of Health, U.S. Public Health Service.
The project described was supported by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
Abbreviations
- CD
celiac disease
- CI
confidence interval
- EMA
endomysial antibody
- tTGA
tissue transglutaminase
Footnotes
Conflicts of interest: No conflicts of interest exist.
Jonathan D. Godfrey: study concept and design, acquisition of data, analysis and interpretation of the data, drafting of the manuscript
Tricia L. Brantner: acquisition of data, technical support
Waleed Brinjikji: acquisition of data, critical revision of the manuscript for important intellectual content.
Kevin N. Christensen: acquisition of data, critical revision of the manuscript for important intellectual content
Deanna L. Brogan: acquisition of data
Carol T. Van Dyke: acquisition of data
Brian D. Lahr: analysis and interpretation of data, critical revision of the manuscript for important intellectual content, statistical analysis
Joseph J. Larson: analysis and interpretation of data, statistical analysis
Alberto Rubio-Tapia: drafting of the manuscript, critical revision of the manuscript for important intellectual content
L. Joseph Melton III: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Alan R. Zinsmeister: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Robert A. Kyle: study concept and design, critical revision of the manuscript for important intellectual content
Joseph A. Murray: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cook HB, Burt MJ, Collett JA, et al. Adult coeliac disease: prevalence and clinical significance. J Gastroenterol Hepatol. 2000;15:1032–1036. doi: 10.1046/j.1440-1746.2000.02290.x. [DOI] [PubMed] [Google Scholar]
- 2.West J, Logan RF, Hill PG, et al. Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut. 2003;52:960–965. doi: 10.1136/gut.52.7.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohi S, Mustalahti K, Kaukinen K, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26:1217–1225. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 4.Corrao G, Corazza GR, Bagnardi V, et al. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001;358:356–361. doi: 10.1016/s0140-6736(01)05554-4. [DOI] [PubMed] [Google Scholar]
- 5.West J, Logan RF, Smith CJ, et al. Malignancy and mortality in people with coeliac disease: population based cohort study. Bmj. 2004;329:716–719. doi: 10.1136/bmj.38169.486701.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solaymani-Dodaran M, West J, Logan RF. Long-term mortality in people with celiac disease diagnosed in childhood compared with adulthood: a population-based cohort study. Am J Gastroenterol. 2007;102:864–870. doi: 10.1111/j.1572-0241.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- 7.Green PH, Fleischauer AT, Bhagat G, et al. Risk of malignancy in patients with celiac disease. Am J Med. 2003;115:191–195. doi: 10.1016/s0002-9343(03)00302-4. [DOI] [PubMed] [Google Scholar]
- 8.Viljamaa M, Kaukinen K, Pukkala E, et al. Malignancies and mortality in patients with coeliac disease and dermatitis herpetiformis: 30-year population-based study. Dig Liver Dis. 2006;38:374–380. doi: 10.1016/j.dld.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Collin P, Reunala T, Pukkala E, et al. Coeliac disease--associated disorders and survival. Gut. 1994;35:1215–1218. doi: 10.1136/gut.35.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray JA, Watson T, Clearman B, et al. Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. Am J Clin Nutr. 2004;79:669–673. doi: 10.1093/ajcn/79.4.669. [DOI] [PubMed] [Google Scholar]
- 11.Cronin CC, Jackson LM, Feighery C, et al. Coeliac disease and epilepsy. Qjm. 1998;91:303–308. doi: 10.1093/qjmed/91.4.303. [DOI] [PubMed] [Google Scholar]
- 12.Hadjivassiliou M, Chattopadhyay AK, Davies-Jones GA, et al. Neuromuscular disorder as a presenting feature of coeliac disease. J Neurol Neurosurg Psychiatry. 1997;63:770–775. doi: 10.1136/jnnp.63.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu WT, Murray JA, Greenaway MC, et al. Cognitive impairment and celiac disease. Arch Neurol. 2006;63:1440–1446. doi: 10.1001/archneur.63.10.1440. [DOI] [PubMed] [Google Scholar]
- 14.Lubrano E, Ciacci C, Ames PR, et al. The arthritis of coeliac disease: prevalence and pattern in 200 adult patients. Br J Rheumatol. 1996;35:1314–1318. doi: 10.1093/rheumatology/35.12.1314. [DOI] [PubMed] [Google Scholar]
- 15.Not T, Tommasini A, Tonini G, et al. Undiagnosed coeliac disease and risk of autoimmune disorders in subjects with Type I diabetes mellitus. Diabetologia. 2001;44:151–155. doi: 10.1007/s001250051593. [DOI] [PubMed] [Google Scholar]
- 16.Hankey GL, Holmes GK. Coeliac disease in the elderly. Gut. 1994;35:65–67. doi: 10.1136/gut.35.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivarsson A, Persson LA, Juto P, et al. High prevalence of undiagnosed coeliac disease in adults: a Swedish population-based study. J Intern Med. 1999;245:63–68. doi: 10.1046/j.1365-2796.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- 18.Ravikumara M, Nootigattu VK, Sandhu BK. Ninety percent of celiac disease is being missed. J Pediatr Gastroenterol Nutr. 2007;45:497–499. doi: 10.1097/MPG.0b013e31812e5710. [DOI] [PubMed] [Google Scholar]
- 19.Vilppula A, Collin P, Maki M, et al. Undetected coeliac disease in the elderly: a biopsy-proven population-based study. Dig Liver Dis. 2008;40:809–813. doi: 10.1016/j.dld.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Dieterich W, Laag E, Schopper H, et al. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115:1317–1321. doi: 10.1016/s0016-5085(98)70007-1. [DOI] [PubMed] [Google Scholar]
- 21.Sulkanen S, Halttunen T, Laurila K, et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology. 1998;115:1322–1328. doi: 10.1016/s0016-5085(98)70008-3. [DOI] [PubMed] [Google Scholar]
- 22.Dieterich W, Laag E, Bruckner-Tuderman L, et al. Antibodies to tissue transglutaminase as serologic markers in patients with dermatitis herpetiformis. J Invest Dermatol. 1999;113:133–136. doi: 10.1046/j.1523-1747.1999.00627.x. [DOI] [PubMed] [Google Scholar]
- 23.Gomez JC, Selvaggio G, Pizarro B, et al. Value of a screening algorithm for celiac disease using tissue transglutaminase antibodies as first level in a population-based study. Am J Gastroenterol. 2002;97:2785–2790. doi: 10.1111/j.1572-0241.2002.07023.x. [DOI] [PubMed] [Google Scholar]
- 24.Tesei N, Sugai E, Vazquez H, et al. Antibodies to human recombinant tissue transglutaminase may detect coeliac disease patients undiagnosed by endomysial antibodies. Aliment Pharmacol Ther. 2003;17:1415–1423. doi: 10.1046/j.1365-2036.2003.01595.x. [DOI] [PubMed] [Google Scholar]
- 25.Hill PG, Forsyth JM, Semeraro D, et al. IgA antibodies to human tissue transglutaminase: audit of routine practice confirms high diagnostic accuracy. Scand J Gastroenterol. 2004;39:1078–1082. doi: 10.1080/00365520410008051. [DOI] [PubMed] [Google Scholar]
- 26.Lock RJ, Stevens S, Pitcher MC, et al. Is immunoglobulin A anti-tissue transglutaminase antibody a reliable serological marker of coeliac disease? Eur J Gastroenterol Hepatol. 2004;16:467–470. doi: 10.1097/00042737-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Collin P, Kaukinen K, Vogelsang H, et al. Antiendomysial and antihuman recombinant tissue transglutaminase antibodies in the diagnosis of coeliac disease: a biopsy-proven European multicentre study. Eur J Gastroenterol Hepatol. 2005;17:85–91. doi: 10.1097/00042737-200501000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Bottaro G, Cataldo F, Rotolo N, et al. The clinical pattern of subclinical/silent celiac disease: an analysis on 1026 consecutive cases. Am J Gastroenterol. 1999;94:691–696. doi: 10.1111/j.1572-0241.1999.00938.x. [DOI] [PubMed] [Google Scholar]
- 29.Nuti R, Martini G, Valenti R, et al. Prevalence of undiagnosed coeliac syndrome in osteoporotic women. J Intern Med. 2001;250:361–366. doi: 10.1046/j.1365-2796.2001.00895.x. [DOI] [PubMed] [Google Scholar]
- 30.Howard MR, Turnbull AJ, Morley P, et al. A prospective study of the prevalence of undiagnosed coeliac disease in laboratory defined iron and folate deficiency. J Clin Pathol. 2002;55:754–757. doi: 10.1136/jcp.55.10.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metzger MH, Heier M, Maki M, et al. Mortality excess in individuals with elevated IgA anti-transglutaminase antibodies: the KORA/MONICA Augsburg cohort study 1989–1998. Eur J Epidemiol. 2006;21:359–365. doi: 10.1007/s10654-006-9002-4. [DOI] [PubMed] [Google Scholar]
- 33.Lohi S, Maki M, Rissanen H, et al. Prognosis of unrecognized coeliac disease as regards mortality: A population-based cohort study. Ann Med. 2009:1–8. doi: 10.1080/07853890903036199. [DOI] [PubMed] [Google Scholar]
- 34.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 35.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 36.Vilppula A, Kaukinen K, Luostarinen L, et al. Increasing prevalence and high incidence of celiac disease in elderly people: a population based study. BMC Gastroenterol. 2009;9:49. doi: 10.1186/1471-230X-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergamaschi G, Markopoulos K, Albertini R, et al. Anemia of chronic disease and defective erythropoietin production in patients with celiac disease. Haematologica. 2008 doi: 10.3324/haematol.13255. [DOI] [PubMed] [Google Scholar]
- 38.Fickling WE, McFarlane XA, Bhalla AK, et al. The clinical impact of metabolic bone disease in coeliac disease. Postgrad Med J. 2001;77:33–36. doi: 10.1136/pmj.77.903.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray JA, Van Dyke C, Plevak MF, et al. Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003;1:19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 40.Collin P. Should adults be screened for celiac disease? What are the benefits and harms of screening? Gastroenterology. 2005;128:S104–108. doi: 10.1053/j.gastro.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Fasano A. European and North American populations should be screened for coeliac disease. Gut. 2003;52:168–169. doi: 10.1136/gut.52.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mearin ML, Ivarsson A, Dickey W. Coeliac disease: is it time for mass screening? Best Pract Res Clin Gastroenterol. 2005;19:441–452. doi: 10.1016/j.bpg.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Fabiani E, Taccari LM, Ratsch IM, et al. Compliance with gluten-free diet in adolescents with screening-detected celiac disease: a 5-year follow-up study. J Pediatr. 2000;136:841–843. [PubMed] [Google Scholar]