Abstract
Despite the prevalence of smoking among adolescents, few studies have assessed the effects of adolescent nicotine exposure on learning in adulthood. In particular, it remains unclear whether adolescent nicotine exposure has effects on hippocampus-dependent learning that persist into adulthood. The present experiment examined whether there were effects of adolescent nicotine exposure on context conditioning, a form of learning dependent on the integrity of the hippocampus, when tested during adulthood. Rats were exposed to nicotine during adolescence (postnatal days [PD] 28–42) via osmotic minipump (0, 3.0 or 6.0 mg/kg/day). Context conditioning occurred in early adulthood (PD 65–70). Animals were exposed to an experimental context and were given 10 unsignaled footshocks or no shock. Additional groups were included to test the effects of adolescent nicotine on delay conditioning, a form of learning that is not dependent upon the hippocampus. Conditioning was assessed using a lick suppression paradigm. For animals in the context conditioning groups, adolescent nicotine resulted in significantly less suppression of drinking in the presence of context cues compared with vehicle-pretreated animals. For animals in the delay conditioning groups, there was a trend for adolescent nicotine (3.0 mg/kg/day) to suppress drinking compared to vehicle-pretreated animals. There were no differences in extinction of contextual fear or cued fear between rats previously exposed to vehicle or nicotine. The data indicate that adolescent nicotine administration impairs context conditioning when animals are trained and tested as adults. The present data suggest that adolescent nicotine exposure may disrupt hippocampus-dependent learning when animals are tested during adulthood.
Keywords: adolescence, context conditioning, extinction, hippocampus, minipump, nicotine
1. Introduction
The 2007 Monitoring the Future Survey found that 21.6% of 12th graders reported smoking within the last 30 days and 46.2% reported smoking some time during their life (Johnston et al., 2008). Given these prevalence rates, it is important to ascertain any effects, particularly persistent effects, of adolescent nicotine exposure. Nicotinic cholinergic receptors are concentrated in brain regions that are critical for learning and memory, including the hippocampus and prefrontal cortex (Sargent, 1993). Adolescent nicotine administration leads to an upregulation of nicotinic receptor sites in the hippocampus, cerebral cortex and midbrain (Doura et al., 2008; Trauth et al., 1999). Adolescent nicotine exposure decreases cell packing density (measured by DNA concentration) and increases cell loss in the hippocampus and cerebral cortex, effects that persist for four weeks following cessation of nicotine (Abreu-Villaca et al., 2003). Alterations of dendritic structure in some medial prefrontal cortical neurons have been reported following adolescent nicotine administration (Bergstrom et al., 2008). Moreover, adolescent nicotine exposure has been shown to produce changes in gene expression in the hippocampus and prefrontal cortex (Polesskaya et al., 2007). Collectively, these findings suggest that nicotine may induce long-term neural changes that could impact cognitive processes involving the hippocampus and prefrontal cortex.
Context conditioning is one form of hippocampus dependent learning. The hippocampus is thought to be critical for binding together the variety of cues that constitute contextual information (Eichenbaum, 1999; Fanselow, 1990; Fanselow, 2000; Rudy & O’Reilly, 1999). Contextual fear conditioning occurs when a previously neutral environment is paired with an aversive unconditioned stimulus (US), often footshock. Following this treatment, the environment (or “context”) alone elicits a fear state which can be measured as changes in fear-relevant behavior. Young rats that have a relatively immature hippocampus are incapable of forming a long-term memory for contextual cues (Rudy, 1996; Rudy & Morledge, 1994), and lesions of the hippocampus have been shown to impair context conditioning in adult animals (Kim & Fanselow, 1992; Phillips & LeDoux, 1992). Thus, alterations of hippocampal functioning appear to disrupt context conditioning. Despite known alterations in hippocampus function that result from adolescent nicotine exposure (Abreu-Villaca et al., 2003), deficits in hippocampus-dependent tasks, such as context conditioning, have not been found (Smith et al., 2006). The demonstration of nicotine-related effects on hippocampus-dependent task performance may depend on a number of factors related to drug administration and on the exact task being employed (Kenney & Gould, 2008a). Relatedly, we have observed that the effects of acute nicotine administration on fear conditioning vary across different dependent measures (Hunt et al., 2007). Thus, the lack of effect of adolescent nicotine exposure on context conditioning in adulthood (Smith et al., 2006) may have been due to factors related to drug administration, the parameters used for training or testing, or the dependent measure used to assess fear conditioning.
Few experiments have assessed the impact of adolescent nicotine exposure on cognition tested in adulthood. Generally, these experiments have found that adolescent nicotine exposure disrupts performance in tasks thought to require the prefrontal cortex, including visual attentional processing (Counotte et al., 2009) and extinction (Smith et al., 2006). Extinction of fear is the reduction in the fear response as the organism is repeatedly exposed to the fear-eliciting stimulus in the absence of the US. Lesions of the prefrontal cortex disrupt extinction (Morgan et al., 1993; Quirk et al., 2006). More recent experiments provide evidence that firing rates in the prelimbic portion of the prefrontal cortex correlate with extinction (Burgos-Robles et al., 2009). Thus, extinction is one testing procedure that can be used to assess the functioning of the prefrontal cortex.
The purpose of the current experiment was to test whether chronic adolescent nicotine exposure would affect various types of fear conditioning and extinction in adulthood. During adolescence, rats were implanted with osmotic minipumps that delivered nicotine (or saline) for a two-week period, after which the pumps were removed. Subcutaneous osmotic minipumps were employed to avoid the stress associated with repeated drug injections (Matta et al., 2007). Following minipump removal, animals were allowed to age and become adults at which time behavioral training was initiated. In the present research, context conditioning and extinction were assessed during early adulthood, using a lick suppression procedure known to be sensitive to phenomena of classical fear conditioning including acquisition, extinction, context conditioning, contextual blocking, as well as variation in level of context conditioning produced by manipulations thought to affect context learning (Barnet et al., 1993, 1995, 1997, 2006, 2008). The lick suppression procedure additionally allowed us to evaluate any drug-induced changes in baseline behavior (drinking) prior to conditioning treatment with shock. We hypothesized that, because adolescent nicotine exposure is known to alter hippocampal and prefrontal cortical functioning, nicotine exposed rats would exhibit impaired context conditioning and extinction compared to vehicle-treated animals when tested in adulthood. Another group of animals was tested in delay conditioning, in which the presentation of a cue (e.g., tone) co-terminates with the US. Delay conditioning is not dependent upon the integrity of the hippocampus, but rather, is critically affected by manipulations of the amygdala (LeDoux, 2000). Adolescent nicotine, at similar doses to those employed in the present experiment, does not affect delay conditioning (Smith et al., 2006). We therefore expected that delay conditioning would be unaffected by adolescent nicotine administration. This finding would provide evidence that any nicotine-induced deficits in context conditioning were not due to differences in nonspecific factors (e.g., pain sensitivity) and more clearly demonstrate that context-US associations, rather than discrete CS-US, are impaired by adolescent nicotine exposure.
2. Materials and Methods
2.1. Subjects
Litters of Sprague-Dawley-descended rats from our breeding colony were used. Male and female breeder pairs were housed together in polycarbonate cages with wire lids. Pine chip bedding was provided and food (Formulab Diet 5008; W.F. Fisher & Son, Somerville, NJ) and water were available ad libitum. Cages were checked daily for new births and the day of birth was designated as Postnatal Day (PD) 0. Litters were culled to 8–10 pups on PD 2. On PD 21 rats were weaned and maintained in 50.8 × 40.6 × 21.6 cm (l × w × d) clear polycarbonate cages with wire lids. Rats were housed as a litter until PD 42 when males and females were kept in separate polycarbonate cages. The vivarium was temperature controlled and maintained on a 14:10 light/dark cycle, with light onset at 0600 h. In the current study, the subjects were 166 Sprague-Dawley rats (85 males and 81 females) representing 31 separate litters. Male and female rats were used in these experiments to increase the generalizability of the results and to make our design comparable to closely related experiments (Smith et al., 2006) and to minimize genetic bias that may contribute to any group differences. Experimental groups for context conditioning consisted of 12–13 rats each and for delay conditioning consisted of 9 rats each. All experimental procedures were consistent with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee at the College of William and Mary.
2.2. Drug Administration
Drug treatments were administered by subcutaneous osmotic minipump infusions beginning on PD 28. Each animal was anesthetized with an intraperitoneal injection of ketamine (90.0 mg/kg) and xylazine (10.0 mg/kg). A small area on the back was shaved and an incision was made to permit the subcutaneous insertion of osmotic minipumps (Alzet micro-osmotic pump model 1002, DURECT Corporation, Cupertino, CA). Pumps were prepared with nicotine bitartrate (Sigma Chemical Co., St. Louis, MO; doses based on free base) dissolved in saline to deliver an initial dose rate of 0 (saline), 3.0 or 6.0 mg/kg of nicotine per day, based on estimated weight on PD 35 (the midpoint of the dosing period). Weight estimates were derived from normative weight data collected from naïve, randomly sampled animals of our rodent colony, separately for males and females (male = 169.5 g, female = 134.9 g). The nicotine doses were chosen to approximate plasma levels reported in moderate and heavy smokers, respectively (Matta et al., 2007). Osmotic minipumps delivered nicotine at a constant rate (average pumping rate (Q) = 0.25 μl/hr) for 14 days and were removed on PD 42. The incision was closed with wound clips and the animals were permitted to recover in their home cages.
2.3. Apparatus
2.3.1. Context conditioning
Training and testing for context conditioning and extinction occurred in identical Med Associates (St. Albans, VT) modular conditioning chambers measuring 30.5 × 24.1 × 21.0 cm. The front and back walls and the ceiling of the chambers were constructed of clear Plexiglas. The two shorter side walls were constructed of aluminum. The floor consisted of parallel stainless steel rods that were connected by an electrical grid. The rods were 0.7 cm in diameter and were spaced 1.5 cm apart, center to center. The grids could be electrified to deliver scrambled 1.0-mA, 1.0-sec footshock produced by Med Associates shocker modules (ENV 414). The chamber was brightly illuminated by a 100-mA, 28-V DC houselight centered on the left aluminum wall and positioned 2.5 cm below the ceiling. The houselight bulb was contained within a cylindrical diffuser that projected light toward the top of the chamber. Background noise from a ventilation fan was 74 dB (C scale). Each of the twelve chambers was contained within a separate sound attenuating chamber.
Each chamber could be equipped with a water-filled lick tube. When inserted, the lick tube protruded 2.0 cm into a square drinking recess located on the right aluminum wall. Each recess consisted of a 5.1 cm2 aperture that was 3.0 cm deep. The recess was centered on the aluminum wall with its center 3.5 cm above the chamber floor. An infrared photobeam was projected across the tip of the lick tube. Subjects had to insert their heads approximately 1 cm into the recess in order to drink from the lick tube, thereby breaking the beam. The duration that subjects were accessing the lick tube and delivery of stimulus events were recorded and controlled by computer using MED-PC software, v.IV.
2.3.2. Delay conditioning
Training and testing for delay groups occurred in two different contexts. Training for delay conditioning occurred in the same chambers used for context conditioning as already described (and referred to here as the “main” chamber). Testing for delay conditioning occurred in a different context. The testing context for delay conditioning was created by installing a small rectangular Plexiglas insert into each main chamber. The insert measured 24.5 cm × 8.5 cm × 15.5 cm (l × w × h). The floor, one side wall, and the rear wall were constructed of clear Plexiglas. The ceiling and other side wall of the insert were constructed of steel wire mesh. The insert was positioned such that the front end of the insert would seat tightly against the front wall of the main chamber to permit access to the lick tube that was present in the chamber. The context for delay CS testing was dimly illuminated by a 100-mA, 28-V DC, 2.5-cm diameter panel light centered on the left panel of the right wall of the chamber. The panel light bulb projected light through a white opaque light diffuser creating dim diffuse illumination. A 2900-Hz, pure tone with an amplitude of 82 dB could be delivered by means of a Med Associates ™ sonalert tone module (ENV-223AM). The tone module was positioned on the left aluminum wall of the main chamber 2.0 cm from the ceiling and 4.1 cm from the rear wall.
2.4. Procedure
2.4.1. Context conditioning
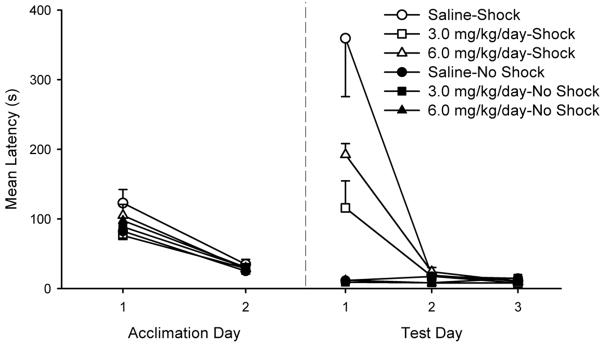
Animals in the context conditioning and extinction groups were removed from their home cages on PD 60 and placed in individual hanging wire cages. From PD 60–65, animals were handled daily and progressively deprived of water. By the beginning of behavioral training (PD 65) animals were limited to 20 min of water access per day that was provided after experimental sessions. On PD 65 and 66, all subjects were acclimated to experimental chambers used for context conditioning. On each acclimation day, subjects were placed in the chamber for 60 min and were allowed to drink from the water-filled lick tubes. The latency (s) it took for each subject to drink for 5 cumulative seconds was recorded and provided a baseline measure of drinking behavior in the lick suppression paradigm (cf. Jacobs et al., 1988). As later indicated, nicotine treated animals did not significantly differ from saline treated animals on either pre-conditioning acclimation day. All animals were performing at the same level prior to shock training.
Context conditioning was conducted on PD 67 during which all subjects were placed in the chamber for 23 minutes with water-filled lick tubes removed. There were six groups which differed in PD 67 conditioning treatment and prior adolescent drug exposure. Three “No Shock” groups were exposed to the context only with no shock presented during the conditioning session. Group Saline-No Shock had been exposed previously to saline and Groups 3.0 mg/kg/day-No Shock and 6.0 mg/kg/day-No Shock had been exposed previously to nicotine. Three “Shock” groups were exposed to 10 unsignaled shocks (1.0-mA, 1.0 s) during the session with a mean ITI of 100 s (range: 65 – 135 s). Importantly, the integrity of the hippocampus is known to be necessary for normal context conditioning even when up to 12 shocks are given in a single session (Lehmann et al., 2009). Groups Saline-Shock had been exposed previously to saline and Groups 3.0 mg/kg/day-Shock and 6.0 mg/kg/day-Shock had been exposed previously to nicotine.
The tests for context conditioning and extinction occurred on PD 68–70. During each of the three 60-min sessions a water-filled lick tube was available and suppression of drinking in the presence of context cues was assessed. On each of the three test days, latency to complete the first five cumulative seconds of drinking from placement in the chamber was recorded. Higher lick latencies reflect proportionally more suppression of drinking (i.e., higher context-elicited fear). Test 1 served as the primary test for context conditioning. Test 2 and Test 3 were identical to Test 1 and were intended to assess extinction of learned context fear across continued nonreinforced exposure to the contextual cues.
2.4.2. Delay conditioning
Delay group animals previously given 0.0, 3.0 or 6.0 mg/kg/day nicotine were removed from their home cages on PD 52 and placed in individual hanging wire cages. From PD 52–59, animals were handled daily and progressively deprived of water. By the beginning of behavioral training (PD 60) animals were limited to 20 minutes of water access per day that was provided after experimental sessions. On PD 60 animals were acclimated to the context to be used for CS testing for 60 min and had access to the lick tube. The first five cumulative seconds of drinking was recorded. On PD 61–64 animals were acclimated to training context for 60 min per day and did not have access to the lick tube. Acclimation days in the training chamber were intended to attenuate any unconditioned/neophobic responses to the context prior to conditioning with the target (tone) CS.
On PD 65 delay conditioning occurred in the training context for all animals. During the single 60-min session, animals were exposed to ten tone-shock conditioning trials with a mean ITI of 315 sec (range: 255 –375 s). The tone was a 15-sec, 2900-Hz, pure tone with an amplitude of 82 dB (C) and shock was 1.0-mA, 1.0-sec in duration. Shock occurred during the last second of each tone presentation. Animals did not have access to lick tubes during delay conditioning.
On PD 66–67, animals were exposed to one 60-min recovery session in the test context (Plexiglas insert). During the recovery session, rats were allowed to drink from water-filled lick tubes. No discrete CS or US was presented. The purpose of recovery sessions was to restabilize drinking behavior following shock sessions prior to target CS testing.
On PD 68–70, conditioning to the tone was assessed in the test context. Animals were placed in the test context with access to the lick tube. After drinking for five cumulative seconds (pre-CS period), the tone CS was presented (CS period) and remained on until the animal completed an additional five cumulative seconds of drinking in the presence of the tone CS. Suppression of drinking in the presence of the tone was taken as a measure of learned fear. Interest was in whether suppression to the tone following delay conditioning would differ as a result of prior adolescent nicotine exposure.
2.5. Statistical Analyses
To control for litter effects, no more than two animals from each litter (one female, one male) was represented in each treatment group. When more than one male or female from a litter was assigned to a particular group, a mean from those animals was computed and served as the unit for data analysis in order to avoid overrepresentation of any litter. Mixed factor ANOVAs were conducted on test data followed by planned comparisons using the overall error term from each analysis. A level of α = 0.05 was used to determine statistical significance.
3. Results
3.1. Context Conditioning and Extinction
None of the groups differed in lick latency on either preconditioning acclimation day (Fs ≤ 1.20; Figure 1). An initial analysis failed to demonstrate any effects or interactions with sex (all p > .36), however, assessment of sex differences was not a primary goal of this experiment and this analysis likely lacks sufficient sample size. The reported analyses reflect data combined for males and females. A 2 (Condition) X 3 (Drug) X 3 (Test Day) mixed factor ANOVA was subsequently conducted on test data. The between-subjects factors were Condition (Shock, No Shock) and Drug (Saline, 3.0 mg/kg/day, 6.0 mg/kg/day) and the within-subjects factor was Test Day (Test 1, Test 2, Test 3). The analysis revealed significant main effects of Condition (F(1, 64) = 29.44, p < .001), Drug (F(2, 64) = 3.67, p < .03), and Test Day (F(2, 128) = 31.15, p < .001), as well as significant interactions between Condition X Test Day (F(2, 128) = 31.79, p < . 001), Drug X Test Day (F(4, 128) = 3.65, p < .008) and a Condition X Drug interaction (F(2, 64) = 2.96, p = .059) that approached statistical reliability. The three-way Condition X Drug X Test Day interaction was also reliable, (F(4, 128) = 3.91, p < .005). Interaction contrasts and subsequent planned comparisons were conducted separately on data from each test day. Condition X Drug ANOVAs revealed significant effects of Condition (F(1, 64) = 31.53, p < .001), and Drug (F(2, 64) = 3.73, p < .03) as well as a significant Condition X Drug interaction on Test 1 (F(2, 64) = 3.62, p < .03). No effects or interactions were significant on subsequent tests.
Fig. 1.
Mean latency to complete five cumulative seconds of drinking in the presence of context cues on each of the two acclimation days and the three consecutive test days in subjects tested as adults (PD 68–70). Lower values reflect comparatively weaker context conditioning. Shock groups received 10 unsignaled shocks on the conditioning day and No Shock groups received context exposure in the absence of shock. The groups further differed in whether they had received saline or nicotine (3.0 mg/kg/day, or 6.0 mg/kg/day) during adolescence (PD 28–42). Error bars represent SEMs.
As can be seen in Figure 1, shock exposed groups showed greater suppression to the context compared to no-shock groups. Test 1 latencies in Groups Saline-Shock and 6.0 mg/kg/day-Shock were significantly higher than in corresponding no-shock groups, (Fs(1, 64) ≥ 30.84 and 7.97, respectively, ps < .006) indicating shock treatment was effective at establishing context conditioning in these groups. Animals in Group 3.0 mg/kg/day-Shock had higher latencies than animals in Group 3.0 mg/kg/day-No Shock but this difference was not significant, (F(1, 64) = 2.43, p > .10). The three no-shock groups did not differ (Fs(1, 64) < 1).
Of critical interest, the figure also suggests that levels of context learning assessed on Test 1 were not the same in groups exposed to different drug treatments during adolescence. Both shock groups receiving nicotine during adolescence, Group 3.0 mg/kg/day and Group 6.0 mg/kg/day, had significantly lower latencies compared with the Saline-Shock group, (F(1, 64) = 14.53, p < .0003, and F(1, 64) = 6.83, p < . 011, respectively). Therefore, adolescent nicotine treatment impaired later adult context fear learning. Finally, Group 3.0 mg/kg/day demonstrated lower levels of suppression compared to Group 6.0 mg/kg/day but this difference was not reliable (F (1, 64) = 1.44, p > .20).
Patterns of context suppression across subsequent extinction testing on Test 2 and Test 3 suggest that extinction of context fear in all groups was relatively rapid and nearly complete by Test 2 (see Figure 1). A Drug X Test Day ANOVA conducted on data from shock-exposed animals revealed significant effects of drug (F(2, 33) = 3.56, p < .04), Test Day (F(2, 66) = 34.09, p < .001), and a significant Drug X Test Day interaction (F(4, 66) = 4.05, p < .005). Groups Saline-Shock and 6.0 mg/kg/day-Shock had significantly shorter latencies on Test 2 compared to Test 1 (Fs(1, 66) ≥ 11.15, ps < .001) and there was no further reduction in latencies on Test 3 compared to Test 2, (Fs (1, 66) < 1) for these groups. Group 3.0 mg/kg/day-Shock had numerically lower latencies on Test 2 and 3 compared to Test 1 but these differences were not reliable. Comparable analyses conducted on data from No Shock groups failed to reveal any significant effect or interaction (Fs < 1).
3.2. Delay Conditioning
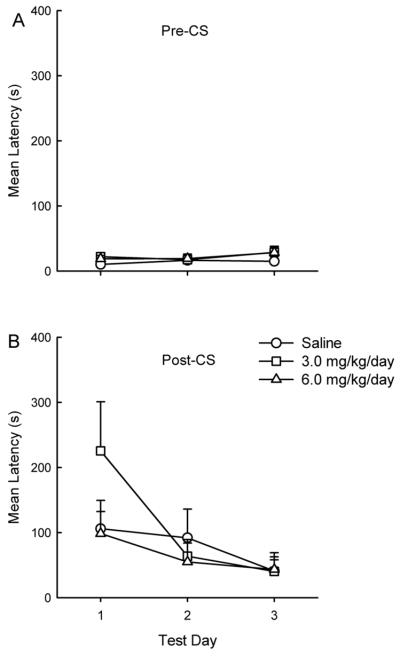
Data from the CS tone test of delay conditioning are shown in Figure 2. Preliminary analyses of test data with sex as a factor failed to reveal any effect or interaction with sex (Fs < 1, p > .44), although, as with context conditioning, testing sex differences was not a primary goal of the present experiment. Subsequent analyses are reported that combine data across sex. Pre-conditioning baseline latencies did not differ among groups (F s < 11 p > .35) and no differences were seen in pre-CS latency scores on CS test days (F s < 1.59 p > .22). A Drug X Test Day ANOVA conducted on the test data revealed significant effects of Test Day (F(2, 48) = 10.30, p < .001), and a Drug X Test Day interaction that approached, but did not reach statistical significance (F(4, 48) = 2.45, p = .059). As Figure 2 suggests, CS suppression in the 3.0 mg/kg/day group was higher than in the remaining groups on the first test after which suppression extinguished uniformly in all groups. It is notable that the drug exposure condition which produced the strongest negative impact on context conditioning (see Figure 1), similarly had the strongest effect on delay conditioning (see Figure 2). Group 3.0 mg/kg/day differed significantly from Groups Saline and 6 mg/kg/day on Test 1 (Fs(1, 48) > 8.91, p < .005) with no further group differences on subsequent test days (F < 1.0).
Fig. 2.
Mean latency to complete five cumulative seconds of drinking in the presence of the tone in subjects tested as adults. Animals were exposed to ten tone-shock pairings on the delay conditioning day. The top panel (Pre-CS; A) depicts the latency to complete five cumulative seconds of drinking prior to CS presentation on test days. The bottom panel (Post-CS; B) depicts the latency to complete five cumulative seconds of drinking in the presence of the CS on test days. The groups differed in whether they had received saline or nicotine (3.0 mg/kg/day, or 6.0 mg/kg/day) during adolescence (PD 28–42). Error bars represent SEMs.
4. Discussion
The current experiment assessed the consequences of chronic adolescent nicotine exposure on learning and memory in adulthood. We found that adolescent nicotine exposure produced deficits in context conditioning, a form of learning dependent upon the hippocampus (Kim & Fanselow, 1992; Phillips & LeDoux, 1992). Rats exposed to both moderate (3.0 mg/kg/day) and high (6.0 mg/kg/day) doses of nicotine for a two-week period during adolescence displayed evidence of impaired context learning when tested later as adults, compared to rats that were not exposed to nicotine. Although not statistically reliable, we also observed that 3.0 mg/kg/day nicotine produced a greater impairment in context conditioning than 6.0 mg/kg/day nicotine. However, there were no differential effects of adolescent nicotine exposure during subsequent extinction testing sessions. We also did not observe any evidence that adolescent nicotine exposure decreased delay conditioning. Importantly, the present experiment did not include a comparison group of animals that received nicotine in adulthood and were tested one month later. Thus, we cannot state that the present effects of nicotine on context conditioning are specific to adolescent administration. Collectively, these findings suggest that associations between the context and the US are specifically impaired by prior nicotine exposure, rather than a general impairment in associative learning involving any type of stimulus that is paired with the US. If anything, the results from the delay conditioning experiment suggest that 3.0 mg/kg/day nicotine during adolescence may strengthen delay conditioning when trained and tested in adulthood. The latter observation is interesting because it suggests that under some circumstances nicotine may shift the relative strength of associations between the context and cue to a weaker association with the context and a stronger association with a cue (see Marlin, 1981 for an example of weaker responding to context correlated with stronger responding to CS). Future research examining the role of specific nicotinic receptors, including the α4β2 subtype which has a higher affinity for nicotine, may be useful for understanding why the 3.0 mg/kg/day dose appears to have more substantial effects on learning than the 6.0 mg/kg/day dose.
None of the groups differed in lick latency during the preconditioning acclimation sessions of the context conditioning procedure. Furthermore, no-shock controls previously exposed to nicotine (3.0 mg/kg/day-No Shock, 6.0 mg/kg/day-No Shock) versus saline (Saline-No Shock) did not differ on any test day. The lack of group differences in lick latencies prior to shock exposure and between groups not exposed to shock suggest that the nicotine-related shorter lick latencies observed on the initial context test day cannot be attributed to changes in locomotor activity or in motivation for water induced by adolescent nicotine administration. The lack of effects of nicotine exposure on delay conditioning further indicates that the nicotine-treated animals were not differentially sensitive to the shock (see also Carstens et al., 2001; Yang et al., 1992). Smith et al. (2006) found that adolescent nicotine exposure decreases time spent in the center of an open field when tested during adulthood. This finding suggests that adolescent nicotine exposure may have increased anxiety. If increases in anxiety occurred in the present experiment, it would be expected that nicotine-exposed animals would demonstrate higher levels of context conditioning. The opposite was observed in the present experiment, although the present experiment used higher nicotine doses compared to Smith et al. (2006). Based on the available data, it seems unlikely that the nicotine-induced decrease in context conditioning is due to concomitant effects on anxiety.
Of the few studies that have examined the effects of adolescent nicotine exposure on hippocampus dependent memory tested in adulthood, Smith et al. (2006) did not find evidence of impaired context conditioning. There are many possibilities for the different results obtained in the present experiment and that of Smith et al. (2006). First, we used higher doses of nicotine (2.0 mg/kg/day was the highest dose in Smith et al., 2006). Second, we employed a lick suppression paradigm for measuring fear conditioning, as opposed to Smith et al.’s (2006) use of freezing as the dependent measure. It may be, as we have previously observed (Hunt et al., 2007), that there are differences in the sensitivity of dependent measures of fear to the effects of nicotine (see also Kenney & Gould, 2008a). Similarly, the failure to demonstrate differences in extinction following adolescent nicotine exposure in the present experiment may have been due to task parameters. Specifically, with the test sessions lasting 60 min, the rats were in the chambers for a relatively long time during the first test session with no shock exposure. As a result, extinction likely proceeded rapidly and the procedures may have not been sufficiently sensitive to detect the effects of adolescent nicotine exposure on extinction behavior.
Contextual fear conditioning involves multiple processes that are assumed to involve different brain substrates. First, a representation of the context is formed when a subject is exposed to a novel environment. The representation of context as a configuration of cues involves the hippocampus (Fanselow, 2000; Rudy & O’Reilly, 1999), although it is believed that the representation is stored elsewhere in the brain. Next, the representation of the context is associated with the aversive US. The amygdala is the critical region involved in this association (Matus-Amat et al., 2007). And finally, upon re-exposure to the context during the test phase the memory is retrieved and elicits a variety of fear responses, including freezing and the suppression of ongoing behavior (e.g. licking). Gould and colleagues have rather extensively examined the effects of acute and chronic nicotine given to adult mice on context conditioning. Acute nicotine can facilitate context conditioning (Kenney & Gould, 2008b) whereas withdrawal from chronic nicotine exposure impairs context conditioning (Davis et al., 2005). The latter finding is reminiscent of the present results. On the basis of his and other research, Gould and colleagues have hypothesized that nicotine affects the function of the hippocampus and the consequences of nicotine for context conditioning are primarily on the first phase, the formation of the representation of the context (Kenney & Gould, 2008b). In the present experiment, all phases of training and testing occurred after the termination of chronic nicotine treatment. Therefore, it remains possible that nicotine may have affected initial representational or later associative processes. Precisely how nicotine impairs complex cognitive processes has not yet been resolved, and remains important to the study of neural mechanisms underlying nicotine’s memory-impairing action. In the present experiment, the effects of nicotine on context conditioning occurred after a substantial time period between drug termination and initiation of behavioral testing. Thus, these deficits may be due to nicotine-induced cell loss. Nicotine has been shown to decrease the number of cells in the hippocampus, as measured by decreases in DNA content (Abreu-Villaca et al., 2003).
In conclusion, these data suggest that the effects of adolescent nicotine exposure are sufficient to lead to deficits in hippocampus-dependent processing when tested in adulthood. Future experiments testing the effects of adolescent nicotine exposure on other hippocampus-dependent tasks, such as trace conditioning, would help to assess the generalizability of the impairments in hippocampus-dependent processing following adolescent nicotine exposure. Findings of this experiment further suggest the importance of specific task parameters chosen to detect impairments in hippocampus dependent memory following adolescent nicotine exposure (cf. Smith et al., 2006). Collectively, these data contribute to a growing literature demonstrating cognitive deficits that can be measured in adulthood following exposure to nicotine during the adolescent period (Counotte et al., 2009; Fountain et al., 2008; Smith et al., 2006).
Research highlights.
Adolescent nicotine exposure disrupted context conditioning
Adolescent nicotine exposure did not significantly affect delay conditioning
Adolescent nicotine exposure may disrupt hippocampus-dependent learning
Acknowledgments
This research was supported by grants from the Virginia Tobacco Settlement Foundation and the National Institute on Alcohol Abuse and Alcoholism (AA015343 to P.S.H.). The authors wish to thank Johanna Smyth for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu-Villaca Y, Seidler FJ, Tate CA, Slotkin TA. Nicotine is a neurotoxin in the adolescent brain: critical periods, patterns of exposure, regional selectivity, and dose thresholds for macromolecular alterations. Brain Res. 2003;979:114–128. doi: 10.1016/s0006-8993(03)02885-3. [DOI] [PubMed] [Google Scholar]
- Barnet RC, Cole RP, Miller RR. Temporal integration in second-order conditioning and sensory preconditioning. Anim Learn Behav. 1997;25:221–233. [Google Scholar]
- Barnet RC, Fields NR, Smigel E. Temporal specificity of the US preexposure effect produced by excitatory status of local context. Paper presented at the annual meeting of the Eastern Psychological Association; Baltimore, MD. 2006. [Google Scholar]
- Barnet RC, Grahame NJ, Miller RR. Local context and the comparator hypothesis. Anim Learn Behav. 1993;21:1–13. [Google Scholar]
- Barnet RC, Grahame NJ, Miller RR. Trial spacing effects in Pavlovian conditioning: A role for local context. Anim Learn Behav. 1995;23:340–348. [Google Scholar]
- Barnet RC, Mullis CE. Temporally specific context fear Is not lost after extinction. Poster presented at the annual meeting of the Eastern Psychological Association; Philadelphia, PA. 2008. [Google Scholar]
- Bergstrom HC, McDonald CG, French HT, Smith RF. Continuous nicotine administration produces selective, age-dependent structural alteration of pyramidal neurons from prelimbic cortex. Synapse. 2008;62:31–39. doi: 10.1002/syn.20467. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens E, Anderson KA, Simons CT, Carstens MI, Jinks SL. Analgesia induced by chronic nicotine infusion in rats: differences by gender and pain test. Psychopharmacology. 2001;157:40–45. doi: 10.1007/s002130100770. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34:29–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behav Brain Res. 1999;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Factors governing one trial context conditioning. Anim Learn Behav. 1990;18:264–270. [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD, Kelley BM, Willey AR, Nolley EP. Adolescent exposure to nicotine impairs adult serial pattern learning in rats. Exp Brain Res. 2008;187:651–656. doi: 10.1007/s00221-008-1346-4. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Barnet RC, Burk JA, Smyth JC. Effects of acute nicotine administration on Pavlovian fear conditioning in rats as measured by freezing and potentiated startle. Presented at the meeting of the Eastern Psychological Association; 2007. [Google Scholar]
- Jacobs WJ, Buttrick M, Kennedy D. A rapid and sensitive method for measuring the conditional emotional response: II. On-the-baseline excitatory conditioning and extinction. Pavlov J Biol Sci. 1988;23:29–34. doi: 10.1007/BF02910542. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national survey results on adolescent drug use: overview of key findings, 2007 (NIH Publication No. 08-6418) Bethesda, MD: National Institute on Drug Abuse; 2008. [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008a;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Nicotine enhances context learning but not context-shock associative learning. Behav Neurosci. 2008b;122:1158–1165. doi: 10.1037/a0012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Sparks FT, Spanswick SC, Hadikin C, McDonald RJ, Sutherland RJ. Making context memories independent of the hippocampus. Learn Mem. 2009;16:417–420. doi: 10.1101/lm.1385409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin NA. Contextual associations in trace conditioning. Anim Learn Behav. 1981;9:519–523. [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121:721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Polesskaya OO, Fryxell KJ, Merchant AD, Locklear LL, Ker KF, McDonald CG, Eppolito AK, Smith LN, Wheeler TL, Smith RF. Nicotine causes age-dependent changes in gene expression in the adolescent female rat brain. Neurotoxicol Teratol. 2007;29:126–140. doi: 10.1016/j.ntt.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Scopolamine administered before and after training impairs both contextual and auditory-cue fear conditioning. Neurobiol Learn Mem. 1996;65:73–81. doi: 10.1006/nlme.1996.0008. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci. 1999;113:867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Smith LN, McDonald CG, Bergstrom HC, Brielmaier JM, Eppolito AK, Wheeler TL, Falco AM, Smith RF. Long-term changes in fear conditioning and anxiety-like behavior following nicotine exposure in adult versus adolescent rats. Pharmacol Biochem Behav. 2006;85:91–97. doi: 10.1016/j.pbb.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Yang C-Y, Wu W-H, Zbuzek VK. Antinociceptive effect of chronic nicotine and nociceptive effect of its withdrawal measured by hot-plate and tail-flick in rats. Psychopharmacology. 1992;106:417–420. doi: 10.1007/BF02245428. [DOI] [PubMed] [Google Scholar]