Abstract
Purpose
Several studies have examined the association between diabetes mellitus (DM) and prostate cancer (PCa) risk and progression, however nearly all of these studies have compared diabetic vs. non-diabetic men. We sought to investigate the role of glycemic control, as measured by HbA1c, on PCa aggressiveness and prognosis in men with DM and PCa from the Shared Equal Access Regional Cancer Hospital (SEARCH) database.
Methods
We identified 247 men in SEARCH with DM and a recorded HbA1c value twelve months prior to radical prostatectomy between 1988 and 2009. We divided men into tertiles by HbA1c level. The association between HbA1c tertiles and risk of adverse pathology and biochemical recurrence were tested using multivariate logistic regression and Cox proportional hazards models, respectively.
Results
Median HbA1c level was 6.9. On multivariate analysis, HbA1c tertiles were predictive of pathological Gleason score (p-trend=0.001). Relative to the first tertile, men in the second (OR 5.90, p=0.002) and third tertile (OR 7.15, p=0.001) were more likely to have Gleason score ≥ 4+3. HbA1c tertiles were not associated with margin status, node status, extracapsular extension or seminal vesicle invasion (all p-trend>0.2). In the multivariate Cox proportional hazards model, increasing HbA1c tertiles were not significantly related to risk of biochemical recurrence (p-trend=0.56).
Conclusion
Men with higher HbA1c levels presented with more biologically aggressive prostate tumors at radical prostatectomy. Although risk of recurrence was unrelated to HbA1c levels, further studies are needed to better explore the importance of glycemic control on long-term outcomes in diabetic men with PCa.
Keywords: prostate cancer, diabetes, hba1c
Introduction
Diabetes mellitus (DM) is one of the most prevalent chronic diseases in the United States, affecting nearly 24 million Americans, or 7.8 percent of the population.(1) A growing body of epidemiology literature suggests that DM increases the risk of various malignancies,(2) with a particular emphasis on pancreatic,(3) bladder,(4) endometrial,(5) breast,(6) and colorectal cancer.(7) Meanwhile, the association between DM and risk of prostate cancer (PCa) – the most common malignancy in American men(8) – appears to exhibit an opposite trend. Two recent meta-analyses suggest an inverse relationship between DM and PCa risk, with diabetic men having a lower risk of developing PCa than their non-diabetic peers.(9,10) In two separate studies, Basara et al. and Baradaran et al. observed the same trend, with a greater reduction of PCa risk in men with longer standing DM diagnoses.(11,12)
Equally important is the question of whether DM is associated with PCa aggressiveness and prognosis. A recent study of the SEER population found no association between DM and disease aggressiveness, which included pathological Gleason score and local vs. regional disease.(13) Similarly, a CaPSURE study found no association between DM diagnosis and either disease aggressiveness or biochemical recurrence after radical prostatectomy (RP).(14) Contrarily, a prior study from the SEARCH database found that while DM was not associated with biochemical recurrence after RP, men with DM had over a 70 percent higher risk of high-grade disease (OR 1.73, IQR 1.22–2.45, p=0.002) and seminal vesicle invasion (OR 1.73, IQR 1.04–2.90, p=0.04) compared to men without DM.(15)
While several studies of PCa risk and prognosis among diabetic vs. non-diabetic men exist in the literature, there is a relative shortage of information on the association between PCa risk and prognosis within a cohort of diabetic men. This is perhaps a more compelling research question, as it is reasonable to suggest that prostate tumors that arise despite the protective milieu afforded by DM possess unique clinical features. In short, we are aware of only one study that has investigated the severity of DM – using HbA1c as a proxy – on the aggressiveness of prostate cancer. Hong et al. compared pre-RP HbA1c levels with several measures of disease aggressiveness and observed that elevated HbA1c predicted higher pathological Gleason score and extraprostatic tumor extension even after adjusting for body mass index (BMI).(16) This study was limited, however, in its small sample size (n=89) and atypically homogeneous population of South Korean men. Moreover, the study did not address the clinical outcome of disease recurrence. We therefore sought to validate these findings in the SEARCH database, utilizing a larger number of subjects from a more diverse population. To our knowledge, this is the first study to test the association between HbA1c level and PCa progression.
Methods
Study Population
After obtaining institutional review board approval from each institution, data were collected from 2,123 men who underwent RP between 1988 and 2009 at four Veterans Affairs Medical Centers (West Los Angeles and Palo Alto, CA; Augusta, GA; Durham, NC) and compiled into the SEARCH database. This database excludes men who received pre-operative hormone therapy or radiation therapy. Within this population, we identified a cohort of 349 men with a confirmed diagnosis of DM at the time of RP. Data on the diabetic status of study subjects were ascertained from medical records and based on clinical diagnosis from an evaluating physician. Of these men, 247 had a recorded HbA1c level within the twelve months prior to undergoing surgery.
Clinical and Pathological Variables
We divided the 247 men in our cohort into tertiles by HbA1c level, with limits of <6.3, 6.3–7.7, and ≥ 7.8. Metformin usage was obtained from medical records and determined from the year preceding surgery. Patient age and year of surgery were defined at the time of RP and evaluated as continuous variables. BMI was abstracted from pre-operative height and weight. Center and race were defined as categorical variables, with race including white, black, and other. Prostate-specific antigen (PSA) (ng/mL) values were obtained from the measurement closest to the date of, but preceding RP. Extracapsular extension, surgical margin status, seminal vesicle invasion, and lymph node metastasis were determined at the time of surgery. Gleason score was obtained from the surgical specimen and placed into one of three categories: ≤ 6, 3+4, and ≥ 4+3. Biochemical recurrence was defined as a single PSA value >0.2ng/mL, two values of 0.2ng/mL, or secondary treatment for an elevated post-operative PSA.
Statistical Analysis
The Stata® 10 software package (StataCorp; College Station, TX) was used for all statistical analysis. The distribution of demographic and clinicopathological characteristics among the HbA1c tertiles was performed using x2, ANOVA, and Kruskal-Wallis test, as appropriate. Normally-distributed variables were described using means with standard deviations, and non-normally-distributed variables were described using medians with interquartile ranges. All P values were 2-sided with a threshold of p < 0.05 for statistical significance. The association between HbA1c tertiles and risk of binary adverse pathological features (Gleason ≥ 4+3; extracapsular extension, seminal vesicle invasion, and lymph node positivity) and biochemical recurrence were tested using a multivariate logistic regression and multivariate Cox proportional hazards model, respectively. Controlling variables included age, race, center, BMI, PSA, and metformin usage. P-trends were calculated by assigning all members of a HbA1c tertile the median HbA1c value of that respective tertile. All regression analyses were then recalculated with HbA1c as a continuous variable.
Results
The median HbA1c level for the entire cohort of men was 6.9 (IQR: 6.1–8.1). Mean age was 62 ± 6 years. The majority of men were black (48%) or white (44%), with a small minority of other races (8%). Mean BMI was 30.1 ± 4.5 kg/m2.
On univariate analysis (Table 1), HbA1c tertiles were not associated with the pre-operative characteristics of age, race, center, BMI or PSA (all p>0.1). Men in the third HbA1c tertile underwent RP earlier (2002) than men in the first two tertiles (2004) (p=0.006). On examination of post-operative characteristics, increasing HbA1c tertiles were associated with a higher pathological Gleason score: 11 percent of men in the first tertile had ≥ 4+3 disease, compared to 28 percent of men in the second tertile and 31 percent of men in the third tertile (p=0.006). HbA1c tertiles were not associated with surgical margin status, seminal vesicle invasion, or lymph node metastasis (all p>0.2). There was a non-significant trend (p=0.14) towards extracapsular extension with increasing HbA1c tertile, with 16, 25, and 29 percent of men in the tertiles, respectively, having extracapsular extension of tumor.
Table 1.
Pre-operative and post-operative characteristics of diabetic men in the SEARCH Database, sorted by HbA1c level tertiles.
| HbA1c < 6.3 | 6.3 ≥ HbA1c < 7.8 | HbA1c ≥ 7.8 | p | |
|---|---|---|---|---|
| Number of Patients (247) | 82 | 81 | 84 | |
| HbA1c | ||||
| Median (IQR) | 5.9 (5.6–6.1) | 6.9 (6.6–7.2) | 8.9 (8.1–9.9) | |
| Age | 0.61† | |||
| Mean (SD) | 62 (±6) | 62 (±6) | 62 (±6) | |
| Year | 0.006† | |||
| Median (IQR) | 2004 (02–06) | 2004 (01–07) | 2002 (98–06) | |
| Race (n=246) | 0.19 | |||
| White | 37 (46%) | 43 (53%) | 29 (34%) | |
| Black | 38 (47%) | 33 (41%) | 46 (55%) | |
| Other | 6 (7%) | 5 (6%) | 9 (11%) | |
| Center | 0.28 | |||
| 1 | 14 (17%) | 12 (15%) | 14 (17%) | |
| 2 | 9 (11%) | 14 (17%) | 17 (20%) | |
| 3 | 7 (9%) | 15 (19%) | 12 (14%) | |
| 4 | 52 (63%) | 40 (49%) | 41 (49%) | |
| BMI (kg/m2) (n=230) | 0.40† | |||
| Mean (SD) | 29.5 (±4.2) | 30.5 (±4.3) | 30.3 (±5.1) | |
| PSA (ng/mL) (n=245) | 0.76‡ | |||
| Median (IQR) | 6.3 (4.8–9.3) | 6.1 (4.5–9.5) | 6.2 (4.7–9.2) | |
| Metformin | 0.84 | |||
| Yes | 45 (55%) | 48 (59%) | 47 (56%) | |
| No | 37 (45%) | 33 (41%) | 37 (44%) | |
| Biopsy Gleason Score | 0.06 | |||
| < 4+3 | 71 (87%) | 67 (83%) | 61 (73%) | |
| ≥ 4+3 | 11 (13%0 | 14 (17%) | 23 (27%) | |
| Pathological Gleason Score | 0.006 | |||
| ≤ 6 | 25 (30%) | 29 (36%) | 25 (30%) | |
| 3+4 | 48 (59%) | 29 (36%) | 33 (29%) | |
| ≥ 4+3 | 9 (11%) | 23 (28%) | 26 (31%) | |
| Extracapsular Ext. (n=245) | 0.14 | |||
| Negative | 68 (84%) | 61 (75%) | 59 (71%) | |
| Positive | 13 (16%) | 20 (25%) | 24 (29%) | |
| Surgical Margins | 0.99 | |||
| Negative | 42 (51%) | 41 (51%) | 43 (51%) | |
| Positive | 40 (49%) | 40 (49%) | 41 (49%) | |
| Seminal Vesicle Invasion | 0.98 | |||
| Negative | 73 (89%) | 72 (89%) | 74 (88%) | |
| Positive | 9 (11%) | 9 (11%) | 10 (12%) | |
| Lymph Node Status | 0.29 | |||
| Negative | 48 (59%) | 48 (59%) | 55 (65%) | |
| Positive | 0 (0%) | 2 (2%) | 0 (0%) | |
| Unknown | 34 (41%) | 31 (38%) | 29 (35%) | |
All analyses are x2 unless otherwise noted:
ANOVA
Kruskal-Wallis
On multivariate analysis (Table 2), HbA1c tertiles were an independent predictor of higher pathological Gleason score (p-trend=0.001) (Table 2). Relative to men in the first tertile, men in the second tertile (OR 4.68, 95% CI 1.68–13.04) and third tertile (OR 6.60, 95% CI 2.28–19.08) were significantly more likely to have a pathological Gleason score ≥ 4+3. In additional analyses, increasing HbA1c tertiles were not significantly associated with extracapsular extension, surgical margin status, seminal vesicle invasion, or lymph node metastasis (all p-trend>0.2).
Table 2.
Multivariate adjusted association between HbA1c tertiles and pathological disease characteristics.
| HbA1c Logistic | OR | 95% CI | P* | P-trend | |
|---|---|---|---|---|---|
| Pathological Gleason ≥ 4+3 | 0.001 | ||||
| Tertile 2 (relative to Tertile 1) | 4.68 | 1.68 | 13.04 | 0.003 | |
| Tertile 3 (relative to Tertile 1) | 6.60 | 2.28 | 19.08 | <0.001 | |
| Extracapsular Extension | 0.26 | ||||
| Tertile 2 | 1.41 | 0.55 | 3.65 | 0.48 | |
| Tertile 3 | 1.76 | 0.69 | 4.45 | 0.24 | |
| Positive Surgical Margin | 0.99 | ||||
| Tertile 2 | 1.10 | 0.52 | 2.33 | 0.81 | |
| Tertile 3 | 1.01 | 0.47 | 2.21 | 0.97 | |
| Seminal Vesicle Invasion | 0.66 | ||||
| Tertile 2 | 0.37 | 0.09 | 1.48 | 0.16 | |
| Tertile 3 | 0.58 | 0.15 | 2.28 | 0.44 | |
| Lymph Node Metastasis | 0.37 | ||||
| Tertile 2 | 1.86 | 0.83 | 4.16 | 0.13 | |
| Tertile 3 | 1.58 | 0.68 | 3.68 | 0.29 | |
adjusted for age, year, center, race, BMI, PSA, and metformin usage.
Overall, HbA1c tertiles were not significantly associated with time to biochemical recurrence (log-rank, p=0.39, Figure 1). After adjusting for multiple pre-operative features, HbA1c tertiles remained not significantly associated with biochemical recurrence (p-trend=0.56). When examined individually, relative to the first tertile, men in the second (HR=1.46, CI=0.81–2.62, p=0.21) and third tertiles (HR=1.28, CI=0.68–2.38, p=0.45) had similar risks of biochemical recurrence.
Figure 1.
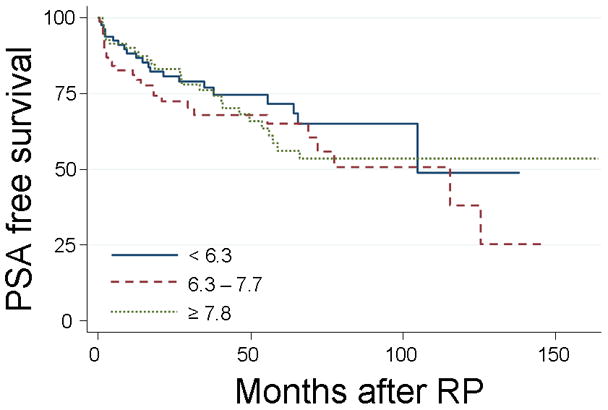
Actuarial Kaplan-Meier estimates of biochemical recurrence rates of patients treated with radical prostaectomy segregated by HbA1c tertiles (%). Log-rank, p=0.39.
Discussion
Although DM has been implicated as a risk factor for several malignancies,(2) studies suggest that men with DM have a reduced risk of developing PCa.(9,10,12) A number of theories exist to explain this protective phenomenon, but the most prevalent argues that DM may lower PCa risk through a reduction of essential serum growth factors – insulin, insulin-like growth factor (IGF-1), and testosterone.(17) Decreases in testosterone are accompanied by a concomitant rise in estradiol levels independent of BMI,(17,18) which could explain why DM paradoxically increases the risk of endometrial(5) and breast cancer(6) in women while reducing the risk of prostate cancer in men.
Prior studies on the association between DM and PCa risk and prognosis have compared diabetic vs. non-diabetic men. Given that DM is thought to be protective of PCa, we sought to investigate various clinical and pathological features of PCa occurring in diabetic men only, as this presented an opportunity to study a potentially unique tumor phenotype. In sum, we investigated the association between HbA1c level and disease aggressiveness and recurrence. We thus posed the question: how does glycemic control in diabetic men affect the aggressiveness and prognosis of their PCa?
In our study of 247 men with DM and PCa, we observed that increasing HbA1c levels were significantly associated with a greater risk of pathological Gleason score ≥ 4+3. This association remained after multivariate adjustment. In contrast, we did not find any statistically significant association between HbA1c tertiles and extracapsular extension, positive surgical margins, seminal vesicle invasion, or nodal metastasis. Though given our small sample size we can not rule out a moderate association with these other variables. We cannot explain the statistically significant association between the third HbA1c tertile and later year of RP. Multivariate adjustment for year of surgery, evaluated as either a continuous or categorical variable, did not alter the statistically significant association between increasing HbA1c tertile and higher pathological Gleason score.
These observations partially validate the findings of Hong et al., who performed a similar analysis in a smaller cohort (n=89) of South Korean men with DM and PCa. That study found that men with HbA1c ≥ 6.5 demonstrated a significantly higher rate of pathological Gleason score ≥ 7 and extraprostatic extension of tumor – defined as extracapsular extension, seminal vesicle invasion, or nodal metastasis – compared to men with HbA1c < 6.5.(16) In the present study, we investigated three tertiles of HbA1c levels and found a similar association between increasing HbA1c and higher pathological Gleason score. In contrast to the results presented by Hong et al., however, we did not observe a statistically significant association between HbA1c level and extraprostatic extension of tumor.
Finally, we did not find a significant association between increasing HbA1c tertile and poor prognosis, defined by biochemical recurrence. Although we did note that the hazard ratio for recurrence among men in the higher HbA1c tertiles was greater than 1.0 suggesting a slight increased risk, this observation did not reach statistical significance. Yet again, however, due to our small sample size, we cannot rule out the possibility of a modest, yet clinically relevant, association between increasing HbA1c levels and PCa recurrence. As such, we conclude that further investigation on glycemic control and PCa outcomes is warranted.
We can not fully explain why increasing HbA1c tertiles were associated with higher pathological Gleason score. Given that DM produces an environment unfavorable to the development of PCa, one possible explanation is that prostate tumors that do arise in diabetic men are of a more aggressive phenotype – recapitulating a theme of natural selection. Whether this increased selection pressure comes from reductions in serum testosterone, insulin, or other hormonal influences requires further investigation. If this is hypothesis is accurate, however, it may be the case that the selected tumors may grow even more aggressive due to higher serum glucose levels reflective of poor glycemic control.
There is increasing evidence that DM,(17,18) and more importantly poor glycemic control, is correlated with low serum testosterone. El-Sakka et al. observed that diabetic men with low serum total testosterone were significantly more likely to have HbA1c values >7 than diabetic men with normal total testosterone levels,(19) while Kapoor et al. reported that testosterone replacement therapy significantly reduced HbA1c levels in diabetic men with low testosterone.(20) Moreover, there are a number of reports suggesting that low testosterone may serve as a marker of more aggressive PCa. Hoffman et al. showed that men with low serum free testosterone were significantly more likely to have biopsy Gleason scores ≥ 8 than men with normal free testosterone,(21) and several studies have found that low total testosterone levels are predictive of extraprostatic disease, although not significantly predictive of biochemical recurrence.(22–24)
This study has several limitations. First, like any retrospective analysis, our study relies on a reading of clinical notes and is therefore vulnerable to inaccuracies. This was particularly the case in determining the DM status of our subjects, as this diagnosis is one of clinical judgment by respective physicians. However, there is no reason to think that a man with aggressive PCa would be more likely to have his physician determine that he has DM than a man with non-aggressive PCa. Secondly, the SEARCH database is but a subset of the total population of men with PCa. Men in SEARCH, by the virtue of the fact that they all received RP at a VA medical center, are typically younger and healthier than the average PCa patient. Finally, our study does not include subject data on serum insulin, IGF-1, or testosterone levels, as these data are not part of the SEARCH database.
Conclusion
In men with DM and PCa, increasing HbA1c tertile was significantly associated with higher pathological Gleason score. This observation confirms the findings of Hong et al. in a smaller cohort of South Korean men,(16) although we did not observe a similar association between HbA1c tertile and extraprostatic extension of tumor. Given that these findings have now been validated in an external dataset, with greater power and racial heterogeneity, we recommend further investigation to elucidate the biological mechanisms underlying this phenomenon. We hypothesize that poorly controlled DM, as measured by high HbA1c, produces an environment low in testosterone, thereby inhibiting the growth of low-grade PCa and selectively allowing the growth of more aggressive, high-grade PCa. This selection pressure does not necessarily portend poorer prognosis, although more elaborate studies on outcome are required.
Acknowledgments
Supported by the Department of Veterans Affairs, National Institute of Health R01CA100938 (WJA), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (WJA), the Georgia Cancer Coalition (MKT), the Department of Defense, Prostate Cancer Research Program, (LLB/SJF), and the American Urological Association Foundation/Astellas Rising Star in Urology Award (SJF). Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
References
- 1.Prevention CfDCa. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. 2010:2008. [Google Scholar]
- 2.Mori M, Saitoh S, Takagi S, Obara F, Ohnishi H, Akasaka H, Izumi H, Sakauchi F, Sonoda T, Nagata Y, Shimamoto K. A Review of Cohort Studies on the Association Between History of Diabetes Mellitus and Occurrence of Cancer. Asian Pac J Cancer Prev. 2000;1(4):269–276. [PubMed] [Google Scholar]
- 3.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273(20):1605–1609. [PubMed] [Google Scholar]
- 4.Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia. 2006;49(12):2819–2823. doi: 10.1007/s00125-006-0468-0. [DOI] [PubMed] [Google Scholar]
- 5.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50(7):1365–1374. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121(4):856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 7.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(22):1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 9.Bonovas S, Filioussi K, Tsantes A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia. 2004;47(6):1071–1078. doi: 10.1007/s00125-004-1415-6. [DOI] [PubMed] [Google Scholar]
- 10.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 11.Baradaran N, Ahmadi H, Salem S, Lotfi M, Jahani Y, Mehrsai AR, Pourmand G. The protective effect of diabetes mellitus against prostate cancer: role of sex hormones. Prostate. 2009;69(16):1744–1750. doi: 10.1002/pros.21023. [DOI] [PubMed] [Google Scholar]
- 12.Kasper JS, Liu Y, Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer. 2009;124(6):1398–1403. doi: 10.1002/ijc.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce BL, Plymate S, Ostrander EA, Stanford JL. Diabetes mellitus and prostate cancer risk. Prostate. 2008;68(10):1126–1132. doi: 10.1002/pros.20777. [DOI] [PubMed] [Google Scholar]
- 14.Chan JM, Latini DM, Cowan J, Duchane J, Carroll PR. History of diabetes, clinical features of prostate cancer, and prostate cancer recurrence-data from CaPSURE (United States) Cancer Causes Control. 2005;16(7):789–797. doi: 10.1007/s10552-005-3301-z. [DOI] [PubMed] [Google Scholar]
- 15.Jayachandran J, Aronson WJ, Terris MK, Presti JC, Jr, Amling CL, Kane CJ, Freedland SJ. Diabetes and outcomes after radical prostatectomy: are results affected by obesity and race? Results from the shared equal-access regional cancer hospital database. Cancer Epidemiol Biomarkers Prev. 2010;19(1):9–17. doi: 10.1158/1055-9965.EPI-09-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong SK, Lee ST, Kim SS, Min KE, Byun SS, Cho SY, Choe G, Lee SE. Significance of preoperative HbA1c level in patients with diabetes mellitus and clinically localized prostate cancer. Prostate. 2009;69(8):820–826. doi: 10.1002/pros.20932. [DOI] [PubMed] [Google Scholar]
- 17.Kasper JS, Liu Y, Pollak MN, Rifai N, Giovannucci E. Hormonal profile of diabetic men and the potential link to prostate cancer. Cancer Causes Control. 2008;19(7):703–710. doi: 10.1007/s10552-008-9133-x. [DOI] [PubMed] [Google Scholar]
- 18.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 19.El-Sakka AI, Sayed HM, Tayeb KA. Type 2 diabetes-associated androgen alteration in patients with erectile dysfunction. Int J Androl. 2008;31(6):602–608. doi: 10.1111/j.1365-2605.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- 20.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154(6):899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman MA, DeWolf WC, Morgentaler A. Is low serum free testosterone a marker for high grade prostate cancer? J Urol. 2000;163(3):824–827. [PubMed] [Google Scholar]
- 22.Massengill JC, Sun L, Moul JW, Wu H, McLeod DG, Amling C, Lance R, Foley J, Sexton W, Kusuda L, Chung A, Soderdahl D, Donahue T. Pretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomy. J Urol. 2003;169(5):1670–1675. doi: 10.1097/01.ju.0000062674.43964.d0. [DOI] [PubMed] [Google Scholar]
- 23.Isom-Batz G, Bianco FJ, Jr, Kattan MW, Mulhall JP, Lilja H, Eastham JA. Testosterone as a predictor of pathological stage in clinically localized prostate cancer. J Urol. 2005;173(6):1935–1937. doi: 10.1097/01.ju.0000158040.33531.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imamoto T, Suzuki H, Fukasawa S, Shimbo M, Inahara M, Komiya A, Ueda T, Shiraishi T, Ichikawa T. Pretreatment serum testosterone level as a predictive factor of pathological stage in localized prostate cancer patients treated with radical prostatectomy. Eur Urol. 2005;47(3):308–312. doi: 10.1016/j.eururo.2004.11.003. [DOI] [PubMed] [Google Scholar]


