Summary
Background
Myosin II is an essential component of the contractile ring that divides the cell during cytokinesis. Previous work showed that regulatory light chain (RLC) phosphorylation is required for localization of myosin at the cellular equator [1, 2]. However, the molecular mechanisms that concentrate myosin at the site of furrow formation remain unclear.
Results
By analyzing spatiotemporal dynamics of mutant myosin subunits in Drosophila S2 cells, we show that myosin accumulates at the equator through stabilization of interactions between the cortex and myosin filaments, and the motor domain is dispensable for localization. Filament stabilization is tightly controlled by RLC phosphorylation. However, we show that regulatory mechanisms other than RLC phosphorylation contribute to myosin accumulation at three different stages; 1) turnover of thick filaments throughout the cell cycle, 2) MHC-based control of myosin assembly at the metaphase-anaphase transition, and 3) redistribution/activation of myosin binding sites at the equator during anaphase. Surprisingly, the third event can occur to a degree in a Rho-independent fashion, gathering pre-assembled filaments to the equatorial zone via cortical flow.
Conclusions
Multiple regulatory pathways cooperate to control myosin localization during mitosis and cytokinesis to ensure that this essential biological process is as robust as possible.
Keywords: cytokinesis, myosin II, myosin regulatory light chain, FRAP, Drosophila S2 cell
Results and Discussion
Thick filament assembly but not actin binding are required for myosin stabilization at the cortex
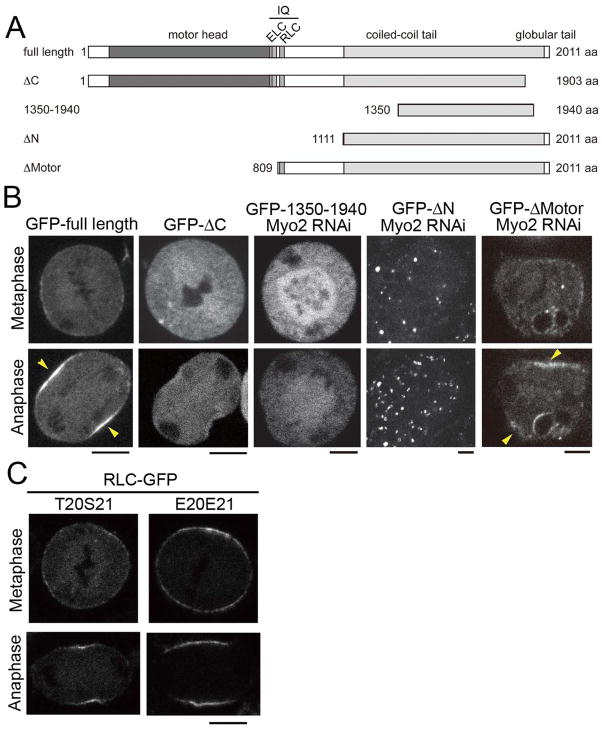
Accumulation of myosin at the equatorial cortex is one of the earliest events of cytokinesis onset [3], and is tightly regulated spatially and temporally. Previously, we showed that the actin-binding motor head domain of myosin is not required for localizing the protein to the site of furrow formation in Dictyostelium and fission yeast cells [4–6]. However, it has not been determined if the motor domain is required for localizing myosin in higher eukaryotic organisms. We therefore made a GFP-tagged Drosophila myosin construct that started at the beginning of the second IQ domain and extended to the end of the C-terminus (GFP-ΔMotor; aa 809–2011)(Figure 1A), and we transfected this into Drosophila S2 cells. To ensure that this protein did not form heterogeneous filaments with the endogenous protein, we treated these cells with a dsRNA that targets the motor domain. We found that GFP-ΔMotor was capable of enriching at the equatorial cortex at anaphase onset (Figure 1B; Movie S1), and cells expressing only this motorless myosin could not complete furrow ingression, indicating that the endogenous heavy chain had been efficiently depleted. We therefore decided to determine which other domains of myosin were required for its localization to the equatorial cortex.
Figure 1. Thick filament assembly and RLC phosphorylation, but not actin binding are necessary for myosin stabilization at the cortex.
(A) Schematic representation of five versions of the myosin heavy chain protein. All deletions but the ΔC construct possess the full domain for self assembly which has been described in [9]. IQ domain consists of ELC-binding domain (ELC) and RLC-binding domain (RLC). (B) The full-length, and GFP-ΔMotor proteins showed enrichment at the equatorial cortex during anaphase (indicated by yellow arrowheads), but the GFP-ΔC, GFP-ΔN, and GFP-1350–1940 proteins did not show cortical accumulation at the equator of anaphase S2 cells. See also Figure S1A and Movie S1. Bars, 5 μm. (C) The phosphomimetic RLC[E20E21]-GFP concentrates on the cortex prior to anaphase onset but does not localize to the equator until anaphase onset.
The last 28-residue repeat at the C-terminal end of the coiled-coil region of the MHC is critical for thick filament formation [7, 8]. Consistent with C-terminal deletion mutants of mammalian non-muscle myosin [8] and also a recent report by Liu et al. in S2 cells [9], we found that a GFP-MHC that lacks this C-terminus (GFP-ΔC) was mainly distributed in the cytoplasm throughout mitosis and anaphase (Figure 1A, B). In addition, FRAP (fluorescence recovery after photobleaching) analysis revealed that GFP-ΔC was not stabilized at the cortex (Figure S1A). Therefore, we conclude that thick filament formation is required for myosin stabilization at the equatorial cortex.
We next tested a truncated myosin (GFP-MHC (GFP-1350–1940; see Figure 1A)) that contains the critical assembly domain and was previously shown by Liu and coworkers to form filaments in vitro and target to the equatorial cortex in anaphase Drosophila S2 cells [9]. However, in their study, they did not deplete the endogenous heavy chain, and were not able to eliminate the possibility that the cortically targeted truncated myosin was incorporated into heterogeneous filaments with the endogenous MHC. In agreement with Liu and coworkers, we found that GFP-1350–1940 targets to the equatorial cortex in cells exposed to a control dsRNA (not shown), but when we depleted the endogenous MHC using a dsRNA that targets the motor domain, we found that GFP-1350–1940 localized diffusely throughout mitosis with no indication of cortical localization or filament formation (Figure 1B).
To determine if the defect in cortical localization of GFP-1350–1940 was due only to a lack of filament formation, we created a construct that begins at the coiled-coil rod domain (GFP-ΔN; aa 1111–2011) and contains all of the filament assembly and globular tail domains. This protein localized to the equatorial cortex during anaphase in cells containing the endogenous MHC (not shown). However, when we depleted the endogenous myosin, the protein was diffusely localized with some small aggregates of bright fluorescence in metaphase. Curiously, during anaphase many evenly distributed fluorescent rings and punctae appeared (Figure 1B; Movie S1) but no protein accumulated at the equatorial cortex, suggesting that this form of this MHC can form higher order structures and react to the change in cell physiology brought about at the metaphase to anaphase transition and that the formation of these structures may be suppressed during metaphase. Post-translational modifications of the MHC and binding to accessory proteins have been shown to regulate myosin assembly, independent of RLC phosphorylation, in other systems [6, 10–13]. The inability of this construct to target to the furrow indicates that the 202 amino acid region between the motor head and the coiled-coil portions of the MHC are essential for localizing to the cytokinetic furrow during anaphase. However, we do not know if this domain is essential for forming filaments of the proper geometry or because it interacts with some component at the equatorial cortex.
The mechanism by which RLC phosphorylation controls myosin accumulation in the cortex is not well understood. In vitro, RLC phosphorylation results in the stabilization of the myosin thick filament [14], which is the main form of myosin within the contractile ring [15, 16]. Therefore, we wished to investigate the localization of RLC mutants, in which phosphorylation sites at threonine 20 and serine 21 were replaced with glutamate or alanine to mimic the phosphorylated or unphosphorylated forms of RLC [17, 18] and thus potentially stabilize or destabilize filament formation respectively. When we compared the localization of RLC[E20E21]-GFP to the wild-type protein in mitosis, we found that it was more abundantly localized to the cortex in metaphase; however, equatorial enrichment did not begin until anaphase onset (Figure 1C; [2]). In contrast, the non-phosphorylated RLC[A20A21]-GFP did not show any apparent accumulation at the cortex when the endogenous RLC was specifically depleted by RNAi treatment for 4–5 days (not shown; [2]). This suggests that RLC phosphorylation promotes redistribution of cytoplasmic myosin to the cortex, but that the particular binding sites that concentrate myosin at the cortex do not redistribute or become active at the equator until anaphase onset when microtubules and/or Rho-dependent activities localize to the site of cytokinetic furrow formation.
Taken together these data show that filament formation is tightly controlled and necessary but not sufficient for localizing myosin to the equatorial cortex. The GFP-ΔN-MHC forms filaments at anaphase onset but lacks some critical region that directs myosin to the equatorial cortex. Additionally, expressing a constitutively phosphorylated RLC drives filament formation and premature localization of myosin to the mitotic cortex, but these filaments cannot localize to the equator until after anaphase onset when the rest of the cytokinetic machinery is targeted to this zone.
Myosin is progressively stabilized at the equatorial cortex as cells progress from metaphase to anaphase by RLC phosphorylation
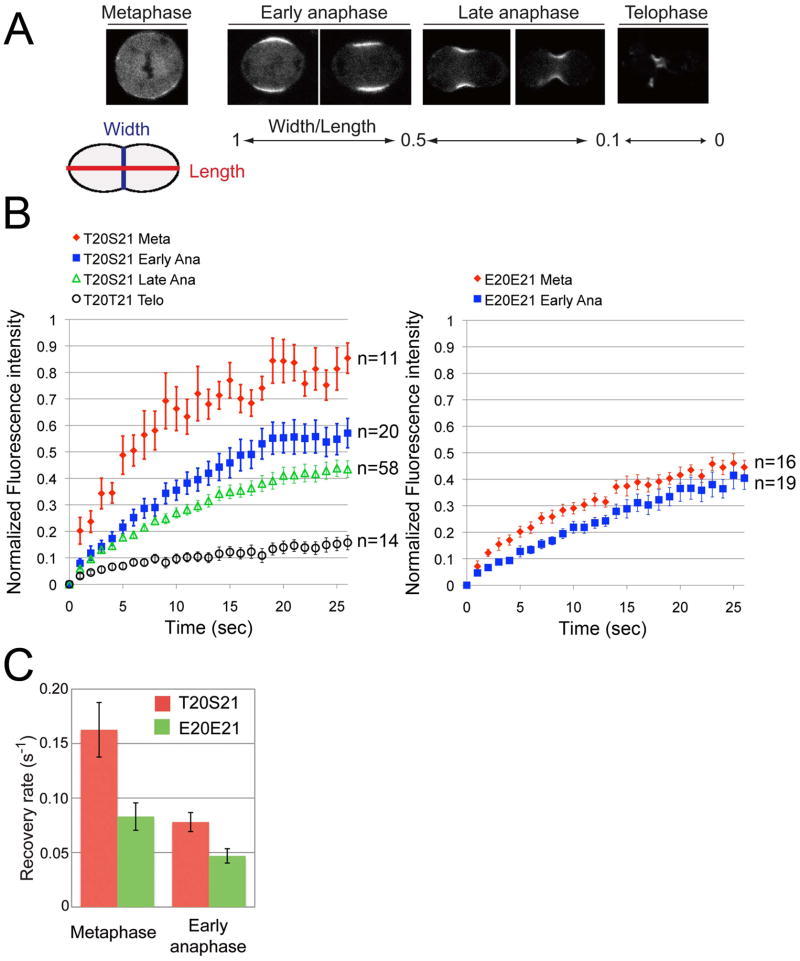
Observing that the phosphomimetic RLC can target to the cortex prior to anaphase onset suggests that Rho-kinase dependent phosphorylation may stimulate the binding of myosin to the equatorial cortex as cells enter anaphase. We would therefore predict that as cells progress through anaphase and the level of Rho activity increases there would be progressive stabilization of myosin at the equatorial cortex. To test this possibility, we measured the turnover of myosin at the equatorial cortex from metaphase to anaphase by FRAP analysis of GFP-tagged RLC. We divided the contraction process into three temporal phases according to the degree of contraction - early anaphase, late anaphase, and telophase (Figure 2A). FRAP of the RLC-GFP revealed that the amount and rate of myosin turnover in the equatorial cortex dramatically decreases upon the metaphase-anaphase transition, and further decreases as constriction of the contractile ring proceeds (Figure 2B, C). Similar, but somewhat slower recovery was observed for the GFPMHC (Figure S1B-C).
Figure 2. Stable association of myosin to the equatorial cortex after anaphase is driven by RLC phosphorylation.
(A) GFP-myosin localization from metaphase to telophase. We divided anaphase/telophase into three phases according to the ratio of cell width over length. (B) FRAP of RLC-GFP and RLC[E20E21]-GFP. We observed slower recovery rates as anaphase progresses for both constructs and at each stage the recovery of the phosphomimetic RLC was slower. Error bars represent SEM. Observed cell number is indicated for each sample. (C) Mean recovery rate of RLC-GFP and RLC[E20E21]-GFP in metaphase and early anaphase, with SEM. See also Figure S1.
To investigate the impact of RLC phosphorylation on myosin turnover, we compared the FRAP rate of GFP-tagged wild-type RLC[T20S21] with that of the phosphomimetic RLC[E20E21]. In metaphase, we found that FRAP recovery of RLC[E20E21]-GFP was much slower than the wild-type RLC (Figure 2B). The rate of recovery of RLC[E20E21]-GFP in metaphase (0.08 ± 0.01s−1, n= 16) was very similar to the rate of wild-type RLC in anaphase (0.07 ± 0.01 s−1, n=20), suggesting that for the phosphomimetic RLC case the myosin-cortical interaction becomes “anaphase-like” in its kinetics of turnover during metaphase (Figure 2C).
To gain insight into how myosin reaches the equatorial cortex in anaphase, we determined the diffusion coefficient of myosin in the cytoplasm by FRAP. We photobleached GFP-MHC in the cytoplasm, and monitored fluorescence recovery. By applying the two-dimensional diffusion equation [19] we obtained about 0.8 μm2 s−1 as the diffusion coefficient of myosin containing GFP-MHC (Figure S1F-G), which is similar to the value recently reported through a different approach in C. elegans [20]. This suggests that myosin freely diffuses in the cytoplasm, and that a significant number of myosin molecules could localize to the equatorial cortex by a random diffusion mechanism during early anaphase.
Thus, myosin accumulation at the equatorial cortex can be explained by a diffusion-trapping mechanism, in which myosin molecules reach the equatorial cortex by Brownian motion, and are induced to form filaments by Rok-dependent phosphorylation of the RLC. These filaments become anchored at the cortex and incorporated into the cytokinetic ring. Then, our results suggest that as the cytokinetic ring ingresses, myosin’s turnover is reduced by a combination of factors including high local activity of Rho-dependent kinases that phosphorylate the RLC, mechanosensory effects that require RLC phosphorylation and regulate myosin localization in Dictyostelium [21], and by the shrinkage of the ring by the shedding of “contractile units” [22] which would inhibit the exchange of unbleached filaments.
Pre-assembled myosin filaments can localize to the equatorial cortex in the absence of Rho
Our earlier observations showed that Rho kinase is not required for recruitment of myosin to the equatorial cortex if the Drosophila RLC is replaced with the phosphomimetic RLC [2]. Taking one step upstream in the signaling pathway, we now asked whether Rho itself is required for the localization of myosin filaments at the equatorial cortex.
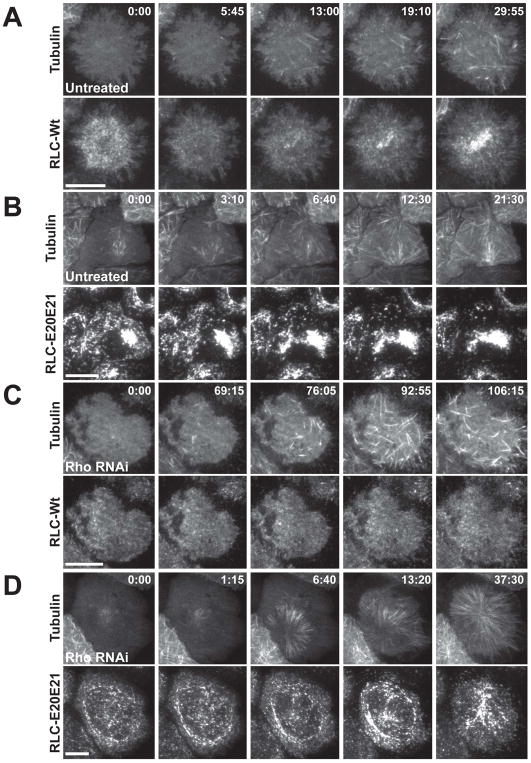
We observed S2 cells expressing mCh-α-tubulin and RLC-GFP plated on conA by total internal reflection fluorescence (TIRF) microscopy. In control cells, we detected the characteristic decrease in polar myosin and subsequent increase in equatorial myosin as cells entered anaphase, consistent with our previous observation (Figure 3A: Movie S2; Figure S2A; [15]). In cells expressing only the phosphomimetic RLC, we observed large cortical aggregates of myosin that were rapidly incorporated into the equatorial band of myosin (Figure 3B; Movie S2) as well as clearance of polar myosin. When we measured total myosin fluorescence at the cortex, we only observed a very slight and transient increase after anaphase onset, which is consistent with the expression of RLC[E20E21] leading to constitutive filament formation (data not shown). However, we still observe myosin filament assembly and disassembly in interphase and mitotic cells expressing only the phosphomimetic RLC (not shown). Beach and Egelhoff also observed that filaments made from an MHC mutant that favors bipolar thick filament formation were still dynamic in mitosis and cytokinesis, which is consistent with the presence of an RLCindependent mechanism that promotes filament turnover during mitosis [23].
Figure 3. Preassembled myosin filaments can target the equatorial cortex in the absence of Rho.
(A) An S2 cell expressing RLC-GFP and mCh-tubulin was plated on a conA coated dish and then imaged from metaphase until late anaphase using TIRF microscopy. (B) Cells expressing only the RLC[E20E21]-GFP and mCh-tubulin were imaged as above. (C) Cells expressing the RLC-GFP were depleted of Rho and imaged in TIRF. In 20% of cells some transient bursts of myosin filament formation were observed. However, in 80% of cells no myosin filament formation was observed (n > 10). (D) In cells depleted of Rho and expressing exclusively the phosphomimetic RLC[E20E21]-GFP, higher levels of cortical myosin are observed prior to anaphase and the protein slowly enriches at the equatorial cortex after anaphase (indicated by yellow arrows) See also Figures S2, S3, and S4 and Movies S2, S3, and S4. Bar, 10 μm.
When we imaged cells expressing the wild-type RLC-GFP treated with a dsRNA targeting Rho, we saw very transient enrichment of myosin in dispersed patches on the equatorial cortex in approximately 20% of cells (indicative of some residual Rho activity due to incomplete depletion), but the distribution of cortical myosin did not noticeably change after anaphase onset in most cells (n > 10 cells observed; 3 representative cells were analyzed; Figure 3C; Movie S3; Figure S2B).
In cells expressing only the phosphomimetic RLC which lack Rho, myosin particles slowly accumulated in regions of the equatorial cortex after anaphase onset, and the filaments were less tightly packed compared to what was observed in cells with active Rho (n > 10 cells observed; Figure 3D; Movie S4; Figure S2C show results from 3 representative cells). The observed myosin accumulation could have been driven by residual Rho activity in cells depleted of Rho and the endogenous RLC, but Western blotting confirmed that the Rho-depletion was as complete in these cells as in the cells expressing the wild-type RLC (Figure S3A). The Rho-independent myosin accumulation was less robust and less stably anchored than what was seen in cells with Rho, possibly due to a lack of active Anillin that can act as a scaffold to link proteins in the cytokinetic ring. Interestingly, in cells lacking Rho, it appeared that cortical flow was able to drive myosin filaments to the equatorial cortex and thus to contribute myosin redistribution (see Movie S4). Kymograph analysis confirmed the lateral movement of these assembled cortical myosin particles (Figure S3B-B’). In sharp contrast, in untreated cells, we observed no evidence of cortical flow in kymographs generated along the cortex during contractile ring formation (Figure S4), which is consistent with previous studies showing that myosin can accumulate at the equatorial cortex without flow [15, 16, 24]. In untreated cells, it is only after myosin filaments have accumulated at the equator that we see some evidence for cortical flow at the edges of the equatorial band of myosin in the TIRF field ([15]; data not shown). Therefore, it is possible that cortical flow may be active in these cells, but may normally only serve to reinforce the spatial distribution of myosin filaments that is achieved by localized filament formation and retention regulated by the Rho-Rok pathway. If the Rho-dependent phosphorylation of the RLC is bypassed, cortical flow of myosin and/or myosin binding sites may be sufficient to concentrate some myosin at the equatorial cortex, which would explain why the accumulation of the phosphomimetic myosin filaments was significantly delayed compared to cells containing active Rho. However, this concentration of myosin was insufficient to drive cytokinesis; Rho-depleted cells expressing only the phosphomimetic RLC plated on poly-D-Lysine coated glass-bottom dishes were unable to form functional furrows (data not shown).
In an unperturbed state, cortical flow could contribute to myosin retention at the equator in an indirect fashion, via redistribution of myosin binding sites, which could be required for the maturation of the actomyosin ring. However, several lines of evidence show that cortical flow requires assembled myosin filaments [16, 24, 25], suggesting that this type of movement cannot trigger the initial concentration of myosin at the cortex. To investigate dynamic redistribution of myosin binding sites, it will be essential to identify their components.
In summary, we have shown that myosin requires several different inputs in order to reach the site of cytokinetic furrow formation. It does not require its actin binding capability but it must be competent for filament formation and contain an essential region between the RLC binding domain and the coiled-coil domain. It also appears that there is a mechanism that targets the MHC to inhibit filament formation during mitosis. We also observed that phosphorylation of the RLC stimulates myosin’s association with the cortex but that it cannot concentrate at the equator until after anaphase onset. Additionally, we found that after anaphase, the equatorial myosin is progressively stabilized within the cytokinetic ring, but that constitutively phosphorylating the RLC can drive an anaphase-like association with the cortex when the cell is still in metaphase. Finally, we demonstrated that pre-assembled myosin filaments can accumulate at the equatorial cortex in the absence of Rho, but that other Rho-dependent activities are required for assembling a functional cytokinetic ring.
Overall, our data and the data from other labs and systems are consistent with a model where multiple redundant mechanisms serve to control myosin activity to make the construction of the cytokinetic ring as robust as possible. During mitosis, multiple mechanisms work together to inhibit filament formation and generate a large soluble pool of myosin [26, 27]. Then, at anaphase onset, these inhibitory mechanisms are turned off [27], and freely diffusing individual myosin molecules can reach the equatorial cortex where Rho-dependent kinases phosphorylate the RLC to stimulate filament formation and cortical attachment [2, 20, 24, 28]. At the equatorial cortex, myosin interacts with the rest of the Rho-activated cytokinetic machinery to become more tightly anchored to the cortex [29, 30]. Assembled filaments at the equatorial cortex can then generate flow to bring peripheral myosin into the equator to further reinforce the spatial restriction of myosin to the cell’s equator [16]. The timing and degree to which each of these mechanisms contribute to myosin’s localization at the equatorial cortex likely varies from organism to organism and even cell type to cell type depending upon variables such as cell size, but together they ensure that cytokinesis and therefore the final segregation of genomes occurs as reliably as possible.
Supplementary Material
Figure 4. A model for myosin regulation during mitosis and cytokinesis.
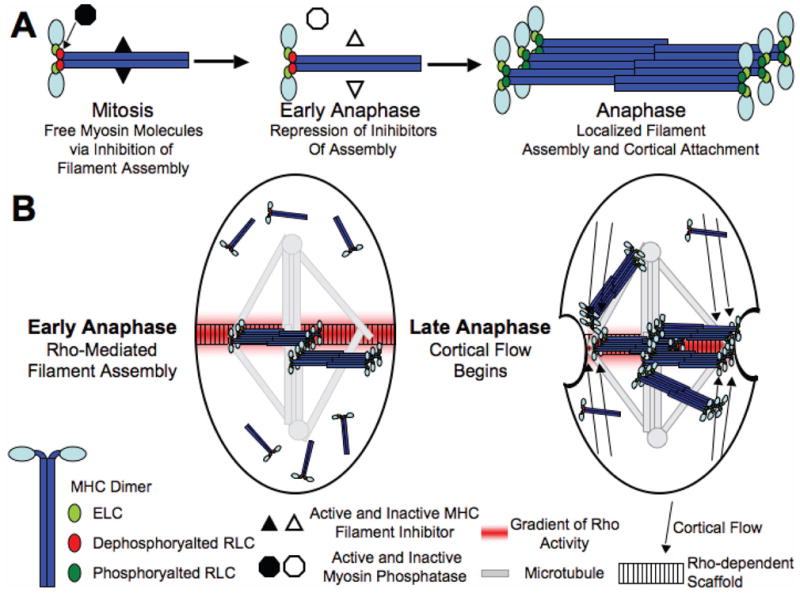
(A) During mitosis myosin phosphatase (via dephosphorylating the RLC, red ovals) and an unknown mechanism (via and interaction with the MHC) both work to keep myosin as single myosin molecules. At the metaphase to anaphase transition, both inhibitors of filament assembly are inactivated. Then when myosin reaches the equatorial cortex, the activity of ROCK phosphorylates the RLC (green ovals) to locally stimulate filament formation. (B) Localized activation of filament assembly and binding to the cortex and the rest of the cytokinetic machinery occurs at equatorial sites that correspond to the zones of microtubule attachment and overlap. Then after the formation of filaments occurs, cortical flow can drive any peripheral myosin molecules and filaments to the equator.
Acknowledgments
This work originated in the Physiology Course 2006 and was continued in the 2008 course at the Marine Biological Laboratory, Woods Hole. We thank the members of the course and David Altman, Sara Dean and Hirofumi Onishi for stimulating discussions and technical instructions. We are also thankful for the helpful comments of our reviewers. The anti-Rho monoclonal antibody p1D9 developed by S. Parkhurst was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA. This work was supported by NIH grant (GM33289) to JAS, the Special Coordination Funds for Promoting Science and Technology (MEXT, Japan) to GG and Grants-in-aid for Scientific Research (MEXT) to IM and GG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15:371–377. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Dean SO, Spudich JA. Rho kinase’s role in myosin recruitment to the equatorial cortex of mitotic Drosophila S2 cells is for myosin regulatory light chain phosphorylation. PLoS ONE. 2006;1:e131. doi: 10.1371/journal.pone.0000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Hostetter D, Rice S, Dean S, Altman D, McMahon PM, Sutton S, Tripathy A, Spudich JA. Dictyostelium myosin bipolar thick filament formation: importance of charge and specific domains of the myosin rod. PLoS Biol. 2004;2:e356. doi: 10.1371/journal.pbio.0020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zang JH, Spudich JA. Myosin II localization during cytokinesis occurs by a mechanism that does not require its motor domain. Proc Natl Acad Sci U S A. 1998;95:13652–13657. doi: 10.1073/pnas.95.23.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motegi F, Mishra M, Balasubramanian MK, Mabuchi I. Myosin-II reorganization during mitosis is controlled temporally by its dephosphorylation and spatially by Mid1 in fission yeast. J Cell Biol. 2004;165:685–695. doi: 10.1083/jcb.200402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohn RL, Vikstrom KL, Strauss M, Cohen C, Szent-Gyorgyi AG, Leinwand LA. A 29 residue region of the sarcomeric myosin rod is necessary for filament formation. J Mol Biol. 1997;266:317–330. doi: 10.1006/jmbi.1996.0790. [DOI] [PubMed] [Google Scholar]
- 8.Ikebe M, Komatsu S, Woodhead JL, Mabuchi K, Ikebe R, Saito J, Craig R, Higashihara M. The tip of the coiled-coil rod determines the filament formation of smooth muscle and nonmuscle myosin. J Biol Chem. 2001;276:30293–30300. doi: 10.1074/jbc.M101969200. [DOI] [PubMed] [Google Scholar]
- 9.Liu SL, Fewkes N, Ricketson D, Penkert RR, Prehoda KE. Filament-dependent and -independent localization modes of Drosophila non-muscle myosin II. J Biol Chem. 2008;283:380–387. doi: 10.1074/jbc.M703924200. [DOI] [PubMed] [Google Scholar]
- 10.Egelhoff TT, Lee RJ, Spudich JA. Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell. 1993;75:363–371. doi: 10.1016/0092-8674(93)80077-r. [DOI] [PubMed] [Google Scholar]
- 11.Sabry JH, Moores SL, Ryan S, Zang JH, Spudich JA. Myosin heavy chain phosphorylation sites regulate myosin localization during cytokinesis in live cells. Mol Biol Cell. 1997;8:2605–2615. doi: 10.1091/mbc.8.12.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg M, Ravid S. Protein kinase Cgamma regulates myosin IIB phosphorylation, cellular localization, and filament assembly. Mol Biol Cell. 2006;17:1364–1374. doi: 10.1091/mbc.E05-07-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami N, Kotula L, Hwang YW. Two distinct mechanisms for regulation of nonmuscle myosin assembly via the heavy chain: phosphorylation for MIIB and mts 1 binding for MIIA. Biochemistry. 2000;39:11441–11451. doi: 10.1021/bi000347e. [DOI] [PubMed] [Google Scholar]
- 14.Scholey JM, Taylor KA, Kendrick-Jones J. Regulation of non-muscle myosin assembly by calmodulin-dependent light chain kinase. Nature. 1980;287:233–235. doi: 10.1038/287233a0. [DOI] [PubMed] [Google Scholar]
- 15.Vale RD, Spudich JA, Griffis ER. Dynamics of myosin, microtubules, and Kinesin-6 at the cortex during cytokinesis in Drosophila S2 cells. J Cell Biol. 2009;186:727–738. doi: 10.1083/jcb.200902083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yumura S, Ueda M, Sako Y, Kitanishi-Yumura T, Yanagida T. Multiple mechanisms for accumulation of myosin II filaments at the equator during cytokinesis. Traffic. 2008;9:2089–2099. doi: 10.1111/j.1600-0854.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- 17.Royou A, Sullivan W, Karess R. Cortical recruitment of nonmuscle myosin II in early syncytial Drosophila embryos: its role in nuclear axial expansion and its regulation by Cdc2 activity. J Cell Biol. 2002;158:127–137. doi: 10.1083/jcb.200203148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 19.Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976;16:1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrasek Z, Hoege C, Mashaghi A, Ohrt T, Hyman AA, Schwille P. Characterization of protein dynamics in asymmetric cell division by scanning fluorescence correlation spectroscopy. Biophys J. 2008;95:5476–5486. doi: 10.1529/biophysj.108.135152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren Y, Effler JC, Norstrom M, Luo T, Firtel RA, Iglesias PA, Rock RS, Robinson DN. Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin I. Curr Biol. 2009;19:1421–1428. doi: 10.1016/j.cub.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalho A, Desai A, Oegema K. Structural memory in the contractile ring makes the duration of cytokinesis independent of cell size. Cell. 2009;137:926–937. doi: 10.1016/j.cell.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Beach JR, Egelhoff TT. Myosin II recruitment during cytokinesis independent of centralspindlin-mediated phosphorylation. J Biol Chem. 2009 doi: 10.1074/jbc.M109.028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou M, Wang YL. Distinct pathways for the early recruitment of myosin II and actin to the cytokinetic furrow. Mol Biol Cell. 2008;19:318–326. doi: 10.1091/mbc.E07-08-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng MM, Chang F, Burgess DR. Movement of membrane domains and requirement of membrane signaling molecules for cytokinesis. Dev Cell. 2005;9:781–790. doi: 10.1016/j.devcel.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Foe VE, von Dassow G. Stable and dynamic microtubules coordinately shape the myosin activation zone during cytokinetic furrow formation. J Cell Biol. 2008;183:457–470. doi: 10.1083/jcb.200807128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Totsukawa G, Yamakita Y, Yamashiro S, Hosoya H, Hartshorne DJ, Matsumura F. Activation of myosin phosphatase targeting subunit by mitosis-specific phosphorylation. J Cell Biol. 1999;144:735–744. doi: 10.1083/jcb.144.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, Inagaki M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene. 2000;19:6059–6064. doi: 10.1038/sj.onc.1203987. [DOI] [PubMed] [Google Scholar]
- 29.Hickson GR, O’Farrell PH. Rho-dependent control of anillin behavior during cytokinesis. J Cell Biol. 2008;180:285–294. doi: 10.1083/jcb.200709005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piekny AJ, Glotzer M. Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr Biol. 2008;18:30–36. doi: 10.1016/j.cub.2007.11.068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.