Abstract
Drug addiction research requires but lacks a valid and reliable way to measure both the risk (propensity) to develop addiction and the severity of manifest addiction. This paper argues for a new measurement approach and instrument to quantify propensity to and severity of addiction, based on the testable assumption that these constructs can be mapped onto the same dimension of liability to addiction. The case for this new direction becomes clear from a critical review of empirical data and the current instrumentation. The many assessment instruments in use today have proven utility, reliability, and validity, but they are of limited use for evaluating individual differences in propensity and severity. The conceptual and methodological shortcomings of instruments currently used in research and clinical practice can be overcome through the use of new technologies to develop a reliable, valid, and standardized assessment instrument(s) to measure and distinguish individual variations in expression of the underlying latent trait(s) that comprises propensity to and severity of drug addiction. Such instrumentation would enhance our capacity for drug addiction research on linkages and interactions among familial, genetic, psychosocial, and neurobiological factors associated with variations in propensity and severity. It would lead to new opportunities in substance abuse prevention, treatment, and services research, as well as in interventions and implementation science for drug addiction.
Keywords: tobacco, cannabis assessment, individual differences, adolescents
1. Introduction
Drug addiction research spans the gamut from neuroscience and genetics to prevention, treatment and services. As in other biobehavioral disciplines, this research requires a valid and reliable measure of both the propensity to develop addiction and the severity of manifest addiction. This paper examines the need for such a measure as well as the assumption on which it is based, namely that these constructs share the same dimension. It provides a critical review of theory, data, and current instrumentation to show how a new measurement tool could impact the field of addiction research. Finally, it considers the next steps required for the development and fruition of a new instrument for measuring addiction.
Fueled by technological innovations and multidisciplinary scientific collaborations, addiction science has advanced rapidly over the past 20 years. The notion that addiction “runs in families” (Merikangas and Conway, 2009; Bierut et al., 1998; Merikangas et al., 1998; Reich et al., 1988; Schuckit et al., 1972; Winokur et al., 1970) is now attributed in large part to additive genetic factors (Kendler et al., 2003a; Tsuang et al., 1996). This has lead scientists to search for genes that influence variation in the risk for addiction, with a number of replication studies now emerging on candidate genes (Foll et al., 2009; Kreek et al., 2005; Li and Burmeister, 2009a; Saxon et al., 2005; Schuckit, 2009; Uhl, 2004; Vanyukov and Tarter, 2000; Li and Burmeister, 2009b). Scientific advances in neuroscience have shown that addiction is a chronic and often relapsing disease that is linked to pathological changes in neural circuitry, including those involved in reward and motivation, learning and memory, cognitive control, decision making, mood, and interoceptive awareness (Adinoff, 2004; Hyman et al., 2006; Kalivas and Volkow, 2005; Kalivas and O’Brien, 2007; Koob and Le, 1997; O’Brien, 2003; Volkow et al., 2003; Volkow et al., 2004). Importantly, these changes involve the same structures and processes that contribute to the mechanisms of behavior regulation and its deviations, predating drug use (Vanyukov et al., 2003b). Impairment of the addicted brain provides a neurological basis for the cardinal behavioral manifestation of drug addiction – persistent drug use despite serious adverse consequences. Longitudinal observations of addicts demonstrate the chronic relapsing nature of addiction and the need for long-term treatment strategies (Dennis and Scott, 2007; Dennis et al., 2005). However, the malleability of the liability phenotype, including “maturing-out” of conditions that meet diagnostic criteria for dependence (Dawson et al., 2006; Newcomb et al., 2001), underscores the nonspecific character and construct validity of liability while still holding promise for effective intervention. In contrast to such static traits as stature in adults, liability to addiction is dynamic over time and development. It can be viewed as forming an ontogenetic trajectory that can be tracked and measured, and given its inherent dynamism, potentially changed (Tarter and Vanyukov, 1994; Tarter and Vanyukov, 1991).
Advances in these and other important areas of addiction science have outpaced progress in the measurement of addiction, and the ability to quantify and measure this construct accurately remains a looming methodological challenge (Conway et al., 2006; Craddock et al., 2008; Merikangas and Avenevoli, 2000; Merikangas and Conway, 2008; Neale et al., 2006). Currently, research usually measures drug addiction in the broadest sense by classifying and contrasting “addicts” to “non-addicts” according to diagnostic criteria. Genetic risk for addiction is similarly categorized based on a parent’s diagnosis or family history. This approach implicitly assumes categorical distinctions between groups and categorical similarities among the individuals within each group regarding their symptoms and group-identified features of addiction. Few studies have focused on variations among individuals within each group, despite the common knowledge that meaningful individual differences exist within any given cohort of addicts and controls. Such heterogeneity is reflected in the hundreds of different combinations of addiction symptoms that meet diagnostic criteria (Vanyukov et al., 2003b). Although needed for clinical practice, the diagnosis per se is less than optimal when it comes to genetic and other etiology research, or for informing efforts in the primary prevention of addiction.
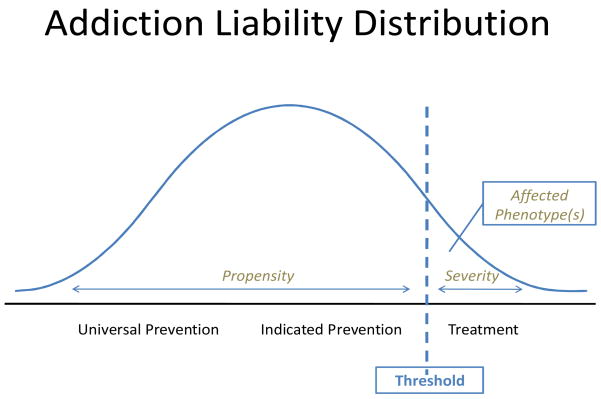
A precise method and instrument for measuring individual variation in liability to addiction is therefore needed to build on and advance the science and understanding of the multifactorial nature of drug abuse and addiction. A term introduced in human genetics by Falconer (Falconer, 1965) (p. 52), liability is a latent (unobservable) quantitative trait that, if measured, “would give us a graded scale of the degree of affectedness or of normality”, with these two categories divided by a threshold. The “gradations of normality” (the subthreshold liability phenotypes) correspond to variation in the risk (propensity), whereas “gradations of affectedness” (the suprathreshold phenotypes, likely to be assigned a clinical diagnosis) correspond to variation in severity, comprising the two portions of the liability distribution. Applied to addiction, severity refers to the degree of maladaptive compulsive drug-seeking and using behavior displayed by an individual, corresponding to variation in liability above the diagnostic threshold. Propensity refers to the probability of the disorder’s onset, corresponding to liability variation below the diagnostic threshold. It follows that individual differences in severity manifest as different degrees of maladaptive drug-seeking and drug-using behavior. Similarly, variation in propensity manifest as behavioral precursors of addiction. The ultimate desirable goal of a new instrument of addiction would be to provide a single scale (see Figure 1) by which an individual’s liability to addiction (propensity or severity) can be quantified using a numeric score.
Figure 1.
Addiction liability distribution
The plausibility of such a single common (versus drug-specific) liability dimension (latent trait) and the feasibility of its measurement are supported by clinical, neurobiological, genetic, and statistical findings (Vanyukov et al., 2003a). Liabilities to addictions to specific drugs are both phenotypically and genetically highly correlated, with minimal specific genetic variance; most of the variance in addiction liability is associated with common (thus, likely, brain-behavioral) mechanisms rather than with drug action per se (Kendler et al., 2007; Tsuang et al., 1998). Indeed, neurobiological data pertaining to drug reward suggest that many drug effects involve the dopaminergic and other major neurobiological systems, despite differences in specific routes of administration, the biotransformation pathways of different drugs, or in their primary targets (Koob and Volkow, 2009). These same systems substantially overlap with those involved in mechanisms of natural reward and incentive motivation, stress response, and social behavior as well (Goldstein and Volkow, 2002; Koob and Volkow, 2009; Vanyukov et al., 2003a). Moreover, data increasingly show that drug addiction can be located on the same dimension as premorbid (and even pre-drug-use) behaviors that indicate a highly heritable latent trait variably known as dysregulation, disinhibition, behavior undercontrol or externalizing behavior, including risks for disruptive behavior disorders (Button et al., 2006; Hicks et al., in press; Kendler et al., 2007; Kirisci et al., 2009; Krueger et al., 2002; Tarter et al., 1990, 2003, 2004; Vanyukov et al., 2009; Young et al., 2009). Further support comes from research on an instrument designed to measure variation in propensity to addiction (the transmissible liability index, TLI; described in detail below). Comprised largely of items indicating dysregulation, scores on the TLI have been found to be elevated among offspring of drug abusers (Tarter et al., 2003), highly heritable (Vanyukov et al., 2009; Hicks et al., in press), predictive of addiction subsequent to drug use initiation (Vanyukov et al., 2009; Kirisci et al., 2009, under review), and prior to exposure to cannabis, higher in boys who later develop cannabis use disorder compared to those who used cannabis but did not develop this disorder (Kirisci et al., under review). It is noteworthy that no shared environment component was detected for TLI (Vanyukov et al., 2009), a finding that is consistent with studies showing that liability to drug use initiation is affected by shared and non-shared environmental influences, whereas liability to addiction is largely influenced by genetic factors (Han et al., 1999; Kendler et al., 2000) and phenotype- (and genotype-) environment correlation (i.e., when individuals with certain characteristics are more likely to seek, and be found in, environments conducive to accessing and using drugs) (e.g., Kirillova et al., 2008). These findings are part of the cumulative science on the biobehavioral foundations of addiction. They reinforce the plausibility of mapping the propensity for and severity of addiction along a common dimension of liability, in the Falconer sense, i.e., extending across a graded scale whose degrees signify gradations between sub- and suprathreshold liability phenotypes, respectively.
Genes found to be associated with addiction-related characteristics, both in candidate gene and whole genome scan studies (Uhl et al., 2008), are not specific to a particular drug, or even to addictions as such, given their involvement in basic neurological mechanisms and signal transduction in the central nervous system. It is thus likely that these associations are mediated by pleiotropic nonspecific effects of these genes on neurobiological processes involved in behavior regulation and control. This hypothesis is supported, for instance, by significant genetic correlations between an index of behavior disinhibition (locating symptoms of conduct and attention-deficit hyperactivity disorder, novelty seeking, and substance use on the same dimension) and an index of performance on neuropsychological tasks measuring attentional control and response inhibition (Young et al., 2009). A similar brain-behavior connection is noted for disinhibition/externalizing and the reduced amplitude of the P300 event-related potential (Iacono et al., 2002; Justus et al., 2001), particularly its main time-frequency components, theta and delta, indexing, respectively, memory encoding and attention processes, and signal matching, decision-making, and memory updating processes (Gilmore et al., 2009). The association between P300 amplitude and an index of the latent externalizing trait (accounting for variance shared between liabilities to alcohol, nicotine and drug dependence, conduct disorder and adult antisocial behavior) is of genetic origin (Hicks et al., 2007). As shown by genetic studies of phenotypes based on neuroimaging, genetic associations of behavioral response regulation and personality characteristics comprising the behavior dysregulation construct are mediated by the function of neural circuitry involving targets of drug action (Hariri et al., 2008; Hariri, 2009).
Statistically, substance use disorders and related symptoms have been shown to best fit a model whereby their covariances are substantially accounted for by a single dominant factor (Kirisci et al., 2002; Kirisci et al., 2006; Compton et al., 2009; Saha et al., 2007; Wu et al., 2009). Unidimensionality was also established for items that comprise the TLI, the instrument (described above) that measures transmissible liability to illicit drug-related substance use disorder in children. Interestingly, the TLI items are largely related to a set of externalizing problems (e.g., conduct disorder, antisocial personality disorder) and temperament-like indicators of nonspecific addiction risk (e.g., behavioral disinhibition, constraint, sensation seeking, and novelty seeking) collectively referred to as behavioral undercontrol, dysregulation, or externalizing psychopathology (Cadoret et al., 1995; Chassin et al., 1991; Conway et al., 2002; Conway et al., 2003; Elkins et al., 2004; Iacono et al., 1999; Iacono et al., 2008; Krueger et al., 2002; Krueger et al., 2007; Sher et al., 2000). Evidence suggests a common genetic variance between these traits and addiction (Mustanski et al., 2003). Some studies suggest that the broad externalizing factor is more heritable than the constituent individual disorders (80–85% versus 40–70%) (Hicks et al., 2004; Moffitt, 2005; Young et al., 2000), as well as being more useful for gene identification (Dick et al., 2008).
Variation in common (nonspecific) liability to addiction could help explain the typical pattern of progression “softer” to “harder” types of substances used (Tarter et al., 2006). Variation in externalizing traits could also mediate heritability of liability to addiction (Button et al., 2006; Kirillova et al., 2008; Slutske et al., 2002; Swendsen et al., 2002; Tarter et al., 2004; Vanyukov et al., 2007). From a genetic-risk perspective, familial antisocial addiction may be particularly potent (or severe). Studies of adopted-away offspring of fathers who are antisocial addicts (compared to either antisocial or addicted), for example, have found the offspring to be at greatest risk for substance abuse themselves (Langbehn et al., 2003). Prevention research has demonstrated how interventions that directly or indirectly target externalizing problems can effectively prevent drug abuse (Hawkins et al., 2008; Kellam et al., 2008; Poduska et al., 2008), perhaps through impacting underlying neuroregulation systems (Romer and Walker, 2007) and familial processes that enhance behavioral regulation (Brody et al., 2009).
2. Severity Measurement Instruments
The following briefly reviews instruments that measure addiction propensity and severity (see Table 1). The instruments in this review were identified through a literature search of several databases (e.g., PubMed) for key terms such as “drug abuse severity”, “drug addiction severity”, “substance abuse severity”, “substance addiction severity”. To be included, an instrument had to be explicitly designed to measure severity of drug or alcohol addiction. Excluded were instruments that measure nicotine addiction1, screening instruments, and those that measure only one specific domain of addiction (e.g., craving). The review includes the purpose and content of each instrument and other information on the applicability of the instrument for liability measurement.
Table 1.
Instruments designed to measure addiction severity
| Instrument | What it Measures | Operationalization of Severity | Item-Selection Methodology |
|---|---|---|---|
| Addiction Severity Instrument (ASI) | Severity of alcohol and drug use | Need for treatment across 6 domains | Clinical judgment |
| Alcohol Dependence Scale (ADS) | Severity of alcohol dependence | DSM symptomatology, loss of control, obsessive drinking style, two aspects of withdrawal | Clinical judgment, item/factor correlation |
| Benzodiazepine Questionnaire (BDEQ) | Severity of benzodiazepine dependence | DSM symptomatology, pleasureable effects of drug, perceived need for drug in order to function properly | Clinical judgment, item/factor correlation |
| Chemical Use, Abuse, and Dependence Scale (CUAD) | Severity of alcohol and drug use | DSM symptomatology | Clinical judgment |
| Drug Use Screening Inventory (DUSI) | Severity of alcohol and drug use | Consequences of drug use | Clinical judgment |
| Global Appraisal of Individual Needs (GAIN) | Severity of alcohol and drug use | DSM symptomatology, substance use frequency, behavioral complexity | Clinical judgment |
| Severity of Alcohol Dependence Questionnaire (SADQ) | Severity of alcohol dependence | DSM symptomatology, three aspects of withdrawal, rapidity of reinstatement after abstinence | Clinical judgment, item/factor correlation |
| Severity of Amphetamine Dependence Questionnaire (SamDQ) | Severity of amphetamine dependence | DSM symptomatology, three aspects of withdrawal, rapidity of reinstatement after abstinence, depression, lethargy | Clinical judgment, item/factor correlation |
| Severity of Dependence Scale (SDS) | Severity of drug dependence | DSM symptomatology, compulsivity of drug use | Clinical judgment, item/factor correlation |
| Severity of Opiate Dependence Questionnaire (SODQ) | Severity of opiate dependence | DSM symptomatology, three aspects of withdrawal, rapidity of reinstatement after abstinence | Clinical judgment, item/factor correlation |
| Substance Dependence Severity Scale (SDSS) | Severity of drug dependence | DSM symptomatology | Clinical judgment |
| Substance Use Involvement Index (SUII) | Severity of alcohol and drug use | DSM symptomatology, liability of substance use | Clinical judgment, item/factor correlation, item-response theory |
2.1. Addiction Severity Index (ASI)
The ASI (McClellan et al., 1980) is a widely used instrument that assesses the need for alcohol or drug treatment across six domains of functioning (Chemical Abuse, Medical, Psychological, Legal, Family/Social, and Employment/Support). Within each domain, the interviewer provides global ratings of severity (referred to as Interviewer Severity Ratings, or ISRs) based on the patient’s responses to objective items as well as the patient’s assessment of how bothered he/she is by problems in each domain. The ISRs, which range from 0 to 9, form the basis for the patient’s profile and treatment plan. Revised versions of the ASI (McDermott et al., 1996) use more advanced psychometric methods, partially in response to studies that failed to replicate high inter-rater reliabilities of the original version of the ASI (McLellan et al., 1985; Hodgins and Elguebaly, 1992). The ASI is one of the most frequently used instruments in addiction research and practice, and it serves as one of the core measures in the NIDA Clinical Trials Network. The reliability and validity of the ASI is well documented, but less is known about its dimensionality or whether its items reflect differences in severity.
2.2 Drug Use Screening Inventory (DUSI)
The DUSI (Kirisci et al., 1995; Tarter et al., 1990) was developed as a diagnostic instrument for the treatment of alcohol or drug problems in adolescents. Despite the word “screening” in its name, this is a phenotypic assessment instrument comprising a series of scales measuring severity of substance abuse as reflected in various life domains: Substance Use, Behavior Patterns, Health Status, Psychiatric Disorder, Social Skills, Family System, School Adjustment, Work, Peer Relationships, Leisure/Recreation. Kirisci et al. (1995) found that the items are scalable as indicators of a unidimensional latent trait, that the scores among individuals with high or moderate substance use were measured with greater precision than those at lower levels, and that scores accurately predicted the level of substance use and consequences at 6-month follow-up.
2.3. Global Appraisal of Individual Needs (GAIN)
The GAIN (Dennis et al., 2003) is a large set of questionnaires designed to provide diagnostic and severity information for both adolescents and adults seeking treatment for drug or alcohol problems. The GAIN’s 16-item Substance Problem Scale (SPS) scale consists of the DSM-IV criteria for substance use diagnosis plus five screener items that measure variation in severity (i.e., weekly use, family and friends complaining about use, continued use despite fights, and use being time consuming). Acceptable reliability and validity estimates have been reported (1999 manual and Dennis, Chan, and Funk 2006). Riley and colleagues (Riley et al., 2007) found that the five screener items had relatively low location parameters, indicating that the items reflect variation at the lower (or “milder”) end of a severity continuum.
2.4. Severity of Alcohol Dependence Questionnaire (SADQ) and Alcohol Dependence Scale (ADS)
The SADQ (Stockwell et al., 1979) and the ADS (Skinner and Allen, 1982) were explicitly designed to extend the Edwards and Gross (Edwards and Gross, 1976) conceptualization of alcoholism to severity of addiction to illicit drugs. The SADQ includes four subscales: physical withdrawal, affective withdrawal, drinking to relieve withdrawal, and rapidity of reinstatement after abstinence. The ADS includes four slightly broader subscales: psychophysical withdrawal, psychoperceptual withdrawal, loss of behavioral control, and obsessive drinking style. Reliability and validity estimates were high for both instruments. Kahler and colleagues (Kahler et al., 2003a; Kahler et al., 2003b) reported that the ADS items primarily measure a single dimension of alcoholism severity, with some items corresponding to greater severity than others.
2.5. Severity of Opioid Dependence Questionnaire (SODQ), the Severity of Amphetamine Questionnaire (SamDQ), and the Benzodiazepine Dependence Questionnaire (BDEPQ)
The SODQ and the SamDQ adapted the SADQ for opioids and amphetamines, respectively. These instruments consist of subscales designed to measure the severity of physical withdrawal symptoms, affective withdrawal symptoms, the extent to which drugs are used to relieve withdrawal symptoms, and the rapidity of reinstatement after periods of abstinence. The BDEPQ (Baillie and Mattick, 1996) assesses aspects of benzodiazepine dependence including the extent to which pleasurable effects were anticipated as a result of drug use, the extent to which drug use is needed to complete daily life activities, and a general dependence factor. The reliability and validity of these instruments are well documented (Baillie and Mattick, 1996; Churchill et al., 1993; Sutherland et al., 1986; Topp and Mattick, 1997), but information is limited regarding dimensionality and whether some items reflect greater severity than others.
2.6. Chemical Use Abuse and Dependence (CUAD)
The CUAD scale (McGovern and Morrison, 1992) is a semi-structured interview designed to provide DSM-III-R substance use diagnoses as well as severity indicators in both clinical and research settings. For each substance used, weights based on clinical judgment are assigned for each symptom to reflect increasing clinical severity, and total severity scores are computed by summing the weights across symptoms. The CUAD has demonstrated reliability and validity (McGovern and Morrison, 1992), but it is unknown whether it is unidimensional or whether some items reflect greater severity than others.
2.7. Substance Dependence Severity Scale (SDSS)
The SDSS (Miele et al., 2000) is a semi-structured interview designed to provide DSM-IV diagnoses and severity indicators in both clinical and research settings. For each substance used, the SDSS provides DSM-IV diagnoses and four different 30-day severity indicators; two for intensity of symptoms (SEV, WORST SEV), and two for frequency of symptoms (DAYS, WORST DAYS). The reliability and validity of the SDSS is documented, but information is lacking about its dimensionality and ability to assess severity.
2.8. Severity of Dependence Scale (SDS)
The SDS (Gossop et al., 1995) was developed as a brief scale to measure the degree of dependence experienced by different types of drug users. In contrast to the other instruments discussed here, the five-question SDS “is primarily a measure of compulsive use and it does not include items to measure tolerance, withdrawal, or reinstatement” (p. 612). Responses are self rated on a 4-point Likert scale, and summed to form a total score. Scale reliability and validity has been reported and factor analytic methods have confirmed unidimensionality of the five items.
2.9. Substance Use Involvement Index (SUII)
The Substance Use Involvement Index (SUII; Kirisci et al., 2002), derived using item-response theory (IRT; described below), is based on the respondent’s endorsement of lifetime use (yes/no) of ten categories of drugs: alcohol, cannabis, cocaine/crack, opiates, amphetamines, methylphenidate, sedatives, tobacco, hallucinogens, PCP and inhalants. One study reported that a unidimensional factor model adequately fit the data for both adult males and adult females, and the factor loadings were not significantly different between gender groups (Kirisci et al., 2002). Interestingly, Kirisci and colleagues also reported that equated item-location parameters were higher in females than males, suggesting that endorsement of substance use by a male is (by itself) indicative of a lower level of severity than the same endorsement by a female. These results are consistent with data showing that females have a higher liability threshold (Kendler et al., 2003b), i.e., they require higher factor scores in order to manifest a disorder.
3. Propensity Measurement Instruments
Measurement of propensity to addiction has rarely been attempted and is considerably more difficult than measurement of addiction severity, especially given the absence of face-value indicators of risk with high construct validity. Obviously, only premorbid characteristics may be safely used as indicators of propensity because drug use and addiction have behavioral and psychological effects. Despite knowledge of many “risk” and “protection” factors (psychological and psychopathological variables associated with addiction risk) (Glantz et al., 2005; Hawkins et al., 1992), it is not evident on an a priori basis that premorbid indicators can be used, or how, in constructing an index of propensity to addiction.
3.1. Transmissible Liability Index (TLI)
One approach to the selection of propensity indicators has been used in the derivation of the index of transmissible liability to addiction related to illicit drugs (the transmission liability index, TLI), based on a high-risk/family design and IRT in the NIDA-funded Center for Education and Drug Abuse Research (CEDAR). Inasmuch as addiction risk is transmissible within families (mostly due to its high heritability), characteristics that discriminate groups of children with affected vs. unaffected parents (high- and low-average-risk groups, HAR and LAR, respectively) are likely to be indicators of the transmissible component of children’s addiction liability/propensity (the variance component correlated between relatives/generations).
The TLI derivation method involves using a large set of items from numerous psychological and psychiatric instruments that were originally selected based on their potential for measuring variables related to addiction risk and psychopathology. Through a variety of statistical methodologies (e.g., factor analysis, IRT), the items were selected and transformed into a set of unidimensional constructs characterizing individual behavior/personality (e.g., antisociality, attention, mood) (see measurement model and other details in Vanyukov et al., 2003a). The resulting 45-item set selected for sons of the probands at age 10–12 was used to assess the quality of items and estimate TLI. The findings show that the TLI is a valid and reliable scale, and highly predictive (e.g., O.R. = 1.81, 95% C.I.: 1.12–2.30) of substance use disorder (Vanyukov et al., 2009; Kirisci et al., 2009, under review). Twin studies have also shown that TLI is highly heritable (H2 = 0.79) (Vanyukov et al., 2009; Hicks et al., in press), further supporting its derivation as a measure of a transmissible trait.
The longitudinal design of the CEDAR study permits further refinement of the method used in the creation of the TLI, such that the liability/propensity indicator constructs and items will be referenced to the offspring’s own, rather than parental, outcomes such as drug use and drug addiction. Such referencing, aside from being based entirely on the offspring’s own phenotype, directly connects indicators and a given manifest trait, which will enable indexing of the resultant total, rather than only transmissible, liability. This extended liability index methodology has potential as a prototype for development of an index to cover the entire range of phenotypic distribution (i.e., both propensity and severity) and identify candidate biologic and genetic mechanisms of drug use and its precursors and antecedents.
4. Summary and Future Directions
4.1. Limitations of Existing Instruments
The assessment instruments in use today for measuring addiction severity (described above and appearing in Table 1) have proven utility, reliability, and validity, but they are of limited use for evaluating individual differences in propensity/severity of the hallmark characteristic(s) of drug addiction, i.e., compulsivity in seeking and using drugs despite harmful consequences. First, existing instruments are based on a variety of related but different constructs of addiction severity including behavioral and social consequences, quantity or type of DSM symptoms endorsed, use patterns within and across substances, and number of different DSM diagnoses. Only one, the SDS, focuses on compulsive drug taking and seeking. Second, the content of existing measures is unlikely to reflect the full range of addiction severity. This limitation can be partly attributed to the fact that the content of many instruments derives from the DSM, which virtually by design, is not optimal for measuring variability in the severity of addiction. Indeed, clinical and epidemiologic studies applying IRT models show that DSM symptoms are redundant and fail to capture variability across the full range of the addiction continuum (Compton et al., 2009; Gillespie et al., 2007; Langenbucher et al., 1995; Saha et al., 2007; Wu et al., 2009). Many existing instruments also rely on clinical judgment in the absence of item-selection techniques, thereby increasing the likelihood of including items that are redundant, collinear, or weakly related to the construct of interest.
Third, only one extant instrument (TLI) is currently available to measure propensity to addiction, let alone both the propensity and the severity of addiction on the same metric. It should be noted that the latent liability phenotype assessed by the TLI, while pertaining to the risk in the nonaffected individuals and thereby to prospective prediction, is nevertheless a cross-sectional measure. Substance use or lack thereof, or consumption with multiple graded categories (e.g., none, low, heavy) are observed phenotypic outcomes that may or may not be used as indicators of latent liability. Using items immediately related to drug use may be problematic, of course, because that would preclude the index’s use in studying respective behaviors as outcomes. Although substance use is a necessary condition for addiction development, it seems likely that the psychological/behavioral components of liability such as personality largely influence substance use initiation and/or, more importantly, development of addiction. Substance use can thus be viewed as a manifestation of liability forming an ontogenetic trajectory that can be tracked and measured.
Fourth, most extant instruments were constructed using classical test theory methodology, which has its own psychometric limitations that directly bear on severity measurement. The use of factor analysis, for example, does not utilize the full power of modern scaling techniques (Embretson and Reise, 2000; Hambleton and Swaminathan, 1985; Lord and Novick, 1968) that take item properties into account (e.g., whether one item indicates greater “severity” than another item). While producing scales with high levels of internal consistency, the use of factor analytic methods alone does not allow one to build instruments to discriminate individuals along selected ranges of an underlying trait.
4.2. Advantages of Item Response Theory (IRT) for Measuring Propensity and Severity
Whereas various methods are available to analyze latent traits, IRT appears particularly suited for the derivation of an index of liability to addiction. Briefly, IRT (e.g., Embretson and Reise, 2000) is a psychometric test theory that relates the performance of an examinee on a test item to a latent trait (ability) that the test is intended to measure. This relationship (e.g., in a simple case, between the trait and the probability of a correct response) is described by an item response function (IRF). While ability level is a characteristic of the examinee, the examinee’s performance will also depend on parameters that characterize the test items themselves. The widely used two-parameter model includes location (difficulty) or b, the trait value at which the probability of a correct response exceeds 0.5, and discrimination or a, which is proportional to the slope of the IRF at point b on the trait scale. In this model, the parameters provide for gradients in item difficulty and capacity to discriminate different values of the trait. In contrast to the classical psychometric test theory, which was used to develop most of the instruments reviewed herein, IRT provides testable models. A data-fitting IRT model yields estimates that have unique value for trait measurement; i.e., item parameters are invariant across samples (subpopulations) of subjects (the trait distribution does not influence the estimates), and trait estimates are invariant across items used.
The many advantages of IRT have been underutilized in severity instrument development, though it has successfully been used in the measurement of transmissible addiction risk (Vanyukov et al., 2003, 2009; Kirisci et al., et al., 2006, 2009, under review). Selection of items based on the information they contribute is a key advantage of item response theory (Hambleton et al., 1991), which is likely to inform the development of the liability index. The most difficult question centers on the problem of selecting and constructing items that are highly informative for measuring low propensity values. Nevertheless, some such items (e.g., “I don’t move around much at all in my sleep”, “I usually eat the same amount each day”, and “Changes in plans make my child restless”) have been identified and applied in an existing propensity measurement instrument, the TLI (Kirisci et al., 2009, under review; Vanyukov et al., 2009). These and other items are potential candidates for an instrument that measures both propensity and severity on the same scale.
How and to what extent the propensity and severity phenotypes can be characterized by a common, underlying, and continuous unidimensional trait of liability to addiction (Chan et al., 2008) remain large, empirical challenges suitable for IRT analyses. Dimension-sharing between propensity and severity need not be perfect for the derivation of a unidimensional index to quantify sub- and suprathreshold liability; showing that a unidimensional model offers the best fit for the data is sufficient. The unidimensionality of a trait essentially refers to the structure of covariance between items used in its measurement (and testable for their dominance in accounting for the covariance) rather than to a single causal influence or the total number of influences potentially determining the shared variance. Even if there is specificity to the unaffected and affected phenotypic distributions (i.e., those of propensity and severity), the variance they share with the common liability dimension, based on genetic data, will likely be large and sufficiently informative to be targeted for measurement. If such tests fail and the two constructs do not share the same dimension to an appreciable degree, continuous indices will need to be developed that quantitate these constructs separately and are applied to the nonaffected (or asymptomatic) and affected (symptomatic) populations. Measurement of common rather than drug-specific liability is also desirable from a practical standpoint, given its universal application.
4.3. Advantages of an Instrument Measuring Propensity to and Severity of Addiction
The conceptual and methodological shortcomings of instruments currently used in research and clinical practice can be overcome through the use of emerging technologies to develop a reliable, valid, and standardized assessment instrument to measure and distinguish variations in phenotypic expression of the underlying latent trait that comprises propensity and severity of drug addiction. The distribution of the hypothesized latent trait in the population is depicted in Figure 1. Key requirements of this assessment instrument include the ability to accurately and comprehensively measure gradations along the propensity/severity continuum using broad and discriminating content that captures the essence of addiction; to detect meaningful variation between, within, and across individuals over time that is scalable along the underlying dimension; and to allow for efficient assessment of the construct with minimal burden for administration, training, and cost to the researcher, clinician, research participant, or patient. Coupling modern psychometric methodology with sophisticated computer technology (e.g., IRT-based computerized adaptive testing [CAT]) enables measurement covering the full phenotypic scale, with maximum flexibility, accuracy, and efficiency (Drasgow and Olson-Buchanan, 1999; Kirisci and Hsu, 1992). CAT-based instruments, for example, can administer different sets of items to different people optimal for their liability phenotypes, can provide analyses to explore the relative difficulty of items, and can generate a single score to represent an individual’s location along an underlying trait (the liability phenotype). This technique is routinely applied in the education field (e.g., the administration of the Graduate Record Examination applies IRT analyses), and can certainly be adapted for use in the field of addiction.
Whether propensity to and severity of addiction are locatable on essentially the same dimension remains to be determined, yet the cumulative evidence on neurobiological mechanisms of addiction, combined with psychometric evidence, gives merit to a common metric of liability to addiction extending across subclinical to clinical diagnostic thresholds. The concept of addiction liability as an unidimensional trait that is complex, i.e., contributed to by many diverse influences, and dynamic is evidenced from a number of other complex, multifactorial traits, such as stature (an observed trait) or the Intelligence Quotient (IQ, an index of a latent trait). Such an instrument, as a continuous measure of addiction propensity and severity at the behavioral level, would enhance the capacity, statistical power, and precision of research on the etiology, neurobiology, and genetics of addiction (Embretson and Reise, 2000; Hambleton and Swaminathan, 1985; Lord and Novick, 1968; Neale et al., 2006), and for use in developing improved approaches in primary prevention, treatment, and intervention. For instance, individuals with high severity scores may require different types of treatments than those with lower scores, and individuals with elevated propensity scores may require preventive interventions tailored to their particular needs. In addition, severity measures have been rarely applied to identify neurobiological correlates of addiction. Volkow et al. (Volkow et al., 2006) found that cue-induced dopamine changes in cocaine-dependent subjects positively correlated with the average ratings on the seven domains of the ASI. Patkar et al. (Patkar et al., 2008) reported that blunted responses to a serotonergic challenge among cocaine-dependent subjects positively correlated with the drug subscale scores of the ASI. Clearly, research of this kind can be enhanced by improved measurement of individual differences in the propensity and severity of addiction. Finally, from a translational perspective, such an instrument would unify and potentially transform research on the addiction liability phenotype by using a single instrument. The field of addiction science has advanced in remarkable ways from discoveries in psychometrics, computerization, etiology, neurobiology, genetics, prevention, and treatment. The challenge before us now is to build on these advances to develop a new instrument that measures the propensity and severity of addiction.
Footnotes
Disclaimer: The views and opinions expressed in this paper are those of the authors and do not necessarily represent the views of the National Institute on Drug Abuse, National Institutes of Health, or any other governmental agency.
Considerable research attention has been focused on the severity of nicotine addiction, and results suggest a latent trait for nicotine dependence (Strong et al., 2003a; Strong et al., 2003b; Strong et al., 2007; Strong et al., 2009). Less is known about the severity of alcohol and/or illicit drug addiction, however, which underscores the need for additional research of this kind.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harvard Review of Psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie AJ, Mattick RP. The benzodiazepine dependence questionnaire: Development, reliability and validity. British Journal of Psychiatry. 1996;169:276–281. doi: 10.1192/bjp.169.3.276. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Dinwiddie SH, Begleiter H, Crowe RR, Hesselbrock V, Nurnberger JI, Jr, Porjesz B, Schuckit MA, Reich T. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch Gen Psychiatry. 1998;55:982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- Brooner RK, King VL, Kidorf M, Schmidt CW, Jr, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- Button TM, Hewitt JK, Rhee SH, Young SE, Corley RP, Stallings MC. Examination of the causes of covariation between conduct disorder symptoms and vulnerability to drug dependence. Twin Res Hum Genet. 2006;9:38–45. doi: 10.1375/183242706776402993. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. Adoption study demonstrating two genetic pathways to drug abuse. Arch Gen Psychiatry. 1995;52:42–52. doi: 10.1001/archpsyc.1995.03950130042005. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Miele GM, Hasin DS. Does motivation to change mediate the effect of DSM-IV substance use disorders on treatment utilization and substance use? Addict Behav. 2002;27:207–225. doi: 10.1016/s0306-4603(00)00178-7. [DOI] [PubMed] [Google Scholar]
- Chan YF, Dennis ML, Funk RR. Prevalence and comorbidity of major internalizing and externalizing problems among adolescents and adults presenting to substance abuse treatment. J Subst Abuse Treat. 2008;34:14–24. doi: 10.1016/j.jsat.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Rogosch F, Barrera M. Substance use and symptomatology among adolescent children of alcoholics. J Abnorm Psychol. 1991;100:449–463. doi: 10.1037//0021-843x.100.4.449. [DOI] [PubMed] [Google Scholar]
- Churchill AC, Burgess PM, Pead J, Gill T. Measurement of the Severity of Amphetamine Dependence. Addiction. 1993;88:1335–1340. doi: 10.1111/j.1360-0443.1993.tb02019.x. [DOI] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Tarter RE. Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Depend. 1998;49:115–121. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- Compton WM, Saha TD, Conway KP, Grant BF. The role of cannabis use within a dimensional approach to cannabis use disorders. Drug Alcohol Depend. 2009;100:221–227. doi: 10.1016/j.drugalcdep.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Kane RJ, Ball SA, Poling JC, Rounsaville BJ. Personality, substance of choice, and polysubstance involvement among substance dependent patients. Drug and Alcohol Dependence. 2003;71:65–75. doi: 10.1016/s0376-8716(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Conway KP, Swendsen JD, Rounsaville BJ, Merikangas KR. Personality, drug of choice, and comorbid psychopathology among substance abusers. Drug and Alcohol Dependence. 2002;65:225–234. doi: 10.1016/s0376-8716(01)00168-5. [DOI] [PubMed] [Google Scholar]
- Conway KP, Compton WM, Miller PM. Editorial: Novel approaches to phenotyping drug abuse. Addictive Behaviors. 2006;31:923–928. [Google Scholar]
- Craddock N, O’Donovan MC, Owen MJ. Genome-wide association studies in psychiatry: lessons from early studies of non-psychiatric and psychiatric phenotypes. Mol Psychiatry. 2008;13:649–653. doi: 10.1038/mp.2008.45. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS. Maturing out of alcohol dependence: the impact of transitional life events. J Stud Alcohol. 2006;67:195–203. doi: 10.15288/jsa.2006.67.195. [DOI] [PubMed] [Google Scholar]
- Dennis M, Scott CK. Managing addiction as a chronic condition. Addict Sci Clin Pract. 2007;4:45–55. doi: 10.1151/ascp074145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Titus J, Unsicker J, Hodkgins D. Global Appraisal of Individual Needs: Administration Guide for the GAIN and Related Measures. Chestnut Health Systems; Bloomington, IL: 2003. [Google Scholar]
- Dennis ML, Chan YF, Funk RR. Development and validation of the GAIN short screener (GSS) for internalizing, externalizing and substance use disorders and crime/violence problems among adolescents and adults. American Journal on Addictions. 2006;15:80–91. doi: 10.1080/10550490601006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ML, Scott CK, Funk R, Foss MA. The duration and correlates of addiction and treatment careers. J Subst Abuse Treat. 2005;28(Suppl 1):S51–S62. doi: 10.1016/j.jsat.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Wang JC, Grucza RA, Schuckit M, Kuperman S, Kramer J, Hinrichs A, Bertelsen S, Budde JP, Hesselbrock V, Porjesz B, Edenberg HJ, Bierut LJ, Goate A. Using dimensional models of externalizing psychopathology to aid in gene identification. Arch Gen Psychiatry. 2008;65:310–318. doi: 10.1001/archpsyc.65.3.310. [DOI] [PubMed] [Google Scholar]
- Drasgow F, Olson-Buchanan JB. Innovations in Computerized Assessment. Lawrence Erlbaum Associates; Mahwah: 1999. [Google Scholar]
- Edwards G, Gross MM. Alcohol Dependence - Provisional Description of A Clinical Syndrome. British Medical Journal. 1976;1:1058–1061. doi: 10.1136/bmj.1.6017.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Malone S, Iacono WG. The effect of parental alcohol and drug disorders on adolescent personality. Am J Psychiatry. 2004;161:670–676. doi: 10.1176/appi.ajp.161.4.670. [DOI] [PubMed] [Google Scholar]
- Embretson SE, Reise WSP. Item response theory for psychologists. Lawrence Erlbaum Associates; Mahwah, New Jersey: 2000. [Google Scholar]
- Falconer DS. The inheritance of liability to certain diseases, estimate from the incidence among relatives. Annals of Human Genetics. 1965;29:51–71. [Google Scholar]
- Foll BL, Gallo A, Le SY, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- Ford JD, Gelernter J, DeVoe JS, Zhang W, Weiss RD, Brady K, Farrer L, Kranzler HR. Association of psychiatric and substance use disorder comorbidity with cocaine dependence severity and treatment utilization in cocaine-dependent individuals. Drug Alcohol Depend. 2009;99:193–203. doi: 10.1016/j.drugalcdep.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Neale MC, Prescott CA, Aggen SH, Kendler KS. Factor and item-response analysis DSM-IV criteria for abuse of and dependence on cannabis, cocaine, hallucinogens, sedatives, stimulants and opioids. Addiction. 2007;102:920–930. doi: 10.1111/j.1360-0443.2007.01804.x. [DOI] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Bernat EM, Iacono WG. Relationship between the P3 event-related potential, its associated time-frequency components, and externalizing psychopathology. Psychophysiology. 2009 doi: 10.1111/j.1469-8986.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz MD, Conway KP, Colliver JD. Drug abuse heterogeneity and the search for subtypes. In: Sloboda Z, editor. Epidemiology of Drug Abuse. New York: Klewer Academic/Plenum Publishers; 2005. pp. 15–28. [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Darke S, Griffiths P, Hando J, Powis B, Hall W, Strang J. The Severity of Dependence Scale (Sds) - Psychometric Properties of the Sds in English and Austrian Samples of Heroin, Cocaine and Amphetamine Users. Addiction. 1995;90:607–614. doi: 10.1046/j.1360-0443.1995.9056072.x. [DOI] [PubMed] [Google Scholar]
- Gottheil E, Weinstein SP, Sterling RC, Lundy A, Serota RD. A randomized controlled study of the effectiveness of intensive outpatient treatment for cocaine dependence. Psychiatr Serv. 1998;49:782–787. doi: 10.1176/ps.49.6.782. [DOI] [PubMed] [Google Scholar]
- Grella CE, Hser YI, Joshi V, Rounds-Bryant J. Drug treatment outcomes for adolescents with comorbid mental and substance use disorders. J Nerv Ment Dis. 2001;189:384–392. doi: 10.1097/00005053-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Hambleton RK, Swaminathan HS. Item response theory principles and applications. Kluwer Nijhoff Publishing; Boston: 1985. [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. Divergent Effects of Genetic Variation in Endocannabinoid Signaling on Human Threat- and Reward-Related Brain Function. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JD, Catalano RF, Miller JY. Risk and Protective Factors for Alcohol and Other Drug Problems in Adolescence and Early Adulthood - Implications for Substance-Abuse Prevention. Psychological Bulletin. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Kosterman R, Catalano RF, Hill KG, Abbott RD. Effects of social development intervention in childhood 15 years later. Arch Pediatr Adolesc Med. 2008;162:1133–1141. doi: 10.1001/archpedi.162.12.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Bernat E, Malone SM, Iacono WG, Patrick CJ, Krueger RF, McGue M. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44:98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Iacono WG, McGue M. Index of the transmissible common liability to addiction: heritability and prospective associations with substance abuse and related outcomes. Addiction. doi: 10.1016/j.drugalcdep.2011.12.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin-family study. Arch Gen Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Hodgins DC, Elguebaly N. More Data on the Addiction Severity Index -Reliability and Validity with the Mentally-Ill Substance-Abuser. Journal of Nervous and Mental Disease. 1992;180:197–201. doi: 10.1097/00005053-199203000-00009. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural Mechanisms of Addiction: The Role of Reward-Related Learning and Memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Dev Psychopathol. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Justus AN, Finn PR, Steinmetz JE. P300, disinhibited personality, and early-onset alcohol problems. Alcohol Clin Exp Res. 2001;25:1457–1466. doi: 10.1097/00000374-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Strong DR, Hayaki J, Ramsey SE, Brown RA. An item response analysis of the alcohol dependence scale in treatment-seeking alcoholics. Journal of Studies on Alcohol. 2003a;64:127–136. doi: 10.15288/jsa.2003.64.127. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Strong DR, Stuart GL, Moore TM, Ramsey SE. Item functioning of the alcohol dependence scale in a high-risk sample. Drug Alcohol Depend. 2003b;72:183–192. doi: 10.1016/s0376-8716(03)00199-6. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug Addiction as a Pathology of Staged Neuroplasticity. Neuropsychopharmacology. 2007;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The Neural Basis of Addiction: A Pathology of Motivation and Choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Volpicelli JR, Oslin DM, Lipkin C, Sparkman T, O’Brien CP. Cocaine dependence severity predicts outcome in outpatient detoxification from cocaine and alcohol. Am J Addict. 2004;13:74–82. doi: 10.1080/10550490490265389. [DOI] [PubMed] [Google Scholar]
- Kellam SG, Brown CH, Poduska JM, Ialongo NS, Wang W, Toyinbo P, Petras H, Ford C, Windham A, Wilcox HC. Effects of a universal classroom behavior management program in first and second grades on young adult behavioral, psychiatric, and social outcomes. Drug Alcohol Depend. 2008;95(Suppl 1):S5–S28. doi: 10.1016/j.drugalcdep.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003a;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003b;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kirillova GP, Vanyukov MM, Kirisci L, Reynolds M. Physical maturation, peer environment, and the ontogenesis of substance use disorders. Psychiatry Res. 2008;158:43–53. doi: 10.1016/j.psychres.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisci L, Hsu T. Ability estimation using only observable quantities in adaptive testing. ERIC Document Reproduction Service No. TM 018393 1992 [Google Scholar]
- Kirisci L, Mezzich A, Tarter R. Norms and Sensitivity of the Adolescent Version of the Drug-Use Screening Inventory. Addictive Behaviors. 1995;20:149–157. doi: 10.1016/0306-4603(94)00058-1. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Tarter R, Mezzich A, Ridenour T, Reynolds M, Vanyukov M. Prediction of cannabis use disorder between boyhood and young adulthood clarifying the phenotype and environtype. The American Journal on Addictions. 2009;18:36–47. doi: 10.1080/10550490802408829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisci L, Tarter RE, Vanyukov M, Martin C, Mezzich A, Brown S. Application of item response theory to quantify substance use disorder severity. Addict Behav. 2006;31:1035–1049. doi: 10.1016/j.addbeh.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Tarter RE, Vanyukov M, Ridenour T, Reynolds M. Quantifying transmissible risk for cannabis use disorder from childhood to adulthood under review. [Google Scholar]
- Kirisci L, Vanyukov M, Dunn M, Tarter R. Item response theory modeling of substance use: an index based on 10 drug categories. Psychol Addict Behav. 2002;16:290–298. [PubMed] [Google Scholar]
- Koob GF, Le MM. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychol. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Nichol PE, Hicks BM, Markon KE, Patrick CJ, Iacono WG, McGue M. Using latent trait modeling to conceptualize an alcohol problems continuum. Psychol Assess. 2004;16:107–119. doi: 10.1037/1040-3590.16.2.107. [DOI] [PubMed] [Google Scholar]
- Langbehn DR, Cadoret RJ, Caspers K, Troughton EP, Yucuis R. Genetic and environmental risk factors for the onset of drug use and problems in adoptees. Drug Alcohol Depend. 2003;69:151–167. doi: 10.1016/s0376-8716(02)00310-1. [DOI] [PubMed] [Google Scholar]
- Langenbucher JW, Morgenstern J, Miller KJ. Dsm-Iii, Dsm-Iv and Icd-10 As Severity Scales for Drug-Dependence. Drug and Alcohol Dependence. 1995;39:139–150. doi: 10.1016/0376-8716(95)01152-o. [DOI] [PubMed] [Google Scholar]
- Li MD, Burmeister M. New insights into the genetics of addiction. Nat Rev Genet. 2009a;10:225–231. doi: 10.1038/nrg2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Burmeister M. New insights into the genetics of addiction. Nat Rev Genet. 2009b;10:225–231. doi: 10.1038/nrg2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord FM, Novick MR. Statistical theories of mental test scores. Addison-Welsley Publishing Company; Reading, MA: 1968. [Google Scholar]
- Lynch M, Walsh B. Genetics and Analysis of Quantitative Traits. Sunderland, MA: Sinauer Associates Inc; 1998. [Google Scholar]
- McDermott PA, Alterman AI, Brown L, Zaballero A, Snider EC, Mckay JR. Construct refinement and confirmation for the addiction severity index. Psychological Assessment. 1996;8:182–189. [Google Scholar]
- Mcgovern MP, Morrison DH. The Chemical Use, Abuse, and Dependence Scale (Cuad) - Rationale, Reliability, and Validity. Journal of Substance Abuse Treatment. 1992;9:27–38. doi: 10.1016/0740-5472(92)90007-b. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, Obrien CP. New Data from the Addiction Severity Index - Reliability and Validity in 3 Centers. Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Conway KP. Genetic epidemiology of substance use disorders. In: Charney DS, Nester EJ, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 2009. pp. 786–800. [Google Scholar]
- Merikangas KR, Conway KP. Genetic Epidemiology of Substance Use Disorders. In: Charney DS, Nester EJ, editors. Neurobiology of Mental Illness. 3 2008. [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Familial Transmission of Substance Use Disorders. Arch Gen Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Avenevoli S. Implications of genetic epidemiology for the prevention of substance use disorders. Addictive Behaviors. 2000;25:807–820. doi: 10.1016/s0306-4603(00)00129-5. [DOI] [PubMed] [Google Scholar]
- Miele GM, Carpenter KM, Cockerham MS, Trautman KD, Blaine J, Hasin DS. Substance Dependence Severity Scale (SDSS): reliability and validity of a clinician-administered interview for DSM-IV substance use disorders. Drug and Alcohol Dependence. 2000;59:63–75. doi: 10.1016/s0376-8716(99)00111-8. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Genetic and environmental influences on antisocial behaviors: evidence from behavioral-genetic research. Adv Genet. 2005;55:41–104. doi: 10.1016/S0065-2660(05)55003-X. [DOI] [PubMed] [Google Scholar]
- Mustanski BS, Viken RJ, Kaprio J, Rose RJ. Genetic influences on the association between personality risk factors and alcohol use and abuse. J Abnorm Psychol. 2003;112:282–289. doi: 10.1037/0021-843x.112.2.282. [DOI] [PubMed] [Google Scholar]
- Neale MC, Aggen SH, Maes HH, Kubarych TS, Schmitt JE. Methodological issues in the assessment of substance use phenotypes. Addictive Behaviors. 2006;31:1010–1034. doi: 10.1016/j.addbeh.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Neale M, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht; Boston: Kluwer Academic Publishers; 1992. [Google Scholar]
- Newcomb MD, Galaif ER, Locke TF. Substance use diagnoses within a community sample of adults: Distinction, comorbidity, and progression over time. Professional Psychology: Research and Practice. 2001;32:239–247. [Google Scholar]
- O’Brien CP. Research advances in the understanding and treatment of addiction. American Journal on Addictions. 2003;12:S36–S47. [PubMed] [Google Scholar]
- Patkar AA, Mannelli P, Peindl K, Hill KP, Wu LT, Lee T, Kuhn C. Relationship of the serotonin transporter with prolactin response to meta-chlorophenylpiperazine in cocaine dependence. J Psychiatr Res. 2008;42:1213–1219. doi: 10.1016/j.jpsychires.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Poduska JM, Kellam SG, Wang W, Brown CH, Ialongo NS, Toyinbo P. Impact of the Good Behavior Game, a universal classroom-based behavior intervention, on young adult service use for problems with emotions, behavior, or drugs or alcohol. Drug Alcohol Depend. 2008;95(Suppl 1):S29–S44. doi: 10.1016/j.drugalcdep.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich T, Cloninger CR, Van EP, Rice JP, Mullaney J. Secular trends in the familial transmission of alcoholism. Alcohol Clin Exp Res. 1988;12:458–464. doi: 10.1111/j.1530-0277.1988.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Riley BB, Conrad KJ, Bezruczko N, Dennis ML. Relative precision, efficiency and construct validity of different starting and stopping rules for a computerized adaptive test: the GAIN substance problem scale. J Appl Meas. 2007;8 [PMC free article] [PubMed] [Google Scholar]
- Romer D, Walker EF. Adolscent psychopathology and the developing brain: Integrating brain and prevention science. Oxford University Press; New York: 2007. [Google Scholar]
- Saha TD, Stinson FS, Grant BF. The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Depend. 2007;89:82–92. doi: 10.1016/j.drugalcdep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon AJ, Oreskovich MR, Brkanac Z. Genetic determinants of addiction to opioids and cocaine. Harv Rev Psychiatry. 2005;13:218–232. doi: 10.1080/10673220500243364. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–14. [PubMed] [Google Scholar]
- Schuckit MA, Goodwin DA, Winokur G. A study of alcoholism in half siblings. Am J Psychiatry. 1972;128:1132–1136. doi: 10.1176/ajp.128.9.1132. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Wood MD. Personality and substance use disorders: a prospective study. J Consult Clin Psychol. 2000;68:818–829. [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol Dependence Syndrome - Measurement and Validation. Journal of Abnormal Psychology. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Madden PA, Bucholz KK, Statham DJ, Martin NG. Personality and the genetic risk for alcohol dependence. J Abnorm Psychol. 2002;111:124–133. [PubMed] [Google Scholar]
- Stockwell T, Hodgson R, Edwards G, Taylor C, Rankin H. Development of A Questionnaire to Measure Severity of Alcohol Dependence. British Journal of Addiction. 1979;74:79–87. doi: 10.1111/j.1360-0443.1979.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Strong DR, Brown RA, Ramsey SE, Myers MG. Nicotine dependence measures among adolescents with psychiatric disorders: evaluating symptom expression as a function of dependence severity. Nicotine Tob Res. 2003a;5:735–746. doi: 10.1080/1462220031000158609. [DOI] [PubMed] [Google Scholar]
- Strong DR, Kahler CW, Abrantes AM, MacPherson L, Myers MG, Ramsey SE, Brown RA. Nicotine dependence symptoms among adolescents with psychiatric disorders: using a Rasch model to evaluate symptom expression across time. Nicotine Tob Res. 2007;9:557–569. doi: 10.1080/14622200701239563. [DOI] [PubMed] [Google Scholar]
- Strong DR, Kahler CW, Colby SM, Griesler PC, Kandel D. Linking measures of adolescent nicotine dependence to a common latent continuum. Drug Alcohol Depend. 2009;99:296–308. doi: 10.1016/j.drugalcdep.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong DR, Kahler CW, Ramsey SE, Brown RA. Finding order in the DSM-IV nicotine dependence syndrome: a Rasch analysis. Drug Alcohol Depend. 2003b;72:151–162. doi: 10.1016/s0376-8716(03)00201-1. [DOI] [PubMed] [Google Scholar]
- Sutherland G, Edwards G, Taylor C, Phillips G, Gossop M, Brady R. The Measurement of Opiate Dependence. British Journal of Addiction. 1986;81:485–494. doi: 10.1111/j.1360-0443.1986.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Conway KP, Rounsaville BJ, Merikangas KR. Are personality traits familial risk factors for substance use disorders?. Results of a controlled family study. Am J Psychiatry. 2002;159:1760–1766. doi: 10.1176/appi.ajp.159.10.1760. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug Alcohol Depend. 2004;73:121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Laird SB, Kabene M, Bukstein O, Kaminer Y. Drug-Abuse Severity in Adolescents Is Associated with Magnitude of Deviation in Temperament Traits. British Journal of Addiction. 1990;85:1501–1504. doi: 10.1111/j.1360-0443.1990.tb01636.x. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Vanyukov M. Theoretical and operational framework for research into the etiology of substance use disorders. Journal of Child and Adolescent Substance Abuse. 1991;10:1–12. [Google Scholar]
- Tarter RE, Vanyukov M. Alcoholism: a developmental disorder. J Consult Clin Psychol. 1994;62:1096–1107. doi: 10.1037//0022-006x.62.6.1096. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Vanyukov M, Kirisci L, Reynolds M, Clark DB. Predictors of marijuana use in adolescents before and after licit drug use: examination of the gateway hypothesis. Am J Psychiatry. 2006;163:2134–2140. doi: 10.1176/ajp.2006.163.12.2134. [DOI] [PubMed] [Google Scholar]
- Thomas DC. Statistical Methods in Genetic Epidemiology. Oxford Univ. Press; 2004. [Google Scholar]
- Todorov AA, Suarez BK. Liability model. In: Elston R, Olson J, Palmer L, editors. Biostatistical Genetics and Genetic Epidemiology. New York, NY: John Wiley & Sons; 2002. pp. 430–435. [Google Scholar]
- Topp L, Mattick RP. Validation of the amphetamine dependence syndrome and the SAmDQ. Addiction. 1997;92:151–162. [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. Am J Med Genet. 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Molecular genetics of substance abuse vulnerability: Remarkable recent convergence of genome scan results. NEW YORK ACAD SCIENCES; NEW YORK: 2004. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Liu QR, Johnson C, Walther D, Komiyama T, Harano M, Sekine Y, Inada T, Ozaki N, Iyo M, Iwata N, Yamada M, Sora I, Chen CK, Liu HC, Ujike H, Lin SK. Genome-wide association for methamphetamine dependence: convergent results from 2 samples. Arch Gen Psychiatry. 2008;65:345–355. doi: 10.1001/archpsyc.65.3.345. [DOI] [PubMed] [Google Scholar]
- Vanyukov M, Kirisci L, Moss L, Tarter RE, Reynolds M, Maher BS, Kirillova GP, Ridenour T, Clark DB. Measurement of the risk for substance use disorders: Phenotypic and genetic analysis of an index of common liability. Behav Genet. 2009;39:233–244. doi: 10.1007/s10519-009-9269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyukov MM, Kirisci L, Tarter RE, Simkevitz HF, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 2. A measurement approach. Neurosci Biobehav Rev. 2003a;27:517–526. doi: 10.1016/j.neubiorev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Maher BS, Devlin B, Kirillova GP, Kirisci L, Yu LM, Ferrell RE. The MAOA promoter polymorphism, disruptive behavior disorders, and early onset substance use disorder: gene-environment interaction. Psychiatr Genet. 2007;17:323–332. doi: 10.1097/YPG.0b013e32811f6691. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE. Genetic studies of substance abuse. Drug and Alcohol Dependence. 2000;59:101–123. doi: 10.1016/s0376-8716(99)00109-x. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neurosci Biobehav Rev. 2003b;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. Journal of Clinical Investigation. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Molecular psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winokur G, Reich T, Rimmer J, Pitts FN., Jr Alcoholism.3 Diagnosis and familial psychiatric illness in 259 alcoholic probands. Arch Gen Psychiatry. 1970;23:104–111. doi: 10.1001/archpsyc.1970.01750020008002. [DOI] [PubMed] [Google Scholar]
- Wu LT, Pan JJ, Blazer DG, Tai B, Stitzer ML, Brooner RK, Woody GE, Patkar AA, Blaine JD. An item response theory modeling of alcohol and marijuana dependences: a National Drug Abuse Treatment Clinical Trials Network study. J Stud Alcohol Drugs. 2009;70:414–425. doi: 10.15288/jsad.2009.70.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am J Med Genet. 2000;96:684–695. [PubMed] [Google Scholar]