Abstract
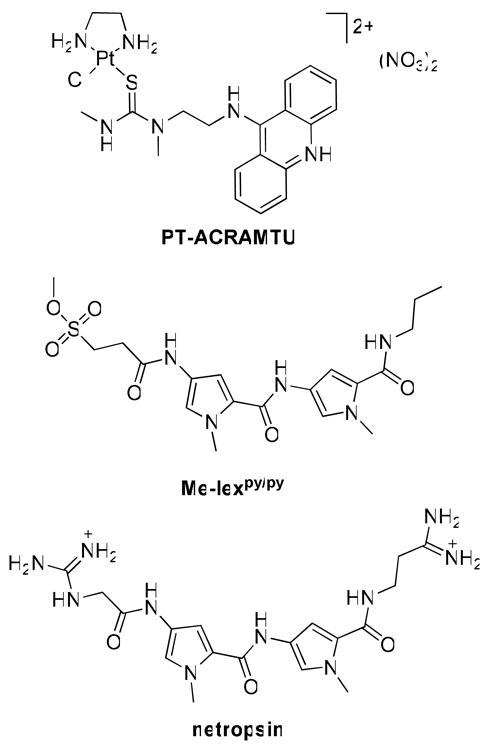
Me-lexpy/py, an adenine-N3-selective alkylating agent, and the reversible minor-groove binder netropsin were used to probe the formation of unusual minor-groove adducts by the cytotoxic hybrid agent PT-ACRAMTU ([PtCl(en)(ACRAMTU)](NO3)2; en = ethane-1,2-diamine, ACRAMTU = 1-[2-(acridin-9-ylamino)ethyl]-1,3-dimethylthiourea). PT-ACRAMTU was found by chemical footprinting to inhibit specific Me-lex-mediated DNA cleavage at several adenine sites, but not at nonspecific guanine, which is consistent with platination of adenine-N3. In a cell proliferation assay, a significant decrease in cytotoxicity was observed for PT-ACRAMTU, when cancer cells were pretreated with netropsin, suggesting that minor-groove adducts in cellular DNA contribute to the biological activity of the hybrid agent.
The development of non-classical platinum-containing drugs in recent years that interact with DNA radically differently than cisplatin1 (cis-diamminedichloroplatinum(II)) (1) requires novel biochemical tools to study the DNA damage caused by these agents and their biological effects. One such compound, PT-ACRAMTU ([PtCl(en)(ACRAMTU)](NO3)2; en = ethane-1,2-diamine, ACRAMTU = 1-[2-(acridin-9-ylamino)ethyl]-1,3-dimethylthiourea); Chart 1) and its derivatives have demonstrated promising activity in chemoresistant cancers in vitro and in vivo (2, 3). Structure–activity relationship studies suggest that the cytotoxic effect of the platinum–acridines is mediated by DNA adducts (2, 4–6). Unlike cisplatin, PT-ACRAMTU and its derivatives do not induce nucleobase–nucleobase cross-links but form monofunctional–intercalative DNA adducts. As a consequence of the unique DNA binding mechanism, which is dominated by the acridine intercalator, the DNA damage profile of PT-ACRAMTU differs significantly from that of clinical platinum-based drugs. While the latter agents primarily target sequences of contiguous guanine (G) bases (7), the hybrid agent’s adducts form with single G bases at pyrimidine–purine steps, and with adenine (A), mainly at 5′-TA sites (5, 8). The most striking DNA-binding feature of PT-ACRAMTU proved to be its ability to form adducts with A-N3, a binding mode previously unknown in platinum–DNA interactions (3).
Chart 1.
Using a combination of enzymatic and acidic digestion of platinum-treated DNA in conjunction with electrospray mass spectrometry and high-resolution structural methods we were able to demonstrate that more than 30% of the adenine adducts are formed with the minor-groove site, A-N3, but the local sequence context of these adducts remained elusive (3). Based on the presence of the platinated deoxydinucleotide fragment d(TpA*) in mixtures of enzymatically digested drug-modified DNA, we proposed that PT-ACRAMTU forms adducts in the minor groove in this specific sequence (9). The reasoning behind this explanation was that platination of A-N3 might interfere with the DNA cleavage chemistry of DNase I, the minor-groove binding endonuclease (10) used in our assay, resulting in the undigested, platinated deoxydinucleotide.
To test our hypothesis of minor-groove platination in TA-rich sequences, we took advantage of two DNA minor-groove directed agents, the dipyrroledipeptide–methylsulfonate ester conjugate Me-lexpy/py, a minor groove-specific alkylating agent (11–14), and netropsin, the classical dipyrroledipeptide natural product (15) (Chart 1). The data presented here suggest that in a synthetic 46-base-pair DNA fragment treated with PT-ACRAMTU, platinum efficiently protects A-N3 from methylation by Me-lexpy/py, providing evidence for platinum binding to this site. Additionally, a cell proliferation assay was performed, demonstrating that netropsin partially protects cancer cells from PT-ACRAMTU (but not from cisplatin), corroborating the notion that A-N3 adducts form intracellularly and contribute to the cytotoxic effect of the hybrid agent.
Alkylating agents have potential applications in probing the sequence and base selectivity of DNA interactive molecules. Dimethyl sulfate (DMS), for instance, which predominantly alkylates strongly nucleophilic G-N7 to form 7-methylguanine (7-MeG) residues, has been used as a chemical footprinting agent to study the base selectivity of cross-links formed by the anticancer drug cisplatin (cis-diamminedichloroplatinum(II)) and related complexes (16–18). Me-lexpy/py methylates A-N3 in double-stranded DNA with high selectivity (14). The proposed mechanism of this agent involves (i) recognition of three consecutive adenine–thymine (AT) base pairs through minor-groove H-bond readout, (ii) methyl group transfer to the 3′-A base directly adjacent to the recognition sequence, and (iii) dissociation of the dipyrroledipeptide carrier from the DNA (14). This produces 3-methyladenine (3-MeA) in TA-rich sequence contexts. 3-MeA is a labile adduct, which undergoes thermal depurination. Subsequent base treatment leads to single-strand breaks, which can be monitored in radioactively labeled DNA fragments by polyacrylamide gel electrophoresis (PAGE).
The footprinting assay reported here is based on the expectation that platination of A-N3 will protect this minor groove site from alkylation by Me-lexpy/py, ultimately inhibiting the DNA-cleavage chemistry of the methylating agent. Thus, platination of Me-lex-specific A-N3 prior to treatment of the probe sequence with the minor groove alkylator should result in a weakening, or complete disappearance, of the corresponding band on the gel. (Unlike 3-MeA, heat-induced depurination of platinated adenine is not observed under the conditions of this assay (19).) Varadarajan et al. demonstrated that Me-lexpy/py preferentially alkylates A-N3 in 5′-(T)n(A)m (n = 2/m = 3 or n = 3/m = 2) sequences in which the A base directly adjacent to T showed the highest propensity for 3-MeA formation (14). Since the 5′-TA step is also targeted by PT-ACRAMTU (8, 9), we designed a DNA fragment containing the above high-affinity sites to detect the formation of A-N3 adducts in this sequence context.
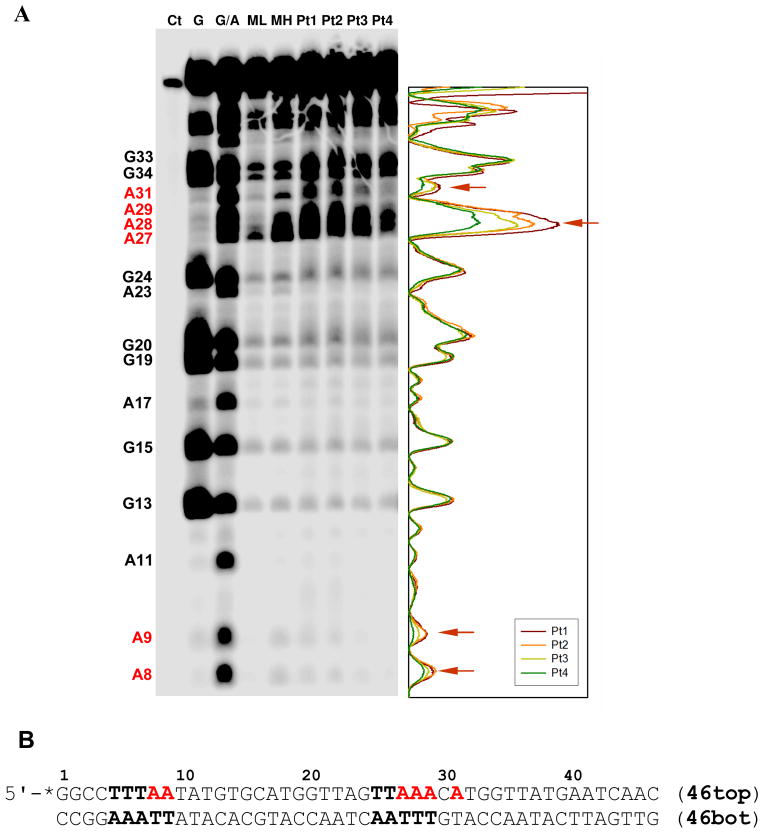
The phosphorimage of a representative footprinting gel and the sequence designed for this assay are shown in Figure 1. In this experiment, the 46-base-pair sequence, whose top strand (46top, Figure 1B) was 5′ end-labeled with 32P, was incubated with four different concentrations of PT-ACRAMTU, treated with Me-lexpy/py, and subjected to piperidine cleavage after removal of bound platinum. The resulting fragments were then identified and quantified by running the samples on a denaturing polyacrylamide gel along G and G+A sequencing lanes, as well as Me-lex-only footprinting lanes. The DNA fragment was modified with platinum at drug-to-nucleotide ratios of 0.022, 0.044, 0.087, and 0.13 (lanes Pt1–Pt4, Figure 1A), assuming that platinum binding is complete after 24 h (8), which corresponds to a total of 6 adducts per double-stranded fragment at the highest drug concentration. These low levels of modification were used to ensure selective binding of platinum.
Figure 1.
Chemical footprinting analysis of a 46-base-pair double-stranded DNA fragment reacted with PT-ACRAMTU and subsequently subjected to Me-lexpy/py-mediated DNA cleavage chemistry. A. Polyacrylamide gel giving integrated band intensities for lanes Pt1–Pt4. Lane assignments: Ct: control, full-length fragment; G: guanine sequencing reaction; G/A: guanine and adenine sequencing reaction; ML: 20 μM Me-lexpy/py; MH: 50 μM Me-lexpy/py; Pt1: 10 μM PT-ACRAMTU, 50 μM Me-lexpy/py; Pt2: 20 μM PT-ACRAMTU, 50 μM Me-lexpy/py; Pt3: 40 μM PT-ACRAMTU, 50 μM Me-lexpy/py; Pt4: 60 μM PT-ACRAMTU, 50 μM Me-lexpy/py. B. Sequence of the 46-base-pair fragment with residues in the top strand numbered. Me-lexpy/py target sites and the sites of cleavage inhibition are highlighted in bold and red, respectively. The asterisk denotes the 32P label.
To test the alkylation activity of Me-lexpy/py in the absence of platinum, the 46-base-pair sequence was incubated with 20 μM and 50 μM alkylating agent, respectively (lanes ML and MH in Figure 1A). The strongest damage caused by Me-lexpy/py in the portion of top46 suitable for monitoring nucleobase alkylation (A8–G33) was observed at adenine residues A27–A29. When the fragment was incubated at a concentration of 50 μM, the minor-groove alkylator had a higher affinity for the adenine triplet than for the two adjacent A bases, A8 and A9. The agent also caused damage at A31 and at several G bases, the latter resulting from nonspecific alkylation, which is in agreement with the damage profile reported in the literature (14).
Pretreatment of the probe sequence with PT-ACRAMTU has a dramatic effect on the alkylation chemistry of Me-lexpy/py at A8, A9, A27–A29, and A31. Cleavage is significantly reduced at these sites as evidenced by a gradual decrease of the corresponding band intensities on the gel with increasing platinum concentration (Pt1–Pt4, Figure 1A). This confirms that PT-ACRAMTU and Me-lexpy/py target adenine bases with the same sequence specificity and comparable affinity. The pronounced inhibitory effect of PT-ACRAMTU on A-N3 alkylation is consistent with the formation of platinum adducts with the minor-groove donor. By contrast, the bands resulting from nonspecific, Me-lex-initiated cleavage at G residues show no decrease in intensity. Cleavage at G13 and G15, for instance, is completely unaffected by platination, which is surprising because PT-ACRAMTU has been shown to target 5′-TG sites (5). Since G is the major target for PT-ACRAMTU (~80%) (9) but only a minor target for Me-lexpy/py (~10%) (14), platinum might be expected to interfere with the alkylation chemistry of the minor groove binder, which is not the case. This suggests that depurination of G in this sequence context is mediated by minor-groove alkylation of G-N3, which does not seem to be affected by the platinum adducts formed exclusively with G-N7 in the major groove. (While methylation of G-N3 by Me-lexpy/py is feasible (14), platination of this site is sterically prohibited (3).)
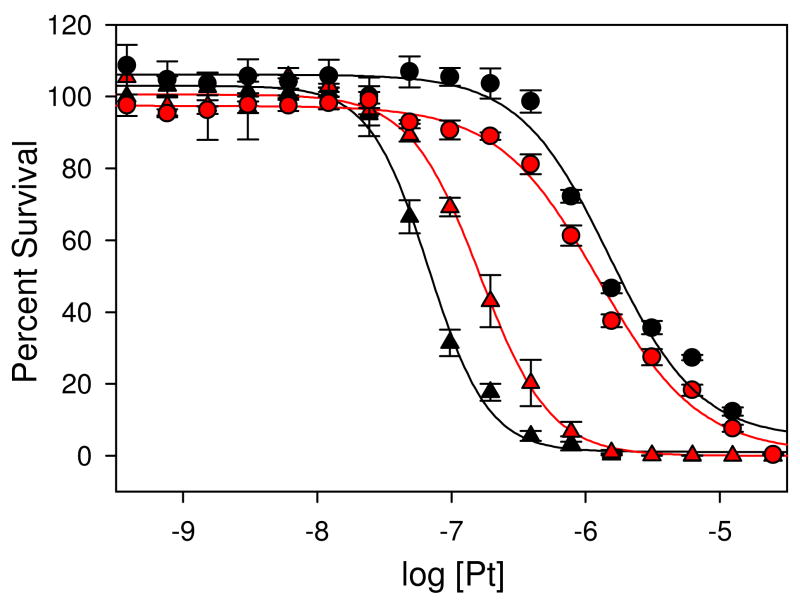
To test our hypothesis that PT-ACRAMTU forms minor groove adducts in nuclear DNA, which might contribute to the cytotoxic effect of this agent, we designed a cell protection assay. The design is based on the supposition that minor groove binders should compete with PT-ACRAMTU for minor-groove binding sites, thereby preventing platination of A-N3. Since Me-lexpy/py itself is very cytotoxic (13), a less toxic, noncovalent binder, netropsin (Chart 1), was chosen in this study. (Netropsin has previously been used to demonstrate that methylation of A-N3 by Me-lexpy/py is the major cause of the alkylating agent’s cell toxicity (20).) The cell line chosen for this experiment is NCI-H460, a non-small cell lung cancer, which has proven highly sensitive to PT-ACRAMTU-type agents. To determine if netropsin can be used as a modulator of cytotoxicity in this assay, NCI-H460 cells were incubated with the minor-groove binder for 72 h in the absence of platinum drug. It was confirmed that netropsin itself does not affect H460 cell proliferation at concentrations below 50 μM. H460 cells were treated for 72 h with PT-ACRAMTU, and, in a control experiment, with cisplatin, both in the absence of netropsin and at three concentrations (6.25, 12.5, and 25 μM) of netropsin that have no effect on cell survival. Selected drug–response curves are shown in Figure 2. In the absence of netropsin, an IC50 value of 0.068±0.003 μM was calculated for PT-ACRAMTU. In the presence of the minor-groove binder, PT-ACRAMTU’s cell kill efficiency decreases, resulting in higher IC50 values. At 25 μM netropsin, a statistically significant (P < 0.05) higher concentration of PT-ACRAMTU (0.163±0.013 μM) is needed compared to netropsin-free H460 to inhibit cell proliferation by 50%. These results suggest that a relationship exists between PT-ACRAMTU’s ability to interact with the minor groove in nuclear DNA and the drug’s cell kill potential. By contrast, cisplatin in netropsin-treated cells shows the opposite trend: a slightly lower IC50 value of 1.245±0.090 μM was determined for cisplatin in cells treated with 25 μM netropsin compared to untreated cells (IC50 = 1.506±0.16 μM). These findings are in agreement with the fact that while PT-ACRAMTU and netropsin target common regions in nuclear DNA, cisplatin (G, major groove) and netropsin (A, minor groove) target DNA at mutually exclusive sites.
Figure 2.
Drug–response curves for cell proliferation assays with NCI-H460 cells treated with PT-ACRAMTU (triangles) and cisplatin (spheres) in the absence of netropsin (black traces) and in the presence of 25 μM netropsin (red traces). The plotted data are averages of three independent experiments (triplicates) for PT-ACRAMTU and two independent experiments (triplicates) for cisplatin. Error bars represent ± standard errors.
Me-lexpy/py was used as a chemical footprinting agent to detect minor-groove adducts formed by the cytotoxic platinum–acridine agent, PT-ACRAMTU. Taking advantage of this sequence- and groove-specific alkylating agent, we were able to demonstrate that the minor groove is a major target site of intercalator-driven platination. PT-ACRAMTU is the only platinum agent known to date that targets TA-rich sequences. However, several bioactive A-N3 alkylating natural and synthetic DNA binders exist, such as bizelesin, a hypercytotoxic bifunctional chloromethyl derivative of the family of cyclopropylpyrroloindole antitumor antibiotics (21). Bizelesin induces long-range A-N3/A-N3 interstrand cross-links in untranscribed, adenine-rich regions (“AT islands”) of the genome known as matrix attachment regions (MARs) (22–24). MARs play important roles in chromatin organization and gene expression, and disruption of MAR function has been shown to stall cell proliferation (22). The potent antiproliferative properties of PT-ACRAMTU may be, in part, due to formation of adducts in these sequences. The decrease in cytotoxicity of PT-ACRAMTU in the presence of netropsin at noncytotoxic concentrations supports this notion. Future work will explore the possibility of using Me-lexpy/py to detect platinum-modified A-N3 in nuclear DNA extracted from drug-treated cells.
Supplementary Material
Acknowledgments
We thank Prof. Barry Gold (Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh) for a generous sample of Me-lexpy/py, and Prof. Sridhar Varadarajan (Department of Chemistry and Biochemistry, University of North Carolina Wilmington) for helpful discussions. This research was supported by the National Institutes of Health (Grant CA101880).
Footnotes
Abbreviations: ACRAMTU, 1-[2-(acridin-9-ylamino)ethyl]-1,3-dimethylthiourea; cisplatin, cis-diamminedichloroplatinum(II); DMS, dimethyl sulfate; IC50, 50% inhibitory concentration; MAR, matrix attachment region; Me-lexpy/py, methyl-3-(1-methyl-5-(1-methyl-5-(propylcarbamoyl)-1H-pyrrol-3-ylcarbamoyl)-1H-pyrrol-3-ylamino)-3-oxopropane-1-sulfonate; PAGE, polyacrylamide gel electrophoresis.
Supporting Information Available: Experimental details; summary of cytotoxicity data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lovejoy KS, Lippard SJ. Non-traditional platinum compounds for improved accumulation, oral bioavailability, and tumor targeting. Dalton Trans. 2009:10651–10659. doi: 10.1039/b913896j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Z, Choudhury JR, Wright MW, Day CS, Saluta G, Kucera GL, Bierbach U. A non-cross-linking platinum-acridine agent with potent activity in non-small-cell lung cancer. J Med Chem. 2008;51:7574–7580. doi: 10.1021/jm800900g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guddneppanavar R, Bierbach U. Adenine-N3 in the DNA minor groove - an emerging target for platinum containing anticancer pharmacophores. Anticancer Agents Med Chem. 2007;7:125–138. doi: 10.2174/187152007779313991. [DOI] [PubMed] [Google Scholar]
- 4.Guddneppanavar R, Choudhury JR, Kheradi AR, Steen BD, Saluta G, Kucera GL, Day CS, Bierbach U. Effect of the diamine nonleaving group in platinum-acridinylthiourea conjugates on DNA damage and cytotoxicity. J Med Chem. 2007;50:2259–2263. doi: 10.1021/jm0614376. [DOI] [PubMed] [Google Scholar]
- 5.Guddneppanavar R, Saluta G, Kucera GL, Bierbach U. Synthesis, biological activity, and DNA-damage profile of platinum-threading intercalator conjugates designed to target adenine. J Med Chem. 2006;49:3204–3214. doi: 10.1021/jm060035v. [DOI] [PubMed] [Google Scholar]
- 6.Ackley MC, Barry CG, Mounce AM, Farmer MC, Springer BE, Day CS, Wright MW, Berners-Price SJ, Hess SM, Bierbach U. Structure-activity relationships in platinum-acridinylthiourea conjugates: effect of the thiourea nonleaving group on drug stability, nucleobase affinity, and in vitro cytotoxicity. J Biol Inorg Chem. 2004;9:453–461. doi: 10.1007/s00775-004-0541-4. [DOI] [PubMed] [Google Scholar]
- 7.Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 8.Budiman ME, Alexander RW, Bierbach U. Unique base-step recognition by a platinum-acridinylthiourea conjugate leads to a DNA damage profile complementary to that of the anticancer drug cisplatin. Biochemistry. 2004;43:8560–8567. doi: 10.1021/bi049415d. [DOI] [PubMed] [Google Scholar]
- 9.Barry CG, Baruah H, Bierbach U. Unprecedented monofunctional metalation of adenine nucleobase in guanine- and thymine-containing dinucleotide sequences by a cytotoxic platinum-acridine hybrid agent. J Am Chem Soc. 2003;125:9629–9637. doi: 10.1021/ja0351443. [DOI] [PubMed] [Google Scholar]
- 10.Weston SA, Lahm A, Suck D. X-Ray Structure of the Dnase I-D(Ggtatacc)(2) Complex at 2.3-Angstrom Resolution. J Mol Biol. 1992;226:1237–1256. doi: 10.1016/0022-2836(92)91064-v. [DOI] [PubMed] [Google Scholar]
- 11.Monti P, Traverso I, Casolari L, Menichini P, Inga A, Ottaggio L, Russo D, Iyer P, Gold B, Fronza G. Mutagenicity of N3-methyladenine: a multi-translesion polymerase affair. Mutat Res. 2010;683:50–56. doi: 10.1016/j.mrfmmm.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo D, Fronza G, Ottaggio L, Monti P, Inga A, Iyer P, Gold B, Menichini P. High frequency of genomic deletions induced by Me-lex, a sequence selective N3-adenine methylating agent, at the Hprt locus in Chinese hamster ovary cells. Mutat Res. 2009;671:58–66. doi: 10.1016/j.mrfmmm.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fronza G, Gold B. The biological effects of N3-methyladenine. J Cell Biochem. 2004;91:250–257. doi: 10.1002/jcb.10698. [DOI] [PubMed] [Google Scholar]
- 14.Varadarajan S, Shah D, Dande P, Settles S, Chen FX, Fronza G, Gold B. DNA damage and cytotoxicity induced by minor groove binding methyl sulfonate esters. Biochemistry. 2003;42:14318–14327. doi: 10.1021/bi0353272. [DOI] [PubMed] [Google Scholar]
- 15.Lown JW. DNA recognition by lexitropsins, minor groove binding agents. J Mol Recognit. 1994;7:79–88. doi: 10.1002/jmr.300070205. [DOI] [PubMed] [Google Scholar]
- 16.Colombier C, Lippert B, Leng M. Interstrand cross-linking reaction in triplexes containing a monofunctional transplatin-adduct. Nucleic Acids Res. 1996;24:4519–4524. doi: 10.1093/nar/24.22.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ourliac Garnier I, Bombard S. GG sequence of DNA and the human telomeric sequence react with cis-diammine-diaquaplatinum at comparable rates. J Inorg Biochem. 2007;101:514–524. doi: 10.1016/j.jinorgbio.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Ourliac-Garnier I, Elizondo-Riojas MA, Redon S, Farrell NP, Bombard S. Cross-Links of Quadruplex Structures from Human Telomeric DNA by Dinuclear Platinum Complexes Show the Flexibility of Both Structures. Biochemistry. 2005;44:10620–10634. doi: 10.1021/bi050144w. [DOI] [PubMed] [Google Scholar]
- 19.Barry CG, Day CS, Bierbach U. Duplex-promoted platination of adenine-N3 in the minor groove of DNA: challenging a longstanding bioinorganic paradigm. J Am Chem Soc. 2005;127:1160–1169. doi: 10.1021/ja0451620. [DOI] [PubMed] [Google Scholar]
- 20.Shah D, Gold B. Evidence in Escherichia coli that N3-methyladenine lesions and cytotoxicity induced by a minor groove binding methyl sulfonate ester can be modulated in vivo by netropsin. Biochemistry. 2003;42:12610–12616. doi: 10.1021/bi035315g. [DOI] [PubMed] [Google Scholar]
- 21.Reddy BS, Sharma SK, Lown JW. Recent developments in sequence selective minor groove DNA effectors. Curr Med Chem. 2001;8:475–508. doi: 10.2174/0929867003373292. [DOI] [PubMed] [Google Scholar]
- 22.Woynarowski JM. Targeting critical regions in genomic DNA with AT-specific anticancer drugs. Biochim Biophys Acta. 2002;1587:300–308. doi: 10.1016/s0925-4439(02)00093-5. [DOI] [PubMed] [Google Scholar]
- 23.Woynarowski JM, Trevino AV, Rodriguez KA, Hardies SC, Benham CJ. AT-rich islands in genomic DNA as a novel target for AT-specific DNA-reactive antitumor drugs. J Biol Chem. 2001;276:40555–40566. doi: 10.1074/jbc.M103390200. [DOI] [PubMed] [Google Scholar]
- 24.Woynarowski JM, Beerman TA. Effects of bizelesin (U-77,779), a bifunctional alkylating minor groove binder, on replication of genomic and simian virus 40 DNA in BSC-1 cells. Biochim Biophys Acta. 1997;1353:50–60. doi: 10.1016/s0167-4781(97)00046-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.