Abstract
Background
Intestinal bacteria have been implicated in colorectal cancer pathology for a long time and a large number of reports point to a close linkage between Streptococcus bovis biotype I (recently renamed Streptococcus gallolyticus) infections and tumors of the human colon. This work aims to investigate the humoral immune response to this bacterium during different stages of colorectal cancer.
Method
The presence of serum antibodies against S. bovis antigen RpL7/L12, previously assigned as a potential diagnostic antigen, was evaluated in Dutch (n=209) and American (n=112) populations using a newly developed ELISA assay.
Results
The analyses consistently showed that an immune response against this bacterial antigen was increased in polyp patients and stage I/II colorectal cancer patients as compared to asymptomatic individuals. This was not paralleled by increased antibody production to endotoxin, an intrinsic cell wall component of the majority of intestinal bacteria, which implicates that the humoral immune response against RpL7/L12 is not a general phenomenon induced by the loss of colonic barrier function. Notably, increased anti-RpL7/L12 levels were not or only mildly detected in late stage colorectal cancer patients having lymph node or distant metastasis.
Conclusion
These findings are indicative for an increased exposure to antigen RpL7/L12 during early stages of colon carcinogenesis and suggest that intestinal bacteria, such as S. bovis, constitute a risk factor for the progression of pre-malignant lesions into early stage carcinomas. Clearly, the current findings emphasize the necessity for further studies on the possible etiologic relationship between intestinal bacteria and human colorectal cancer.
Keywords: Colorectal Cancer, Bacterial Infection, Humoral Immune Response, Intestinal Bacteria, Streptococcus bovis, Streptococcus gallolyticus, Carcinogenesis
INTRODUCTION
The human gastrointestinal tract is the habitat for a large and dynamic bacterial community, which is essential for digestion of food and the control of intestinal epithelial homeostasis and human health. Gut flora may, however, also be a critical factor in gastrointestinal diseases such as colorectal cancer (CRC) [1]. Although hundreds of microbial species reside in the human intestinal tract, only a systemic infection with the Gram-positive gut bacterium Streptococcus bovis (biotype I), recently renamed Streptococcus gallolyticus, has a well-known clinical association with CRC [2–6]. This opportunistic pathogen is normally detected in the gastrointestinal tract of about 10% of the human population [7]. Due to its relative low virulence, S. bovis can only establish a systemic infection in immunocompromised hosts (bacteremia) and/or individuals with damaged heart valves (endocarditis) together resulting in a ~1% incidence of pathological S. bovis infections in CRC patients. Interestingly, fecal carriage of S. bovis was shown to be increased about 5-fold in patients with CRC [2], and a colon tumor was detected up to 60% of patients with an S. bovis endocarditis or bacteremia [3]. Despite these observations, clear implications of this infection have not yet been established. There are several possible interpretations that are not necessarily mutually exclusive. First, it has been hypothesized that colorectal neoplastic sites provide a specific niche for S. bovis resulting in sustained colonization, survival, and the establishment of a local tumor-associated (clinically silent) infection. Second, S. bovis itself may promote colorectal carcinogenesis, which has been supported by experimental studies [8, 9].
A previous pilot study showed that S. bovis infection profiles generated by mass spectrometry, which consisted of antigens that were specifically bound by patient serum IgG, could be observed in CRC patients. The most abundant antigen peaks in these profiles belonged to a cluster of proteolytic fragments from ribosomal protein (Rp)L7/L12 [10,11]. The aim of the current study was to determining the relative serum anti-RpL7/L12 (IgG) levels during different stages of CRC using a newly developed quantitative ELISA assay. Cases and control samples from both Dutch and American institutes were used to determine geographical influence. Importantly, these analyses consistently showed an increased humoral immune response against RpL7/L12 in polyp and early CRC patients as compared to healthy controls. In contrast, no increased anti-RpL7/L12 levels were found in late stage CRC patients having lymph node or distant metastasis. Together, this provides the first clinical support for a temporal association between S. bovis and human colon tumors during CRC progression.
MATERIAL & METHODS
Patient material
Serum samples from 82 CRC and polyp patients and 10 patients with a systemic bacterial infection who had been admitted to the Radboud University Nijmegen Medical Centre (Nijmegen, The Netherlands) were used in this study. Patients suffering from infection with S. bovis (Gram-positive) or E. coli (Gram-negative) strains were recognized by a positive blood culture and routine microbial typing. Anonymized serum samples from 100 healthy volunteers (age 18–25 years), which were collected as part of the Nijmegen Biomedical Study [12, 13] and 27 healthy blood donors (>50 years) were used as controls. In addition, plasma samples from 64 CRC/polyp patients and 48 healthy controls who participated in a population-based case-control study in Metropolitan Detroit (USA), were included as a second independent study population; Detroit cancer cases included 7 with stage unknown. The use of the samples was approved by the local medical ethical committees and informed consent was obtained when required. Serum and plasma samples were stored at −80 °C until use.
RpL7/L12 overproduction and purification
To construct the RpL7/L12-His production vector pET11-RpL7/L12-His, chromosomal DNA from S. gallolyticus subsp. gallolyticus strain UCN34 (previously classified as S. bovis biotype I), was used as a template to amplify the rpL7/L12 gene. First a fragment comprising the complete open reading frame of the rpL7/L12 gene was amplified by PCR using the primers RpL7-u (5′-TCGACATATGGCATTGAACATTGAAAACATTATTGC-3′) containing an NdeI cleavage site and RpL7-d (5′-TCGAGGATCCTTAATGGTGATGGTGATGGTGTTTAAGAGTAACTGAAGCTCCAGC-3′) that inserts a hexahistidine (6xHis) tag upstream of the rpL7/L12 gene stop codon and contains a BamHI cleavage site. Next, the amplified fragment was cleaved with NdeI and BamHI and ligated into the corresponding sites of pET11a (Novagen) and used to transform Escherichia coli DH5α (Invitrogen) after which plasmid containing cells were selected on 50 μg/ml ampicillin [14]. Intact pET11-RpL7/L12-His was transferred to E. coli BL21 (DE3; Novagen) and cells were grown aerobically at 37°C in liquid Luria Broth (LB) medium containing Bacto-tryptone (1%), Bacto-yeast extract (0.5%), NaCl (1%) and 50 μg/ml ampicillin. RpL7/L12-His production was induced in exponentially growing cells by 500 nM isopropyl-D-thiogalacto-pyranoside (IPTG). Cells were harvested after 4 hours and lysed by two freeze-thaw cycles after which native His-tagged RpL7/L12 was purified (>95%) by Nickel-affinity chromatography using the Ni-NTA mini spin kit from Qiagen [15]. Cell lysates and isolated protein fractions were analyzed by Tricine SDS-polyacrylamide gel electrophoresis (SDS-PAGE) [16]. The identity purified RpL7/L12-His was confirmed by in-gel tryptic digestion followed by tandem mass spectrometry [17].
ELISA measurements
To measure anti-RpL7/L12 IgG levels, ELISA microtiter plates (Maxisorp Nunc) were first coated with purified RpL7/L12-His (0.3 μg/ml) in 50 mM NaHCO3 (pH9.5) during a 48 hour incubation at 4°C. Next, wells were washed 3 times with 50 mM KH2PO4/130 mM NaCl buffer (pH7) containing 0.01% Tween-20 (wash/incubation buffer) after which the immobilized antigen was incubated with 100-fold diluted serum samples for 18 hours at 4°C. Wells were washed (3 times) after which incubation was prolonged for 90 min in the presence of Horseradish peroxidase (HRP)-labeled goat anti-human IgG (1:15.000; Jackson Immuno Research) at room temperature. After washing, 100 mM citric phosphate buffer containing O-phenylenediamine dihydrochloride substrate (DAKO) and 0.0125% H2O2 was added. The subsequent coloration reaction was terminated by the addition of 11% sulfuric acid when appropriate. The optical density of HRP-mediated substrate conversion was quantified at a wavelength of 492 nm in a spectrophotometer. Titers were expressed in arbitrary optical density (OD) units. The correlation of the anti-RpL7/L12 ELISA and the previously used RpL7/L12 immunocapture MS assay (peak m/z 7888) on 24 samples from CRC patients and controls was 0.42 (Pearson; p=0.018) [10]. The average coefficient of variation of the ELISA assay was determined to be 2.5% by repeated measurements of 48 samples included within this study. Anti-endotoxin IgG levels in serum were determined by the EndoCab ELISA assay (Hycult Biotechnology) according to the manufacturers instructions. Titers were expressed as median units (MU)/ml [17]. For the latter analysis, 180 samples, including 120 polyp and CRC samples and 60 controls, were randomly selected from the Nijmegen and Detroit populations.
Statistical Analyses
Routine statistical analysis was performed by SPSS version 16. Obtained data sets were normally distributed. Detailed analyses by SAS version 9.1 revealed that age was correlated with anti-RpL7/L12 IgG titer levels in each population at marginal levels (p=0.09 for Nijmegen and p=0.07 for Detroit), while gender did not show any associations. Therefore also age-adjusted analyses were performed. Age was imputed for 6 polyp cases in the Nijmegen group using the overall mean of known values in this disease group. For all tumors combined and their subgroups, age-adjusted means and standard errors (SE) of the titers were calculated using a one-way analysis of covariance [18]. Age-adjusted odds ratios (OR) associated with the above-median titer level, which was determined separately for the Nijmegen and Detroit populations, were calculated by unconditional logistic regression model [19].
RESULTS
Measurement of anti-RpL7/L12 serum levels in Dutch patients and controls
To evaluate whether serum anti-RpL7/L12 levels were positively associated with CRC as found in our previous experiments [10, 11], the anti-RpL7/L12 IgG levels were determined in 12 polyp patients, 36 stage I/II, 18 stage III and 16 stage IV CRC patients using a new ELISA assay (see Material and Methods Section). These levels were compared to the anti-RpL7/L12 IgG levels of 27 healthy blood donors (>50 years) and 100 healthy youthful blood donors between 18–25 years of age to investigate the effect of age on the serum anti-RpL7/L12 levels.
At first sight, no noticeable differences in the median RpL7/L12 levels were observed when all cases were combined and compared with healthy controls. However, detailed analysis revealed a positive correlation of anti-RpL7/L12 levels with age and the presence of colon polyps (Fig. 1A), thus showing a positive association with increased risk for CRC. In contrast, a negative association of anti-RpL7/L12 levels was observed between patients with early stage (I/II) tumors and those with advanced stage (III and IV) disease. Both polyp patients and stage I/II CRC patients (here together defined as early stage CRC; Fig. 1C) contained the highest anti-RpL7/L12 titers, which were significantly different from those of healthy individuals (p=0.013) and advanced stage CRC patients (p=0.025). The age-adjusted odds ratios (ORs) for colorectal tumors associated with above-median RpL7/L12 titers ranged from 0.7 for stage III/IV tumors to 4.04 for polyps (Table 1). It should be noted that although the relative anti-RpL7/L12 levels between polyp and early stage CRC patients and healthy individuals are significantly different (Fig. 1C); this significance is lost with respect to ORs as these are calculated in an age-adjusted manner (see Materials & Methods section).
FIGURE 1.
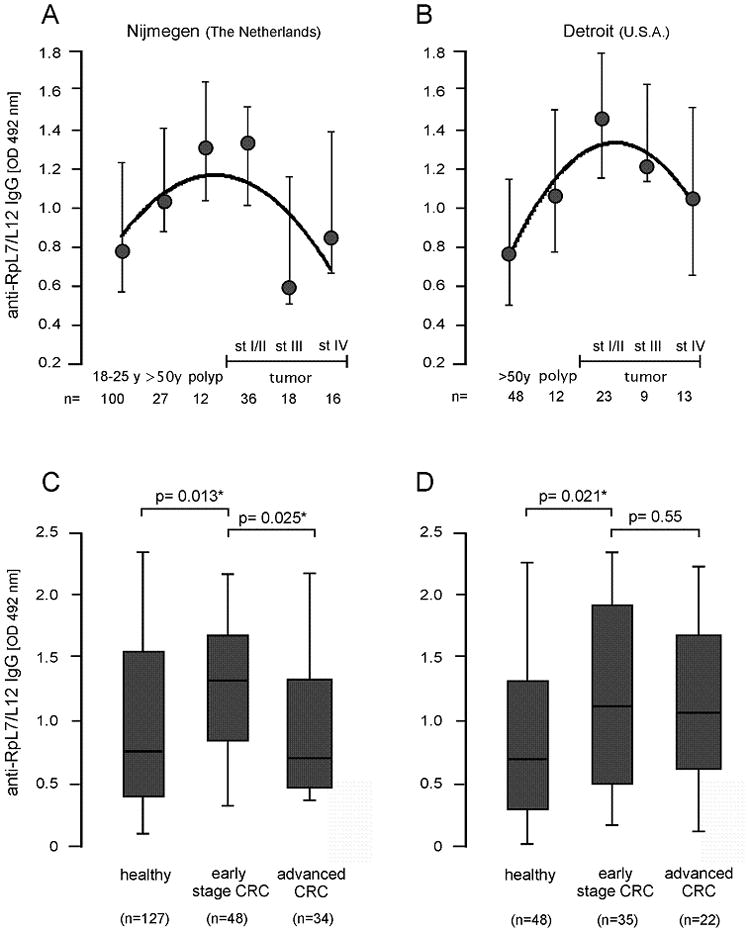
Humoral immune response against bacterial antigen RpL7/L12. Median serum anti-RpL7/L12 IgG levels in healthy individuals older than 50 years of age (>50y), colon polyp patients, and CRC patients with stage (st) I/II, stage III and stage IV tumors in the Nijmegen (A) and Detroit (B) populations. The Nijmegen population contains an additional healthy control group of individuals between 18 and 25 years (18–25y) of age. Relative anti-RpL7/L12 IgG levels were expressed as optical density units measured at 492 nm (OD 492 nm); standard deviations are indicated. The Nijmegen (C) and Detroit (D) populations were divided into “healthy”, “early stage CRC” (polyp and local stage I/II tumors) and “advanced CRC” (tumors with regional and distant metastases) for statistical analysis. Median levels, second and third quartile (boxes), ranges (lines) and numbers (n) of included subjects are indicated; significant differences of p<0.05 are indicated with “*”.
Table 1.
Age-adjusted mean anti-RpL7/12 titer levels and age-adjusted odds ratios (ORs) for colorectal tumors associated with above-median titers
| Population | Disease status | N | Mean | SE | P-values | ORa | 95% CI |
|---|---|---|---|---|---|---|---|
| Nijmegen | Controls | 127 | 1.06 | 0.07 | - | 1.00 | - |
| Colorectal tumors | 82 | 1.05 | 0.10 | 0.993 | 1.90 | 0.49 – 2.84 | |
| Polyps | 12 | 1.28 | 0.20 | 0.309 | 4.04 | 0.45–36.45 | |
| Cancer | 70 | 1.02 | 0.10 | 0.836 | 1.07 | 0.44 – 2.61 | |
| Early stage | 36 | 1.15 | 0.13 | 0.518 | 1.50 | 0.48 – 4.62 | |
| Advanced stage | 34 | 0.90 | 0.12 | 0.352 | 0.70 | 0.25 – 1.98 | |
| Detroit | Controls | 48 | 0.95 | 0.10 | - | 1.00 | - |
| Colorectal tumors | 64 | 1.29 | 0.08 | 0.011 | 2.30 | 1.06 – 5.00 | |
| Polypsb | 12 | 1.20 | 0.20 | 0.245 | 1.53 | 0.43 – 5.49 | |
| Cancerc | 52 | 1.30 | 0.09 | 0.011 | 2.58 | 1.14 – 5.83 | |
| Early stage | 23 | 1.31 | 0.14 | 0.042 | 2.75 | 0.96 – 7.88 | |
| Advanced stage | 22 | 1.22 | 0.15 | 0.130 | 1.90 | 0.68 – 5.35 |
SE: Standard errors; P-values: in comparison with controls; CI: Confidence intervals
Median cutoff points were 0.93 for the Nijmegen (n=209) and 1.00 for the Detroit (n=112) populations (all groups combined); above-median titers (> median cutoff) for each disease group were used to calculate OR’s.
Detroit polyp cases were self-reported
Detroit cancer cases include 7 cases with stage unknown
Nevertheless, these data show that an increased humoral immune response to bacterial antigen RpL7/L12 is associated with increased risk for CRC in a Dutch population, whereas the immune response against this antigen in advanced stage CRC patients is similar to that of healthy controls.
Measurement of anti-RpL7/L12 serum levels in patients and controls from the U.S.A
To confirm the apparent temporal association of the humoral immune response against RpL7/L12 and CRC progression as observed in the Nijmegen population, a similar but completely independent set of samples from CRC patients and controls from Detroit (Michigan, USA) was included in this study. These comprised 12 polyp patients, and 23 stage I/II, 9 stage III, and 13 stage IV CRC patients. The serum anti-RpL7/L12 levels were compared to those of 48 healthy control subjects. This showed the same overall trend as observed for the Nijmegen samples (compare Figs. 1A and B). Compared to controls, early stage CRC patients contained significantly increased serum anti-RpL7/L12 levels (p=0.021; Fig. 1D). However in this population the anti-RpL7/L12 levels were not significantly lower in patients with stage III and IV compared to early stage CRC patients. The age-adjusted OR for colorectal tumors associated with above-median titers was the highest for early stage CRC (2.75), but also for all CRC cases the OR of 2.58 was significantly increased compared to the population controls (Table 1).
Thus, these data show that increased anti-RpL7/L12 levels in patients from the U.S.A. are significantly associated with an increased risk for CRC and that increased anti-RpL7/L12 levels are associated with early stage CRC irrespective of geographical location.
Comparison of anti-endotoxin and anti-RpL7/L12 serum levels in CRC patients
To investigate whether the increased humoral immune response against RpL7/L12 is related to the fact that colon tumors make the intestinal tract more prone to bacterial infiltration in general, the humoral immune response to endotoxin was determined in 120 polyp and CRC patients and 60 healthy controls, randomly selected from the Nijmegen and Detroit populations, by an ELISA assay. Endotoxin is an intrinsic component of the cell wall from the majority of Gram-negative intestinal bacteria, and increased humoral immune response against endotoxin is known to be associated with decreased colonic barrier function [20,21]. As shown in Figure 2A, the anti-endotoxin levels displayed a similar tendency as the anti-RpL7/L12 levels. However, in contrast to anti-RpL7/L12, the relative endotoxin antibody expression in patients with stage I/II tumors was markedly lower than in polyp patient. Moreover, no significant differences were observed in anti-endotoxin IgG levels between healthy, early stage CRC and advanced CRC (Fig. 2B). This suggests that increased anti-RpL7/L12 levels in early CRC patients cannot be fully attributed to a general loss of intestinal barrier function.
FIGURE 2.
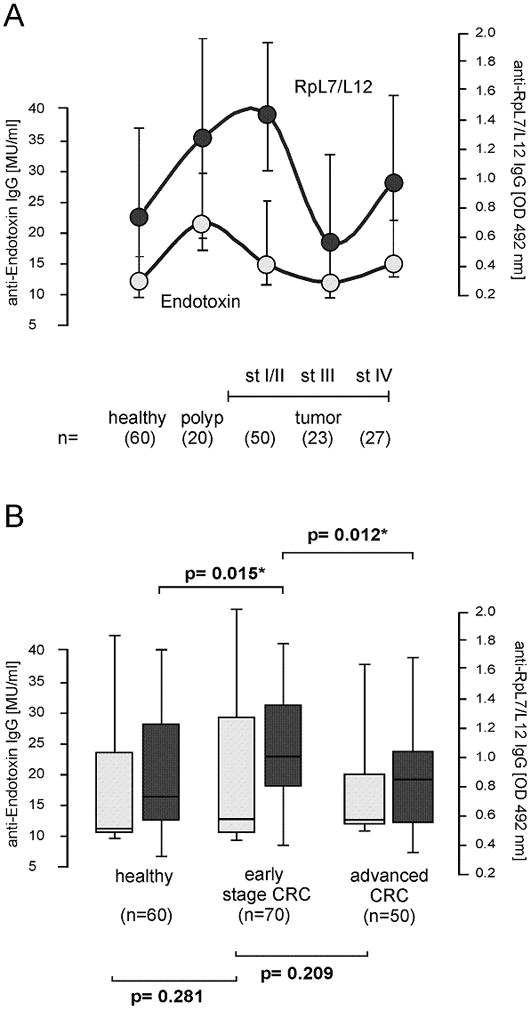
Humoral immune response against endotoxin. (A) Median serum anti-endotoxin IgG levels in healthy individuals older than 50 years of age (>50y), colon polyp patients, and CRC patients with stage (st) I/II, stage III and stage IV tumors in samples that were randomly selected from the Nijmegen and Detroit populations. Relative anti-endotoxin IgG levels were expressed as Median Units (MU/ml) according to calibration standards of the EndoCab assay (open circles). The levels were compared with the anti-RpL7/L12 IgG levels of the same set of serum samples (filled circles); relative anti-RpL7/L12 IgG levels were expressed as optical density units measured at 492 nm (OD 492 nm); standard deviations are indicated. No significant overall correlation between anti-RpL7/L12 and anti-endotoxin levels was observed (Pearson = 0.10). (B) The study population was divided into “healthy”, “early stage CRC” (polyp and local stage I/II tumors) and “advanced CRC” (tumors with regional and distant metastases) for statistical analysis. Median anti-endotoxin levels and ranges (open bars), were compared with anti-RpL7/L12 IgG levels and ranges (filled bars); lines represent upper and lower quartiles. Numbers (n) of included subjects are indicated; significant differences of p<0.05 are indicated with “*”.
To further evaluate specificity of the observed increased anti-RpL7/L12 levels in CRC patients, these levels were also determined in 5 patients with a systemic S. bovis infection and 5 patients with an E. coli (Gram-negative) infection. As shown in Figure 3, anti-RpL7/L12 levels in S. bovis-infected patients displayed a higher tendency than those in E. coli-infected individuals. In fact, these levels matched to those of CRC patient with high anti-RpL7/L12 levels. On the other hand, RpL7/L12 antibody levels in E. coli-infected patients were higher than those of individuals with low anti-RpL7/L12 titers, which is indicative for a certain extent of cross reactivity between antibodies elicited by ribosomal proteins of E. coli and the RpL7/L12 antigen of S. bovis. Taken together, the increased anti-RpL7/L12 levels observed in early stage CRC patients strongly point towards a selective interaction of S. bovis and colonic tumors, however, it cannot be excluded that this is also the case for related bacterial species with highly homologous RpL7/L12 proteins.
FIGURE 3.
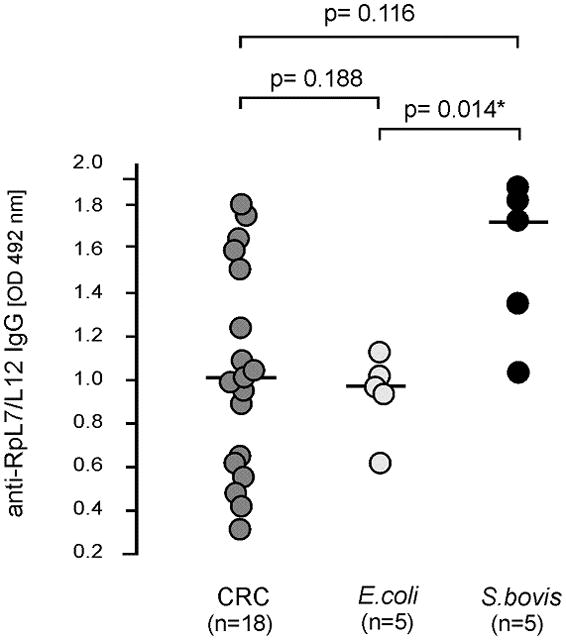
RpL7/L12 antibody levels in S. bovis and E. coli infected patients. Anti-RpL7/L12 IgG levels in serum samples from patients with proven systemic E. coli or S. bovis infections were compared with those in serum of a representative set of CRC patients. Relative anti-RpL7/L12 IgG levels were expressed as optical density units measured at 492 nm (OD 492 nm); median and numbers (n) of included subjects are indicated and significant differences of p<0.05 are indicated with “*”.
DISCUSSION
Inspired by the results of our previous pilot study showing an enhanced immune response against RpL7/L12 in CRC patients [10], we developed an ELISA-based quantitative serological assay for S. bovis. The application of this anti-RpL7/L12(IgG) ELISA on two independent sample collections demonstrates that anti-RpL7/L12 response is the most pronounced in patients with early stage colonic tumors, which cannot be fully attributable to impaired colonic barrier function. To our knowledge the present data show for the first time that specific intestinal bacteria constitute a risk factor during the early stages of colon carcinogenesis and may actively contribute to the progression of pre-malignant lesions into early stage carcinomas. However, due to the observed cross reactivity of the anti-RpL7/L12 ELISA, we can currently not yet pinpoint the increased humoral immune response to specific intestinal pathogens. It should be noted that an increased humoral immune response does not linearly reflect increased exposure on the individual level as there are many host factors that determine the strength of an immune response. However, RpL7/L12 antibody expression is preceded by exposure to this antigen, and we therefore feel that it is justified to regard the increased RpL7/L12 serum antibody levels as a marker for increased antigen exposure at the population level. As our observations are indicative for increased exposure to S. bovis and possibly a subset of related intestinal bacteria, our current data will be discussed in view of what is known about the clinical association between S. bovis and CRC. We hypothesize the following events to take place during CRC development, although we want to emphasize that most questions still remain to be answered. Furthermore, it should be realized that although S. bovis biotype I (most strongly associated with CRC) was recently renamed S. gallolyticus, we use S. bovis in this paper because it is unclear whether bacterial strains have been properly classified into biotype I or biotype II (renamed Streptococcus infantarius) in many of the previous investigations [4,6].
In healthy individuals, S. bovis incidentally enters the gastrointestinal tract where the protective mucus layer prevents direct interaction of these bacteria with colon epithelial cells [22], whereas the established intestinal flora may prevent S. bovis to be a general inhabitant of the human gastrointestinal tract (detectable in ~10% of the healthy population) [7]. Increased anti-RpL7/L12 antibody prevalence in older subjects compared with younger subjects observed in our control group may be explained by cohort effect or by impaired local immunity to clear bacteria from the gastrointestinal tract associated with aging. It should also be worth noting that about 20% of the population over age 50 years carries asymptomatic colon polyps [23], and thereby this group may acquire higher anti-RpL7/L12 titers as described below.
Pre-malignant lesions can be initiated and progressed by carcinogenic (dietary) factors that diffuse through the colonic mucus layer and induce mutations within the APC or B-catenin genes [24]. These thereby immortalized epithelial cells are prone to the accumulation of other mutations and as a side effect, the aberrant epithelial physiology disturbs the mucus layer covering the epithelial cells [25] and makes it susceptible to bacterial infiltration (Fig. 4). Pre-malignant epithelial sites may also provide a selective bacterial microenvironment, for instance by the excretion of specific metabolites, recruitment of immune cells and/or production of selective anti-microbial substances. Bacteria, such as S. bovis, which are unable to effectively colonize the healthy colon may have a competitive advantage in this microenvironment and survive for prolonged periods of time. These pre-malignant lesions may also provide a portal of entry for S. bovis, which explains the increased anti-RpL7/L12 antibody and the increased occurrence of S. bovis endocarditis in colon adenoma patients [6] Late stage tumors entering the metastatic phase seem to change in such a way that bacterial survival on the tumor surface is diminished or antibody expression due to bacterial interaction is reduced. The possibility that tumor progression may drive bacteria out of the cancerous tissue is similar to what has been reported for H. pylori during gastric cancer progression [26, 27]. If true, this phenomenon may partly account for a wide range (10–60%) of the prevalence of S. bovis reported for CRC patients that is comprised of various stages of the disease.
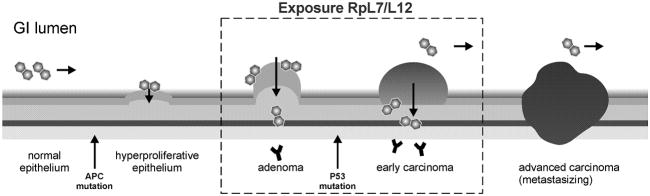
FIGURE 4.
Temporal association between bacterial infection and CRC progression. The development of colorectal tumors is schematically depicted from left (healthy) to right (invasive and metastasizing carcinomas). Hallmarks are a first mutation, mostly in the APC gene, resulting in immortal adenomas and a following mutation that transforms adenomas into carcinomas, often in the P53 gene. We hypothesize that adenomas and early carcinomas provide a preferred niche for S. bovis, which leads to an increased exposure to RpL7/L12 as monitored by increased serum antibodies against this bacterial antigen. By this, S. bovis and possibly related intestinal bacteria may specifically interfere with early colon carcinogenesis, whereas tumor progression may drive these bacteria out of advanced cancerous tissue (see text for details).
The current data suggest that the exposure to bacterial antigen RpL7/L12 is accompanied by an increased risk factor for CRC during the early stages of tumor development. This may be explained by the fact that the interaction between bacteria and colorectal epithelial cells can induce inflammatory pathways [28, 29] or carcinogenic pathways that stimulate progression of adenomas into carcinomas. Increased mutation rate may for instance be achieved by the bacterial conversion of dietary constituents into carcinogens or the toxic effects of specific bacterial metabolites [30, 31]. On the other hand, increased bacterial interaction by for instance probiotic bacteria may also have a negative effect on tumor cell development [32]. Thus, bacterial interference may positively and negatively affect tumor cell progression, which may be balanced by the composition of the gut flora and dietary intake. The present observations, however, point towards a net increased risk for colon tumor development upon increased humoral immune response against bacterial antigen RpL7/L12. Although this risk seems low, a small increase may be relevant for the progression of colon adenomas to carcinomas (accumulation of mutations), a process which can take over a decade to take place. In this respect, it is interesting to note that the calculated ORs of 1.50 and 2.75 for early stage CRC are within the range of those calculated for the serological response to a panel of Helicobacter pylori antigens in patients with early stage gastric cancer (ORs ranging from 1.0 – 8.9) [33].
A drawback of the current approach is the fact that the RpL7/L12 antigen is conserved within the bacterial community, resulting in a certain extent of cross reactivity of our serological assay. Furthermore, the strength of the humoral immune response to a specific antigen is both dependent on the immune status of the host and may vary between different infective strains. Finally, no data are yet available that correlate bacterial colonization of tumor tissue with the humoral immune response to bacterial antigens. Accordingly, our future plans include the identification of new S. bovis-specific antigens and the development of a panel of preferably less conserved antigens for the detection of S. bovis exposure in CRC patients, which will be correlated with S. bovis carriage in the intestinal tract. We anticipate that a broader approach will yield a more specific assay that may be applied on an individual level. Finally, and most important, cross-sectional and retrospective studies, including the current study and others, are not able to address the temporal relationship between an exposure and a disease outcome directly. Thus, prospective studies are essential to elucidate the etiological roles of S. bovis and other intestinal bacteria in colorectal carcinogenesis. These, altogether, would provide a more detailed insight in the relation between intestinal bacteria and the development and progression of CRC.
Acknowledgments
We thank Coby Laarakkers, Erwin Wiegerinck, Siem Klaver, Stan de Klein, Bart Kiemeney, Femmie de Vegt, Theo Ruers, and our other colleagues from the Departments of Laboratory Medicine, Epidemiology and Biostatistics, and Surgery and the members of the Nijmegen Biomedical Study project group for stimulating discussions, technical support and/or collection/selection of serum samples from cases and controls.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
Financial support: AB and RS were supported by the Dutch Cancer Society (KWF; project KUN 2006-3591) and IK was supported in part by the US National Institutes of Health (Research Grant R01-CA93817).
References
- 1.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 2.Klein RS, Recco RA, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. Association of Streptococcus bovis with carcinoma of the colon. N Engl J Med. 1977;297:800–802. doi: 10.1056/NEJM197710132971503. [DOI] [PubMed] [Google Scholar]
- 3.zur Hausen H. Streptococcus bovis: causal or incidental involvement in cancer of the colon? Int J Cancer. 2006;119:xi–xii. doi: 10.1002/ijc.22314. [DOI] [PubMed] [Google Scholar]
- 4.Corredoira J, Alonso MP, Coira A, et al. Characteristics of Streptococcus bovis endocarditis and its differences with Streptococcus viridans endocarditis. Eur J Clin Microbiol Infect Dis. 2008;27:285–291. doi: 10.1007/s10096-007-0441-y. [DOI] [PubMed] [Google Scholar]
- 5.Burnett-Hartman AN, Newcomb PA, Potter JD. Infectious agents and colorectal cancer: A review of Helicobacter pylori, Streptococcus bovis, JC Virus, and Human Papillomavirus. Cancer Epidemiol Biomarkers Prev. 2008;17:2970–2979. doi: 10.1158/1055-9965.EPI-08-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boleij A, Schaeps RMJ, Tjalsma H. Association between Streptococcus bovis and colon cancer. J Clin Microbiol. 2009;47:516. doi: 10.1128/JCM.01755-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlegel L, Grimont F, Collins MD, Régnault B, Grimont PA, Bouvet A. Streptococcus infantarius sp. nov., Streptococcus infantarius subsp. infantarius subsp. nov. and Streptococcus infantarius subsp. coli subsp. nov. isolated from humans and food. Int J Syst Evol Microbiol. 2000;50:1425–1434. doi: 10.1099/00207713-50-4-1425. [DOI] [PubMed] [Google Scholar]
- 8.Ellmerich S, Schöller M, Duranton B, et al. Promotion of intestinal carcinogenesis by Streptococcus bovis. Carcinogenesis. 2000;21:753–756. doi: 10.1093/carcin/21.4.753. [DOI] [PubMed] [Google Scholar]
- 9.Ellmerich S, Djouder N, Schöller M, Klein JP. Production of cytokines by monocytes, epithelial and endothelial cells activated by Streptococcus bovis. Cytokine. 2000;12:26–31. doi: 10.1006/cyto.1999.0521. [DOI] [PubMed] [Google Scholar]
- 10.Tjalsma H, Schöller-Guinard M, Lasonder E, Ruers TJ, Willems HL, Swinkels DW. Profiling the humoral immune response in colon cancer patients: diagnostic antigens from Streptococcus bovis. Int J Cancer. 2006;119:2127–2135. doi: 10.1002/ijc.22116. [DOI] [PubMed] [Google Scholar]
- 11.Tjalsma H, Schöller-Guinard M, Lasonder E, Swinkels DW. Shotgun immunoproteomics to identify disease-associated bacterial antigens: application to human colon cancer. Proteomics Clin Appl. 2007;1:429–434. [Google Scholar]
- 12.Hoogendoorn EH, Hermus AR, de Vegt F, et al. Thyroid function and prevalence of anti-thyroperoxidase antibodies in a population with borderline sufficient iodine intake: influences of age and sex. Clin Chem. 2006;52:104–111. doi: 10.1373/clinchem.2005.055194. [DOI] [PubMed] [Google Scholar]
- 13.Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int. 2007;72:632–637. doi: 10.1038/sj.ki.5002374. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 15.Boleij A, Schaeps RMJ, de Kleijn S, et al. Surface-exposed Histone-like protein A modulates adherence of Streptococcus gallolyticus to colon adenocarcinoma cells. Infect Immun. 2009;77:5519–5527. doi: 10.1128/IAI.00384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schägger H. Tricine-SDS-PAGE. Nat Protoc. 2006;1:16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- 17.Barclay GR. Endogenous endotoxin-core antibody (EndoCAb) as a marker of endotoxin exposure and a prognostic indicator: a review. Prog Clin Biol Res. 1995;392:263–272. [PubMed] [Google Scholar]
- 18.Littell RC, Stroup WW, Freund RJ. Statistical Analysis System (SAS) for linear models. 4. SAS Institute; Cary, NC: 2002. [Google Scholar]
- 19.Breslow NE, Day NE. IARC Scientific Publications No. 32. IARC; Lyon: 1980. Statistical Methods in Cancer Research. Vol. I. The Analysis of Case-Control Studies. [PubMed] [Google Scholar]
- 20.Gardiner KR, Halliday MI, Barclay GR, et al. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995;36:897–901. doi: 10.1136/gut.36.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardiner KR, Anderson NH, Rowlands BJ, Barbul A. Colitis and colonic mucosal barrier dysfunction. Gut. 1995;37:530–535. doi: 10.1136/gut.37.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bond JH. Colon polyps and cancer. Endoscopy. 2005;37:208–212. doi: 10.1055/s-2004-826236. [DOI] [PubMed] [Google Scholar]
- 24.Cho KR, Vogelstein B. Genetic alterations in the adenoma--carcinoma sequence. Cancer. 1992;15:1727–1731. doi: 10.1002/1097-0142(19920915)70:4+<1727::aid-cncr2820701613>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000;47:589–594. doi: 10.1136/gut.47.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang HY, Kim N, Park YS, et al. Progression of atrophic gastritis and intestinal metaplasia drives Helicobacter pylori out of the gastric mucosa. Dig Dis Sci. 2006;51:2310–2315. doi: 10.1007/s10620-006-9276-0. [DOI] [PubMed] [Google Scholar]
- 27.Brenner H, Rothenbacher D, Weck MN. Epidemiologic findings on serologically defined chronic atrophic gastritis strongly depend on the choice of the cutoff-value. Int J Cancer. 2007;121:2782–2786. doi: 10.1002/ijc.22992. [DOI] [PubMed] [Google Scholar]
- 28.Biarc J, Nguyen IS, Pini A, et al. Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S. bovis) Carcinogenesis. 2004;25:1477–1484. doi: 10.1093/carcin/bgh091. [DOI] [PubMed] [Google Scholar]
- 29.Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009 doi: 10.1038/nm.2015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huycke MM, Abrams V, Moore DR. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis. 2002;23:529–536. doi: 10.1093/carcin/23.3.529. [DOI] [PubMed] [Google Scholar]
- 31.Rescigno M. The pathogenic role of intestinal flora in IBD and colon cancer. Curr Drug Targets. 2008;9:395–403. doi: 10.2174/138945008784221125. [DOI] [PubMed] [Google Scholar]
- 32.Capurso G, Marignani M, Delle Fave G. Probiotics and the incidence of colorectal cancer: when evidence is not evident. Dig Liver Dis. 2006;38(Suppl 2):S277–282. doi: 10.1016/S1590-8658(07)60010-3. [DOI] [PubMed] [Google Scholar]
- 33.Gao L, Weck MN, Michel A, Pawlita M, Brenner H. Association between chronic atrophic gastritis and serum antibodies to 15 Helicobacter pylori proteins measured by multiplex serology. Cancer Res. 2009;69:2973–2980. doi: 10.1158/0008-5472.CAN-08-3477. [DOI] [PubMed] [Google Scholar]