Abstract
Background & Aims
Factors that regulate enterocyte apoptosis in necrotizing enterocolitis (NEC) remain incompletely understood, although Toll like receptor-4 (TLR4) signaling in enterocytes plays a major role. Nucleotide-binding-oligomerization domain-2 (NOD2) is an immune receptor that regulates other branches of the immune system, although its effects on TLR4 in enterocytes and role in NEC remain unknown. We now hypothesize that activation of NOD2 in the newborn intestine inhibits TLR4, and that failure of NOD2 signaling leads to NEC through increased TLR4-mediated enterocyte apoptosis.
Methods
The effects of NOD2 on enterocyte TLR4 signaling and intestinal injury and repair were assessed in enterocytes lacking TLR4 or NOD2, in mice with intestinal-specific wild-type or dominant-negative TLR4 or NOD2, and in mice with NEC. A protein array was performed on NOD2-activated enterocytes to identify novel effector molecules involved.
Results
TLR4 activation caused apoptosis in newborn but not adult small intestine or colon, and its intestinal expression was influenced by NOD2. NOD2 activation inhibited TLR4 in enterocytes – but not macrophages – and reversed the effects of TLR4 on intestinal mucosal injury and repair. Protection from TLR4-induced enterocyte apoptosis by NOD2 required a novel pathway linking NOD2 with SMAC-diablo, both in vitro and in vivo. Strikingly, activation of NOD2 reduced SMAC-diablo expression, attenuated the extent of enterocyte apoptosis and reduced the severity of NEC.
Conclusions
These findings reveal a novel inhibitory interaction between TLR4 and NOD2 signaling in enterocytes leading to the regulation of enterocyte apoptosis and suggest a therapeutic role for NOD2 in the protection of intestinal diseases like NEC.
Keywords: TLR4, NOD2, NEC
INTRODUCTION
The regulation of enterocyte apoptosis in the newborn intestine plays an important role in determining the balance between mucosal homeostasis and disease1. Such regulation may be of particular relevance to the development of necrotizing enterocolitis (NEC), a devastating illness of premature neonates that is characterized by exaggerated enterocyte apoptosis leading to severe intestinal injury2. In seeking to define the pathways that lead to NEC, we and others have recently identified a critical role for the innate immune receptor Toll like receptor 4 (TLR4) in the regulation of enterocyte apoptosis and the development of this disease3–4. Since the newborn intestine does not spontaneously develop NEC, we now posit that counter-regulatory pathways may exist within enterocytes to restrain TLR4 signaling, and that the failure of such pathways lead to NEC development. However, the existence of pathways that could restrain TLR4 signaling within the newborn intestine, and any role in the pathogenesis of NEC, remains largely undefined.
The nucleotide-binding oligomerization domain-containing-2 (NOD2) protein is a member of the NOD like receptor protein family, which regulates the intracellular recognition of bacterial components by immune cells5. In response to the bacterial ligand muramyl-di-peptide (MDP), NOD2 activation recruits a platform of proteins called the inflammasome, resulting in the activation of caspase-1 and release of IL-1β and IL-186. Although primarily considered to be a receptor for bacterial peptides within immune cells, NOD2 has also been shown to be expressed in intestinal epithelial cells, and a role for NOD2 in intestinal inflammation is suggested as NOD2 mutations are associated with Crohn’s disease7–8. Recently, NOD2 was shown to inhibit TLR4 signaling in dendritic cells9, raising the intriguing possibility that NOD2 could regulate TLR4 signaling in enterocytes also. However, the effects – if any – of NOD2 activation on TLR4 signaling in enterocytes and TLR4-mediated apoptosis in the induction of NEC remain largely unknown.
We now hypothesize that NOD2 activation in the newborn intestine inhibits TLR4 signaling within enterocytes, and that failure of appropriate NOD2 signaling leads to the development of NEC through increased TLR4-mediated enterocyte apoptosis. In support of this hypothesis, we now show that TLR4 within enterocytes is influenced by NOD2, demonstrate a novel link between NOD2 activation in the intestine and TLR4-induced apoptosis, and after examining NOD2-activated enterocytes using protein expression array technology, have identified an unexpected mechanistic role for the oncogenic protein SMAC-diablo in mediating this effect.
METHODS
Cell culture, mice and reagents
IEC-6 enterocytes were obtained from the American Type Culture Collection (ATCC, Manassas, VA), and maintained as described10. Replication-deficient recombinant adenoviruses that express GFP-tagged mouse wild-type or dominant negative TLR4-cDNA and control adenovirus expressing GFP, were prepared with the Adeno-X-Expression System2 kit (Clontech) as described11; adenoviral wild-type and dominant negative NOD2 were from Cell Biolabs (San Diego, CA). TLR4−/− mice were generated as described11. C57Bl-6 and NOD2−/− mice were from Jackson labs. TLR4-deficient IEC-6 enterocytes were generated by transduction of lentiviral particles (Invitrogen) containing TLR4 shRNA (Open Biosystems, Huntsville, AL), using the four plasmid lentiviral packaging system in HEK 293 cells. Stable integration of lentivirus was obtained by selection of cells using puramycin-containing media(5 μg/ml), and complete knockdown of TLR4 was verified by SDS-PAGE.
Protein array
To discover novel signaling pathways by which MDP may alter enterocyte apoptosis, IEC-6 enterocytes were treated with MDP (10μg/ml, 1h) and subjected to a Panorama Antibody Microarray (Sigma-Aldrich) as per manufacture’s protocol. The array was read using a GenePix 4000B scanner utilizing GenePix Pro 6.1.0.4 software. Protein expression intensity at 635nm from duplicate spots were averaged, 635nm channel background was subtracted; data were z-transformed with high inter-array correlation (R2=0.98). Differential expression was measured using the J5 test T>2.0 as described12.
Induction of experimental NEC and endotoxemia
All procedures were approved by the Children’s Hospital of Pittsburgh Animal Care and Use Committee and the Institutional Review Board of the University of Pittsburgh. Endotoxemia was induced by the intraperitoneal injection of LPS (Escherichia coli 0111:B4 >99% pure, 5mg/kg, Sigma-Alderich) and/or MDP (1mg/kg). Animals were then euthanized, and samples were obtained from blood and the distal 2cm of terminal ileum. Experimental NEC was induced in 10–14 day old mice as described13 using formula gavage (Similac Advanced infant formula (Ross Pediatrics):Esbilac canine milk replacer 2:1) five times/day, and hypoxia (5%O2, 95%N2) for 10min in a hypoxic chamber (Billups-Rothenberg, DelMar, CA) twice daily for 4d. Where indicated, mice were enterally administered adenoviruses as described above twice daily for three days (240uL, 1012 PFU). NEC severity was determined by a blinded pediatric pathologist who reviewed histological specimens obtained from the terminal ileum of mice with and without NEC, and who was unaware of the experimental condition. Each slide was then assigned a NEC severity score based upon the extent on mucosal inflammation, necrosis, leukocyte infiltration, edema of the lamina propria and thrombosis as described 3, 11, 13–14. The expression of GFP (for transfected TLR4) or human NOD2 in mucosal scrapings of the intestine, lung and liver were assessed by RT-PCR and confirmed to be expressed in the intestinal mucosa with no detectable expression in the liver or lung13. Intestinal samples were obtained from human neonates undergoing intestinal resection for NEC, for unrelated indications (control), or at time of stoma closure were processed as described13.
Determination of intestinal injury and repair
Enterocyte apoptosis was determined in wild-type or TLR4-deficient IEC-6 cells 14h after LPS treatment (50 μg/ml), and in the terminal ileum 6h after injection with LPS (1mg/kg). Enterocyte migration was determined using a scrape-wounding assay in serum-starved IEC-6 cells after treatment with LPS (50μg/ml), MDP (10μg/ml), or MDPC (10μg/ml) and imaged using a live cell Axiovert 200 microscope (Carl Zeiss), and in vivo by determining the extent of migration of BrdU-labeled enterocytes along the crypt-villus axis in mice injected for 18h with LPS (1mg/kg) and/or MDP (1mg/kg) as described15. Enterocyte proliferation was determined in IEC-6 cells using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (XTT) proliferation assay (Sigma-Aldrich), and in vivo by determining the expression of the proliferation marker pCNA as described11. To determine enterocyte apoptosis in vitro, IEC-6 cells were stained for cleaved caspase-3 and the number of caspase-3 positive cells were identified in a blinded fashion using Metamorph software and expressed as a percentage of caspase-3 positive cells per high power field, with over 100 fields/experiment studied, and over 100 cells/field. Experiments were repeated in triplicate, and the data was expressed as mean±SEM.
Statistical analysis
Data are mean+/−SEM, and comparisons are by two-tailed Student’s t-test or ANOVA, statistical significance p<0.05. All experiments were repeated in at least triplicate, with >200 cells/high power field, and at least five mice/group. Chi-squared was performed for analysis NEC incidence.
RESULTS
TLR4 activation leads to enterocyte apoptosis in the newborn small intestine and is negatively influenced by NOD2 expression
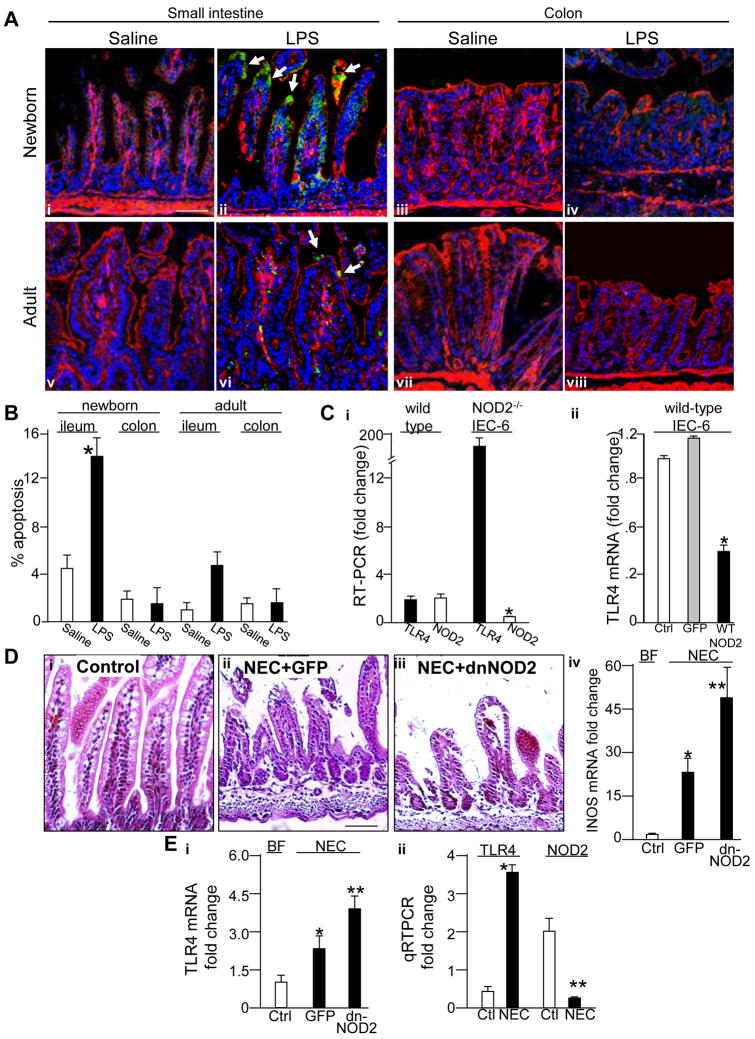
A central hypothesis of the current study is that TLR4 signaling leads to enterocyte apoptosis in the newborn intestine, that NOD2 inhibits TLR4 signaling within enterocytes, and that impaired NOD2 signaling in the premature intestine leads to exaggerated TLR4 signaling and the development of NEC. To test this directly, we first assessed the effects of TLR4 activation on enterocyte apoptosis in the newborn versus adult ileum and colon. As shown in Figure 1 panels A and B, LPS injection led to an increase in enterocyte apoptosis in the newborn but not adult ileum and rarely if at all in the colon at either age. To test next whether TLR4 expression may be influenced by NOD2, we generated NOD2-deficient IEC-6 cells and assessed TLR4 expression. As shown in Figure 1 panel Ci, deletion of NOD2 led to a significant increase in TLR4. In further support of a relationship between TLR4 and NOD2 expression in enterocytes, transfection of IEC-6 cells with wild-type NOD2 resulted in a significant loss of TLR4 expression (Figure 1 panel Cii). These findings suggest the possibility that the inverse relationship between TLR4 and NOD2 may affect the development of NEC. To test this directly, newborn mice were induced to develop NEC through the combination of formula gavage and intermittent hypoxia over four days. For the first two days, mice were also administered by enteral gavage adenoviruses that express either GFP, or dominant-negative NOD2, which resulted in significant expression of transfected protein in the enterocytes with minimal detectable expression in the lung or liver13. As is shown in Figure 1 panel D–E, administration of mutant-NOD2 resulted in a marked increase in the severity of NEC as manifest by histological disruption of villous architecture and increased iNOS expression (D), and increased expression of TLR4 (panel E i) compared to mice administered adenoviral GFP. Further evidence for the relevance of these findings is demonstrated as the development of NEC in mice was associated with a significant loss of NOD2 and a concomitant increase in TLR4 expression in the premature intestine (panel E ii). These findings demonstrate that TLR4 expression was influenced by NOD2 in the gut, and suggest that activation of NOD2 could limit signaling in enterocytes.
Figure 1. TLR4 activation leads to enterocyte apoptosis in the newborn small intestine and is negatively influenced by NOD2 expression.
A. Immuno-confocal micrographs showing cleaved caspase 3 (green, arrows indicate apoptotic enterocytes), actin (red) and nuclei (blue) in the small bowel or colon of newborn or adults treated with saline or LPS (1mg/kg, 16hrs) as indicated. B: Quantification of apoptosis under conditions in A. *p<0.05 vs. saline. C. Quantitative RT-PCR showing expression of i: TLR4 (solid bars) and NOD2 (open bars) in wild-type or NOD2-deficient IEC-6 cells *p<0.05 vs. open bars; ii: TLR4 in IEC-6 cells transfected with adenoviruses expressing either GFP (grey) or wild-type-NOD2 (black), *p<0.05vs GFP. D i–iii: Micrographs of the terminal ileum in newborn mice that were either breast fed (control, i) or induced to develop NEC and administered adenoviruses expressing GFP (ii) or dominant-negative NOD2 (iii); iv: RT-PCR showing expression of iNOS pertaining to i–iii. *p<0.05 vs. ctrl, **p<0.05 vs. GFP. E. i: RT-PCR showing expression of TLR4 pertaining to panel D; ii: quantitative RT-PCR for TLR4 and NOD2 in mice with and without NEC,*p<0.05 vs. control. All mice experiments performed over 3 times with> 8 mice/group.
NOD2 activation limits TLR4 signaling in enterocytes in vivo and in vitro
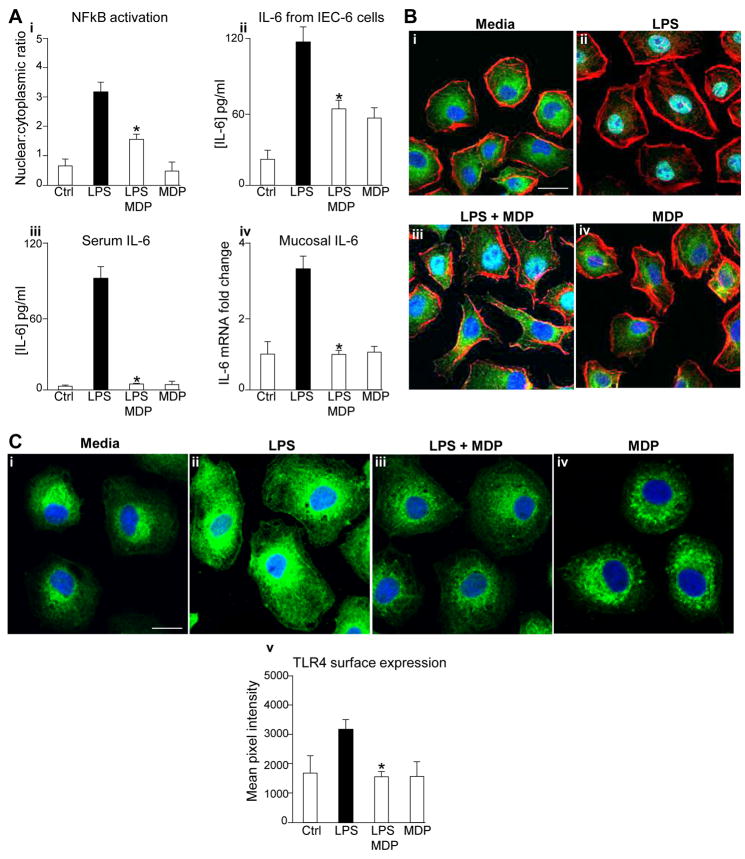
To test directly whether activation of NOD2 could limit TLR4 signaling in enterocytes, we used the NOD2 agonist MDP, and assessed TLR4 signaling as the extent of nuclear translocation of NFkB and the release of IL-6. As shown in Figure 2, treatment of IEC-6 enterocytes with MDP reduced the extent of TLR4-mediated NFkB activation (panel Ai and B) and release of IL-6 (Panel Aii). MDP also inhibited TLR4 signaling in the newborn intestinal mucosa, as the injection of MDP into mice reduced the extent of LPS-mediated increase in serum IL-6 and ileal mucosal IL-6 (Panel Aiii–iv). MDP did not reduce LPS signaling in macrophages (Supplementary Figure 1, panels A–B), confirming an enterocyte-specific effect. In seeking to determine whether NOD2 signaling affects the subcellular distribution of TLR4, IEC-6 cells were treated with LPS and/or NOD2 and examined by confocal microscopy using antibodies to TLR4. In control cells, TLR4 is predominantly localized within a diffuse, subcellular compartment, with a distribution that closely resembles that which we have reported previously 16. The addition of LPS resulted in an increase in the total expression levels of TLR4, as we have shown previously3, as well as a relative shift of TLR4 to the cell surface. Importantly, the addition of the NOD2 agonist MDP restored the levels of TLR4 to the diffuse subcellular distribution that more closely resembled untreated cells, consistent with inhibitory effects of NOD2 activation of TLR4 responses. The addition of MDP alone did not have any detectable effect on the localization of TLR4 within enterocytes (See quantification of surface TLR4 pixel distribution in Figure 2, panel Cv). Taken together, these findings demonstrate that NOD2 activation limits TLR4 signaling in enterocytes and in the newborn intestinal mucosa, and when considered in light of the findings of Figure 1, suggest that a loss of NOD2 responsiveness in enterocytes may contribute to the pathogenesis of NEC.
Figure 2. NOD2 activation limits TLR4 signaling in enterocytes both in vivo and in vitro.
A. IEC-6 cells or wild-type mice were treated with LPS±MDP as indicated. i: extent of NFkB activation IEC-6 cells as measured by ratio NFkB p65-subunit in nucleus vs. cytoplasm, ii: IL-6 release from IEC-6 cells by ELISA; iii: serum IL-6; iv: RT-PCR showing fold change in IL-6 in small intestinal mucosa. *p<0.05 vs. LPS alone in each case B: Confocal micrographs of IEC-6 cells corresponding to panel Ai immunostained for NFkB (green), actin(red), nucleus(blue), size bar=10μm. C i–iv: Confocal micrographs of IEC-6 cells treated as indicated and immunostained for TLR4 (green) and nuclei(blue); v: quantification of the surface expression of TLR4 using ImageJ software representative of 3 separate experiments. *p<0.05 vs. LPS alone.
We next sought to assess whether post-translational mechanisms may mediate the inhibitory effects of NOD2 activation with MDP on TLR4 signaling. We first sought to determine whether MDP could activate the inflammasome in enterocytes, and whether activation of the inflammasome could mediate the inhibition of TLR4 signaling in enterocytes by MDP. As shown in Supplemental Figure 1 panel C, both MDP and LPS activated the inflammasome in IEC-6 cells as demonstrated by increased expression of cleaved caspase-1. However, prevention of inflammasome activation did not alter the ability of MDP to attenuate TLR4-induced translocation of NFkB in enterocytes (Supplemental Figure 1 panel D). Several intracellular inhibitors have been shown to limit the extent of TLR4 activation, including IRAK-M, A20, PPARγ, Tollip, and IRF417. However, MDP did not alter the expression of these inhibitors at any of the time-points studied (Supplemental Figure 2). Having excluded these potential mechanistic pathways, we next sought to evaluate the physiological relevance of the inhibitory effects of MDP on enterocyte TLR4 signaling on intestinal injury and repair, and in the pathogenesis of NEC.
MDP reverses the inhibitory effects of TLR4 on intestinal injury and repair
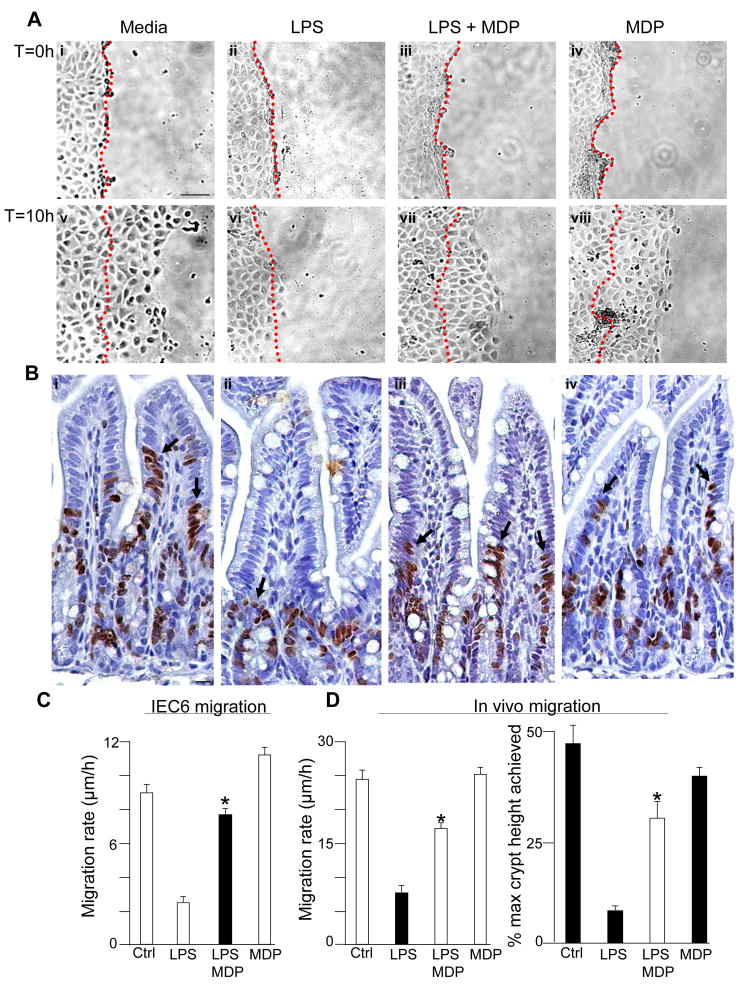
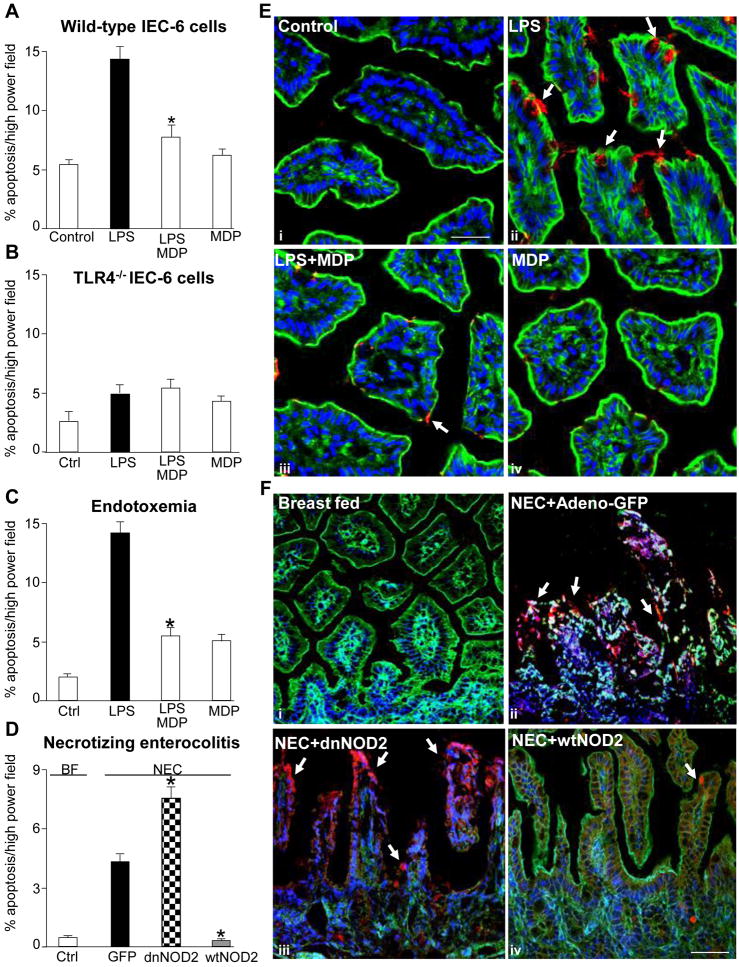
We have shown that activation of TLR4 within the newborn small intestinal mucosa leads to increased intestinal injury through increased apoptosis, and reduced healing capacity through inhibitory effects on enterocyte migration and proliferation, and that together these factors lead to the development of NEC3. In order to further define the potential significance of the interactions between NOD2 and TLR4 signaling, we next investigated the effects of MDP on TLR4-mediated intestinal injury and repair. As is shown in Supplemental Figure 3, treatment of IEC-6 cells with MDP reversed the inhibitory effects of TLR4 activation with LPS on enterocyte proliferation, both in vitro and in vivo. MDP also reversed the TLR4-induced inhibition of enterocyte migration, both in cultured enterocytes (Figure 3 panels A, C) and in the small intestine of newborn mice (Figure 3 panel B, D). MDP did not alter the extent of phosphorylation of focal adhesion kinase – a factor that we have previously shown to mediate the inhibition of enterocyte migration in response to TLR4 activation3 - suggesting that MDP reverses the TLR4-mediated inhibition in enterocyte migration through alternate pathways (not shown). As shown in Figure 4 panel A, NOD2 activation with MDP also reversed the effects of LPS on the induction of enterocyte apoptosis in vitro. The effect of LPS on the induction of enterocyte apoptosis was specific for TLR4, as LPS caused no change in the degree of enterocyte apoptosis in IEC-6 cells that were deficient in TLR4 expression (Figure 4, Panel B). In support of the physiological relevance of these findings, the injection of MDP into mice reversed the LPS-mediated increase in apoptosis observed in the small intestinal mucosa (Figure 4 panels C, see micrographs in E). These findings raised the exciting possibility that NOD2 signaling in the intestinal mucosa may play a role in the regulation of enterocyte apoptosis in NEC. To test this directly, newborn mice were induced to develop NEC and administered by enteral gavage adenoviruses that express either dominant-negative or wild-type NOD2, or control viruses expressing GFP. As shown in Figure 4 panel D (see micrographs in F), newborn mice with NEC that were administered adenoviral-GFP demonstrated a significant increase in enterocyte apoptosis as compared with breast-fed control mice (open bars). Importantly, the administration of wild-type NOD2 to mice with NEC significantly decreased enterocyte apoptosis (grey bars) while administration of dominant-negative NOD2 bearing an inhibitory frame-shift mutation (checker bars) significantly increased enterocyte apoptosis in mice with NEC compared with control NEC-mice administered adenoviral-GFP. These findings demonstrate NOD2 activation not only inhibits TLR4 signaling, but also reverses the inhibitory effects of TLR4 activation on mucosal injury within the intestine. We next sought to evaluate further the molecular mechanisms that could mediate these effects.
Figure 3. Activation of NOD2 reverses the inhibitory effects of TLR4 on enterocyte migration in vitro and in vivo.
A: Micrographs demonstrating IEC-6 cells migrating into a scraped wound after treatment with media or LPS±MDP, at the beginning (T=0) and the end (T=0) of migration. The position of the scrape is indicated by a dashed line. B. Micrographs of terminal ileum immunostained with BrdU 24h after injection of BrdU into newborn mice that were also injected with saline (i), LPS (ii), LPS+MDP (iii) or MDP (iv). Arrows show leading BrdU-positive enterocytes. C–D: Quantification of migration in vitro (C) and in vivo (D). *p<0.005 vs. LPS alone in each case;bar=100μm.
Figure 4. Activation of NOD2 reverses the effects of TLR4 activation on enterocyte apoptosis in vitro and in vivo and in NEC.
A–B: Quantification of apoptosis in either (A) wild-type or (B) TLR4−/− IEC-6 cells treated with LPS±MDP as indicated. C–D: Apoptosis in the terminal ileum of newborn mice injected with C: saline or LPS±MDP, or D: induced to develop NEC and administered adenoviruses expressing GFP, dominant-negative (dn)-NOD2 or wild-type (wt)-NOD2. *p<0.05 vs. solid bar, 3 separate experiments. E: Confocal micrographs of terminal ileum of newborn mice corresponding to C stained with cleaved-caspase-3 (red), nuclei (blue), actin (green). Arrows=apoptotic cells; bar=100μm. F: Confocal micrographs of terminal ileum corresponding to D; i, tissue stained with actin (green), nuclei (blue); ii, tissue stained with GFP (green), cleaved-caspase-3 (red), nuclei (blue); iii–iv, tissue stained with cleaved-caspase-3(red), actin(green), nuclei(blue), size bar=100μm;arrows=apoptotic cells; representative of 3 separate experiments, over 8 mice/group.
MDP reverses the TLR4-induced enterocyte apoptosis through activation of the regulatory protein SMAC-diablo
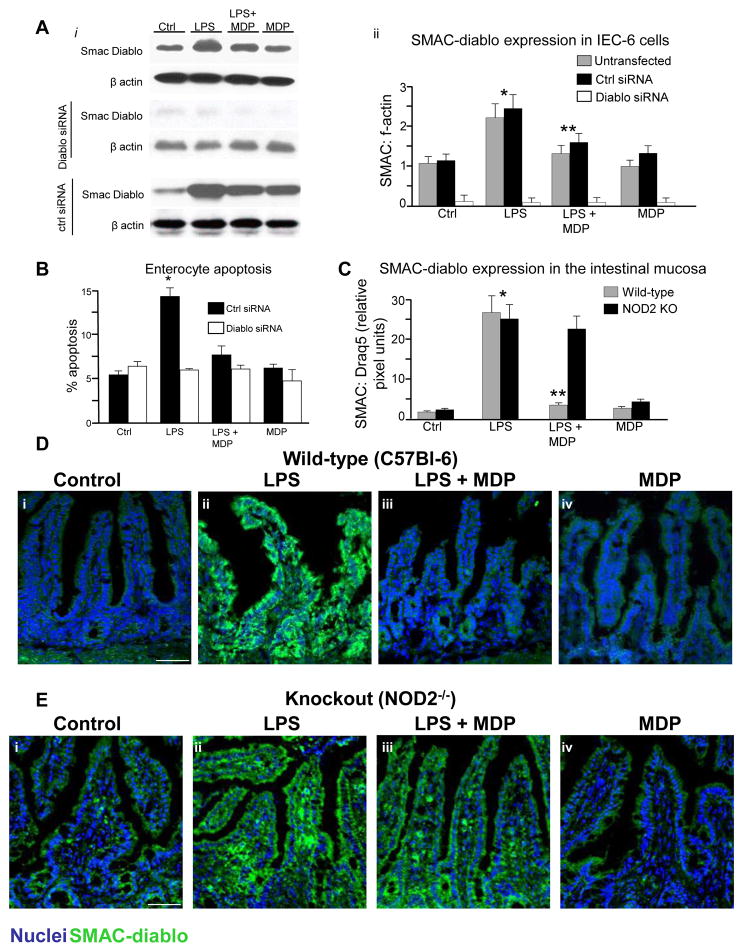
To identify the potential molecular mechanisms by which MDP may reduce the extent of TLR4-induced apoptosis in enterocytes, we performed a protein array on enterocytes in the presence or absence of MDP. We were particularly interested in identifying proteins that were down-regulated by MDP so as to identify novel counter-regulatory pathways. As is shown in Supplemental Figure 4, although a variety of proteins demonstrated increased expression, the protein that was most significantly downregulated by MDP was the apoptosis regulatory protein SMAC-diablo. Although not yet shown to play a role in enterocytes during inflammation, SMAC-diablo regulates cancer cell apoptosis by blocking the inhibitor of apoptosis protein (IAP) family through c-termini interactions18. As shown in Figure 5 panel A, LPS increased the expression of SMAC-diablo in IEC-6 cells, and this was reduced in the presence of MDP, confirming the array data (quantification in Aii). The induction in enterocyte apoptosis by LPS, and the protection by MDP, were significantly reduced after knockdown of SMAC-diablo using siRNA (Figure 5, Panel B). There was no effect after treatment with control siRNA against no known targets. In support of the physiological relevance of these findings, SMAC-diablo expression was increased in the small intestine of wild-type mice after treatment with LPS, and this was reversed in the presence of MDP (Figure 5 Panel C,D). The reversal in SMAC-diablo expression in response to MDP was not seen in NOD2-deficient mice (Figure 5 panel C,E), confirming the novel link between NOD2 signaling and the inhibition of SMAC-diablo expression in the pathways leading to TLR4-induced enterocyte apoptosis.
Figure 5. MDP reverses TLR4-induced enterocyte apoptosis through activation of SMAC-diablo.
A. i: Expression of SMAC-diablo and beta-actin by SDS-PAGE in IEC-6 cells LPS±MDP after being either untransfected or transfected with siRNA to SMAC-diablo or control-siRNA. ii: Quantification of SMAC-diablo:f-actin in 3 separate blots similar to those in i,*p<0.05 vs control; **p<0.05 vs. LPS. B: Apoptosis in IEC-6 cells transfected with control-siRNA or SMAC-diablo-siRNA and treated with LPS±MDP. *p<0.005 vs. control, both transfection conditions. C: quantification of intestinal mucosal expression of SMAC-diablo in wild-type and NOD2 KO pertaining to D and E,*p<0.05 vs control; **p<0.05 vs. LPS. D–E: Confocal micrographs of terminal ileum of wild-type(D) or NOD2−/− mice(E) treated with saline (i), LPS (ii), LPS±MDP(iii) or MDP (iv) and stained for SMAC-diablo(green) and nuclei(blue). Size bar=100μm. representative of 3 separate experiments, over 8 mice/group.
NOD2 activation in enterocytes regulates SMAC-diablo expression and enterocyte apoptosis in NEC
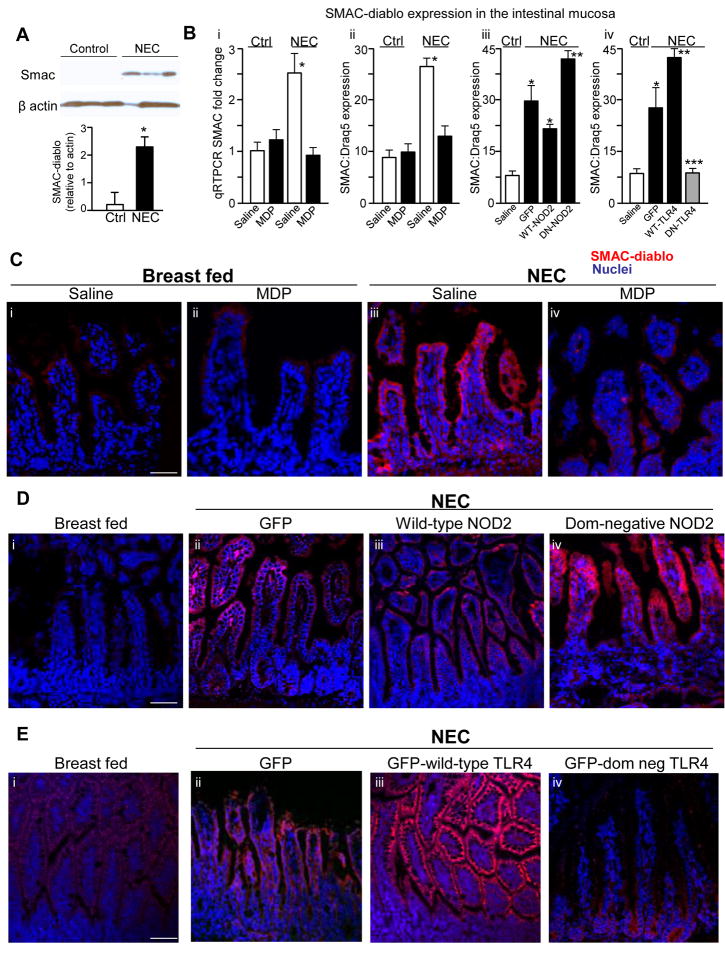
The above findings raise the intriguing possibility that MDP activation of NOD2 may regulate the expression of SMAC-diablo in NEC, a disease whose development we have shown to require TLR4-mediated enterocyte apoptosis3. To evaluate this possibility, the expression of SMAC-diablo was assessed in the terminal ileum of newborn mice that were induced to develop NEC, and the extent of NOD2 and TLR4 signaling in the intestinal mucosa was experimentally manipulated. As shown in Figure 6 panel A, newborn mice with NEC were found to have increased SMAC-diablo expression compared with breast fed controls by SDS-PAGE. The administration of MDP to mice on each day of the experimental model reversed the increase in SMAC-diablo expression in the intestinal mucosa, as demonstrated both by RT-PCR (Figure 6 panel Bi) and immunohistochemistry (Figure 6 panels Bii and C). To test directly whether NOD2 activation in enterocytes could regulate the expression of SMAC-diablo in NEC, newborn mice were induced to develop NEC and administered by enteral gavage adenoviruses that express either dominant negative or wild-type NOD2, or control viruses that express GFP. As is shown in Figure 6 panels Biii and D, the expression of SMAC-diablo was increased in the terminal ileum of newborn mice with NEC compared with breast fed controls, yet was markedly attenuated after adenoviral expression of wild-type NOD2 - correlating with a marked reduction in enterocyte apoptosis (see Figure 4D). Strikingly, expression of mutant (inhibitory) NOD2 led to a striking increase in SMAC-diablo expression (Figure 6 panel D iv versus i) – correlating with an increase in enterocyte apoptosis (see Figure 4D), and providing further evidence that NOD2 activation can restrain increases in SMAC-diablo expression during intestinal inflammation.
Figure 6. NOD2 activation regulates SMAC-diablo expression in enterocytes in NEC.
A: SDS-PAGE showing SMAC-diablo expression in terminal ileum from 3 separate mice with and without NEC, blots were re-probed for actin; quantification is shown; *p<0.05, n=3 similar blots. B: i: SMAC-diablo quantitative RT-PCR in terminal ileum mucosa in mice without (control) and with NEC that were injected with saline or MDP. *p<0.05 vs. solid bar; ii–iv: quantification of SMAC-diablo expression in enterocytes corresponding to panels C–E; *p<0.05 **p<0.005 black vs. white bar; *** black vs. grey bar. C–E: Confocal micrographs of terminal ileum of newborn mice either breast fed or induced to develop NEC and stained for SMAC-diablo (red) or nuclei (blue). As indicated, mice with NEC were either treated with MDP or saline (C), administered by gavage adenoviruses expressing either GFP, wild-type or dominant negative NOD2 (D), or administered by gavage adenoviruses expressing either GFP, wild-type or dominant negative TLR4 (E). Size bar=100μm. representative of 3 separate experiments, over 8 mice/group.
Having shown that TLR4 signaling is influenced by NOD2 expression in enterocytes (Figure 1), a possible interpretation of the above findings is that TLR4 activation may also be required for the induction of SMAC-diablo in the setting of NEC. To test this directly, newborn mice were induced to develop NEC, and were administered by enteral gavage adenoviruses that express either wild-type or dominant negative TLR4. As is shown in Figure 6 panels Div and E, transfection with dominant-negative TLR4 blocked the increase in expression of SMAC-diablo in the intestinal mucosa of mice with NEC compared with transfection with wild-type TLR4, in which SMAC-diablo was significantly increased. Based upon these findings, we next sought to evaluate whether activation of NOD2 with MDP would affect the severity of NEC.
NOD2 activation with MDP reduces the extent of intestinal injury and attenuates the severity of NEC
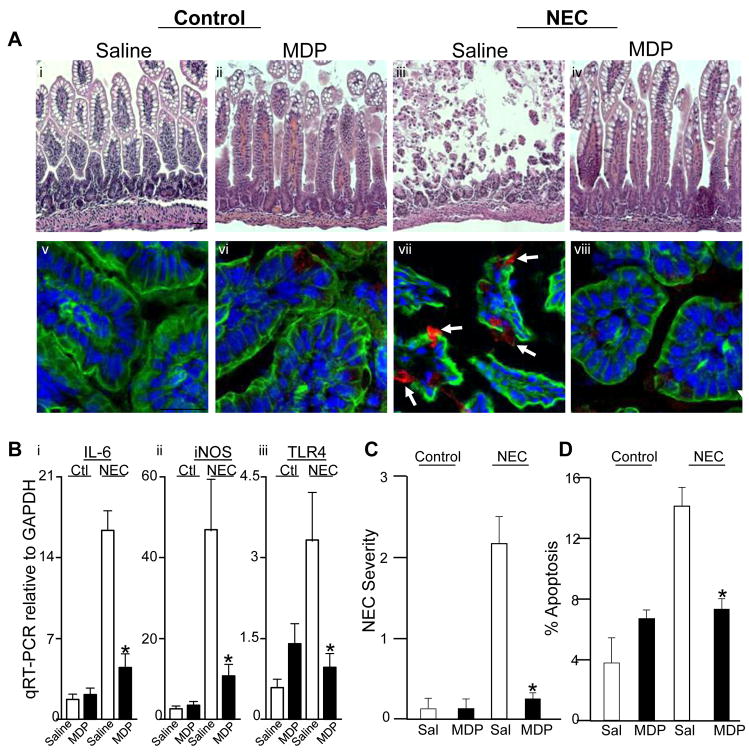
In the final series of studies, we investigated the effects of MDP on the extent and severity of NEC, and the degree of apoptosis in the terminal ileum of newborn mice with disease. To do so, mice were induced to develop NEC and treated with either saline or MDP on each day of the model. As shown in Figure 7 panels A–C, NEC was associated with profound tissue injury as well as marked enterocyte apoptosis, consistent with our previous results3. Strikingly, the treatment with MDP markedly attenuated the degree of intestinal injury (Figure 7 panels A and B), while also reducing the degree of expression of pro-inflammatory cytokines IL-6, iNOS and TLR4 (panel B), as well as reducing enterocyte apoptosis (panels A and D). Taken together, these studies reveal that NOD2 activation inhibits TLR4 signaling in the newborn intestine leading to reduced injury, and as a result, that MDP may serve as a potential novel therapeutic in NEC.
Figure 7. NOD2 activation with MDP reduces intestinal injury and severity of NEC.
A. H&E (i–iv) and confocal micrographs (v–viii, red=cleaved caspase-3, blue=nuclei, green=actin) from terminal ilea of newborn mice with and without NEC and injected with either saline or MDP; arrows=apoptotic cells; size bar=100μm. B–D: Severity of NEC as determined by B: RT-PCR for expression of IL-6, iNOS and TLR4 within the intestinal mucosa, C: Histological grading and D: apoptosis; *p<0.05 vs. NEC-saline. n=5 experiments, >20 mice/group.
DISCUSSION
In the current studies, we describe a novel mechanism by which the interplay between two innate immune receptors in enterocytes, namely NOD2 and TLR4, drives the extent of intestinal injury in the pathogenesis of NEC. We demonstrate that NOD2 activation, as well as the expression of the NOD2 protein itself, limits the extent of TLR4 signaling in enterocytes. In seeking to identify the signaling pathways involve, we now uncover a novel pathway linking these innate immune receptors with the mitochondrial protein SMAC-diablo, which serves to regulate the extent by which NOD2 activation with MDP limits the extent of TLR4-induced intestinal injury and thus serve as a potential therapeutic agent for NEC. This is not only the first demonstration of the importance of SMAC-diablo signaling in enterocytes during inflammation, but also sheds light on a novel signaling effector mechanism linking TLR4 activation with cell death that can be constrained through the activity of NOD2.
Three lines of evidence indicate a role for impaired NOD2 signaling in the pathogenesis of NEC. First, the expression of NOD2 is reduced in NEC compared with controls. Second, inhibition of NOD2 in enterocytes increased NEC severity and increased enterocyte apoptosis. And third, the administration of MDP led to a reduction in enterocyte apoptosis and reduction in NEC severity. In each case, we believe that NOD2 signaling serves to restrain TLR4 signaling in enterocytes, leading to a reduction in the extent of apoptosis, and that the development of NEC represents a failure of this protection. This may in fact represent a general theme by which activation of NOD2 limits TLR4 signaling in other cells also, as has been demonstrated to occur in dendritic cells9. And given that Crohn’s disease is characterized in certain cases by inhibitory mutations in NOD2, it is tempting to speculate that the signaling pathway that we now describe may have relevance to inflammatory bowel disease also.
In terms of seeking to explain the molecular mechanisms by which NOD2 activation limits TLR4 signaling in enterocytes, we were able to exclude various possibilities, and to shed light on others. For instance, although NOD2 activation did lead to the activation of the inflammasome in enterocytes, its activation was not required for the protection of TLR4-induced signaling. Further, NOD2 activation with MDP did not alter the expression of the predominant endogenous inhibitors of TLR4. This finding in enterocytes stands in distinction to the prior work by Watanabe et al who demonstrated that MDP activation of dendritic cells leads to increased IRF4 expression, and that IRF4 mediates the protective effects of MDP in experimental colitis9. And given our novel observation that TLR4 expression is influenced by NOD2, we now suggest that the mechanisms by which activation of NOD2 inhibits TLR4 signaling involves both pre-translational and post-translational factors, and that such modifications lead to the activation of effector signaling pathways that directly affect barrier integrity, including those involving SMAC-diablo.
What is the potential teleological explanation for which NOD2 and TLR signaling would coexist and negatively interact in enterocytes? One possibility is that NOD2 activation may serve to activate the inflammasome in enterocytes and thus alert the host to the presence of invasion by mounting a pro-inflammatory response while concomitantly limiting the extent of TLR4-induced intestinal injury. However, unlike dendritic cells and macrophages, the extent of inflammasome activation by NOD2 in enterocytes would be expected to be relatively small19. An alternative role for the interplay between NOD2 and TLR4 may be found in our observation that TLR4 leads to the internalization of bacteria by enterocytes, a process that may be necessary for antigen sampling and the education of the gut to the presence of various immunologically active peptides16. Such intracellular antigens could then conceivably activate NOD2 and thereby restrain TLR4 signaling, effectively preventing further bacterial entry, while also limiting the effects of TLR4-induced intestinal injury. In a similar manner, we have recently shown that bacterial DNA can inhibit TLR4 signaling via activation of TLR9 – a close homolog of TLR4 that is present on endomembranes within enterocytes13. We now propose that the extent of TLR4 signaling in the newborn intestine – and by extension the predisposition to NEC development in susceptible neonates – is regulated in large part through the combined interactions of TLR4, NOD2 and other innate immune receptors such as TLR9, and that the extent of these interactions is governed by a dynamic interplay between the microbial flora and the inflammatory microenvironment of the host.
In summary, the current work represents a novel mechanism linking NOD2 activation with the inhibition of TLR4 signaling in enterocytes leading to a reduction in apoptosis via SMAC-diablo. Further studies to explore the interplay between TLR4 and NOD2 activation may provide a useful framework in advancing our understanding of the role of the innate immune system in the maintenance of intestinal homeostasis during health, and how dysregulation of these receptors may lead to disease processes such as NEC.
Supplementary Material
Acknowledgments
Grant support: DJH is supported by R01GM078238 and RO1DK08752 from the National Institutes of Health, and The Hartwell Foundation, Memphis, TN. JLW is supported by UL1 RR024153 from the National Center for Research Resources (NCRR) of the National Institutes of Health.
Data analysis was directed by Dr. James Lyons-Weiler in the Bioinformatics Analysis Core at the University of Pittsburgh.
Abbreviations
- NOD2
nucleotide-binding oligomerization domain-2
- TLR4
Toll like receptor-4
- NEC
necrotizing enterocolitis
- SMAC-diablo
second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low PI
Footnotes
Disclosures:
Ward M. Richardson – no conflicts exist; Chhinder P. Sodhi – no conflicts exist; Anthony Russo – no conflicts exist; Steven C. Gribar – no conflicts exist; Matthew D. Neal – no conflicts exist; Shipan Dai – no conflicts exist; Thomas Prindle Jr. – no conflicts exist; Maria Branca – no conflicts exist; Congrong Ma – no conflicts exist; John Ozolek – no conflicts exist; David J. Hackam – no conflicts exist.
Transcript profiling – Not applicable
Writing assistance – Not applicable.
Role in the study: Richardson – study concept and design, data acquisition, data analysis, drafting of manuscript; Sodhi – study concept and design, data acquisition, data analysis; Anthony Russo – data acquisition; Steve Gribar – data acquisition; Matthew Neal – data acquisition, Shipan Dai – data acquisition, Thomas Prindle – data acquisition; Maria Branca – data acquisition; Congrong Ma – data Acquisition; John Ozolek – data acquisition; David Hackam – study concept and design, data analysis, supervision of all experiments, obtaining funding, drafting of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hackam DJ, Upperman JS, Grishin A, Ford HR. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:49–57. doi: 10.1053/j.sempedsurg.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Jilling T, Lu J, Jackson M, Caplan MS. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res. 2004;55:622–9. doi: 10.1203/01.PDR.0000113463.70435.74. [DOI] [PubMed] [Google Scholar]
- 3.Leaphart CL, Cavallo JC, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 4.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, Thomson RB, Soliman A, Arditi M, Caplan MS. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177:3273–82. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franchi L, Warner N, Viani K, Nuñez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–28. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanneganti TD, Lamkanfi M, Núñez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–59. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 8.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe T, Asano N, Murray PJ, Ozato K, Tailor P, Fuss IJ, Kitani A, Strober W. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qureshi FG, Leaphart CL, Cetin S, Jun L, Grishin A, Watkins S, Ford HR, Hackam DJ. Increased expression and function of integrins in enterocytes by endotoxin impairs epithelial restitution. Gastroenterology. 2005;128:1012–22. doi: 10.1053/j.gastro.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 11.Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle TJ, Branca M, Russo A, Gribar SC, Ma C, Hackam DJ. Toll-like Receptor-4 Inhibits Enterocyte Proliferation via Impaired beta-Catenin Signaling in Necrotizing Enterocolitis. Gastroenterology. 2010;138:185–96. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel S, Lyons-Weiler J. A web application for the integrated analysis of global gene expression patterns in cancer. Applied Bioinformatics. 2004;3:49–62. doi: 10.2165/00822942-200403010-00007. [DOI] [PubMed] [Google Scholar]
- 13.Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, Jakub A, Shi XH, Shah S, Ozolek JA, Hackam DJ. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol. 2009;182:636–46. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res. 2000;92:71–7. doi: 10.1006/jsre.2000.5877. [DOI] [PubMed] [Google Scholar]
- 15.Dai S, Sodhi CP, Cetin S, Richardson W, Branca M, Neal MD, Prindle T, Ma C, Shapiro RA, Li B, Wang JH, Hackam DJ. Extracellular high mobility group box1 (HMGB1) inhibits enterocyte migration via activation of toll like receptor 4 and increased cell-matrix adhesiveness. J Biol Chem. 2009 doi: 10.1074/jbc.M109.067454. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176:3070–9. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 17.Shibolet O, Podolsky DK. TLRs in the Gut. IV. Negative regulation of Toll-like receptors and intestinal homeostasis: addition by subtraction. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1469–73. doi: 10.1152/ajpgi.00531.2006. [DOI] [PubMed] [Google Scholar]
- 18.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 19.Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–52. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.