Abstract
Gliosarcoma is a rare central nervous system tumor usually located in the supratentorial area. Here we report a rare case of a gliosarcoma that developed in the cerebellar hemisphere in a 70-year-old woman. Computed tomography (CT) and magnetic resonance imaging (MRI) of the brain revealed an infratentorial mass of which radiological features were similar to those of glioblastoma. The tumor was diagnosed by pathology as a gliosarcoma. Though rare, gliosarcoma should be considered in the differential diagnosis of infratentorial tumors with radiological features of glioblastoma or metastasis in elderly patients.
Keywords: Gliosarcoma, Cerebellum, Posterior cranial fossa, Infratentorial tumor, Computed tomography (CT), Magnetic resonance imaging (MRI)
Gliosarcoma is a rare astrocytic tumor of the central nervous system (CNS) that is considered to be a glioblastoma variant according to the 2007 World Health Organization (WHO) classification (1). While several cases of supratentorial gliosarcomas have been reported in the literature, there are only a few reports of infratentorial gliosarcoma (2, 3). This is, to our knowledge, the 3rd published case report of infratentorial gliosarcoma. In this case report, we present an unusual case of a gliosarcoma that developed in the cerebellar hemisphere.
CASE REPORT
A 70-year-old woman had a headache following a tooth extraction. Although she took medication for several days, the symptom was not relieved. She began vomiting the day before she arrived at the emergency room. Her medical history included a spine operation due to tuberculous spondylitis of the lumbar spine about 10 years ago. However, she had no history of cancer and radiation therapy. She was alert without neurologic symptoms at the time of the hospital visit.
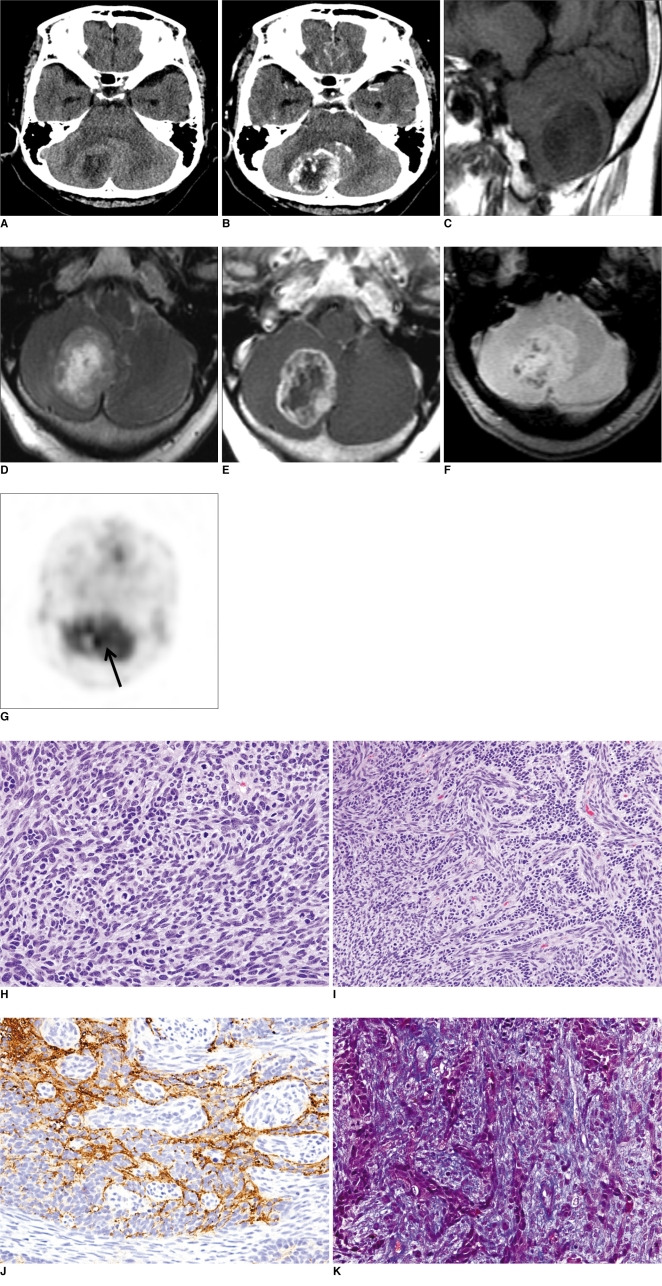
The initial brain computed tomography (CT) scan (Brilliance 64-multislice CT scanner, Philips Medical System, MA) demonstrated a well-defined intra-axial mass in the cerebellum (Fig. 1A, B). The mass was mainly located in the right cerebellar hemisphere, displacing the cerebellar vermis to the left side and compressing the right side of the 4th ventricle and resulting in mild obstructive hydrocephalus. The mass was composed of a peripheral isoattenuation area and a central low attenuation area, which was the central necrotic portion in the precontrast CT scan (Fig. 1A). The isoattenuation area of the mass demonstrated heterogeneous dense enhancement after the injection of contrast materials. Some vascular or linear enhancing portions were observed, but the central low attenuation portion was scarcely enhanced. The mass had little peritumoral edema (Fig. 1B).
Fig. 1.
70-year-old woman with gliosarcoma in right cerebellar hemisphere.
A, B. Precontrast (A) and postcontrast (B) CT scans demonstrate right cerebellar mass with heterogeneous peripheral enhancement.
C-E. Sagittal T1-weighted image (C) demonstrates well-defined hypointense mass, axial T2-weighted image (D) central hyperintense area, and post-contrast axial T1-weighted image (E) peripheral enhancement.
F. This gradient echo image shows multiple dark signal foci in solid portion of mass, which indicate tumoral bleeding.
G. This 18F-FDG-PET image shows increased metabolism in solid portion of tumor (arrow).
H. Sarcomatous component consisted of densely packed spindle cells with nuclear atypia and frequent mitotic figures (Hematoxylin & Eosin staining, original magnification ×400).
I, J. Gliomatous component, which was immunoreactive for glial fibrillary acid protein, intermingles with sarcomatous component (I: Hematoxylin & Eosin staining, J: immunohistochemistry; original magnification ×400).
K. Abundant collagen fibers were found between individual tumor cells in sarcomatous component (Masson-trichrome; original magnification, ×400).
One day after admission, brain magnetic resonance imaging (MRI) (Twin Speed 1.5T MR scanner, GE Healthcare, WI) was performed. The mass was hypointense on precontrast T1-weighted images (repetition time [TR]/echo time [TE], 400/-8 ms) (Fig. 1C). On T2-weighted images (TR/TE, 3700/-1200 ms), the mass showed an intermediate signal intensity at the periphery, and central hyperintensity (Fig. 1D). The outer surface margin was comparatively smooth and slightly lobulated, but the inner surface margin was very irregular. Peritumoral edema was minimal on MRI. After contrast material injection, the periphery showed heterogeneous enhancement as did the brain CT images (Fig. 1E). Gradient echo images (TR/TE, 500/-15 ms) revealed multiple bleeding foci in the periphery as dark signal intensity foci (Fig. 1F). We initially diagnosed the case as malignant glioma or metastasis. 18F-Fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) demonstrated hypermetabolism at the tumor periphery. There was a metabolic defect within the central area of the tumor, which was correlated to the central necrosis of the tumor (Fig. 1G).
Partial resection of the tumor was done. Macroscopically, it was a relatively well-circumscribed and firm mass with areas of necrosis and hemorrhage. Microscopically, it had a biphasic pattern with areas of gliomatous and sarcomatous differentiation (Fig. 1H). The gliomatous component consisted of atypical glial cells with pleomorphic nuclei and frequent mitotic figures. Geographic necrosis and endothelial cell proliferation were noted. The tumor predominantly consisted of spindle cell proliferation forming sarcomatous areas with intersecting fascicular or storiform patterns similar to fibrosarcoma. Extensive necrosis, frequent mitotic figures (> 80/10 HPFs) and abnormal mitoses were observed in the sarcomatous component (Fig. 1H). The gliomatous component showed immunoreactivity for glial fibrillary acid protein (GFAP), in contrast with lack of immunoreactivity for GFAP in the sarcomatous component (Fig. 1I, J). Abundant collagen and reticulin fibers were found between individual tumor cells in the sarcomatous component using Masson-trichrome and reticulin staining (Fig. 1K). No immunoreactivity for cytokeratin was seen in gliomatous and sarcomatous components. These findings were consistent with a gliosarcoma.
DISCUSSION
Almost all previously reported cases of gliosarcoma were located in the supratentorial area, especially in the peripheral region of the temporal lobe (2). Gliosarcoma occurs less commonly in the frontal, parietal or occipital lobes, and in the corpus callosum. Only three cases of infratentorial gliosarcoma have been reported (3, 4). The infratentorial gliosarcoma reported by Nitta et al. (3) showed similar MRI findings to supratentorial gliosarcoma such as peripheral location of the cerebellum, broad base on the meninges, and multiple lesions. Other brain and spinal gliosarcoma cases usually had a medical history of radiation therapy for other cerebellar tumors. Quite a number of gliosarcomas have been reported as secondary tumors after radiation therapy for histologically different primary CNS tumors (5-9). Among several possible mechanisms for gliosarcoma histogenesis, the transformation theory, which states that part of a glial tumor is dedifferentiated to primitive cells and transformed into sarcomatous components, supports the idea that radiation therapy might induce a dedifferentiation of the glial cell to the sarcomatous cells in cases with previous CNS tumors (4, 6, 8, 10, 11). Compared to the previous literature, our case had no history of previous radiation therapy for a CNS tumor. In addition, its location was in a relatively central portion, without abutting the meninges.
Pathologically, gliosarcoma is characterized by a biphasic tissue pattern with alternating areas of glial and mesenchymal differentiation (7). The sarcomatous component shows malignant changes of spindle cells with features of fibrosarcoma or malignant fibrous histiocytoma. Areas of mesenchymal differentiation into cartilage, bone, fat and smooth or skeletal muscle may be associated. In our case, although no specific mesenchymal differentiation was seen, the tumor was predominantly sarcomatous and had a mixture of clearly malignant mesenchymal GFAP-negative areas and GFAP-positive glial areas. In addition, collagen deposition and reticulin fibers were observed in the sarcomatous component. Therefore, we were able to exclude the possibility of glioblastoma with a florid fibroblastic proliferation.
Radiologic findings of gliosarcomas in the previous literature are variable and sometimes the tumors are very aggressive. CT findings are similar to those for an infiltrating glioblastoma. A gliosarcoma may appear as a well-defined hyperdense mass with heterogeneous or ring enhancement due to a fibrous component. In contrast, a glioblastoma usually shows low or intermediate attenuation in a precontrast CT scan. Vasogenic edema often accompanies glioblastoma, but central necrosis is less common due to the predilection of such tumors to develop in a peripheral location. Dural involvement is not uncommon due to its peripheral location (3, 5, 12). On MRI, gliosarcoma appears as a heterogeneous mass both in T1- and T2-weighted images, with strong peripheral enhancement and central hemorrhage or necrosis (5). Metastasis along CSF flow and local recurrence is frequent (13, 14). Extracranial metastasis is also common in gliosarcomas, resulting in a worse prognosis (8, 10).
In our case, the imaging findings were similar to those of an infratentorial glioblastoma, which usually presents as an aggressive heterogeneous tumor with hemorrhage, necrosis, a cystic portion or calcification, and surrounding peritumoral edema (15). But, it is known as a less aggressive and slower growing tumor than a supratentorial glioblastoma, and sometimes there is a lack of peritumoral edema, as in our case (16, 17). In addition, as the most common incidence of both tumors occurs between the 5th and 7th decades of life, it was very difficult to make the differential diagnosis. Common differential diagnoses for gliosarcoma are glioblastoma, metastasis, brain abscess or other infratentorial intraaxial tumors such as hemangioblastoma or medulloblastoma (5, 12, 15-17).
In conclusion, we reported a rare case of infratentorial gliosarcoma. Imaging findings were so similar to those of an infratentorial or supratentorial glioblastoma that it was difficult to distinguish from a glioblastoma. Though a rare entity, gliosarcoma should be considered in the differential diagnosis of infratentorial tumors with radiological features of glioblastoma and metastasis in elderly patients.
Footnotes
This article was supported by the Kyung Hee University Research Fund in 2006 (KHU-20060432).
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melo JR, Souza AL, Reis RC, Almeida MA. Infantile gliosarcoma. Arq Neuropsiquiatr. 2008;66:88–89. doi: 10.1590/s0004-282x2008000100022. [DOI] [PubMed] [Google Scholar]
- 3.Nitta H, Hayase H, Moriyama Y, Yamashima T, Yamashita J. Gliosarcoma of the posterior cranial fossa: MRI findings. Neuroradiology. 1993;35:279–280. doi: 10.1007/BF00602614. [DOI] [PubMed] [Google Scholar]
- 4.Kawaguchi S, Kashiwaba T, Koiwa M, Shimoyama M, Kobayashi N, Fukushi Y, et al. Two autopsied cases of radiation-induced gliosarcoma. No Shinkei Geka. 1991;19:285–290. [Japanese] [PubMed] [Google Scholar]
- 5.Alatakis S, Stuckey S, Siu K, McLean C. Gliosarcoma with osteosarcomatous differentiation: review of radiological and pathological features. J Clin Neurosci. 2004;11:650–656. doi: 10.1016/j.jocn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Malde R, Jalali R, Muzumdar D, Shet T, Kurkure P. Gliosarcoma occurring 8 years after treatment for a medulloblastoma. Childs Nerv Syst. 2004;20:243–246. doi: 10.1007/s00381-003-0850-x. [DOI] [PubMed] [Google Scholar]
- 7.Barresi V, Cerasoli S, Morigi F, Cremonini AM, Volpini M, Tuccari G. Gliosarcoma with features of osteoblastic osteosarcoma: a review. Arch Pathol Lab Med. 2006;130:1208–1211. doi: 10.5858/2006-130-1208-GWFOOO. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman KA, Fuller CE, Caruso RD, Schelper RL. Postradiation gliosarcoma with osteosarcomatous components. Neuroradiology. 2001;43:555–558. doi: 10.1007/s002340000531. [DOI] [PubMed] [Google Scholar]
- 9.Carstens PH, Johnson GS, Jelsma LF. Spinal gliosarcoma: a light, immunohistochemical and ultrastructural study. Ann Clin Lab Sci. 1995;25:241–246. [PubMed] [Google Scholar]
- 10.Beaumont TL, Kupsky WJ, Barger GR, Sloan AE. Gliosarcoma with multiple extracranial metastases case report and review of the literature. J Neurooncol. 2007;83:39–46. doi: 10.1007/s11060-006-9295-x. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez FJ, Scheithauer BW, Jenkins R, Burger PC, Rudzinskiy P, Vlodavsky E, et al. Gliosarcoma arising in oligodendroglial tumors ("oligosarcoma"): a clinicopathologic study. Am J Surg Pathol. 2007;31:351–362. doi: 10.1097/01.pas.0000213378.94547.ae. [DOI] [PubMed] [Google Scholar]
- 12.Rees JH, Smirniotopoulos JG, Jones RV, Wong K. Glioblastoma multiforme: radiologic-pathologic correlation. Radiographics. 1996;16:1413–1438. doi: 10.1148/radiographics.16.6.8946545. [DOI] [PubMed] [Google Scholar]
- 13.Witwer BP, Salamat MS, Resnick DK. Gliosarcoma metastatic to the cervical spinal cord: case report and review of the literature. Surg Neurol. 2000;54:373–378. doi: 10.1016/s0090-3019(00)00315-3. [DOI] [PubMed] [Google Scholar]
- 14.Fischer S, Lee W, Aulisi E, Singh H. Gliosarcoma with intramedullary spinal metastases: a case report and review of the literature. J Clin Oncol. 2007;25:447–449. doi: 10.1200/JCO.2006.07.8527. [DOI] [PubMed] [Google Scholar]
- 15.Demir MK, Hakan T, Akinci O, Berkman Z. Primary cerebellar glioblastoma multiforme. Diagn Interv Radiol. 2005;11:83–86. [PubMed] [Google Scholar]
- 16.Mattos JP, Marenco HA, Campos JM, Faria AV, Queiroz LS, Borges G, et al. Cerebellar glioblastoma multiforme in an adult. Arq Neuropsiquiatr. 2006;64:132–135. doi: 10.1590/s0004-282x2006000100028. [DOI] [PubMed] [Google Scholar]
- 17.Gupta V, Goyal A, Sinha S, Singh AK, Tatke M, Kumar S, et al. Glioblastoma of the cerebellum. A report of 3 cases. J Neurosurg Sci. 2003;47:157–164. [PubMed] [Google Scholar]