Abstract
Pictorial representations of specific environments related to smoking can evoke robust craving to smoke, even in the absence of any proximal cues to smoke (e.g., cigarettes, lighters.) To evaluate the salience of smoking environment cues, we developed a novel procedure for bringing smokers’ real world smoking and nonsmoking environments into the laboratory to compare them with standard (i.e. not personalized) environments within a cue-reactivity paradigm. Seventy-two smokers used digital cameras to take pictures of the environments in which they do and do not smoke. They then completed a cue-reactivity session during which they viewed and rated pictures of smoking and nonsmoking environments, half personal and half standard, all devoid of proximal smoking cues. As hypothesized, personal environments led to a significantly larger smoking-nonsmoking difference in craving, compared with the standard environments. Personalization also enhanced stimuli vividness, relevance, positive affect, and excitement, as well as heart rate changes from baseline. Implications of these findings for exposure-based research and treatment for addiction, as well as other psychological disorders, are discussed.
Keywords: tobacco, cue reactivity, context, conditioning
1. Introduction
Research on human behavior generally has to choose between assessment in the natural environment, which can improve the generalizability of findings but limit experimental control, and assessment in the laboratory, which allows for much greater control but reduces real world generalizability (Anderson, Lindsay, & Bushman, 1999). Recent innovations have brought more control to assessments in the natural environment, such as the replacement of retrospective questionnaires with the use of electronic diaries (Shiffman, Ferguson, Gwaltney, Balabanis, & Shadel, 2006.) However, this approach still, by design, leaves many extraneous factors uncontrolled, as subjects self-select the timing and duration of their exposure and engage in a variety of other behaviors concurrent with completion of responses. As an alternative to conducting research in the real world where “noise” inevitably interferes, the present study was aimed at developing a method for bringing the real world into the laboratory where controlled exposure and assessment can both be realized.
To develop a novel real world/laboratory exposure paradigm, we used craving responses to smoking-related stimuli, as the model clinical behavior. Our first step involved comparing smokers’ cue reactivity to pictorial smoking and nonsmoking proximal cues to distal environment cues (Conklin, Robin, Perkins, Salkeld, & McClernon, 2008). Proximal cues can be conceptualized as cues that are integral to actual smoking behavior (e.g., ashtray, cigarette). Distal cues are not inherently linked to actual drug administration, but over repeated drug use in their presence they can come to elicit similar reactivity to that produced by proximal smoking cues. In our first study, proximal cues depicted smoking items on a completely neutral background. Smoking and nonsmoking environment cues (e.g., a bar, a church; respectively) showed real world places that were not personally familiar to subjects but were identified in a pilot study to be strongly associated with smoking or not smoking (Conklin et al., 2008). Environment cues were completely devoid of any proximal cues to smoke. This work demonstrated that smokers respond to standard smoking-related environment cues, independent of proximal cues, with robust craving. Although smokers were highly reactive to both types of smoking cues, the levels of reactivity generated by the standard smoking environment cues was significantly less than that evoked by proximal smoking cues. This was true not just of craving, but also of smokers’ self-report of the vividness and relevance of the standard smoking and nonsmoking environment cues.
The main goal of personalizing cues is to more closely capture what smokers encounter in the real world. Therefore, the finding from the first study (Conklin et al., 2008), that environments led to less craving than proximal cues and were less vivid and relevant, suggests that we may not have achieved that goal. It may be the case that environments will always produce less craving because they are less reliably linked to smoking than proximal cues; however, the lower ratings of vividness and relevance may also be a key to why environment cues functioned less well. Unlike standard proximal cues, which are salient for smokers regardless of how similar they are to an individual’s own smoking paraphernalia (Conklin & Tiffany, 2001), environments might not generalize as easily. More specifically, whereas a generic cigarette burning in any ashtray may work as well to evoke craving as an individual’s own cigarette and ashtray, a random bar might not be as effective in evoking a response as one’s favorite and highly frequented bar.
In theory, the best way to personalize environments is to physically take individuals to the actual environments and conduct exposure there. This is often done as part of in vivo exposure therapy with anxiety patients. However, even for anxiety disorders this method has proven hard to implement (Cook, Schnurr, & Foa, 2004), and it has proven even more difficult with addiction. Researchers attempting this technique with opiate dependent individuals by providing therapist-accompanied live exposure within each patients’ drug-related environments met with considerable time constraints, safety concerns, and difficulty controlling extraneous factors (i.e., Dawe et al., 1993, Kasvikis, Bradley, Powell, Marks & Gray, 1991.) They concluded that these problems may make live-exposure a largely unviable technique. This might be doubly true with smoking and other clinical presentations in which the target behavior manifests itself across a large number of environments. Hence, our interest in developing a method for bringing a patient’s real world environments into the laboratory or clinic, where safe and controlled assessments or treatments can be conducted.
To better capture personal environments we designed a method for having smokers take pictures, using borrowed digital cameras, of the actual places in which they do and do not smoke. Pictures allow for quick stimulus presentation (compared to other methods such as imagery and in vivo cues) and they allow for a more exact representation of the environment cues we are interested in studying. Using this technique, the present study aimed to test the impact of personalized environment cues on smokers’ cue-reactivity, in the absence of proximal cues to smoke. We expected smoking environments, through their association with past episodes of actual smoking behavior, to produce increases in self-reported craving and heart rate (HR) relative to nonsmoking environments (i.e., main effect of cue.) We also expected personal smoking environments to evoke stronger reactivity than standard smoking environments (i.e., main effect of source).
2. Method
2.1 Participants
Newspaper advertisements and flyers were used to recruit 72 smokers (36 men and 36 women) for the study. Advertisements invited, “healthy men and women smokers, ages 20-65 [ to participate in] a research study investigating smoking cues.” The age range was restricted to 20-65 and the number of cigarettes per day to greater than 15 for the purpose of guarding against a primarily undergraduate student sample of non-dependent or mildly dependent smokers. Participants were on average 28.86 years old (SD = 12.2; range 20-58) and smoked 20.72 cigaretes per day (SD= 5.28; range = 15-40). Additionally, they had to have been smoking regularly for at least a year (M = 15.47 years; SD = 12.13; range = 3-45), and have a carbon monoxide (CO) concentration > 8 ppm (M = 24.4, SD = 12.25). Participants had an average Fagerström Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker & Fagerström, 1991) score of 5.7 (SD = 1.8; range = 2-10). Each participant was paid $100 for completing the study.
2.2Procedure
Session 1
The first session lasted approximately 90 minutes. After signing initial consent forms and registering time since last cigarette, a CO measure was taken using a Vitalograph CO monitor (Vitalograph; Lenexa, KS). Participants then completed several forms a Smoking History Form, the Fagerström Test of Nicotine Dependence scale, and the Balanced Inventory of Desired Responding-Impression Management section (BIDR-IM; Paulhus, 1991.) The BIDR is a factor-analytically derived questionnaire of the extent to which an individual engages in conscious dissimilation of responses in an attempt to create favorable responses. Inclusion in the current study allowed for investigation of possible associations between impression management and participants’ answers on self-report measures.
Participants then listed and ranked ten environments in which they most often smoke during a typical week, as well as ten environments in which they do not. A semi-structured interview was conducted to identify the participant’s top 4 smoking and 4 nonsmoking environments. Smoking environments had to be places in which the individual spent time at least once a week, smoked at least 7 out of 10 times when in that environment (mean = 9.32), found the difficulty of not smoking in that environment to be at least a 5 (0-10 scale; mean = 8.27), and reported feeling negatively if smoking could not occur in that place. Nonsmoking environments also had to be places in which the individual spent time at least once a week. Smoking could only occur 3 or less times out of 10 times in that place (mean = 0.33 out of 10 times.) The strength of thinking about smoking in that place had to be 5 or less on a 0-10 scale (mean = 2.23), and difficulty of refraining from smoking in that place had to be 5 or less on a 0-10 scale (mean = 1.54). For nonsmoking environments, we chose to include places in which only limited smoking occurred, rather than no smoking, due to the difficulty of finding participants who could identify four places where they regularly go and absolutely never smoke. Participants took pictures of their top 4 smoking and 4 nonsmoking environments, but only 3 of each were used. This was done to create a buffer in the event that one set of pictures was not clear enough, or could not be taken by the subject.
Once the environments were selected, participants went through picture-taking training using the experiment room as a sample environment. Prior to leaving, participants received an Olympus Camedia D-390 digital camera to borrow for picture taking (Olympus Optical Co., Ltd; Tokyo, Japan) as well as a written reminder of the environments of which to take pictures and the time of the next session.
Session 2
The second session was scheduled approximately one week after the first (M= 6.9 days [SD 4.7]) to give the participant an adequate amount of time to take pictures. The participant returned with the digital camera, which held the pictures of the environments, and supplied a CO measurement aimed at capturing typical mid-day smoking exposure. The experimenter scheduled the participant’s third session and told him/her to remain abstinent from smoking for 6 hours prior to coming in. This was done to equate exposure to last cigarette across participants and to allow for comparison with our earlier study examining cue reactivity to the standard environment cues following brief deprivation (Conklin et al., 2008.)
Session 3
The third session lasted approximately 2 hours and took place approximately one week after the second session (M = 6.8 days [SD 2.7].) This time was needed to edit subjects’ pictures (e.g., remove any proximal smoking cues, people, or alcohol from the pictures) and set up the individual Powerpoint file for stimuli presentation. To verify 6-hour deprivation from smoking, the participant gave a CO sample, which had to be at least a 50% reduction from the highest CO measurement obtained from either of the previous two sessions. Means for each session were as follows: Session 1: M = 24.44 (SD 12.25); Session 2: M =22.03 (SD 14.73); Session 3: M=10.00 (SD 5.36). Subjects who did not meet the CO criteria were rescheduled once. All rescheduled subjects met CO criteria. Next, participants completed two self-report ratings: The four-item Questionnaire of Smoking Urges (QSU-4; Carter & Tiffany, 2001) and the Diener and Emmons Mood Form (1984). The experimenter then placed the heart rate pulse plethysmograph on the participant’s index finger. The participant was given an overview of the remainder of the session, including being instructed to focus intently on being in each environment that appeared on the screen during the picture trials.
After the instructions, the participant completed a practice trial to ensure that he/she could follow the automated cue-reactivity procedure. The experimenter then left the room and the 12 cue-exposure picture trials began. Each trial followed a standard format: 20 seconds relaxation, 20 seconds baseline, 40 seconds picture viewing (10 seconds for each of the 4 angles within a picture set), and post-trial subjective ratings. The presentation of the pictorial stimuli was controlled by Microsoft PowerPoint 2000 (Microsoft Corporation; Redwood, WA) software on a Compaq Evo computer (Hewlett Packard Company; Palo Alto, CA) and displayed on a 22” monitor (ViewSonic Corporation; Walnut, CA). After the final cue-exposure trial, the experimenter returned to the room, removed the monitors, and debriefed the participant before paying him/her.
2.3 Stimulus Materials
Stimulus materials for session 3 included 12 sets of pictures, with each set depicting one environment shown from four angles. Six picture sets (3 smoking and 3 nonsmoking) were experimenter-generated standard cues created through extensive pilot work. (For full description of stimulus development, please see Conklin et al, 2008.) The other 6 picture sets were the 3 smoking and 3 nonsmoking subject-generated picture sets of environments. Important to note, at the time of the study no public smoking bans were in place in the city of Pittsburgh, PA where the study was conducted.
Order effects were controlled via counterbalancing. We purposefully constrained the randomization to avoid order effects that might occur due to background craving increasing over the course of time. The 12 pictures were counterbalanced across 4 randomizations such that 3 smoking and 3 nonsmoking cues, as well as 3 personal and 3 standard cues had to occur in the first six and last six trials. Additionally, neither a smoking or nonsmoking cue nor a personal or standard cue could occur more than twice in a row. Lastly, each of the four cue types had to occur in each of the 12 positions an equal number of times across the four randomizations.
2.4 Physiological Measures
During cue reactivity, heart rate (HR) was collected using BioPac physiological recording equipment (BioPac Systems; Goleta, CA). Pulse was recorded from a BIOPAC photoelectric pulse plethysmograph transducer attached to the nondominant index finger.
2.5 Post-trial ratings
Following each picture trial, participants answered 12 questions about the environment they just viewed and how they felt while focusing on it. All items were completed on a 0-100 likert-type scale. These included the following: 1) Vividness: One item assessing how vividly the participant was able to focus on the environment, 2) Craving: The 4-item brief Questionnaire on Smoking Urges (Carter & Tiffany, 2001) to measure cigarette craving while viewing that environment, 3) Negative and Positive Affect: A 2-item scale derived from the nine-item Mood Form (Diener & Emmons, 1984) assessing positive and negative affect, 4) Arousal: A two-item scale (Conklin & Tiffany, 2001) measuring the extent to which the participant felt excited and calm. 5) Relevance: A four-item scale (Conklin & Tiffany, 2001; Conklin et al, 2008) measuring how relevant the stimulus was for the participant. Past research has demonstrated the utility of these measures to capture changes as a function of exposure to smoking-related cues (e.g.,Conklin & Tiffany, 2001).
2.6 Data Reduction and Analyses
Using the AcqKnowledge interactive editing program for Windows [Biopac], HR data was visually inspected and artifacts were removed. The peak pulse waves collected via photoplethysmography were automatically detected and converted to heart rate beats per minute. Deviation scores were computed for each trial by subtracting combined data from the stimuli presentation periods (computed from the average of seconds 4 – 36 of the 40 second picture trial) from the average of seconds 6-14 of the baseline period for each trial.
The overall data analytic strategy focused on the impact of picture source (standard, personal) and cue (smoking, nonsmoking) on self-report and physiological indices of reactivity using a 2 (Source) × 2 (Cue) within-subject repeated measures analysis of variance (ANOVA). Simple effects were investigated with pairwise comparisons (p<.05). Additionally, correlational analyses were first conducted to examine possible associations between self-report measures and trait scales of impression management (BIDR-IM) and nicotine dependence (FTND).
3. Results
3.1 Correlational Analyses
Correlations were conducted between self-report measures and the BIDR-IM (impression management scale) to examine if a tendency to engage in impression management, which could bias subjective reporting, was associated with self-report responding. Unlike our previous study in which high impression management scores were associated with lower self-reported craving (Conklin et al., 2008), no significant correlations were found between craving self-report and the BIDR-IM (impression management scale) in the present study. However, BIDR-IM score was negatively associated with self-reported negative mood during exposure to nonsmoking environments, r = −.026, p<.05. Being high on impression management was associated with potentially under-reporting the amount of negative affect a participant was experiencing. Because of this, as described below, BIDR-IM score was entered as a covariate in the negative mood ANOVAs. No other significant correlations between any self-report measures and BIDR-IM were found. No significant correlations between self-report measures and FTND (nicotine dependence) were found. As expected vividness and relevance were highly positively correlated for both personal and standard cue conditions, r = .567, p<.001, and r = .796, p <.001; respectively. However, these measure were only correlated with craving under the personal nonsmoking picture conditions, for which negative correlations were revealed, r = −.273, p <.05, and r = −.332, p <.01, for vividness and relevance respectively.
3.2 Self-report measures
Average post-trial ratings for all self-report measures can be seen in Table 1. Significant source (personal, standard) and cue (smoking and nonsmoking) main effects, as well as significant Source × Cue interactions are noted in Table 1 and are presented below:
Table 1.
Dependent measure means (SD) as a function of Source (Personal, Standard) and Cue (Smoking, Nonsmoking)
| SOURCE | PERSONAL | STANDARD | SIGNIFICANT EFFECTS |
||
|---|---|---|---|---|---|
| CUE | Smoking | Nonsmoking | Smoking | Nonsmoking | S = Source C = Cue I = Interaction |
| Craving | 74.2 (19.7) |
32.3 (22.1) |
65.0 (19.2) |
28.6 (23.1) |
S = p < .001 C = p < .001 I = p < .05 |
|
| |||||
| Vividness | 93.7 (11.2) |
88.9 (15.6) |
67.9 (18.1) |
64.7 (19.7) |
S = p<.001 C = p<.05 |
|
| |||||
| Relevance | 94.9 (8.1) |
91.7 (10.7) |
70.8 (18.6) |
66.3 (19.5) |
S = p<.001 C = p<.001 |
|
| |||||
| Negative Affect | 26.3 (21.8) |
19.2 (18.0) |
24.0 (18.6) |
19.3 (19.0) |
C = p<.001 |
|
| |||||
| Positive Affect | 46.6 (23.5) |
42.3 (21.6) |
36.0 (21.0) |
36.9 (20.8) |
S = p<.001 |
|
| |||||
| Excited | 43.1 (22.8) |
32.5 (20.4) |
35.3 (21.8) |
29.2 (21.8) |
S = p < .05 C = p < .001 |
|
| |||||
| Calm | 46.2 (24.2) |
51.1 (22.0) |
41.5 (21.4) |
51.1 (20.0) |
C = p<.001 |
|
| |||||
| Heart Rate (HR) | 0.42 (2.9) |
0.57 (2.6) |
−.22 (2.6) |
−0.13 (2.7) |
S = p < .05 |
Craving
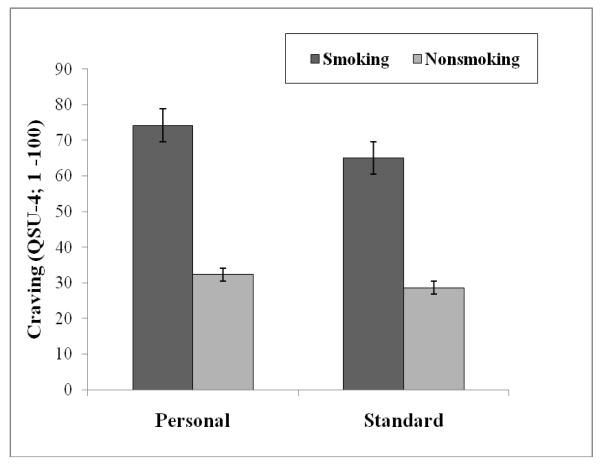
Craving data revealed a significant main effect of source, F (1,71) = 21.46, p<.001, and cue, F (1,71) = 243.08, p<.001. A significant Source × Cue interaction was also revealed, F (1,71) = 4.08, p<.05. Post hoc evaluation via pairwise comparisons revealed that the magnitude of the smoking-nonsmoking difference was greater for personal (mean difference = 41.88; 95% CI 35.9 – 47.8) compared to standard (mean difference = 36.39; 95% CI 30.9 – 41.8) environment cues, t(72) 2.02, p<.05. Although personal cues significantly enhanced self-reported craving, the smoking- nonsmoking difference for both sources of cues led to effect sizes that would be considered large (Cohen, 1988); personal d = 1.99, standard d = 1.70. These craving results are depicted in Figure 1.
Figure 1.
Craving as a function of stimulus cue (smoking, nonsmoking) and source (personal, standard) depicting significant main effects of cue and type, as well as a Cue × Source interaction.
Vividness
The ANOVA of these data revealed a significant main effect of source, F (1,71) = 182.02, p<.001, as well as cue, F (1,71) = 10.55, p<.05. Participants rated smoking cues and personal environments as more vivid than nonsmoking and standard cues.
Negative mood
The ANOVA on negative mood ratings revealed a significant cue effect, F (1,71) = 15.98, p<.001. Exposure to smoking cues led to greater report of negative mood compared to nonsmoking cues. However, as noted above, subjects’ BIDR-IM score was negatively associated with their negative mood ratings to nonsmoking stimuli. When the ANOVA for negative mood was re-run including BIDR-IM score as a covariate, this difference in negative mood as a function of cue disappeared, suggesting a penchant among some subjects to under-report negative mood under specific circumstances.
Positive mood
A significant source effect for positive mood was found, F (1,71) = 18.17, p<.001. Participants rated their positive mood higher after viewing their own personal cues compared to standard cues.
Excited
There was a significant source effect for self-reported excitement, F (1,71) = 9.20, p<.05, as well as a main effect of cue, F (1,71) = 31.95, p<.001. Participants reported greater excitement when viewing personal cues compared to standard cues. Smoking cues also led to greater excitement compared to non-smoking cues.
Calm
Nonsmoking cues led to greater self-reported calmness compared to smoking cues, as evidenced by a significant cue effect, F (1,71) = 23.31, p<.001.
Relevance
There was a significant effect of source on relevance ratings, F (1,71) = 171.18, p<.001, such that smokers rated personal cues as more relevant than standard cues. There was also a significant effect of cue on relevance, F (1,71) = 11.28, p<.001, such that smokers rated smoking cues as significantly more relevant compared to nonsmoking cues.
3.3 Heart Rate (HR)
Average post-trial ratings for heart rate can be seen in Table 1. There was a significant source effect on heart rate, F (1,69) = 5.33, p<.05. Heart rate increased from baseline as function of viewing personal cues and decreased as a function viewing of standard environments, although this effect was small (Cohen’s d= .25.)
4. Discussion
The goal of this research was to develop a method for bringing smokers’ real world smoking and nonsmoking environments into the laboratory and testing smokers’ reactivity to their personalized environment cues compared to standard environment cues. Consistent with our initial hypotheses, smoking-related pictorial environments led to greater craving to smoke than nonsmoking pictorial environments. Likewise, exposure to personal environments led to significantly greater craving to smoke than standard environments. However, the most novel finding of this study was that personalizing the stimuli produced a more robust difference in craving due to smoking versus nonsmoking environments, although both cue sources led to smoking-nonsmoking effect sizes that can be considered large (Cohen, 1988).
This study furthers our previous work showing the importance of environments on craving to smoke (Conklin et al., 2008) by demonstrating that this distal cue type (i.e. environments) can be made even more evocative through personalization. Unlike proximal cues, which are highly vivid and relevant to smokers regardless of personalization (Conklin & Tiffany, 2001; Niaura et al.,1988), the present study supported our supposition that personalizing environment cues can enhance their vividness, relevance, and the level of craving they evoke from smokers. However, vividness and relevance were only associated with craving levels presence of personal nonsmoking cues, where they were negatively associated with craving. Thus, the vividness and relevance of personal nonsmoking cues may have aided craving suppression.
The personalized environment cues also led to enhanced heart rate compared to the standard cues. From a theoretical perspective, there is reason to believe that personalizing cues should enhance reactivity across multiple indices of responding (e.g., Tiffany, 1990, Niaura et. al. 1988). In the present study, personalization enhanced smokers’ heart rate from baseline. However, there was no main effect of cue; that is, no differential heart rate was observed between smoking and nonsmoking stimuli, only between standard and personal cues, and the effect was small. Therefore, this HR increase for personal environments likely reflects a familiarity effect, in that HR tends to decelerate in response to novel stimuli and accelerate upon exposure to familiar stimuli (Graham & Clifton, 1966). Although this finding does not support our anticipated enhanced autonomic reactivity as a function of our cue manipulations, it offers an objective measure of the validity of the picture viewing procedure, in that smokers HR reactivity suggests that they were paying attention to the specificity of the cues.
Maximizing the evocativeness of environment cues and having an effective new method for capturing individuals’ personal real world environmental contexts have important implications for behavioral research in addiction, as well as in other disorders involving learned behavior. This observation becomes more apparent in consideration of what is termed the renewal effect. Best explained through animal learning studies, renewal occurs when an animal learns a behavior in one environment (A), undergoes extinction training in a different environment (B) in which it shows diminished responding to stimuli associated with the target behavior, and is then returned to the original environment (A) where responding to extinguished stimuli is renewed upon this switch in environments (e.g., Bouton & Bolles, 1979; Grahame, Hallam, Geier, & Miller, 1990.) Renewal is a contextual effect that limits the generalizability of extinction training and impedes the reduction in conditioned responding that can be achieved (Conklin & Tiffany, 2002).
Renewal poses a challenge to exposure or extinction-based treatments for any disorder. As Wikler first noted in 1948, when ex-drug-dependent individuals returned to the environments in which they previously used drugs, even if no proximal drug cues are present and physiological withdrawal has long abated, the environments alone served as highly salient cues for drug-seeking behavior. Although not called such, Wikler is essentially describing renewal such that a drug dependent individual uses drugs in the real world (A), undergoes treatment in the clinic (B) where drug-cue responses appear to diminish, then returns home post-treatment (A) where the saliency of the extinguished cues is quickly renewed due to a switch back to the original context of learning (i.e., the exact environments where multiple drug-taking trials routinely occurred.) Extinguishing the most proximal cues associated with a given psychopathology (e.g., lit cigarettes for smokers, physiological arousal in panic patients, trigger foods for bulimics) may be inadequate in light of the numerous distal environmental stimuli that elicit craving and substance use behavior. The present methodology could be applied to allow clinicians to expose patients to proximal cues inherent in any problem behavior in conjunction with representations of the exact distal environments likely to promote relapse. Thus, the individual can practice not only refraining from an undesired behavior (e.g., smoking, panicking, binging) in the face of strong proximal cues to engage in it, but can do so in the presence of the environmental contexts where urges to engage in the behavior are likely to be triggered or be most robust outside of treatment.
This methodology could also be applied to multiple research endeavors. Changes in an individual’s reactivity to environmental contexts could be used to gauge treatment effectiveness over time and offer more specific information about triggers and set backs. On a larger scale, the impact of changes in social acceptability or public policies, which may affect the expression of behavior, could be tracked. For example, as public smoking bans become more widespread, smokers will increasingly smoke more in highly personalized environments, such as their own homes and vehicles. Understanding how smokers’ reactivity to various cues increases, decreases, or shifts between stimuli can shed considerable light on how the conditioning effects of environments develop, function, and change over time.
One limitation of the present work is that we are unable to determine exactly why personalized smoking environments evoke greater reactivity than standard smoking environments. It is easy to assume that the former work better because they capture the exact places in which individuals are smoking, and that their familiarity makes the stimuli more salient. However, there is a second possibility. It may be the case that personalization just eliminates environments to which certain smokers are not reactive, environments that are contained within the standard set of environment cues. A bar may be a smoking environment for most smokers, but not for all. Personalization may function to eliminate ineffective cues for each participant, or ensure that the types of environments that an individual strongly associates with smoking or nonsmoking are presented. If so, personalization may require only that researchers develop a larger set of standard environments from which each smoker can choose, thereby allowing smokers to eliminate any cues that are not relevant for them. Future research is needed to determine which mechanism of personalization is at work. However, it may be the case that a large set of standard environments from which participants can choose may work well in laboratory studies to examine reactivity but might fail to capture the real world contexts needed to extinguish responding and attenuate renewal within an exposure-based treatment. Whether or not increasing the urge-eliciting potential of environment cues and including them in cue-exposure treatment reduces renewal effects and/or enhances treatment efficacy is an empirical question that future research needs to address. This study provides new methodology for beginning to examine those questions.
In summary, this study supports our previous finding that environments associated with smoking can alone, in the absence of proximal smoking stimuli, function as strong cues to smoke (Conklin et al., 2008). It furthers that work by demonstrating that personalizing those environments enhances smokers’ reactivity, not only their craving report, but the vividness and relevance of the stimuli, the level of positive affect and excitement they report, and their heart rate enhancement from baseline. More broadly, the goal of this work was to develop a method of bringing individuals’ real world environments into the laboratory where controlled exposure and assessment could be conducted. The methodology developed through this research can easily be applied not only to addictive behaviors, but to exposure-based treatments for a number of psychological disorders in which both proximal cues and the real world environments in which they are encountered are both key components in the perpetuation and/or relapse of disordered behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CA, Lindsay JJ, Bushman BJ. Research in the Psychological Laboratory: Truth or Triviality? Current Directions in Psychological Science. 1999;8(1):3–9. [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learning and Motivation. 1979;10:445–466. [Google Scholar]
- Carter BL, Tiffany ST. Meta analysis of cue reactivity in addiction research. Addiction. 1999;92:15–26. [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental & Clinical Psychopharmacology. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal Versus Distal Cues to Smoke: The Effects of Environments on Smokers’ Cue-Reactivity. Experimental and Clinical Psychopharmacology. 2008;16:207–214. doi: 10.1037/1064-1297.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA. Environments as cues to smoke: Implication for human extinction-based research and treatment. Experimental & Clinical Psychopharmacology. 2006;14:12–19. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Experimental and Clinical Psychopharmacology. 2001;9:399–408. doi: 10.1037//1064-1297.9.4.399. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Cook JM, Schnurr PP, Foa EB. Bridging the Gap Between Posttraumatic Stress Disorder Research and Clinical Practice: The Example of Exposure Therapy. Psychotherapy: Theory, Research, Practice, Training. 2004;41(4):374–387. [Google Scholar]
- Corty E, McFall RM. Response prevention in the treatment of cigarette smoking. Addictive Behaviors. 1984;9:405–408. doi: 10.1016/0306-4603(84)90042-x. [DOI] [PubMed] [Google Scholar]
- Dawe S, Powell JH, Richards D, Gossop M, Marks I, Strang J, Gray JA. Does post-withdrawal cue exposure improve outcome in opiate addiction? A controlled trial. Addiction. 1993;88:1233–1245. doi: 10.1111/j.1360-0443.1993.tb02146.x. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA. The independence of positive and negative affect. Journal of Personality and Social Psychology. 1984;47:1105–1117. doi: 10.1037//0022-3514.47.5.1105. [DOI] [PubMed] [Google Scholar]
- Graham FK, Clifton RK. Heart-rate change as a component of the orienting response. Psychological Bulletin. 1966;65:305–320. doi: 10.1037/h0023258. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Hallam SC, Geier L, Miller RR. Context as an occasion setter following either CS acquisition and extinction or CS acquisition alone. Learning & Motivation. 1990;21:237–265. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Kasvikis Y, Bradley B, Powell J, Marks I, Gray JA. Postwithdrawal exposure treatment to prevent relapse in opiate addicts: a pilot study. International Journal of Addictions. 1991;26:1187–1195. doi: 10.3109/10826089109062154. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The Psychology of Animal Learning. Academic Press; London: 1974. [Google Scholar]
- Miranda R, Jr., Rohsenow DJ, Monti PM, Tidey J, Ray L. Effects of repeated days of smoking cue exposure on urge to smoke and physiological reactivity. Addictive Behaviors. 2008;33(2):347–353. doi: 10.1016/j.addbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Abrams DB, Shadel WG, Rohsenow DJ, Monti RM, Sirota AD. Cue exposure treatment for smoking relapse prevention: a controlled clinical trial. Addiction. 1999;94:685–695. doi: 10.1046/j.1360-0443.1999.9456856.x. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Paulhus DL. Measures of personality and social psychological attitudes. In: Robinson JP, Shaver PR, editors. Measurement and control of response bias. Vol. 1. Academic Press, Inc.; San Diego, CA: 1991. pp. 17–59. [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology. 2006;184:637–644. doi: 10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Simes R. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: The role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Wikler A. Recent progress in research on the neurophysiologic basis of morphine addiction. American Journal of Psychiatry. 1948;105:329–338. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]