Abstract
Intrahepatic bile duct adenoma is a rare benign epithelial hepatic tumor derived from bile duct cells. We report the imaging findings of a patient with bile duct adenoma, which appeared as a small heterogeneously enhancing mass with focal small cystic change on CT and MRI. Follow-up images at seven months showed a slight increase in tumor size, which could be partly explained by intratumoral hemorrhage on pathologic examination. Although rare, bile duct adenoma should be considered as a differential diagnosis of a small hypervascular tumor located in the periphery of liver. Focal cystic change and intratumoral hemorrhage may occur.
Keywords: Liver, Adenoma, Bile duct, Computed tomography (CT), Magnetic resonance (MR)
Intrahepatic bile duct adenoma (BDA) is a rare benign epithelial liver tumor derived from bile duct cells. BDA represents about 1.3% of all primary liver tumors and it is mainly found incidentally on the surface of the liver at laparotomy or autopsy (1-5). Grossly, BDA is a well-circumscribed, however not encapsulated mass ranging in size from 1 to 20 mm (1). Histologically, BDA is characterized by a confluent proliferation of bile ductules in a connective tissue stroma which show variable degrees of inflammation and fibrosis. BDA has been reported to show benign behavior and have limited growth potential (1, 4).
We report imaging findings of a case of BDA showing mild lesion enlargement on seven-month follow-up images, leading to the preoperative diagnosis of a possible malignant tumor. To the best of our knowledge, this is the first case studied with triphasic dynamic contrast-enhanced CT scans and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MRI.
CASE REPORT
A 59-year-old female patient was referred to our hospital for evaluation of a hepatic mass. The lesion was incidentally found during ultrasound screening at a local clinic. The patient had no symptoms or history of viral hepatitis or excessive alcohol intake. A physical examination and laboratory findings were unremarkable.
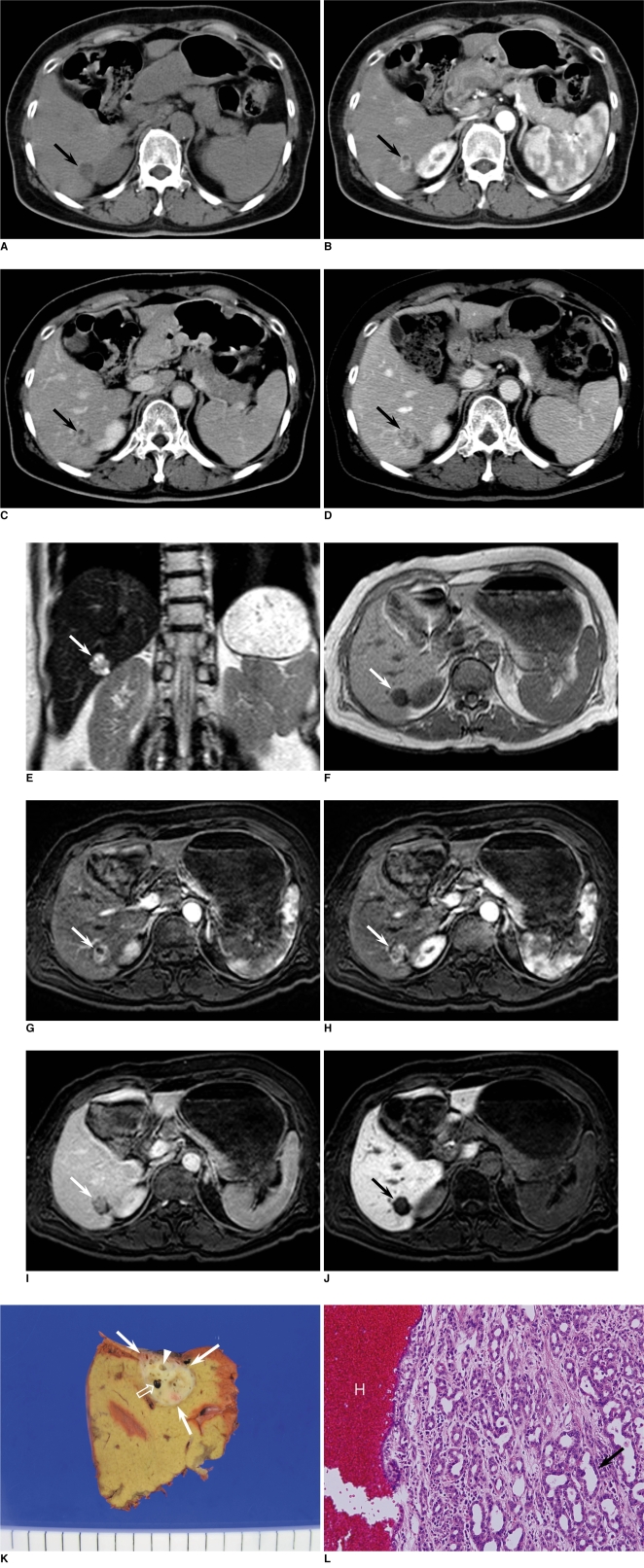
CT scans were performed to evaluate the hepatic mass. Unenhanced CT scan showed a well-defined low density mass measuring about 1.7 cm in the periphery of the posteroinferior segment of the right lobe of the liver (Fig. 1A). On dynamic contrast-enhanced CT, the mass revealed heterogeneous enhancement with an internal non-enhancing small cystic portion on the hepatic arterial phase (Fig. 1B) and persistent enhancement on portal venous and equilibrium phases (Fig. 1C). No other mass was found in the liver. Initially, we diagnosed the mass as a benign lesion such as angiomyolipoma with little fat component or inflammatory pseudotumor. However, the possibility of malignant lesions such as cholangiocarcinoma or atypical hepatocellular carcinoma could not be excluded. Follow-up CT scan was performed two months later and the imaging findings did not change compared to the initial CT scans. However, follow-up CT at seven months revealed a slight increase in the diameter of the mass from 1.7×1.2 cm to 2.1×1.8 cm, with an increase in the size of the internal enhancing portion (Fig. 1D). We performed liver MRI for further evaluation. MRI revealed that the mass showed heterogeneous high signal intensity on T2-weighted images (Fig. 1E) and low signal intensity on T1-weighted images (Fig. 1F). Contrast-enhanced MRI was performed using a breath-hold 3D gradient echo sequence with a fat saturation sequence, after an intravenous bolus injection of 0.025 mmol Gadoxetic acid (Gd-EOB-DTPA; Primovist, Schering AG, Berlin, Germany) per kilogram of body weight followed by a saline flush of 30 mL. The mass showed heterogeneous enhancement and focal cystic change on hepatic arterial phase (Fig. 1G, H), relative hypointensity in comparison with the normal liver parenchyma on portal venous and equilibrium phases (Fig. 1I), and lower signal intensity on hepatobiliary phase obtained 20 minutes after contrast injection (Fig. 1J). Considering the duration of the tumor growth period (7 months), it was difficult to rule out the possibility of low grade malignancy. The patient opted for resecting the mass and hepatic segmentectomy was performed.
Fig. 1.
Dynamic contrast-enhanced CT scans and follow-up MR images in 59-year-old woman with intrahepatic bile duct adenoma.
A. Unenhanced CT scan shows well-defined low density mass measuring about 1.7 cm in periphery of posteroinferior segment of right hepatic lobe (arrow).
B. Dynamic contrast-enhanced CT performed on mass shows heterogeneous enhancement (arrow) during hepatic arterial phase. Focal non-enhancing cystic portion is seen in anterior portion of mass.
C. On equilibrium phase, mass shows relatively persistent enhancement (arrow).
D. On equilibrium phase of follow-up CT scans obtained after seven months, tumor is slightly enlarged, measuring about 2.1 cm in diameter.
E. Coronal T2-weighted half fourier acquisition single shot turbo spin echo (HASTE) image revealed that lesion has heterogeneous high signal intensity (arrow) with several small areas of bright signal intensity similar to that of fluid.
F. On T1-weighted image, lesion shows homogeneous hypointensity (arrow).
G. On dynamic contrast-enhanced T1-weighted images with fat saturation after administration of Gd-EOB-DTPA, upper portion of mass shows focal non-enhancing cystic portion with heterogeneous enhancement in remaining portion (arrow) during hepatic arterial phase.
H. Lower portion of mass located 8 mm caudad to G shows heterogeneous enhancement during hepatic arterial phase.
I. On equilibrium phase, mass shows relative hypointensity in comparison with normal liver parenchyma (arrow).
J. On hepatobiliary phase obtained 20 minutes after contrast injection, mass shows distinct low signal intensity (arrow).
K. Gross specimen shows well-circumscribed, non-encapsulated, yellowish white, subcapsular mass (white arrows). Multiple cystic changes (arrowhead) are seen in mass and focal hemorrhagic component (open arrow) is combined.
L. Microscopically, mass consists of densely packed proliferation of simple tubular ducts (arrow) combined with hemorrhage (H). Cuboidal epithelium resembles that of interlobular bile ducts without cell atypia or mitotic activity (Hematoxylin & Eosin staining, ×200).
Gross specimen showed a well-circumscribed, non-encapsulated, firm, yellowish white mass with multiple cystic changes and a hemorrhagic component (Fig. 1K). Microscopically, the mass consisted of a densely packed proliferation of simple tubular ducts with cuboidal cells embedded in a moderate amount of fibrous stroma (Fig. 1L). The cuboidal cells were uniform and lacked nuclear pleomorphism as well as hyperchromasia without definite mitoses and vascular or lymphatic invasion. The background liver parenchyma showed no fibrosis or fatty change. An immunohistochemical examination demonstrated positive staining for cytokeratin 7 and cytokeratin 19 as well as negative staining for p 53. Expression of cytokeratin 7 and cytokeratin 19 represented the bile duct origin tumor, while the negative reactivity for p 53 helped distinguish BDA from the metastatic pancreatic adenocarcinoma in the liver (6). The histologic diagnosis was an intrahepatic BDA.
DISCUSSION
Intrahepatic BDA is a rare benign epithelial tumor of the liver that is usually incidentally found at surgery or autopsy (1-4). Its incidence was reported to make up 1.3% of primary liver tumors (2). Craig et al. (3) reported only five cases of intrahepatic BDA in 50,000 autopsies, and until now, Allaire et al. (1) have reported the 152 cases between 1943 and 1986, which is the largest series of BDA. Of the 152 cases, most BDAs were asymptomatic nodules discovered incidentally on the surface of the liver during intra-abdominal surgery in 103 cases or at autopsy in 49 cases. They were usually subcapsular, ranging in size from 1 to 20 mm (mean, 5.8 mm), and most of them were less than 1 cm in diameter. The majority of BDAs occurred in individuals between the ages of 20 and 70 years with a mean age of 55 years and no significant difference in sex distribution (1). BDA is a usually solitary nodule, but may occur as multiple nodules throughout the liver (5, 7).
Macroscopically, BDA is a solitary, well-circumscribed, firm, gray-white or tan, subcapsular nodule (8). Microscopically, BDAs are composed of the proliferation of disorganized mature peribiliary gland acini and ductules within a variable amount of connective tissue stroma showing signs of chronic inflammation and collagenization (1, 4). The cells of the bile ductules have low mitotic activity (1).
There has been considerable controversy in the origin, pathogenesis and nomenclature of BDA. In the early literature, BDA was regarded as a true neoplasm and has been called a cholangioma, benign cholangioma, or cholangioadenoma (5). It has also been confused with bile duct hamartoma (von Meyenberg complex) or intrabiliary BDA in some reports (5, 9, 10); however, BDA is a pathologically distinct entity (11). Microscopically, BDA is distinguished from a bile duct hamartoma by its lack of intraluminal bile and the compact nature of its proliferation (8).
Although the pathogenesis of BDA is still under discussion, the most accepted pathogenesis of BDA, based on immunohistochemical studies, is a reactive process to a focal bile ductular injury caused by trauma or inflammation (1). Recently, a study demonstrated a similarity in the secretory gland cell phenotype between BDA and peribiliary glands, suggesting that BDA represents disorganized peribiliary glands (9). In BDA, the acini and tubules fail to organize into a mature gland, draining into a bile duct, possibly because of the absence of appropriate mesenchymal-epithelial signaling. The authors of the aforementioned study suggested that BDA is a misnomer and BDA should be called a peribiliary gland hamartoma (9).
There have been four reports on the radiological features of BDA, of which two are in the Japanese literature. In those four reports, a total of six BDAs were included. Adding our case, we summarized the radiologic features of seven BDAs. The masses were measured from 4 to 21 mm in size (mean: 12 mm) and all lesions were located in the subcapsular or surface of the liver. Four lesions were found during the evaluation of chronic hepatitis and three lesions were found incidentally.
Ultrasound was performed in five cases, in which three cases showed an echogenic nodule with or without a hypoechoic rim (4, 12, 13), while two lesions were not detected by ultrasound. On nonenhanced CT performed in four cases, two lesions showed hyperdense areas in the lesion, probably due to calcifications within the tumor (12, 14). On MRI performed in five cases, three lesions, including our case, showed hypointensity on T1-weighted image (WI) and hyperintensity on T2-WI (4). One lesion showed hyperintensity both on T1- and T2-WI (4), while the other showed hypointensity both on T1- and T2-WI, probably due to calcification (7). Although various imaging techniques were used in the previous reports from different institutions, most BDAs showed hypervascular characteristics consisting of prolonged enhancement on dynamic contrast-enhanced CT and MRI, hepatic artery angiography, CT hepatic arteriography, and CT during arterial portography. Delayed or prolonged enhancement in dynamic studies of CT and MRI may be due to the fibrous stroma within the tumor (15). Our case showed relative hypointensity on equilibrium phase and distinct hypointensity on hepatobiliary phase of dynamic contrast-enhanced MRI, which may be explained by the use of Gd-EOB-DTPA. Since it is distinct from extracellular fluid contrast agents that rapidly equilibrate with the extravascular space following injection, Gd-EOB-DTPA is initially distributed in the extracellular fluid compartment, but is subsequently taken up by functioning hepatocytes and is excreted in the bile (16). Therefore, our case showed low signal intensity compared to background liver enhancement on equilibrium phase of GD-EOB-DTPA-enhanced MRI. Since BDA may have various amounts of fibrous stroma, the signal intensity on T2-weighted MR images and enhancement features on dynamic studies of CT and MRI may be variable according to the amount of fibrous stroma (17).
Our case was a well-defined hypervascular mass in the subcapsular portion of the liver with prolonged enhancement on CT, as previously reported. The previously unreported feature of our case is a focal cystic change in the tumor and increase in size seen in images obtained at the 7-month follow-up point. Although benign tumors may have slow growth potential, the possibility of a low grade malignant tumor cannot be excluded by the imaging findings. Pathologic findings showed multifocal cystic changes and focal hemorrhage within the lesion, which are thought to be the cause of tumor growth.
Previous reports represented that BDA was difficult to detect due to its small size and peripheral location (4, 17). However, due to recently improved imaging techniques and increased use of cross sectional imaging, especially for patients with chronic hepatitis, more BDAs can be identified on imaging studies. Therefore, radiologists should be familiar with the imaging findings of BDAs, even though the accurate diagnosis of BDA still requires a histopathologic examination.
In summary, we experienced a case of BDA showing internal cystic change and slight tumor enlargement at a 7-month follow-up time. The mass showed heterogeneous enhancement during the hepatic arterial phase of dynamic CT and MRI. The mass showed distinct hypointensity on hepatobiliary phase obtained 20 minutes after administration of Gd-EOB-DTPA. BDA should be considered in the differential diagnosis of a small hypervascular tumor located in the subcapsular portion of liver. Focal cystic changes and intratumoral hemorrhage may occur.
References
- 1.Allaire GS, Rabin L, Ishak KG, Sesterhenn IA. Bile duct adenoma. A study of 152 cases. Am J Surg Pathol. 1998;12:708–715. doi: 10.1097/00000478-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Edmondson HA. Atlas of tumor pathology. Washington DC: Armed Forces Institute of Pathology; 1958. Tumors of the liver and intrahepatic bile duct; pp. 19–29. fascicle 25. [Google Scholar]
- 3.Craig JR, Peters RL, Edmondson HA. Atlas of tumor pathology. Washington DC: Armed Forces Institute of Pathology; 1989. Tumors of the liver and intrahepatic bile ducts; pp. 56–62. 2nd series, fascicle 26. [Google Scholar]
- 4.Tajima T, Honda H, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, et al. Radiologic features of intrahepatic bile duct adenoma: a look at the surface of the liver. J Comput Assist Tomogr. 1999;23:690–695. doi: 10.1097/00004728-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Cho C, Rullis I, Rogers LS. Bile duct adenoma as liver nodules. Arch Surg. 1978;113:272–274. doi: 10.1001/archsurg.1978.01370150044007. [DOI] [PubMed] [Google Scholar]
- 6.Hornick JL, Lauwers GY, Odze RD. Immunohistochemistry can help distinguish metastatic pancreatic adenocarcinomas from bile duct adenomas and hamartomas of the liver. Am J Surg Pathol. 2005;29:381–389. doi: 10.1097/01.pas.0000149710.01559.fe. [DOI] [PubMed] [Google Scholar]
- 7.Levin SE, Dail DH, Saik RP. Bile duct adenomatosis of the liver: a misleading finding on surgical exploration of the abdomen. Am Surg. 1975;41:106–108. [PubMed] [Google Scholar]
- 8.Christine AL, Elizabeth M. Gastrointestinal and Liver Pathology. Philadelphia, PA: Churchill Livingstone; 2005. pp. 600–602. [Google Scholar]
- 9.Bhathal PS, Hughers NR, Goodman AD. The so-called bile duct adenoma is a peribiliary gland hamartoma. Am J Surg Pathol. 1996;20:858–864. doi: 10.1097/00000478-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Semelka RC, Hussain SM, Marcos HB, Woosley JT. Biliary hamartomas: solitary and multiple lesions shown on current MR techniques including gadolinium enhancement. J Magn Reson Imaging. 1999;10:196–201. doi: 10.1002/(sici)1522-2586(199908)10:2<196::aid-jmri14>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Govindarajan S, Peters RL. The bile duct adenoma. A lesion distinct from Meyenburg complex. Arch Pathol Lab Med. 1984;108:922–924. [PubMed] [Google Scholar]
- 12.Kobayashi T, Matsui O, Takashima T Abdominal Radiology Study Group of Japan, editors. Atlas of diagnostic abdominal imaging. vol. 1. Osaka, Japan: Nihon Schering; 1993. A case of bile duct adenoma (Japanese) pp. 26–27. [Google Scholar]
- 13.Miyazaki Y, Honda H, Yamamichi K. A case of bile duct adenoma, its features of preoperative imaging (Japanese); 33rd annual meeting of Liver Cancer Study Group; 1997. 175 pp. [Google Scholar]
- 14.Maeda E, Uozumi K, Kato N, Akahane M, Inoh S, Inoue Y, et al. Magnetic resonance findings of bile duct adenoma with calcification. Radiat Med. 2006;24:459–462. doi: 10.1007/s11604-006-0044-z. [DOI] [PubMed] [Google Scholar]
- 15.Honda H, Onitsuka H, Yasumori K, Hayashi T, Ochiai K, Gibo M, et al. Intrahepatic peripheral cholangiocarcinoma: two-phased dynamic incremental CT and pathologic correlation. J Comput Assist Tomogr. 1993;17:397–402. [PubMed] [Google Scholar]
- 16.Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29:1725–1748. doi: 10.1148/rg.296095515. [DOI] [PubMed] [Google Scholar]
- 17.Skelly RT, Lee J, Sloan JM, Diamond T. Incidental bile duct adenomas in a patient with obstructive jaundice. Ulster Med J. 1999;68:114–115. [PMC free article] [PubMed] [Google Scholar]