Abstract
Distinct areas within the visual association cortex are specialized for representing specific stimulus features, such as V4 for color and V5/hMT+ for motion. Recent studies have demonstrated that areas associated with attended features exhibit enhanced cortical activity, whereas those associated with ignored features elicit reduced activity. However, the source of this attentional (or top-down) modulation remains uncertain. A network of fronto-parietal cortical regions has been proposed as the prime candidate underlying this top-down modulation. Here, we evaluate whether there are distinct or overlapping top-down network regions for attention to different stimulus features. To this end, we explored functional magnetic resonance imaging (fMRI) functional connectivity data, electroencephalographic (EEG) source localization, and phase coherence that were obtained while participants attended or ignored motion and color stimuli. Functional connectivity analysis indicated that attention to color relies strongly on prefrontal regions, whereas attention to motion recruits both prefrontal and parietal areas. Although these networks are generally topologically segregated, both color and motion processes recruit right inferior frontal junction (IFJ). However, the IFJ may be more critical for color processing, as only connectivity with V4 predicted the degree of attentional modulation. Source localization at the time range of attentional modulation of the event related potential corroborated the role of the right IFJ and indicated that feature-based, top-down modulation occurs early during processing (< 200 ms post- stimulus onset). Furthermore, long-distance alpha (8–12 Hz) phase coherence between the IFJ and visual cortices may serve as a mechanism underlying anticipatory, top-down modulation of color feature processing.
Keywords: selective attention, neural networks, functional magnetic resonance imaging (fMRI), electroencephalography (EEG), inferior frontal junction (IFJ), visual features
Introduction
Both internally and externally generated attention interact to influence perception of our surrounding environment. Information about stimulus features extracted from environmental stimuli are frequently processed in a bottom-up fashion while top-down processing utilizes endogenous factors, such as expectations and goals, to divert or direct attentional resources to accomplish higher cognitive objectives (Corbetta and Shulman, 2002; Frith, 2001; Posner et al., 1980). In this way, top-down modulation is the driving force behind our ability to ignore distractions and focus attention on relevant information. The ability to focus our attention is manifest as an enhancement of neural activity in selective sensory cortical areas; conversely, the ability to ignore distractors is associated with a suppression of neural activity in these same areas. Although top-down modulation is studied most often for the visual system (Duncan et al., 1997; Fuster, 1990; Gazzaley et al., 2005; Kastner et al., 1998), similar characteristics are found in the auditory (Hillyard et al., 1973), somatosensory (Seminowicz et al., 2004) and olfactory (Zelano et al., 2005) systems.
It is believed that top-down modulation is not an intrinsic property of visual cortices, but rather, this goal directed control is mediated by long-range communication between distal brain regions, or neural networks. Tract-tracing studies in monkeys have identified an anatomical framework of reciprocal cortico-cortical projections linking distributed areas in the parietal and frontal cortices to visual association cortex, with the former presumed to be sources of modulation and the latter presumed to be the site of modulation (Cavada and Goldman-Rakic, 1989; Ungerleider et al., 1989; Webster et al., 1994). Moreover, neuroimaging studies in humans have indicated that top-down modulation during visual processing involves a similar fronto-parietal network (also referred to as the dorsal attention network), including frontal eye fields (FEF), middle frontal gyrus (MFG), superior parietal lobule (SPL), supramarginal gyrus (SMG) and intraparietal sulcus (Corbetta and Shulman, 2002; D’Esposito et al., 1998; Moran and Desimone, 1985; Ungerleider et al., 1998).
Recent research has begun to assess whether independent or shared neural networks exist for selective attention to distinct types of information. For example, both spatial- and feature-based attention utilize a largely overlapping neural network, although spatial attention may recruit additional sub-regions (Corbetta et al., 1995; Giesbrecht et al., 2003; Schenkluhn et al., 2008). To our knowledge, comparisons of networks supporting attention to different visual features, such as color and motion, have not yet been explored using functional connectivity analysis. On the one hand, it seems reasonable to hypothesize that the control networks involved in top-down modulation to these different features are distinct, as motion processing occurs primarily in a dorsal (where) visual pathway, and color processing in a ventral (what) visual pathway (Ungerleider et al., 1998). On the other hand, there is the suggestion that color and motion are under the influence of top-down modulation by a common set of regions in the dorsal attention network (Pollmann et al., 2000; Shulman et al., 1999; Weissman et al., 2002). A review of the literature, however, reveals that much of the evidence supporting this assertion is based on analysis of functional magnetic resonance imaging (fMRI) data using a general linear model (GLM) approach to examine brain regions independently of one another that are coincident with attentional/task demands (i.e. based on univariate analysis). This approach does not provide a measure of the relationship between the source and site of top-down modulation. Thus, there is still much to learn about the dynamic network organization of the source (i.e. frontal/parietal regions) and the site (e.g. visual association cortex (VAC)) of modulation in the context of different types of attention.
In addition to overlapping brain regions controlling attention to different types of information, selective attention and working memory also utilize common neural networks, thereby placing top- down modulation at the mechanistic crossroads between the two processes (Awh and Jonides, 2001; de Fockert et al., 2001; Desimone, 1996; LaBar et al., 1999). It is established that selective attention is necessary for optimal working memory performance by preventing distracting information from overloading limited memory stores (Ploner et al., 2001; Vogel et al., 2005; Zanto and Gazzaley, 2009). Therefore, to explore neural networks involved in top-down modulation, the current study implements a working memory task that includes the presentation of both relevant and distracting stimuli to increase selective demands on attentional networks.
Here, we identified fronto-parietal regions involved in the modulation of separable VAC regions that have selective sensitivity for different visual features, namely V4 for color and V5/hMT+ for motion, by implementing functional connectivity analysis on fMRI data (Gazzaley et al., 2004; Rissman et al., 2004). Additionally, electroencephalographic (EEG) recordings from different participants engaged in the same task were used to illuminate the spatio-temporal properties of feature-based, top-down modulation. It was hypothesized that attention to different visual features is associated with both distinct and common regions within the fronto-parietal network and that the degree of functional connectivity between the source and site of modulation predicts the magnitude of top-down modulation.
Methods
Participants
Fourteen healthy individuals (mean age 25.0 years; range 20–31 years; 5 males) participated in the fMRI study and a non-overlapping group of twenty healthy individuals participated in the EEG version of the experiment (mean age 25.1 years; range 20–33 years; 11 males; previously reported in Zanto and Gazzaley, 2009). All participants gave informed consent to partake in the study according to procedures approved by the University of California. Each participant had normal or corrected to normal vision. One participant from the fMRI study and one from the EEG study were excluded from the data analysis due to excessive data artifacts.
Stimuli
The stimuli consisted of a circular aperture of 290 dots (0.08° × 0.08° each) that subtended 8° of visual angle centered at the fovea. Two types of dots were used during the experiment: 1) gray and moving coherently at 10° per second or 2) stationary and colored along the tritan axis. All colored and gray dots were equated for brightness by minimizing heterochromatic flicker in tests carried out prior to the experiment for each subject. Stimuli were presented with a gray fixation cross in the center of the circular aperture on a black background.
Thresholding
After all stimuli were equated for brightness, participants engaged in two thresholding procedures (one for motion, one for color) in order to minimize perceptual discriminability differences between participants (Zanto and Gazzaley, 2009; Zanto et al., 2010). A staircase procedure required participants to determine whether two stimuli (directions of motion or colors) were different from each other. The two stimuli were presented for 800 ms each and separated by 2000 ms. The procedure continued until a “just 100%” level of performance was reached, meaning if the stimuli were any more similar, performance would drop below 100%. Thresholding determined the twelve possible directions of motion (three per quadrant, cardinal axes excluded) and the six possible colors each participant receive during the experiment.
Experimental procedure
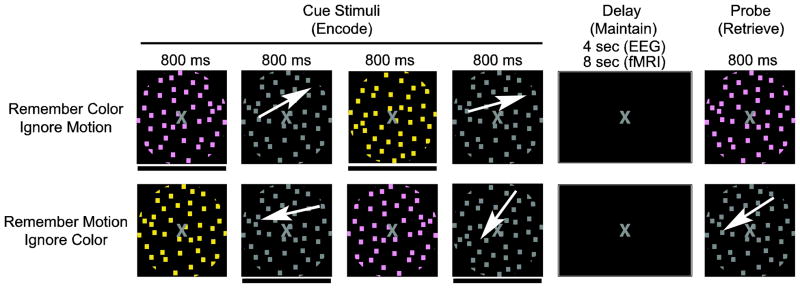
The experimental paradigm is depicted in Figure 1, as previously reported (Zanto and Gazzaley, 2009). Both conditions required viewing four sequentially presented images: two differently colored stimuli and two different directions of motion. Every image was presented for 800 ms with a 1200 ms inter-stimulus-interval (ISI; an 800 ms ISI was used in the EEG experiment). After the four images were presented, there was an eight second delay (4 sec in EEG) followed by a probe stimulus (800 ms duration) when participants determined whether the probe matched any of the items held in memory. Participants were required to either 1) remember the two directions of motion (ignore the two colors) or 2) remember the two colors (ignore motion). Each condition was presented in two blocks, and the four blocks were randomized across the experiment and participants. Prior to beginning each block, participants were given task instructions (i.e. “Remember Motion” or “Remember Color”). Additionally, a brief (1 sec) task reminder was provided at the start of each trial during the EEG session. Participants responded by pressing one of two buttons. One half of the probe stimuli matched a previously attended object. Participants were instructed to respond as quickly as possible and yet retain accuracy during all conditions. Prior to beginning the experiment, participants were given 12 practice trials for each of the two conditions, split into two blocks (6 trials each). During the experiment, participants received 30 trials per condition (60 trials per EEG condition). The stimuli for each trial were randomly selected from predetermined sets of stimuli, constraining the directions of motion to one quadrant. Two other conditions, passively view and remember both (motion and color), were presented in addition to the two conditions displayed in Figure 1, but were not included in the current analyses.
Figure 1.
Experimental Paradigm. White arrows indicate motion and were not present during the experiment. A black bar below a cue stimulus (also not present during the experiment) indicates it is an item to be remembered. The pictures approximate stimuli appearance (see methods for details).
MR imaging
All fMRI data was collected on a Siemens 3T MAGNETOM Trio with stimuli presented on an LCD monitor positioned behind the head of the participants and viewed using a mirror rigidly attached to a 12-channel head-coil. Echo planar imaging data was acquired (FA=90°, TE = 25 ms, TR = 2 sec) with 33 interleaved axial slices (0.5 mm gap) and a 1.8 × 1.8 × 3 mm voxel size (FOV = 23 cm; 128 × 128 matrix). All pre-preprocessing of the data was conducted in SPM5 (Wellcome Department of Imaging Neuroscience, London, England). Raw blood oxygen level dependent (BOLD) data was corrected offline for slice-timing acquisition and motion-artifacts. A 5 mm isotropic Gaussian smoothing kernel was applied prior to modeling the data. To aid in anatomical localizations of BOLD activity, high-resolution T1-MPRAGE images were acquired (1 × 1 × 1 mm voxel size; FOV = 160 × 240 × 256 mm, TR = 2300 ms, TE = 3 ms, FA = 9°).
fMRI region of interest localization
A functional localizer task was run prior to beginning the fMRI experiment in order to identify feature selective regions of interest (ROIs) in the VAC (i.e. V4 for color, V5 for motion) that are known to be modulated by attention (Chawla et al., 1999; Schoenfeld et al., 2007). Participants were instructed to perform a 1-back task where circular apertures of color and motion stimuli (as described above) were presented in separate blocks. Each stimulus type (color and motion) was presented in ten, 16 sec blocks interleaved with 16 sec of rest when participants passively viewed stationary gray dots. Within each block, stimuli were presented for 300 ms with a 500 ms ISI. Upon identifying a 1-back matched stimulus, participants were instructed to press the right-sided button. There were two random matches within each color and motion block. BOLD data from the color and motion localizers were analyzed using a GLM approach and contrasted against each other. ROIs were selected in native space as the most significant cluster of activation (p < 0.01) within the respective anatomical region, fusiform gyrus (specifically, V4) for color and middle temporal gyrus (specifically, V5/hMT+) for motion. For all GLM analyses, epochs spanning the duration of stimulus presentation were convolved with the SPM canonical hemodynamic response function (HRF).
fMRI functional connectivity analysis
Functional connectivity network maps were created for each subject as described previously using a beta series connectivity analysis approach (Gazzaley et al., 2004; Rissman et al., 2004). This method utilizes a GLM approach, but adapts the model to elicit parameter estimates (beta values) on a trial-by-trial basis and then a correlation is conducted between a ROI and other brain regions in order to capitalize on trial-wise variability. For each condition, the encoding and retrieval stages from every trial were modeled within the GLM using separate regressors placed at the onset of each stage for the duration of stimulus presentation. Subsequently, a mean beta value was extracted for each VAC ROI (per trial). Although the encoding and retrieval stages were modeled during each trial, only the encoding period was subject to analysis. Thus, the ROI beta values from the encoding period were then correlated across trials with every voxel in the brain to find regions with covariant activity. This procedure produces a whole brain Pearson’s r-value map for each subject and a Fisher’s r-to-z transformation is applied. The z-values are subsequently normalized to the Montreal Neurological Institute (MNI; 2 × 2 × 2 mm voxel size) template and Gaussian smoothed (5 mm FWHM) for group level analysis. Group contrast maps were created by utilizing a permutation test (Edgington, 1995). Data was corrected for multiple comparisons by thresholding the p-values with a cluster extent determined by a monte carlo simulation, resulting in a corrected p-value of 0.001.
EEG recordings
EEG data reported in Zanto and Gazzaley (2009) were reanalyzed to elucidate the spatio-temporal dynamics of neural networks supporting feature-based top-down modulation. This data set used the same paradigm as the fMRI data, but with a separate group of participants. Electrophysiological signals were recorded at 1,024 Hz through a 24-bit BioSemi ActiveTwo 64-channel Ag-AgCl active electrode EEG acquisition system (Cortech Solutions, LLC). Electrode offsets were maintained between +/− 20 mV. Raw EEG data were referenced to the average off-line. Eye artifacts were removed through an independent component analysis by excluding components consistent with the electrooculogram time-series and topographies for blinks and eye movements. One-second epochs were extracted from the data beginning 200 ms pre-stimulus onset and ending 800 ms post-stimulus onset. This preprocessing was conducted in Brain Vision Analyzer (Cortech Solutions, LLC) and exported to Matlab (The Mathworks, Inc.) for all subsequent analyses. Each trial contained four stimulus epochs, two attended and two ignored, resulting in 120 epochs per stimulus of interest.
EEG source localization
Electrode positions for each subject were acquired via Brainsight (Rogue Research, Inc.) in conjunction with a Polaris optical tracking system (Northern Digital, Inc.). These positions along with event related potential (ERP) data were submitted to standardized low resolution electromagnetic tomography (sLORETA) for source localization (Pascual-Marqui et al., 1994). In previous analyses, ERPs indicated that the P1 (100 ms post stimulus onset) was modulated by attention to motion, whereas the N1 (170 ms post stimulus onset) was modulated by attention to color (Zanto and Gazzaley, 2009). Thus, the time frame associated with the P1 and the N1 served as the temporal windows of interest for whole-brain source localization of top-down modulation during motion and color processing. Data from a 30 ms window centered on the P1 for motion (85–115 ms post stimulus onset) and the N1 for color (155–185 ms post stimulus onset) were submitted to sLORETA for source localization. Paired t-tests were conducted to identify condition-specific differences with a statistical non-parametric mapping to correct for multiple comparisons (Nichols and Holmes, 2002).
EEG phase coherence analysis
Raw epoched data whose voltage exceeded a threshold of +/− 100 μV were rejected. Artifact free trials were then convolved with complex Morlet wavelets (family ratio: fo/σf = 7) to resolve frequencies from 4 to 70 Hz. Whereas Zanto and Gazzaley (2009) utilized wavelet coefficients to measure induced spectral changes, here we use them to compute phase for each ROI within a time- frequency window of interest. The time-frequency window was defined apriori as 70–200 ms and 8–12 Hz. The time window was based on the ERP results from Zanto and Gazzaley (2009) and the frequency window on previous research indicating alpha oscillations as being involved in attentional modulation (Capotosto et al., 2009; Thut et al., 2006). Phase locking values (PLV) between two ROIs were computed by measuring the intertrial variability of the phase difference at each time-frequency point (Lachaux et al., 1999). This procedure yields a PLV measure bound from 0 to 1 such that 0 represents random phase differences across trials while 1 indicates a consistent phase difference. Planned paired t-tests were conducted between conditions.
Results
Behavior
Behavioral data was monitored online to ensure participants understood the task and responded appropriately. Working memory accuracy recorded across conditions was between 75–80% during the fMRI session, similar to previously reported accuracies in the EEG experiment (Zanto and Gazzaley, 2009).
fMRI ROIs
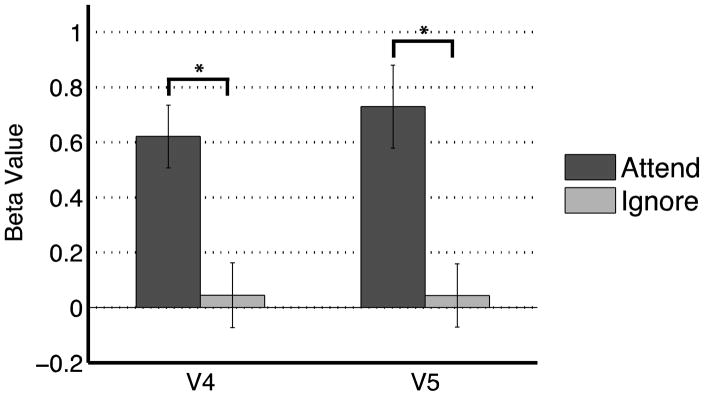
Data from the functional localizer task were used to identify native space (i.e. not normalized) ROIs within the VAC. These ROIs were selective for either color or motion features, and were centered around V4 (mean = 26 voxels, s.e.m. = 2 voxels) and V5 (mean = 20 voxels, s.e.m. = 1 voxel), respectively. In order to evaluate top-down modulation within the ROIs, data from the experimental task were submitted to a GLM for univariate analysis and contrasted across the two experimental conditions (remember motion/ignore color and remember color/ignore motion). Paired t-tests were conducted on the average beta values within each ROI, allowing statistics to be calculated in each participant’s native space. Figure 2 displays significant top-down modulation in the VAC, such that BOLD activity was increased in the ROI when the corresponding feature was attended. Specifically, V4 ROIs showed attention to color elicited greater activity than ignoring color (T(12) = 4.02, p < 0.005), and V5 ROIs showed attention to motion yielded greater activity than ignored motion (T(12) = 3.84, p < 0.005). These results indicate that top-down modulation occurs in stimulus-selective VAC for visual features, as reported previously for color and motion (Corbetta et al., 1991) as well as complex objects (Gazzaley et al., 2005). Moreover, the results suggest the selected ROIs are appropriate seed regions to use in whole-brain functional connectivity analysis.
Figure 2.
Attentional modulation of BOLD fMRI signal for processing color and motion features in areas V4 and V5, respectively. Asterisk indicates p < 0.05.
fMRI Functional Connectivity
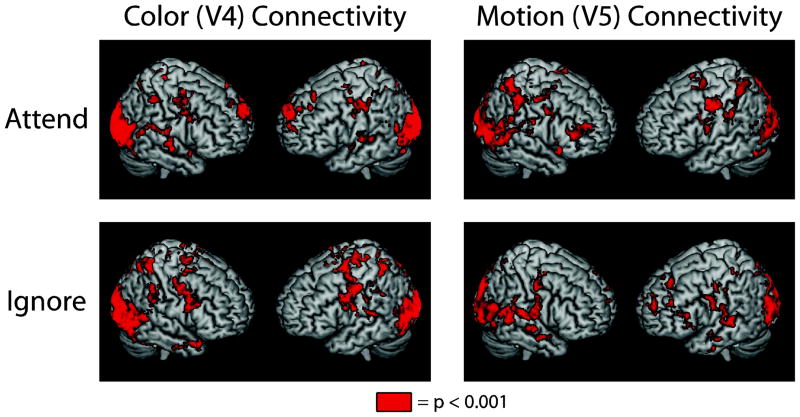
In order to identify cortical control areas that may facilitate top-down modulation of stimulus-selective VAC regions, functional connectivity analysis was conducted utilizing the VAC ROIs as seeds. Four group-level connectivity maps were generated using the encoding period regressor and pairing a seed with a condition: V4 attend (remember color condition), V4 ignore (remember motion condition), V5 attend (remember motion condition) and V5 ignore (remember color condition). Significance was determined via permutation tests conducted for each connectivity map compared to smoothed Gaussian noise (5 mm FWHM). Figure 3 summarizes these results and reveals a large-scale fronto-parietal network of regions functionally connected with the VAC seeds, regardless of whether the feature is attended or ignored (all regions p < 0.001, corrected for multiple comparisons). These results are consistent with previous reports using univariate analysis that describe the dorsal attention network as being involved in attending to color and motion (Corbetta and Shulman, 2002; Giesbrecht et al., 2003) and are, in general, comparable to the network pattern obtained with functional connectivity analysis of attending to and ignoring natural scenes (Gazzaley et al., 2007).
Figure 3.
Extensive fronto-parietal networks are associated with VAC processing for both motion and color feature processing, regardless of whether the feature is attended or ignored. All regions colored in red indicate connectivity at p < 0.001, corrected.
Next, attended and ignored connectivity maps were directly contrasted for each ROI using permutation tests (e.g., V4 attend color > V4 ignore color). Table 1 lists all regions in which the attended feature yielded greater connectivity than ignored features (p < 0.001, corrected for multiple comparisons). Only prefrontal regions exhibited attention-specific connectivity affiliated with modulation of activity in V4 (attend color > ignore color connectivity). These regions included bilateral frontal pole, left frontal orbital cortex and the inferior frontal junction (IFJ). However, both frontal and parietal regions displayed attention-specific connectivity associated with modulating activity in V5 (attend motion > ignore motion connectivity). Frontal regions included bilateral frontal eye fields (FEF), superior frontal gyrus (SFG), paracingulate gyrus (PCG) and bilateral IFJ; parietal regions included bilateral superior parietal lobule (SPL) and right supramarginal gyrus (SMG). Only one region, the IFJ, was involved in modulating both motion and color VAC areas (Figure 4). Although attention to either feature (motion or color) resulted in greater IFJ connectivity, adjacent yet distinct sub-regions within the IFJ were associated with the two types of feature-based, top-down modulation.
Table 1.
Summary of all fronto-parietal regions of connectivity to the VAC involved in top-down modulation. Center-of-mass coordinates listed are in MNI space.
| V4 Connectivity: Attend > Ignore | x | y | z | Cluster size (mm3) | |
|---|---|---|---|---|---|
| Cortex Frontal | left Frontal Pole Superior Frontal Gyrus) | −8 | 62 | 16 | 2832 |
| left Frontal Orbital Cortex (Inferior Frontal Gyrus) | −38 | 34 | −14 | 1472 | |
| right Frontal Pole (Medial Frontal Gyrus) | 8 | 58 | 8 | 880 | |
| right Frontal Pole (Superior Frontal Gyrus) | 26 | 62 | −14 | 600 | |
| right Inferior Frontal Junction | 42 | 0 | 26 | 544 | |
| V5 Connectivity: Attend > Ignore | x | y | z | Cluster size (mm3) | |
| Cortex Frontal | left Frontal Eye Field | −28 | −8 | 58 | 1560 |
| left Inferior Frontal Junction | −48 | 4 | 26 | 1312 | |
| Superior Frontal Gyrus/Paracingulate Gyrus | 0 | 18 | 50 | 752 | |
| right Frontal Eye Field | 26 | 14 | 50 | 704 | |
| right Inferior Frontal Junction | 48 | 10 | 36 | 520 | |
| Cortex al Pariet | left Superior Parietal Lobule | −36 | −50 | 62 | 2864 |
| right Supramarginal Gyrus | 46 | −36 | 44 | 1832 | |
| right Superior Parietal Lobule | 32 | −54 | 62 | 664 | |
| V5 Connectivity: Ignore > Attend | x | y | z | Cluster size (mm3) | |
| Frontal Cortex | left/right Frontal Pole (Superior/Medial Frontal Gyrus) | −6 | 62 | 16 | 2064 |
| Superior Frontal Gyrus/Middle Frontal Gyrus | −24 | 36 | 50 | 824 |
Figure 4.

Only one fronto-parietal region is associated with both motion and color top-down modulation, the inferior frontal junction (IFJ). However, distinct sub-regions within the IFJ distinguish motion from color modulation.
The aforementioned regions were detected in functional connectivity contrast analysis for attending to specific features, however, V5 connectivity when ignoring motion (i.e. attending to color) also revealed two frontal regions that were greater compared to attending to motion: bilateral frontal pole and an area bordering the SFG and MFG. Interestingly, this frontal pole region overlaps with the frontal pole elicited by V4 connectivity during the same task (attend color). This indicates that the same frontal region may be involved in modulating two distinct VAC areas during the same task. No fronto-parietal regions displayed connectivity to V4 when ignoring color greater than attending color (p > 0.001).
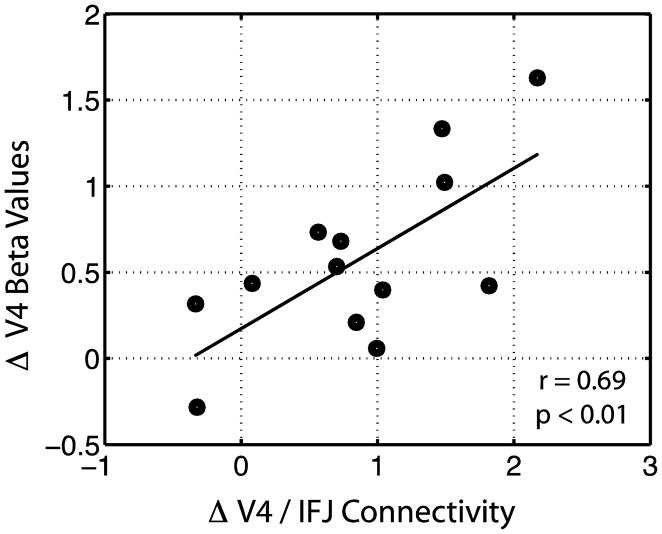
To further evaluate the influence IFJ may have on VAC modulation, an across-participant regression analysis was conducted between VAC/IFJ connectivity (attend – ignore) and VAC BOLD activity (attend – ignore) for each of the ROIs. Figure 5 depicts the relationship between V4/IFJ connectivity and V4 attentional modulation, which shows that the magnitude of attentional modulation observed in V4 is greater when connectivity between V4 and the IFJ is greater (r = 0.69, p < 0.01). This relationship was not observed for V5 and the right IFJ (nor the left IFJ), possibly because the top-down modulation network is more widespread for motion processing, and includes bilateral IFJ and parietal regions, reflective of more distributed control. Although these data are correlational and not causal, they do raise the possibility that top-down modulation of color processing may rely more heavily on focal nodes of its neural network, e.g., the IFJ, as compared to top-down modulation of motion processing.
Figure 5.
Correlation between V4 attentional modulation and V4/IFJ functional connectivity. Values represent the difference between attended and ignored color.
Taken together, these results indicate that during working memory encoding, V4 and V5 are modulated by attention to color and motion, respectively, and that overlapping, but distinct fronto-parietal networks may subserve this modulation. Specifically, color (V4) modulation appears to rely more on prefrontal cortex, whereas motion (V5) modulation utilizes a more distributed fronto-parietal network. Most of the neural networks involved in color and motion feature modulation recruited distinct cortical regions, however, the IFJ appears to be a shared node in both networks.
EEG source localization
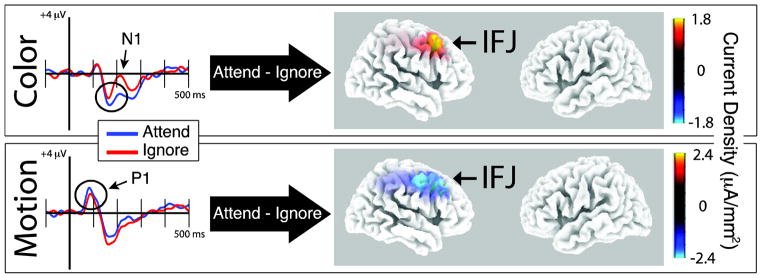
While fMRI is ideal for identifying anatomical regions involved in top-down modulation, it is less informative about the time course over which the modulation occurs and when it begins. The results reported above indicate that neural networks involved in modulating feature-selective VAC occurs during the encoding period. Here, we capitalized on the strength of EEG, being an electrical measure of neural activity, to provide more precise timing of this modulation. Therefore, EEG data reported in Zanto and Gazzaley (2009), which utilized the same paradigm in a separate group of participants, were reanalyzed to elucidate the spatio-temporal dynamics of neural networks supporting feature-based top-down modulation. The left-sided panels in Figure 6 display ERPs from posterior electrodes (average of P4, P6, P8, PO4, PO8) to color (top row) and motion (bottom row) stimuli. Black circles highlight the N1 for color and the P1 for motion, which corresponded to the time windows individually subject to source localization for each condition (attend/ignore).
Figure 6.
ERPs from posterior electrodes are plotted on the left whereas the contrast between EEG source localization from time windows centered around the relevant ERP components to attended and ignored features are on the right. Results suggest that the IFJ is involved in top-down modulation of both color (top row) and motion (bottom row) feature processing at an early processing stage (<200 ms from stimulus onset; P1 for motion, N1 for color).
Localizing the individual ERP peaks yielded similar results to previous reports of feature-selective VAC regions (Schoenfeld et al., 2007). The right-sided panels in Figure 6 depict the source localization contrast between the attended and ignored features at the P1 for motion and N1 for color. Red indicates that the ERP to attended stimuli was more positive than the ERP to ignored stimuli, whereas blue indicates that the ERP to attended stimuli was more negative than the ERP to ignored stimuli. The color difference between the motion and color localization is due to the polarity reversal between the P1 and the N1. EEG localization indicates that the right IFJ is the most robust region associated with top-down modulation for both color (Figure 6, top row; p < 0.05) and motion (Figure 6, bottom row; p = 0.14). Although the localization for motion-related modulation did not reach significance in any region after correcting for multiple comparisons, the result converges with the fMRI data. These data support the results from the fMRI connectivity analysis that highlight the IFJ’s role in top-down modulation for visual features, especially for color processing. Moreover, this analysis indicated that the modulation occurred early during visual processing (< 200 ms post stimulus onset).
EEG phase coherence
Source localization is very useful as a univariate approach to identify localized events in ROIs using EEG data and is parallel to the univariate fMRI analysis. However, it is advantageous to utilize a bivariate approach to analyze the EEG data akin to the functional connectivity analysis implemented on the fMRI data. Previous research has suggested that EEG phase coherence reflects communication between distant, yet functionally related neural populations (reviewed in Sauseng and Klimesch, 2008). It has been suggested that alpha band (8–12 Hz) oscillations are involved in visual attention (Thut et al., 2006) and may be modulated via fronto-parietal networks (Capotosto et al., 2009). Moreover, alpha band activity may have functional similarities to the ERP, such that the ERP frequency components lie in the alpha range and are modulated by attention in a similar manner (Freunberger et al., 2008). Therefore, a phase coherence analysis in the alpha band was conducted to identify signatures of long-distance communication between frontal (around IFJ) and posterior (around VAC) cortices. Based on the fMRI and EEG source localization results, one right frontal ROI and three posterior ROIs were selected apriori for analysis (Figure 7a). The one frontal region was selected based on data presented in Figures 4 and 6. Each ROI was an average of 5 electrodes: F4, FC2, FC4, FC6, C4 (frontal; surrounding the right IFJ region identified by source localization analysis), P3, P5, P7, PO3, PO7 (left-posterior), POZ, OZ, O1, O2, IZ (central-posterior) and P4, P6, P8, PO4, PO8 (right-posterior).
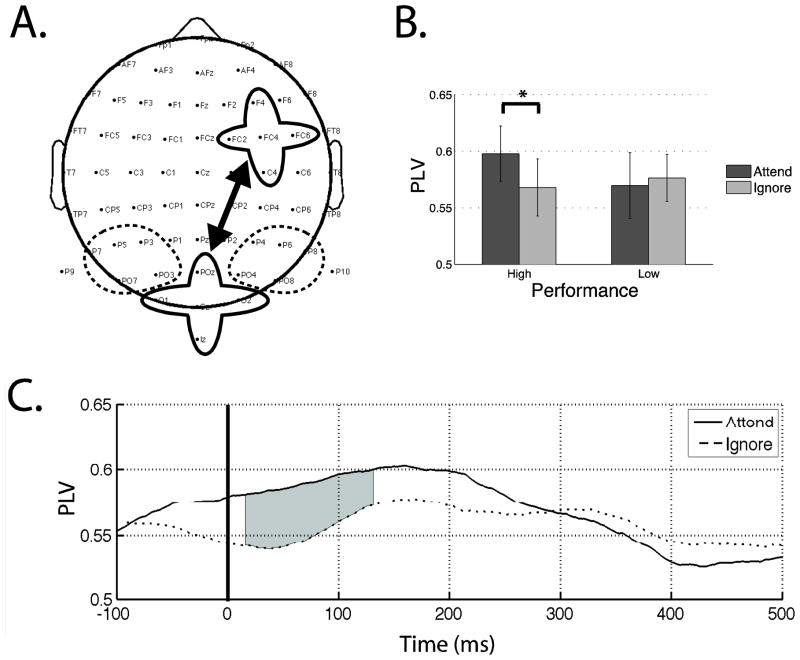
Figure 7.
Phase coherence analysis. (A) One frontal and three posterior regions of interest are circled. Solid lines indicate significant coherence measures observed between regions. (B) Phase locking values (PLVs) during color processing. Attentional modulation of the PLV is observed only during high performance trials (p < 0.05). (C) Time course of the PLV during high performing color processing. Shaded areas indicate significant difference between attended and ignored color (p < 0.05).
Results from Zanto and Gazzaley (2009) indicate that participants performing this experiment yielded greater neural signatures of top-down modulation during high performance trials (fast response times and greater accuracy). Therefore, following previous methodology, trials were first separated by each participant’s median response time prior to calculating phase locking values (PLVs). PLVs were then calculated between the frontal ROI and the three posterior ROIs, as described in the methods section. PLVs were averaged over the time-frequency window that encompassed the alpha frequency band (8–12 Hz) and the ERP of interest time period (70–200 ms). Attended and ignored features were analyzed separately for high and low performance trials. Results indicated that during high performance trials, greater phase locking existed between the frontal and central-posterior ROI for remember color compared to ignore color, while no such difference was observed for the low performance trials (T(18) = 2.32, p < 0.05; Figure 7a,b). Additionally, no differences were observed during motion processing for either performance trials (p > 0.05). To evaluate if the observed phase locking during high performance color processing was due to volume conduction, 5 electrodes (CZ, CPZ, PZ, CP1, CP2) located between the frontal and posterior ROIs were subjected to the same analysis. This analysis revealed no signs of attentional modulation (p > 0.80), suggesting that the reported significant effects were not the result of volume conduction.
To explore whether the observed phase locking effect associated with attention was driven by the ERP modulation occurring during the same time-period, the time course of PLVs during high performance color processing were analyzed via paired t-tests at each time-point from −100 to 500 ms post stimulus onset. Shaded gray regions in Figure 7c indicate that significant phase locking measures of attentional modulation begin as early as 15 ms post stimulus onset and continues throughout the P1 period of the ERP, although the point of non-significant divergence was actually prior to stimulus onset. It should be noted that the temporal resolution in the alpha band was 140 ms. Therefore, the start of modulation at 15 ms incorporates data from −55 to 85 ms post stimulus onset. Although a portion of this window encompasses the onset of the P1, the majority does not. Furthermore, the color ERP was modulated by attention only at the N1, which is well after the onset of phase modulation. Finally, the P1 was modulated by attention to motion, yet it did not show any significant phase modulation with attention. Taken together, these results indicate that the onset of alpha phase modulation was not driven by the ERP modulation, which agrees with previous research indicating a dissociation between alpha oscillations and early ERP components (Thut et al., 2003).
Overall the results of the phase coherence analysis are consistent with our interpretation of the fMRI connectivity data, which suggest that neural networks subserving color modulation rely more heavily on the right inferior frontal junction than for motion processing. Moreover, this data suggests that top-down modulation utilizes alpha phase locking for long-distance communication, which may occur in anticipation of stimulus onset, as phase locking 15 ms post stimulus onset precedes the first neural signature of visual cortical processing (Whittaker and Siegfried, 1983)
Discussion
The current study identified neural networks involved in top-down modulation of feature processing in selective VAC areas for different stimulus features. The fMRI univariate data revealed that areas V4 and V5 were modulated by attention to color and motion features, respectively. The fMRI functional connectivity data revealed that this modulation may be generated by control regions in the prefrontal cortex for color processing, and both prefrontal and parietal regions for motion processing. Although distinct neural networks were associated with different feature types, both color and motion top-down attention networks included the right IFJ. Moreover, the magnitude of IFJ connectivity to V4 predicted the degree of attentional modulation of color processing in V4. Source localization of EEG data also indicated that the right IFJ was involved in feature-based attentional modulation and that this modulation occurred early after stimulus presentation (< 200 ms post stimulus onset). Lastly, EEG phase coherence analysis suggested that long-distance communication in the alpha band might subserve top-down modulation of color processing.
Feature-based neural networks
It has been well documented that areas V4 and V5/hMT+ are selectively responsive to color and motion features, respectively (Corbetta et al., 1991; Watson et al., 1993; Zeki et al., 1991). More recent research has shown that these VAC areas enhance their activity when their respective features are attended (Beauchamp et al., 1997; Chawla et al., 1999; Clark et al., 1997). Here, we corroborate these findings by showing that V4 activity is increased when color is attended, while V5 activity is amplified when motion is the attended feature.
A fronto-parietal network has been hypothesized to serve as a source for top-down modulation of both spatial- and feature-based attentional processing, which may include overlapping cortical control regions (Corbetta and Shulman, 2002; Schenkluhn et al., 2008; Slagter et al., 2007). The current study used a fMRI functional connectivity approach to explore whether attention to different features (i.e. motion and color) that are processed in the dorsal and ventral visual stream respectively, exhibit common or distinct frontal and/or parietal control regions. Results indicated that color processing attentional networks rely more heavily on prefrontal regions, specifically, bilateral frontal pole and the right IFJ. They further indicated that the strength of IFJ connectivity with V4 predicts the degree of neural modulation in that VAC area, emphasizing the importance of this frontal region. Of note, the current findings did not replicate previous reports that described a parietal contribution for color modulation (Egner et al., 2008; Giesbrecht et al., 2003; Slagter et al., 2007; Yeh et al., 2007). There are several possibilities as to why parietal regions in the current experiment were not associated with top-down modulation of color processing. One reason could be due to the analytic approach. Previous research typically used a univariate approach to identify brain regions, whereas the current study implemented functional connectivity analysis. Indeed, widespread fronto-parietal connectivity was shown for both V4 and V5, similar to previous approaches utilizing a GLM for data analysis. However, contrasting connectivity maps between attended and ignored features isolated only a few key cortical sites. Another possibility may involve the task itself. Previous studies utilized either spatial cue processing, target detection or passive viewing, whereas the current experiment required working memory encoding. Much research has highlighted the role of frontal regions in working memory processes (for reviews, see D’Esposito et al., 2000; Owen, 1997), which may have biased the balance of activity within the fronto-parietal network. A third possibility of why parietal region connectivity was not observed may be due to the stimuli being presented in the participant’s central visual field. Stimuli in other experiments often included a spatial component, (e.g. covert attention to periphery) which may explain the observed overlap between spatial and feature-based attentional networks, as spatial-oriented processing largely occurs in the parietal cortex (Dimattia and Kesner, 1988; Posner et al., 1982).
The fMRI data indicated that neural networks subserving attention to motion recruited more widespread frontal-parietal regions, including SFG, bilateral IFJ, bilateral FEF, bilateral SPL and right SMG. Each of these regions has been previously implicated in studies using univariate analysis to explore attention to motion stimuli (reviewed in Corbetta and Shulman, 2002). However, the functional connectivity analysis employed in the present study provides more direct evidence for the role of these regions in top-down modulation. An explanation for why motion (and not color) modulation involves FEF and parietal control regions may be the result of the spatial element inherent in motion processing, as it depends upon at least two distinct spatial locations being occupied at sequential time-points. Indeed, FEF and parietal regions are recruited during both motion and spatial processing, and thus it may be difficult to dissect these overlapping processes (Dumoulin et al., 2003).
It is unclear why motion elicits bilateral IFJ connectivity, whereas color preferentially engages right IFJ connectivity. Previous research has identified hemispheric differences in IFJ activity during switching (bilateral) and stroop (left lateralized) tasks (Derrfuss et al., 2005), however, the significance of these differences remain uncertain. The current findings do support previous univariate analyses indicating differential fronto-parietal regions are involved in attentional shifts between motion and color features (Liu et al., 2003). Furthermore, attention to motion has been shown to recruit bilateral frontal and parietal regions (Culham et al., 1998), whereas the right hemisphere is more dominant in color detection (Sasaki et al., 2007). However, lexical color codes may be utilized in categorical perception, which recruits more left hemisphere activity (Franklin et al., 2008). This would indicate that the participants in the current experiment might have relied more on a color detection strategy rather than categorical perception. Indeed, the colors chosen for the current experimental stimuli fall along the tritan axis and were selected due to the difficulty in attributing names to them. Nonetheless, additional research will be required to delineate hemispheric differences regarding the role of the IFJ.
IFJ and top-down modulation
Although most of the putative control regions identified by the connectivity analysis as being involved in attention to color and motion were mutually exclusive (Table 1), the right IFJ was highly connected with the VAC ROIs in both feature networks. The IFJ is a region ventro-lateral to FEF and is anatomically localized at the intersection of the precentral sulcus and the inferior frontal sulcus. Although IFJ activation is variable in stereotactic coordinates, it is highly reliable based on the anatomy of individual participants (Derrfuss et al., 2009). Due to this variability and because the IFJ boarders the inferior frontal gyrus, middle frontal gyrus and the precentral gyrus, activity in this region is often reported as being located in the closest classically defined area, although it now appears to be cytoarchitectonically and functionally distinct from its neighboring regions (Brass et al., 2005). IFJ activity has been elicited by many different types of paradigms, including task switching, interference control and working memory (Derrfuss et al., 2005; Derrfuss et al., 2004). As such, it has been hypothesized that the IFJ plays a role in updating relevant task representations (Brass and von Cramon, 2004; Bunge, 2004). The current data supports this hypothesis and extends it by suggesting that updating relevant task representations may occur via biasing of neural activity in distal cortical regions.
The IFJ is considered to be part of the dorsal attentional network (Corbetta et al., 2008; labeled MFG), which is comprised of several other frontal and parietal regions. However, only the IFJ appears to reside at the intersection of the dorsal (top-down) and ventral (bottom-up) attentional networks (He et al., 2007; labeled MFG). Our current findings show that color and motion processing both recruit the IFJ, albeit distinct subregions of the IFJ. It is interesting to note that color is processed in the ventral visual processing stream and recruits the ventral portion of the IFJ, whereas motion processing is conducted in the dorsal visual stream and utilizes a dorsal section of the IFJ. Such domain specificity is thought to be maintained from posterior to frontal regions (Ungerleider et al., 1998) and recent research has begun to identify topographic maps within frontal and posterior regions (reviewed in Silver and Kastner, 2009), indicating a potential structure-function organization throughout the cortex.
Attention and working memory are intimately related processes, such that attended items are more likely to be remembered (Ploner et al., 2001; Rainer et al., 1998). Moreover, selective attention and working memory are thought to share common neural substrates (Awh and Jonides, 2001; de Fockert et al., 2001; Desimone, 1996; LaBar et al., 1999). Recent research has implicated the IFJ in both attentional and working memory processes. The IFJ is involved in fixating the locus of attention (Kelley et al., 2008), processing infrequent stimuli and inhibiting behavioral responses (Chikazoe et al., 2009). Additionally, the IFJ is related to working memory performance (Kelly et al., 2006) as part of a fronto-parietal network that updates working memory, regardless of whether the updated information are from sensory stimuli or long term memory (Roth and Courtney, 2007). Given the importance of attention and working memory to our everyday lives, it is not surprising that the IFJ is utilized by children, as well as adults, for attentional shifting (Morton et al., 2009) and may form at early stages of cortical folding (Derrfuss et al., 2009).
Source localization of EEG data supports the role of the IFJ in top-down modulation of visual features and extends this finding to show its involvement occurs within 200 ms of stimulus onset. Although the localization for attention to motion did not reveal significant sources after multiple comparison corrections, it was nonetheless strongest at the right IFJ. Localization may not have reached significance because motion utilizes a more diverse fronto-parietal neural network, and so each node contributes less to modulating VAC compared to color processing that recruits fewer regions. This may also explain why EEG source localization for motion stimuli did not yield bilateral IFJ activity. This may also explain why EEG source localization for motion stimuli did not yield bilateral IFJ activity. Previous research on the same EEG data showed that when participants are encoding color and motion features, neural activity in visual cortical regions within 200 ms of stimulus onset displays attentional modulation only during high performance trials (i.e. fast response times and greater accuracy; Zanto and Gazzaley, 2009). Thus, this temporal window of interest occurs at a time involved in subsequent working memory performance and underscores the relationship between attention and memory processes.
Mechanisms subserving top-down modulation
Long-distance cortical communication is thought to occur via phase information (reviewed in Sauseng and Klimesch, 2008) and alpha band activity appears to be a prime candidate for such processes, as it has been implicated in fronto-posterior attentional networks (Capotosto et al., 2009). Thus, further analysis of the EEG data explored phase coherence in the alpha band during this temporal window of interest (< 200 ms post-stimulus onset) in order to identify signatures of cortico-cortical communication in service of top-down modulation. Results show greater frontal (~ right IFJ) to posterior phase locking when attending, relative to ignoring color stimuli. However, this attentional modulation was only observed during high performance trials, similar to previous ERP results (Zanto and Gazzaley, 2009) suggesting that top-down modulation influences working memory performance. Given that attention to motion did not yield similar findings, the EEG phase coherence data supports the fMRI functional connectivity data in suggesting that color relies more heavily on the right IFJ as a source for attentional modulation.
The onset of the phase locking modulation occurred very near stimulus onset and may thus be anticipatory in nature. Therefore, these results corroborate recent findings that suggest top-down modulation biases activity in sensory cortical regions prior to stimulus onset (Bressler et al., 2008; Capotosto et al., 2009). Although the current data suggests that the IFJ is involved in top-down modulation in an anticipatory manner, recent findings have suggested the right IFJ may operate in both proactive and reactive modes of cognitive control (Braver et al., 2009). In addition to this flexibility in operation, the IFJ also exhibits robust plasticity. Multitasking training has been shown to reduce IFJ activity and this was interpreted to reflect more efficient neural processing that released the cognitive bottleneck via increased processing speed (Dux et al., 2009).
Conclusion
The results of the current study highlight the role of the IFJ in top-down modulation of processing color and motion visual features, and contributes to the evolving role of the IFJ in attention and working memory processes. It should be noted, however, that these findings are correlational in nature and cannot yield strong conclusions of causal inference or the necessity of these putative control regions. Future research will apply repetitive transcranial magnetic stimulation to the right IFJ in order to perturb function in this area, allowing causal inferences to be drawn by observing the impact on modulation in distal cortical regions and behavioral performance.
Acknowledgments
This work was supported by the National Institute of Health Grants 1F32AG030249-01A2 (TZ) and NIH Grant 5R01AG030395 (AG). We would like to thank Steve Bressler for useful discussion as well as Kelly Hennigan, Arul Thangaval and Chips McSteeley for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Cox RW, DeYoe EA. Graded effects of spatial and featural attention on human area MT and associated motion processing areas. Journal of Neurophysiology. 1997;78:516–520. doi: 10.1152/jn.1997.78.1.516. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends in Cognitive Sciences. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Decomposing components of task preparation with functional magnetic resonance imaging. Journal of Cognitive Neuroscience. 2004;16:609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. Journal of Neuroscience. 2008;28:10056–10061. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA. How we use rules to select actions: A review of evidence from cognitive neuroscience. Cognitive Affective & Behavioral Neuroscience. 2004;4:564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal Cortex Controls Spatial Attention through Modulation of Anticipatory Alpha Rhythms. Journal of Neuroscience. 2009;29:5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nature Neuroscience. 1999;2:671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, Miyashita Y, Konishi S. Functional Dissociation in Right Inferior Frontal Cortex during Performance of Go/No-Go Task. Cerebral Cortex. 2009;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- Clark VP, Parasuraman R, Keil K, Kulansky R, Fannon S, Maisog JM, Ungerleider LG, Haxby JV. Selective attention to face identity and color studied with fMRI. Human Brain Mapping. 1997;5:293–297. doi: 10.1002/(SICI)1097-0193(1997)5:4<293::AID-HBM15>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color and speed - Functional anatomy by positron emission tomography. Journal of Neuroscience. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, Petersen SE. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270:802–805. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RBH. Cortical fMRI activation produced by attentive tracking of moving targets. Journal of Neurophysiology. 1998;80:2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Experimental Brain Research. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291:1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: Meta-analyses of switching and stroop studies. Human Brain Mapping. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY. Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control, and working memory. Neuroimage. 2004;23:604–612. doi: 10.1016/j.neuroimage.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY, Lohmann G, Amunts K. Neural Activations at the junction of the Inferior Frontal Sulcus and the Inferior Precentral Sulcus: Interindividual Variability, Reliability, and Association With Sulcal Morphology. Human Brain Mapping. 2009;30:299–311. doi: 10.1002/hbm.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimattia BV, Kesner RP. Role of the posterior parietal association cortex in the processing of spatial event information. Behavioral Neuroscience. 1988;102:397–403. doi: 10.1037//0735-7044.102.3.397. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Baker CL, Hess RF, Evans AC. Cortical specialization for processing first- and second-order motion. Cerebral Cortex. 2003;13:1375–1385. doi: 10.1093/cercor/bhg085. [DOI] [PubMed] [Google Scholar]
- Duncan J, Humphreys G, Ward R. Competitive brain activity in visual attention. Curr Opin Neurobiol. 1997;7:255–261. doi: 10.1016/s0959-4388(97)80014-1. [DOI] [PubMed] [Google Scholar]
- Dux PE, Tombu MN, Harrison S, Rogers BP, Tong F, Marois R. Training Improves Multitasking Performance by Increasing the Speed of Information Processing in Human Prefrontal Cortex. Neuron. 2009;63:127–138. doi: 10.1016/j.neuron.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington ES. Randomization tests. 3. Marcel Dekker; New York: 1995. [Google Scholar]
- Egner T, Monti JMP, Trittschuh EH, Wieneke CA, Hirsch J, Mesulam MM. Neural integration of top-down spatial and feature-based information in visual search. Journal of Neuroscience. 2008;28:6141–6151. doi: 10.1523/JNEUROSCI.1262-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A, Drivonikou GV, Bevis L, Davies IRL, Kay P, Regier T. Categorical perception of color is lateralized to the right hemisphere in infants, but to the left hemisphere in adults. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3221–3225. doi: 10.1073/pnas.0712286105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freunberger R, Holler Y, Griesmayr B, Gruber W, Sauseng P, Klimesch W. Functional similarities between the P1 component and alpha oscillations. European Journal of Neuroscience. 2008;27:2330–2340. doi: 10.1111/j.1460-9568.2008.06190.x. [DOI] [PubMed] [Google Scholar]
- Frith C. A framework for studying the neural basis of attention. Neuropsychologia. 2001;39:1367–1371. doi: 10.1016/s0028-3932(01)00124-5. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Inferotemporal units in selective visual attention and short-term memory. J Neurophysiol. 1990;64:681–697. doi: 10.1152/jn.1990.64.3.681. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D’Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D’Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cerebral Cortex. 2007;17:I125–I135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, D’Esposito M. Functional connectivity during working memory maintenance. Cognitive, Affective and Behavioral Neuroscience. 2004;4:580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Woldorff MG, Song AW, Mangun GR. Neural mechanisms of top-down control during spatial and feature attention. Neuroimage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–179. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- Kelley TA, Serences JT, Giesbrecht B, Yantis S. Cortical mechanisms for shifting and holding visuospatial attention. Cerebral Cortex. 2008;18:114–125. doi: 10.1093/cercor/bhm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Hester R, Foxe JJ, Shpaner M, Garavan H. Flexible cognitive control: Effects of individual differences and brief practice on a complex cognitive task. Neuroimage. 2006;31:866–886. doi: 10.1016/j.neuroimage.2006.01.008. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. Neuroimage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Human Brain Mapping. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Slotnick SD, Serences JT, Yantis S. Cortical mechanisms of feature-based attentional control. Cereb Cortex. 2003;13:1334–1343. doi: 10.1093/cercor/bhg080. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Morton JB, Bosma R, Ansari D. Age-related changes in brain activation associated with dimensional shifts of attention: An fMRI study. Neuroimage. 2009;46:249–256. doi: 10.1016/j.neuroimage.2009.01.037. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM. The functional organization of working memory processes within human lateral frontal cortex: The contribution of functional neuroimaging. European Journal of Neuroscience. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. International Journal of Psychophysiology. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Ostendorf F, Brandt SA, Gaymard BM, Rivaud-Pechoux S, Ploner M, Villringer A, Pierrot-Deseilligny C. Behavioural relevance modulates access to spatial working memory in humans. Eur J Neurosci. 2001;13:357–363. [PubMed] [Google Scholar]
- Pollmann S, Weidner R, Muller HJ, von Cramon DY. A fronto-posterior network involved in visual dimension changes. Journal of Cognitive Neuroscience. 2000;12:480–494. doi: 10.1162/089892900562156. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y, Rafal RD. Neural systems control of spatial orienting. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1982;298:187–198. doi: 10.1098/rstb.1982.0081. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR, Davidson BJ. Attention and the Detection of Signals. Journal of Experimental Psychology-General. 1980;109:160–174. [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Roth JK, Courtney SM. Neural system for updating object working memory from different sources: Sensory stimuli or long-term memory. Neuroimage. 2007;38:617–630. doi: 10.1016/j.neuroimage.2007.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Morimoto A, Nishio A, Matsuura S. Right hemisphere specialization for color detection. Brain and Cognition. 2007;64:282–289. doi: 10.1016/j.bandc.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neuroscience and Biobehavioral Reviews. 2008;32:1001–1013. doi: 10.1016/j.neubiorev.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Schenkluhn B, Ruff CC, Heinen K, Chambers CD. Parietal Stimulation Decouples Spatial and Feature-Based Attention. Journal of Neuroscience. 2008;28:11106–11110. doi: 10.1523/JNEUROSCI.3591-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld MA, Hopf JM, Martinez A, Mai HM, Sattler C, Gasde A, Heinze HJ, Hillyard SA. Spatio-temporal analysis of feature-based attention. Cerebral Cortex. 2007;17:2468–2477. doi: 10.1093/cercor/bhl154. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mikulis DJ, Davis KD. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain. 2004;112:48–58. doi: 10.1016/j.pain.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, Corbetta M. Areas involved in encoding and applying directional expectations to moving objects. Journal of Neuroscience. 1999;19:9480–9496. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends in Cognitive Sciences. 2009;13:488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter HA, Giesbrecht B, Kok A, Weissman DH, Kenemans JL, Woldorff MG, Mangun GR. AM evidence for both generalized and specialized components of attentional control. Brain Research. 2007;1177:90–102. doi: 10.1016/j.brainres.2007.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. alpha-Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. Journal of Neuroscience. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Theoret H, Pfennig A, Ives J, Kampmann F, Northoff G, Pascual-Leone A. Differential effects of low-frequency rTMS at the occipital pole on visual-induced alpha desynchronization and visual-evoked potentials. Neuroimage. 2003;18:334–347. doi: 10.1016/s1053-8119(02)00048-4. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV. A neural system for human visual working memory. Proc Natl Acad Sci U S A. 1998;95:883–890. doi: 10.1073/pnas.95.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Gaffan D, Pelak VS. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp Brain Res. 1989;76:473–484. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Watson JDG, Myers R, Frackowiak RSJ, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area-V5 of the human brain - Evidence from a combined study using positron emission tomography and magnetic-resonance-imaging. Cerebral Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex. 1994;4:470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Mangun GR, Woldorff MG. A role for top-down attentional orienting during interference between global and local aspects of hierarchical stimuli. Neuroimage. 2002;17:1266–1276. doi: 10.1006/nimg.2002.1284. [DOI] [PubMed] [Google Scholar]
- Whittaker SG, Siegfried JB. Origin of wavelets in the visual evoked potential. Electroencephalography and Clinical Neurophysiology. 1983;55:91–101. doi: 10.1016/0013-4694(83)90151-7. [DOI] [PubMed] [Google Scholar]
- Yeh YY, Kuo BC, Liu HL. The neural correlates of attention orienting in visuospatial working memory for detecting feature and conjunction changes. Brain Research. 2007;1130:146–157. doi: 10.1016/j.brainres.2006.10.065. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. Journal of Neuroscience. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Toy B, Gazzaley A. Delays in neural processing during working memory encoding in normal aging. Neuropsychologia. 2010;48:13–25. doi: 10.1016/j.neuropsychologia.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Watson JDG, Lueck CJ, Friston KJ, Kennard C, Frackowiak RSJ. A direct demonstration of functional specialization in human visual cortex. Journal of Neuroscience. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N. Attentional modulation in human primary olfactory cortex. Nat Neurosci. 2005;8:114–120. doi: 10.1038/nn1368. [DOI] [PubMed] [Google Scholar]