Abstract
During late fetal life, Schwann cells in the peripheral nerves, singled out by the larger axons will transit through a promyelinating stage before exiting the cell cycle and initiating myelin formation. A network of extra- and intracellular signaling pathways, regulating a transcriptional program of cell differentiation, governs this progression of cellular changes, culminating in a highly differentiated cell. In this review we focus on the roles of a number of transcription factors not only in myelination, during normal development, but also in demyelination, following nerve trauma. These factors include specification factors involved in early development of Schwann cells from neural crest (Sox10) as well as factors specifically required for transitions into the promyelinating and myelinating stages (Oct6/Scip and Krox20/Egr2). From this description we can glean the first, still very incomplete, contours of a gene regulatory network that governs myelination and demyelination during development and regeneration.
Keywords: CMT, peripheral neuropathy, gene regulation, Pou3f1, Egr2, Sox10, Srebp
Transcriptional control of myelination and demyelination
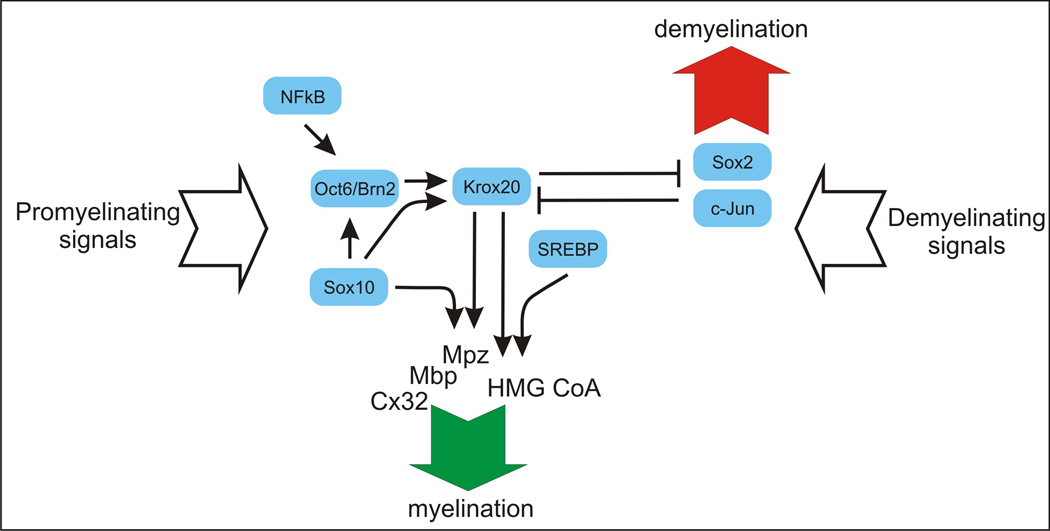
Myelination promoting signals (discussed in other reviews in this special issue of Glia) converge on a number of transcription factors to drive the transition of immature, promyelinating proliferative cells to myelinating cells. Over the last decade several transcription factors were found to play key roles in this transition and the execution of a transcriptional program directing myelination. These transcription factors, their regulatory relationships and the intracellular signaling pathways that modulate their activity have been studied with growing intensity over the last few years. These studies have revealed a number of regulatory circuits that outline the contours of a gene regulatory network of myelination in the peripheral nervous system. A simplified outline of this network is depicted in Figure 1. A myelination promoting circuit, consisting of the POU domain factors Oct6/Scip, Brn2 and Sox10 that regulate Krox20/Egr2 and drives the promyelinating to myelinating transition, forms the backbone of this regulatory network. More recently, a second cross-antagonistic circuit of Krox20/Egr2 versus cJun and Sox2 has been described that governs active demyelination following nerve trauma. In the following sections we will discuss the different transcription factors, their mechanism of action and their regulatory relationships.
Figure 1. Outline of a gene regulatory network of myelin-associated Schwann cell differentiation.
The major transcription factors involved in the promyelinating to myelinating transition are depicted with their regulatory relationship. Arrows indicate activation while blunt lines indicate repression. This regulatory pathway culminates in induction of several genes in myelinating Schwann cells, such as Myelin protein zero (Mpz), Myelin basic protein (Mbp), Connexin 32 (Cx32), and HMG CoA reductase. See text for further details.
Major transcriptional regulators of the myelination process: Oct6/Scip, Krox20/Egr2 and Sox10
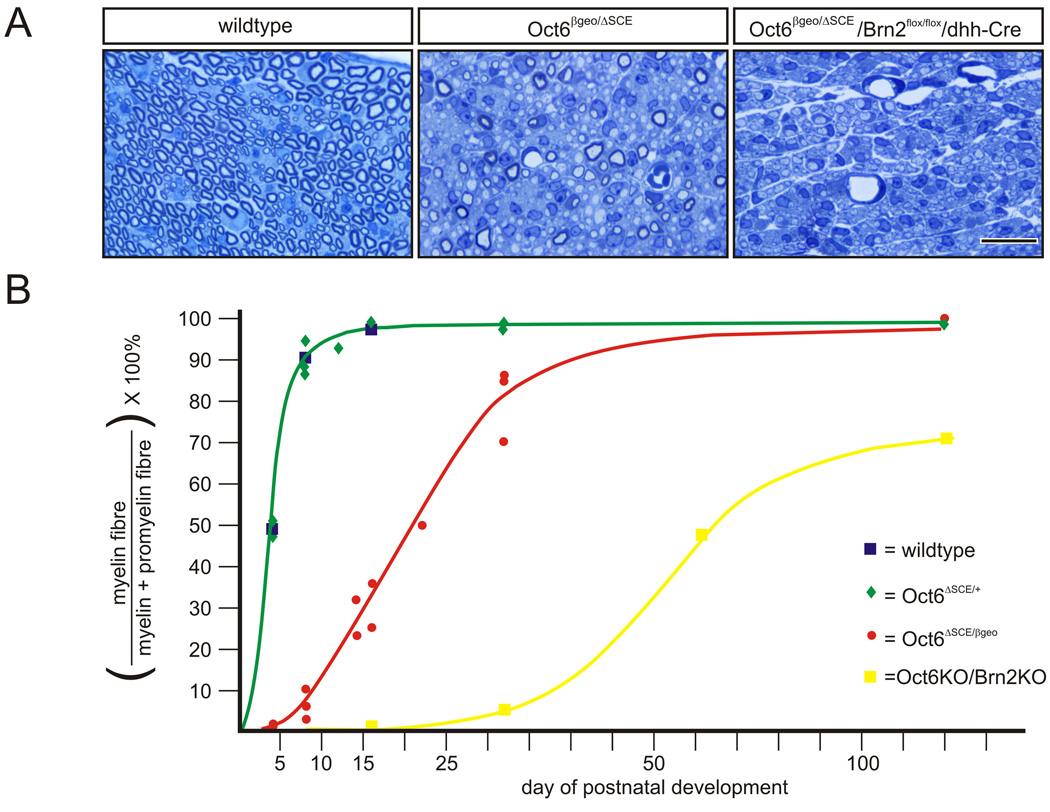
The POU homeo-domain transcription factors Oct6/Scip and Brn2 are important Schwann cell autonomous regulators of the timing and rate of the promyelin to myelinating transition (Bermingham et al. 1996; Jaegle et al. 2003; Jaegle et al. 1996). While in normal rodent nerves, the vast majority of myelination competent Schwann cells make this transition in the first week of postnatal life, Oct6/Scip mutant Schwann cells initiate this transition with 1–2 days delay and do so with altered kinetics (Figure 2). Deletion of both Oct6/Scip and Brn2 results in a more severe phenotype with persistence of promyelin Schwann cells into adulthood and hypomyelinated axons.
Figure 2. The POU domain transcription factors Oct6/Scip and Brn2 regulate the timing and rate of the promyelin to myelinating transition.
A. Toluodine Blue stained cross-sections of wildtype (left panel), Oct6 mutant (middle panel) and Oct6/Brn2 mutant nerves at 16 days of postnatal development. Double Oct6/Brn2 mutant Schwann cells are arrested at the promyelin stage of differentiation while around 70% of Oct6 mutant Schwann cells are stalled at this stage. In wildtype nerves all Schwann cell have gone on to myelinate their associated axon. B. Quantification of the promyelinating to myelinating transition. The graphical representation demonstrates the dramatic reduction in rate and altered kinetics of this cellular transition. Promyelin and myelin figures were counted in electron micrographs at 2600× magnification. At least 250 axons were scored per genotype at the indicated timepoint.
A major target of Oct6/Brn2 regulation is the immediate early gene Krox20/Egr2. Previous mouse knockout studies had identified Krox20/Egr2 as a transcription factor required for the myelinating phase of Schwann cell development (Topilko et al. 1994). Peripheral nerve from Krox20/Egr2 null mice exhibits lower levels of several major myelin genes with Schwann cells arrested at the promyelin stage, failing to enter the myelinating phase of cell differentiation. Independent analysis of a mouse with a hypomorphic Krox20/Egr2 allele (Egr2 Lo/Lo) confirmed these initial observations (Le et al. 2005a). Furthermore, it was recently demonstrated that Krox20/Egr2 is also required for maintenance of peripheral nerve myelination (Decker et al. 2006).
Additional critical insight into the transcriptional regulation of myelination resulted from identification of the HMG box–containing Sox10 transcription factor as an integral mediator of Schwann cell differentiation. Sox10 is specifically expressed in Schwann cells and other neural crest–derived tissues and is required for Schwann cell specification from the neural crest (Britsch et al. 2001; Kuhlbrodt et al. 1998b). Sox10 belongs to the E class of Sox factors, which also includes Sox8 and Sox9 (reviewed in Wegner and Stolt 2005). Indeed, recent work from the Wegner group has uncovered functional redundancy in specific developmental contexts, including oligodendrocyte differentiation (Finzsch et al. 2008; Stolt et al. 2004). However, Sox10 appears to be the preeminent member of this family regulating Schwann cell development.
Feedforward coordination of commitment to myelination
As mentioned above, the zinc finger transcription factor Krox20/Egr2 is a major target of Oct6/Brn2 regulation. Activation of Krox20/Egr2 transcription in Schwann cells is mediated through a cis-acting element in the Krox20/Egr2 locus called the myelin-associated Schwann cell enhancer or mSCE (Ghislain and Charnay 2006; Ghislain et al. 2002). Co-transfection experiments have shown that Oct6/Scip, Brn2 and Sox10 synergistically activate a reporter through this enhancer (Ghislain and Charnay 2006; Kuhlbrodt et al. 1998a). While Oct6/Scip and Brn2 are transiently upregulated during Schwann cell differentiation, Sox10 is expressed at all developmental stages of the Schwann cell lineage (Jaegle et al. 2003; Kuhlbrodt et al. 1998b; Sim et al. 2002). Analysis of a hypomorphic Sox10 mutant mouse line (see below), further confirms the involvement of Sox10 in regulation of Oct6/Scip.
The regulatory relationship between Sox10, Oct6, Brn2 and Krox20/Egr2 (Figure 1) resembles that of a Feed-forward loop in which factor A (Sox10) activates factor B (Oct6/Scip) and subsequently activates, in synergy with B, factor C (Krox20). Feed forward loops are common regulatory motifs that provide temporal control and forward momentum in gene regulatory networks (Shen-Orr et al. 2002; Swiers et al. 2006). That this regulatory circuit receives multiple inputs is suggested by the observation that promyelinating Schwann cells in Lgi4 mutant animals (Claw Paw; clp), which show a developmental delay similar to that observed in Oct6 mutant animals, express Oct6/Scip and Sox10, yet do not express Krox20/Egr2 (Bermingham et al. 2006; Bermingham et al. 2002; Darbas et al. 2004). As Lgi4 is a Schwann cell expressed, secreted molecule it is conceivable that it modulates a signaling pathway that triggers activation of Oct6/Scip or Sox10, for example through modifications such as phosphorylation and acetylation, or the translation or stability of Krox20/Egr2 mRNA. It has recently been shown that Oct6/Scip contains a nuclear export signal that allows the rapid removal of Oct6/Scip from the nucleus (Baranek et al. 2005). It is however unlikely that this mode of regulation plays a role in Lgi4 mutant Schwann cells as Oct6/Scip is mainly nuclear in these cells (Darbas et al. 2004). Any post-translational mechanism operating on Oct6/Scip should operate equally on Brn1 (Pou3f3) as it has been shown that Brn1 can fully substitute for Oct6/Scip function in differentiating Schwann cells of mice in which the Brn1 gene was knocked in the Oct6 locus, replacing the Oct6 gene (Friedrich et al. 2005).
In the regulatory motif described above, Oct6/Scip and Brn2 function as activators of gene expression, in particular Krox20/Egr2. However, co-transfection experiments in primary rat Schwannn cells have suggested that Oct6/Scip can also function as a repressor of major myelin genes such as Myelin protein-zero (Mpz) and Myelin basic protein (Mbp) (Monuki et al. 1993; Monuki et al. 1990). It was suggested that Oct6/Scip represses reporter gene activation through quenching of an activating complex on the Mpz and Mbp promoters. It remains unclear what the physiological relevance of these findings is as analysis of Oct6/Scip mutant animals did not support a repressive role for Oct6/Scip in promyelinating cells as in the absence of Oct6/Scip, steady state levels of Mbp and Peripheral myelin protein-22 (Pmp22) mRNA, as well as Mpz and Pmp22 protein levels, were strongly reduced (Jaegle et al. 1996).
Oct6/Scip regulation
Oct6/Scip was originally described as a POU domain factor that is repressed in primary rat Schwann cell cultures but whose expression is upregulated with intermediate kinetics following administration of cAMP (hence the name Suppressed cAMP inducible POU: Scip; (Monuki et al. 1989). Activation of Protein kinase A (PKA), the main target of the second messenger cAMP, in cultured Schwann cells results in upregulation of myelination associated genes, suggesting a role for PKA in myelination (Morgan et al. 1991; Sobue et al. 1986). Indeed, inhibition of PKA activity results in reduced myelination in DRG co-cultures (Howe and McCarthy 2000). A major target of PKA signaling is the cAMP response element binding protein or Crebp. Crebp is phosphorylated by PKA following cAMP administration to Schwann cells but whether this has a direct effect on Oct6/Scip expression is unclear (Lee et al. 1999). Another target of PKA is NFκB, a transcription factor involved in cell proliferation and differentiation of a variety of cells (Zhong et al. 1998). NFκB is a transcription factor formed by homo- or heterodimerization of 5 subunits (p65/RelB, RelA, c-rel, p52 and p50). Recently it was shown that NFκB is required in Schwann cells for activation of Oct6/Scip and myelination (Nickols et al. 2003). NFκB transcriptional activity can be regulated through phosphorylation by PKA at Ser276 of p65 and PKA inhibition results in reduced myelin formation in DRG co-cultures (Yoon et al. 2008). Although activated NFκB increases Oct6/Scip protein levels in cultured Schwann cells, it did not affect Oct6/Scip mRNA levels suggesting that NFκB regulates Oct6/Scip expression in Schwann cells at the posttranscriptional level.
Oct6/Scip expression in vivo is controlled by axonal signals that include Type III Neuregulin1 (Leimeroth et al. 2002; Scherer et al. 1994; Taveggia et al. 2005). These signals converge on the Oct6/Scip Schwann cell enhancer (SCE) element, located downstream of the gene, to regulate every aspect of Oct6/Scip expression in Schwann cells (Ghazvini et al. 2002; Mandemakers et al. 2000). Ongoing deletion analysis of this enhancer element in differentiating Schwann cells has identified a 500 bp region that is required for activation both in cultured Schwann cells as well as in Schwann cells of transgenic mice (N.B. Jagalur, M. Ghazvini and D.M. in prep). This element contains several Sox protein binding sites suggesting a potential role for Sox proteins in Oct6/Scip regulation. Indeed, a hypomorphic allele of Sox10 that no longer homodimerizes does support Schwann cell development, but these Schwann cells do not upregulate Oct6/Scip in late fetal life (Schreiner et al. 2007). Further analysis should identify elements, in addition to Sox10 binding elements, that confer temporal control of Oct6/Scip gene activation.
During normal nerve development, Oct6/Scip expression is rapidly extinguished in myelinating Schwann cells. The mechanism through which Oct6/Scip expression is down regulated remains unknown. We have previously speculated that Oct6/Scip is involved in its own downregulation (Jaegle and Meijer 1998). Others have suggested that Krox20/Egr2 is involved in extinguishing Oct6/Scip expression in myelinating cells as Krox20/Egr2 deleted Schwann cells fail to downregulate Oct6/Scip (Zorick et al. 1999). This is very well possible as the Oct6/Scip promoter region is in a CpG island and contains multiple Krox20/Egr2 binding sites. Krox20/Egr2 has been shown to function as a transcriptional repressor through its interaction with the Nab (NGFI-A/Egr binding) repressors (see below). However, evidence supporting a direct role for Krox20/Egr2 in Oct6 downregulation is still lacking. Identification of the factors involved in extinguishing Oct6/Scip expression is of importance as these factors could contribute to the development of peripheral neuropathy. Indeed, forced expression of Oct6 in Schwann cells beyond the early myelinating stages result in hypomyelination and axonal loss, emphasizing the need for Oct6 downregulation during normal development (Ryu et al. 2007).
The role of Sox10 in myelinating Schwann cells
Although Sox10 expression is maintained in myelinating Schwann cells, the early embryonic phenotype of the knockout mouse initially precluded testing a definitive role for Sox10 during myelination by mature Schwann cells (Britsch et al. 2001). As noted above, Sox10 regulates entry into the promyelinating stage through its role in Oct6/Scip and Krox20/Egr2 induction. In addition, a number of lines of evidence indicate that Sox10 is required throughout the myelination process. Several myelin genes have conserved Sox10 binding sites that appear to be important for function (Bondurand et al. 2001; Denarier et al. 2005; Peirano et al. 2000), and binding of Sox10 to these sites has been shown in myelinating sciatic nerve in vivo (LeBlanc et al. 2007). Finally, identification of SOX10 mutants in a complex human syndrome that includes peripheral myelin defects—PCWH: peripheral demyelinating neuropathy, central dysmyelinating leukodystrophy, Waardenburg syndrome and Hirschsprung disease (Inoue et al. 2004)—further highlights the importance of Sox10 in not only Schwann cell specification but also initiation of myelination.
Initial characterization of Sox10 deficient mice revealed that it is required for embryonic expression of Mpz and binds to several sites in the Mpz promoter (Peirano et al. 2000). Using these target sites, a number of elegant structure/function studies of Sox10 have further elucidated its mechanism of action. One unique feature of the E class of Sox factors is their ability to bind DNA as either monomers or dimers. Dimer formation can increase apparent affinity as shown by cooperative binding of a Sox10 dimer to two adjacent sites in an inverted orientation (C/C’) in the Mpz promoter (Peirano and Wegner 2000). Another striking aspect of Sox10 is its ability to induce DNA bending, and both Sox10 monomers and dimers can induce bends of ~80° and 100°, respectively. The ultimate significance of the DNA bending has yet to be elucidated (Schlierf et al. 2002), but it would be expected to dramatically change the architecture of target loci, and facilitate interactions between target promoters and more distal regulatory elements.
A knock in of Sox8 into the Sox10 locus revealed that Sox8 could largely fulfill the role of Sox10 in peripheral nerve development if expressed under the same developmental control (Kellerer et al. 2006). Recent in vivo experiments have begun to test the role of protein domains that are shared among the E class Sox factors. A domain N-terminal of the HMG class DNA-binding domain has been shown to mediate Sox10 dimer formation (Peirano and Wegner 2000). As noted above, Sox10 mutant with a defective dimerization domain is able to sustain early Schwann development, but resulting Schwann cells do not enter the promyelinating stage, characterized by expression of Oct6/Scip. In contrast, a hypomorphic Sox10 allele with a deletion of the K2 activation domain can drive Schwann cells to the promyelinating stage, but fails to initiate myelination (Schreiner et al. 2007).
Sites of Krox20/Egr2 action and interactions with Sox10
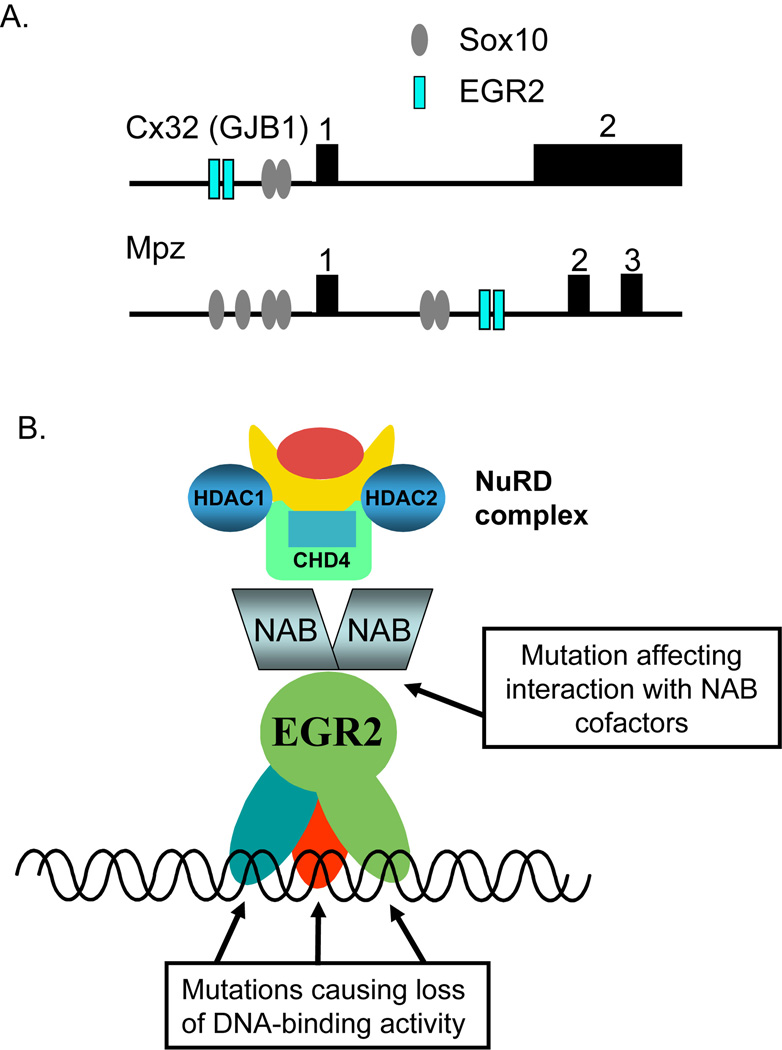
The genes controlled by Krox20/Egr2 have been elucidated by expression analyses of knockout mice (Le et al. 2005a; Topilko et al. 1994), and ectopic expression of Krox20/Egr2 in primary Schwann cells (Nagarajan et al. 2001). These analyses highlighted not only major myelin genes such as Mpz, Mbp, Pmp22, Connexin 32 (Cx32), and Myelin-associated glycoprotein (Mag), but also identified several genes involved in the tremendous increase in lipid biosynthesis during myelination, such as HMG CoA reductase (Leblanc et al. 2005) (see below). Many of the Krox20/Egr2-regulated genes identified in these studies are also controlled by Sox10. One of the first examples of this combinatorial regulation was provided by analysis of the promoter of the Cx32 gene, which itself is a target of an X-linked form of CMT (CMTX). The promoter for Cx32 contains binding sites for both Egr2 and Sox10 (Figure 3A), and transfection assays showed synergistic activation of this promoter by the two factors. Furthermore, a promoter mutation found in a patient with CMT interferes with binding of Sox10 (Bondurand et al. 2001). Another example was found in the analysis of the Schwann cell enhancer of the Mbp gene (Farhadi et al. 2003; Forghani et al. 2001; Taveggia et al. 2004), which contains conserved binding sites for Krox20/Egr2 and Sox10. Mutation of these sites results in diminished ability of this enhancer to drive peripheral nerve expression in transgenic experiments (Denarier et al. 2005). Other occurrences of a composite module consisting of conserved Krox20/Egr2 and Sox10 sites have been recently defined in other myelin genes (Jones et al. 2008)
Figure 3. Sites of Krox20/Egr2 function.
In panel A, the configuration of Krox20/Egr2 binding sites in the Connexin 32 and Mpz genes is diagrammed. The promoter element of Connexin 32 and intron element of Mpz are synergistically activated by Egr2 and Sox10, and similar configurations of sites are found in the Mbp and Mag genes.
In panel B, various EGR2 mutations associated with human peripheral neuropathies are diagrammed. For exact details, refer to the Inherited Peripheral Neuropathies Mutation Database (http://www.molgen.ua.ac.be/CMTMutations/). Dominant mutations have been found in all three zinc fingers, and they generally impair DNA-binding by EGR2. In addition, a recessive mutation in the NAB-binding domain was identified in one family. Loss of NAB binding is expected to result in defective targeting of the NuRD chromatin remodeling complex.
Although Mpz is the most highly expressed myelin gene, its control by Krox20/Egr2 initially proved enigmatic since relatively minor effects on Mpz promoter function were observed (Nagarajan et al. 2001; Peirano et al. 2000; Slutsky et al. 2003; Zorick et al. 1999). However, a transgenic analysis of the Mpz locus had indicated that important control elements resided downstream of the transcription start site (Feltri et al. 1999). Based on comparative genomic analysis, we recently described a conserved element within the first intron of the Mpz gene (LeBlanc et al. 2006), which contains binding sites for both Egr2 and Sox10 (Figure 3A). Reporter assays showed that the isolated intron element responds to treatments that induce endogenous Mpz expression in Schwann cells, and chromatin immunoprecipitation analysis in cell lines and also myelinating sciatic nerve in vivo confirmed binding of both Egr2 and Sox10 to this conserved intron element. Interestingly, intron localization of myelin gene enhancers may prove to be a common theme, since a similar analysis identified another intron-associated enhancer for the Mag gene, with an Egr2 site located proximal to an inverted pair of Sox10 sites (Jang et al. 2006; LeBlanc et al. 2007). Altogether, accumulating evidence indicates that enacting the myelination program in Schwann cells depends upon convergent regulation exerted by the Sox10 specification factor and axon-dependent stimulation by Krox20/Egr2.
Notwithstanding, Sox10 appears to interact promiscuously with a variety of different factors (Wissmuller et al. 2006), including Oct6/Scip and others (Ghislain and Charnay 2006; Kuhlbrodt et al. 1998a). Another example uncovered in studies of the Mpz promoter is the cooperation of Sox10 with the Zbp99 factor, which is induced by gp130-mediating signaling in Schwann cells (Slutsky et al. 2003). Overall, interactions of Sox10 with diverse partners probably underly its ability to foster Schwann cell differentiation at many stages from neural crest specification to myelination.
EGR2 Mutations in Human Peripheral Neuropathies
Several groups identified mutations in the human EGR2 gene associated with peripheral myelinopathies, including Charcot-Marie-Tooth disease, as well as the more severe forms of Dejerine-Sottas Syndrome and Congenital Hypomyelinating Neuropathy (Figure 3B). Dominant neuropathy-associated mutations of EGR2 have been identified in all three zinc fingers of the DNA-binding domain (Bellone et al. 1999; Mikesova et al. 2005; Pareyson et al. 2000; Szigeti et al. 2007; Timmerman et al. 1999; Warner et al. 1998), and these mutations generally impair or prevent DNA-binding (Musso et al. 2001; Musso et al. 2003; Warner et al. 1999). The underlying mechanisms by which dominant EGR2 mutants cause myelinopathies have recently been investigated. One of the affected pathways involves cell cycle regulation, based on observations that Krox20/Egr2 induces cell cycle arrest of Schwann cells by antagonizing regulation of the JNK/c-jun pathway (see below, Parkinson et al. 2004). In contrast, expression of a dominant negative mutant form of Krox20/Egr2 induces Schwann cell proliferation through upregulation of cyclin D1 and reduction of the cyclin-dependent kinase inhibitor, p27 (Arthur-Farraj et al. 2006).
The dominant Egr2 mutants interfere with activation of some endogenous target genes in Schwann cells by wild type Egr2 (Arthur-Farraj et al. 2006; Nagarajan et al. 2001), with the most striking effects observed on Mpz expression. Recent analysis of Mpz regulatory elements indicated that Krox20/Egr2 activation of the intron element, but not the promoter, was sensitive to expression of dominant neuropathy-associated Krox20/Egr2 mutants. Binding of Sox10 to the Mpz intron element was required for the dominant negative effects, and expression of a Krox20/Egr2 dominant mutant displaced Sox10 binding from the intron element. Overall, these data are consistent with a model in which the dominant Egr2 mutants interfere with interactions required for Egr2-dependent binding of Sox10 to the conserved intron element of Mpz (LeBlanc et al. 2007). Cooperation between Krox20/Egr2 and Sox10 probably involves the recently characterized interaction between their DNA-binding domains (Wissmuller et al. 2006), although it appears that other domains are also involved.
Gene repression in the myelination process
Like most transcriptional regulators, the ultimate effect of Krox20/Egr2 on a given target gene is determined by its association with coactivators and corepressors. Krox20/Egr2 has been linked with the p300 Histone acetylase and HCF1 cofactor (Luciano and Wilson 2003), which has been identified as a component of complexes containing the Mll histone H3K4 methylases (Yokoyama et al. 2004). However, screening for Krox20/Egr2-interacting proteins has identified mostly co-repressors such as the Nab co-repressors, which repress Egr-mediated transcription (Russo et al. 1995; Svaren et al. 1996; Svaren et al. 1998). Other interacting co-repressor proteins include the Piasxβ Sumo ligase (Garcia-Dominguez et al. 2006), and the Ddx20 member of the RNA helicase family (Gillian and Svaren 2004), although the functions of these in Schwann cell differentiation have not been explored.
Of these cofactors, the most extensive evidence supports an integral role of the Nab co-repressors in regulation of myelin gene expression. One of the EGR2 mutations associated with a very severe congenital hypomyelinating neuropathy (I268N, Warner et al. 1998) prevents binding of EGR2 to NAB proteins (Figure 3B, Warner et al. 1999). The importance of Nab co-repressors to the regulation of peripheral nerve myelination by Egr2 was confirmed by the demonstration that a double knockout of the Nab1/Nab2 genes results in a phenotype very similar to that of Krox20/Egr2 deficient mice: early lethality and peripheral neuropathy resulting from arrested myelination (Le et al. 2005b). Although Nab co-repressors may interact with other transcriptional regulators, a recent knock in analysis of a Nab-resistant allele of Egr2 confirmed the importance of the interaction of Nab proteins with Egr2/Krox20 (Desmazieres et al. 2008). Nab proteins are active repressors when recruited to a transcriptional template (Svaren et al. 1998; Swirnoff et al. 1998), and recent progress has elucidated the mechanism by which Nab proteins repress transcription. Deletion analysis of Nab2 showed that it had two independent repression domains, one of which was found to interact with the Chromodomain Helicase DNA-binding 4 (CHD4) protein (Srinivasan et al. 2006). CHD4 is an integral subunit of the NuRD (nucleosome remodeling and deacetylase) chromatin-remodeling complex. Repression by this complex involves both histone deacetylation and nucleosome mobilization mechanisms (reviewed in Bowen et al. 2004; Feng and Zhang 2003) and CHD4 is highly expressed in peripheral nerve.
Analysis of Nab expression initially suggested that Nab proteins serve as feedback inhibitors of Egr2. Indeed, characterization of the Nab1 and Nab2 promoters identified multiple Egr binding sites (Le et al. 2005b), and recent analysis indicates that Nab co-repressors are induced by Egr2 and other neuregulin-dependent transcription factors (Srinivasan et al. 2007). However, a negative feedback model would predict that loss of Nab1/Nab2 would cause overexpression of myelin genes. A striking aspect of this knockout is the notable absence of myelin gene overexpression (Le et al. 2005b), and similar results were obtained with knock in of a Nab-resistant allele of Krox20/Egr2 (Desmazieres et al. 2008). Overall, the data indicate that Nab proteins do not play a negative feedback role in activation of major myelin genes by Krox20/Egr2, and may indicate that Nab proteins can act as coactivators in certain contexts.
Recent studies have uncovered a number of genes that are apparently repressed by Krox20/Egr2 (directly or indirectly) in peripheral nerve development. In addition to the proposed repression of Oct6/Scip expression (discussed above, Zorick et al. 1999) Krox20/Egr2 expression in Schwann cells was also found to downregulate the L1 marker of immature Schwann cells (Parkinson et al. 2003) and antagonize expression and activity of c-jun (Parkinson et al. 2008; Parkinson et al. 2004). Subsequent microarray analysis of Krox20/Egr2 hypomorphic mice showed that Egr2 downregulated expression of the Sox2 transcription factor (Le et al. 2005a), which is generally associated with pluripotency and maintenance of a stem cell phenotype in a variety of cell lineages. Moreover, expression of Krox20/Egr2 in primary Schwann cells represses Sox2 expression (Parkinson et al. 2008), and induction of Sox2 and Oct6/Scip was also observed after induced deletion of Krox20/Egr2 in mature Schwann cells (Decker et al. 2006). Repression of at least some of these genes requires interaction of Nab proteins with Krox20/Egr2, as Oct6/Scip, Sox2, Id2 and Id4 are upregulated in the absence of Nab function (Desmazieres et al. 2008; Le et al. 2005b). It should be noted that expression profiling alone does not prove a direct role for Nab-dependent repression of such genes by Krox20/Egr2, and there is so far no evidence of direct repression of either Sox2 or Oct6/Scip. However, analysis of Nab-regulated genes has recently provided evidence of direct repression of some target genes, including Id2 and Id4, by Krox20/Egr2 in vivo (Mager et al. 2008).
Krox20/Egr2 and its close relatives
Krox20/Egr2 is a member of a small family of early growth response (Egr) genes that includes Egr1 and Egr3. Krox20/Egr2 is co-expressed with the highly related Egr1 factor in Schwann cells at the onset of myelination (P1). In contrast by one month of age, Krox20/Egr2 is exclusively expressed in myelinating Schwann cells, whereas Egr1 is confined to nonmyelinating Schwann cells (Topilko et al. 1997). Conversely, after nerve injury, Krox20/Egr2 is downregulated and Egr1 becomes induced. Despite the intriguing expression pattern of Egr1, no deficiency in peripheral nerve myelination has been noted in two independent knockout lines (Lee et al. 1996; Topilko et al. 1998). It had been suggested that Egr1 could regulate expression of the p75 neurotrophin receptor (Nikam et al. 1995), which is expressed by nonmyelinating Schwann cells and is required for effective myelination (Cosgaya et al. 2002). Although p75 expression is not diminished in the Egr1 knockout, Egr3 is also co-expressed in p75-expressing Schwann cells in sciatic nerve. Moreover, a double knockout of Egr1 and Egr3 exhibits lower p75 expression and thinner myelin sheaths comparable to the phenotype caused by p75 deficiency (Gao et al. 2007). Since Egr1 and Egr3 share many properties with Krox20/Egr2, including the ability to interact with NAB corepressors (Sevetson et al. 2000), an important question is how Egr2’s role is distinct from, or overlaps with, the function of Egr1 and Egr3.
Transcriptional regulation of demyelination
If specific transcriptional programs must be repressed to allow myelination, a corollary idea is that demyelination associated with nerve injury (or certain types of hereditary peripheral neuropathy) is also coordinated by a specific transcriptional program. Pathways involved in nonmyelinating Schwann cell formation/maintenance have been reviewed recently (Jessen and Mirsky 2005), and one of the major pathways that has emerged involves the c-jun activator, which had previously been shown to be involved in Schwann cell proliferation (Parkinson et al. 2004). These studies have described a mutually antagonistic relationship between Krox20/Egr2 and c-jun that regulates the transitions between nonmyelinating and myelinating Schwann cells (Figure 1, Parkinson et al. 2008) and have provided a mechanistic basis for previous observations that Schwann cells in Krox20/Egr2 deficient mice not only fail to myelinate, but also exhibit higher levels of proliferation and apoptosis (Le et al. 2005a; Topilko et al. 1994; Zorick et al. 1999). Moreover, c-jun is also able to antagonize myelin gene expression independent of its cell cycle effects (Parkinson et al. 2008).
Another variation on this theme was recently presented in an analysis of a mouse model of CMT1B, which is caused by a gain-of-function mutant of Mpz. Expression of this mutant was shown to provoke a canonical unfolded protein response (UPR), characterized by induction of the Chop transcription factor and Bip chaperone (Pennuto et al. 2008). Interestingly, the same Mpz mutant in a Chop null background exhibited reduced demyelination, indicating that the unfolded protein response plays an important role in demyelination caused by this mutant. Although Chop has been implicated in promoting apoptosis in some systems, its activation in Schwann cells apparently causes demyelination in the absence of apoptosis. These findings indicate that targeting the activity of Chop or other UPR mediators could be an attractive target for treatment for human peripheral neuropathies, and it will be important to determine if Chop activation occurs as a consequence of other myelin gene mutations.
Coordination of Lipid Synthesis with Myelination
An important aspect of myelination is the coordination of myelin gene induction with the high level of lipid synthesis required for production of multiple layers of cholesterol-rich membrane. Indeed, a striking aspect of myelination is that most of the cholesterol required is synthesized locally rather than being absorbed from the circulation (Jurevics and Morell 1994). Gene expression profiling revealed coordinate regulation of various lipid biosynthesis genes during myelination and after nerve injury (Nagarajan et al. 2002; Verheijen et al. 2003). The regulatory element binding proteins (Srebp) are network regulators of genes involved in synthesis of fatty acids and cholesterol and are also expressed in Schwann cells during the myelination process, apparently regulating lipid biosynthetic genes (Verheijen et al. 2003). Preliminary observations in mice carrying a Schwann cell specific deletion of Scap (Srebp activation depends on Scap function) indicate that the function of Srebps is required for proper myelination (M.H.G. Verheijen, personal communication). Srebp controlled gene expression appears to converge with Krox20/Egr2 regulation on several target gene promoters, including that of the HMG CoA reductase gene, encoding a protein that represents the rate-limiting step in cholesterol biosynthesis (Leblanc et al. 2005). Accordingly, ectopic expression of Egr2 in primary Schwann cells activated several known Srebp target genes (Nagarajan et al. 2001). Srebp-dependent gene regulation probably plays a role beyond development, since disregulation of insulin-regulated Srebp1c expression in Schwann cells might play a role in diabetic neuropathy (de Preux et al. 2007).
Transcription-based therapies for human peripheral myelinopathies
Most inherited peripheral myelinopathies are caused by altered gene dosage of myelin genes, i.e. Pmp22 and Mpz (Saifi et al. 2003; Szigeti et al. 2006; Wrabetz et al. 2006). Therefore, transcription-based therapies that effect a relatively subtle (<2-fold) change in expression levels could be a very fruitful mode of treatment for these very common diseases. One active area of investigation is that of steroid receptors, such as the progesterone and glucocorticoid receptors, which play an important role in regulation of myelination. Recent proof-of-principle studies using progesterone antagonists to reduce Pmp22 expression in a rat model of CMT1A have shown beneficial effects (Sereda et al. 2003). Although progesterone has been shown to enhance myelination and elevate expression of Pmp22 and other myelin genes (Koenig et al. 1995; Melcangi et al. 1999), specific binding sites for the progesterone receptor have not been defined in myelin genes. Recent studies indicate that progesterone’s effects may be mediated by stimulated synthesis of Krox20/Egr2 and Sox10 in Schwann cells (Guennoun et al. 2001; Magnaghi et al. 2007), which would be consistent with downregulation of Mpz levels in rat sciatic nerve (Melcangi et al. 2003) after administration of a glucocorticoid/progesterone antagonist (mifepristone).
Concluding remarks
Although substantial progress has been made in identifying the pathways underlying commitment to myelination, considerable challenges remain in defining the mechanisms by which myelin gene regulation is controlled. First of all, the signal transduction pathways by which axon-dependent events give rise to nuclear activation of gene networks remain largely unexplored. Recent elegant work has defined important requirements for Type III neuregulin signaling and activation of PI3 kinase pathways to control the initiation and extent of myelination (Michailov et al. 2004; Taveggia et al. 2005). However, more work will be required to connect axon-dependent signaling pathways to transcription factors that direct myelin gene expression. Second, the interactions between Sox10, Krox20/Egr2, and Oct6/Scip represent probably only a small proportion of the combinatorial interactions that induce and repress gene expression during myelination, and future work promises to elucidate other such examples. Third, Oct6/Scip and Sox2 are prototypes of regulators that are downregulated during myelination, and the recent progress in studies of c-jun and Chop function have opened exciting therapeutic avenues by which demyelination (and subsequent nerve damage) could be prevented. However, the molecular targets of these factors that trigger demyelination (or a nonmyelinating phenotype) have yet to be defined. Finally, the specific roles of chromatin remodeling complexes and histone modifications have not yet emerged from studies of myelin gene regulation, and mechanistic analysis of factors directing myelination have not yet uncovered how they alter the chromatin configuration of their target loci.
Acknowledgements
Work in the laboratory of JS is supported by grants from National Institutes of Health (HD41590), Muscular Dystrophy Association, and the Charcot-Marie-Tooth Association. Work in the laboratory of DM is supported by grants from NWO (Vici 918.66.616), the BSIK program of the Dutch Government (BSIK 03038, Stem Cells in Development and Disease) and the European Union (FP7 NGIDD).
References
- Arthur-Farraj P, Mirsky R, Parkinson DB, Jessen KR. A double point mutation in the DNA-binding region of Egr2 switches its function from inhibition to induction of proliferation: A potential contribution to the development of congenital hypomyelinating neuropathy. Neurobiol Dis. 2006;24(1):159–169. doi: 10.1016/j.nbd.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Baranek C, Sock E, Wegner M. The POU protein Oct-6 is a nucleocytoplasmic shuttling protein. Nucleic Acids Res. 2005;33(19):6277–6286. doi: 10.1093/nar/gki947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone E, Di Maria E, Soriani S, Varese A, Doria LL, Ajmar F, Mandich P. A novel mutation (D305V) in the early growth response 2 gene is associated with severe Charcot-Marie-Tooth type 1 disease. Hum Mutat. 1999;14(4):353–354. doi: 10.1002/(SICI)1098-1004(199910)14:4<353::AID-HUMU17>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Jr, Scherer SS, O'Connell S, Arroyo E, Kalla KA, Powell FL, Rosenfeld MG. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10(14):1751–1762. doi: 10.1101/gad.10.14.1751. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Jr, Shearin H, Pennington J, O'Moore J, Jaegle M, Driegen S, van Zon A, Darbas A, Ozkaynak E, Ryu EJ, et al. The claw paw mutation reveals a role for Lgi4 in peripheral nerve development. Nat Neurosci. 2006;9(1):76–84. doi: 10.1038/nn1598. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Jr, Shumas S, Whisenhunt T, Sirkowski EE, O'Connell S, Scherer SS, Rosenfeld MG. Identification of genes that are downregulated in the absence of the POU domain transcription factor pou3f1 (Oct-6, Tst-1, SCIP) in sciatic nerve. J Neurosci. 2002;22(23):10217–10231. doi: 10.1523/JNEUROSCI.22-23-10217.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurand N, Girard M, Pingault V, Lemort N, Dubourg O, Goossens M. Human Connexin 32, a gap junction protein altered in the X-linked form of Charcot-Marie-Tooth disease, is directly regulated by the transcription factor SOX10. Hum Mol Genet. 2001;10(24):2783–2795. doi: 10.1093/hmg/10.24.2783. [DOI] [PubMed] [Google Scholar]
- Bowen NJ, Fujita N, Kajita M, Wade PA. Mi-2/NuRD: multiple complexes for many purposes. Biochim Biophys Acta. 2004;1677(1–3):52–57. doi: 10.1016/j.bbaexp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15(1):66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgaya JM, Chan JR, Shooter EM. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science. 2002;298(5596):1245–1248. doi: 10.1126/science.1076595. [DOI] [PubMed] [Google Scholar]
- Darbas A, Jaegle M, Walbeehm E, van den Burg H, Driegen S, Broos L, Uyl M, Visser P, Grosveld F, Meijer D. Cell autonomy of the mouse claw paw mutation. Dev Biol. 2004;272(2):470–482. doi: 10.1016/j.ydbio.2004.05.017. [DOI] [PubMed] [Google Scholar]
- de Preux AS, Goosen K, Zhang W, Sima AA, Shimano H, Ouwens DM, Diamant M, Hillebrands JL, Rozing J, Lemke G, et al. SREBP-1c expression in Schwann cells is affected by diabetes and nutritional status. Mol Cell Neurosci. 2007;35(4):525–534. doi: 10.1016/j.mcn.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Decker L, Desmarquet-Trin-Dinh C, Taillebourg E, Ghislain J, Vallat JM, Charnay P. Peripheral myelin maintenance is a dynamic process requiring constant Krox20 expression. J Neurosci. 2006;26(38):9771–9779. doi: 10.1523/JNEUROSCI.0716-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denarier E, Forghani R, Farhadi HF, Dib S, Dionne N, Friedman HC, Lepage P, Hudson TJ, Drouin R, Peterson A. Functional organization of a Schwann cell enhancer. J Neurosci. 2005;25(48):11210–11217. doi: 10.1523/JNEUROSCI.2596-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmazieres A, Decker L, Vallat JM, Charnay P, Gilardi-Hebenstreit P. Disruption of Krox20-Nab interaction in the mouse leads to peripheral neuropathy with biphasic evolution. J Neurosci. 2008;28(23):5891–5900. doi: 10.1523/JNEUROSCI.5187-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadi HF, Lepage P, Forghani R, Friedman HC, Orfali W, Jasmin L, Miller W, Hudson TJ, Peterson AC. A combinatorial network of evolutionarily conserved myelin basic protein regulatory sequences confers distinct glial-specific phenotypes. J Neurosci. 2003;23(32):10214–10223. doi: 10.1523/JNEUROSCI.23-32-10214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltri ML, D'Antonio M, Quattrini A, Numerato R, Arona M, Previtali S, Chiu SY, Messing A, Wrabetz L. A novel P0 glycoprotein transgene activates expression of lacZ in myelin-forming Schwann cells. Eur J Neurosci. 1999;11(5):1577–1586. doi: 10.1046/j.1460-9568.1999.00568.x. [DOI] [PubMed] [Google Scholar]
- Feng Q, Zhang Y. The NuRD complex: linking histone modification to nucleosome remodeling. Curr Top Microbiol Immunol. 2003;274:269–290. doi: 10.1007/978-3-642-55747-7_10. [DOI] [PubMed] [Google Scholar]
- Finzsch M, Stolt CC, Lommes P, Wegner M. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor alpha expression. Development. 2008;135(4):637–646. doi: 10.1242/dev.010454. [DOI] [PubMed] [Google Scholar]
- Forghani R, Garofalo L, Foran DR, Farhadi HF, Lepage P, Hudson TJ, Tretjakoff I, Valera P, Peterson A. A distal upstream enhancer from the myelin basic protein gene regulates expression in myelin-forming schwann cells. J Neurosci. 2001;21(11):3780–3787. doi: 10.1523/JNEUROSCI.21-11-03780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich RP, Schlierf B, Tamm ER, Bosl MR, Wegner M. The class III POU domain protein Brn-1 can fully replace the related Oct-6 during schwann cell development and myelination. Mol Cell Biol. 2005;25(5):1821–1829. doi: 10.1128/MCB.25.5.1821-1829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Daugherty RL, Tourtellotte WG. Regulation of low affinity neurotrophin receptor (p75(NTR)) by early growth response (Egr) transcriptional regulators. Mol Cell Neurosci. 2007;36(4):501–514. doi: 10.1016/j.mcn.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Dominguez M, Gilardi-Hebenstreit P, Charnay P. PIASxbeta acts as an activator of Hoxb1 and is antagonized by Krox20 during hindbrain segmentation. Embo J. 2006;25(11):2432–2442. doi: 10.1038/sj.emboj.7601122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazvini M, Mandemakers W, Jaegle M, Piirsoo M, Driegen S, Koutsourakis M, Smit X, Grosveld F, Meijer D. A cell type-specific allele of the POU gene Oct-6 reveals Schwann cell autonomous function in nerve development and regeneration. Embo J. 2002;21(17):4612–4620. doi: 10.1093/emboj/cdf475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghislain J, Charnay P. Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep. 2006;7(1):52–58. doi: 10.1038/sj.embor.7400573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghislain J, Desmarquet-Trin-Dinh C, Jaegle M, Meijer D, Charnay P, Frain M. Characterisation of cis-acting sequences reveals a biphasic, axon-dependent regulation of Krox20 during Schwann cell development. Development. 2002;129(1):155–166. doi: 10.1242/dev.129.1.155. [DOI] [PubMed] [Google Scholar]
- Gillian AL, Svaren J. The Ddx20/DP103 dead box protein represses transcriptional activation by Egr2/Krox-20. J Biol Chem. 2004;279(10):9056–9063. doi: 10.1074/jbc.M309308200. [DOI] [PubMed] [Google Scholar]
- Guennoun R, Benmessahel Y, Delespierre B, Gouezou M, Rajkowski KM, Baulieu EE, Schumacher M. Progesterone stimulates Krox-20 gene expression in Schwann cells. Brain Res Mol Brain Res. 2001;90(1):75–82. doi: 10.1016/s0169-328x(01)00094-8. [DOI] [PubMed] [Google Scholar]
- Howe DG, McCarthy KD. Retroviral inhibition of cAMP-dependent protein kinase inhibits myelination but not Schwann cell mitosis stimulated by interaction with neurons. J Neurosci. 2000;20(10):3513–3521. doi: 10.1523/JNEUROSCI.20-10-03513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Khajavi M, Ohyama T, Hirabayashi S, Wilson J, Reggin JD, Mancias P, Butler IJ, Wilkinson MF, Wegner M, et al. Molecular mechanism for distinct neurological phenotypes conveyed by allelic truncating mutations. Nat Genet. 2004;36(4):361–369. doi: 10.1038/ng1322. [DOI] [PubMed] [Google Scholar]
- Jaegle M, Ghazvini M, Mandemakers W, Piirsoo M, Driegen S, Levavasseur F, Raghoenath S, Grosveld F, Meijer D. The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev. 2003;17(11):1380–1391. doi: 10.1101/gad.258203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaegle M, Mandemakers W, Broos L, Zwart R, Karis A, Visser P, Grosveld F, Meijer D. The POU factor Oct-6 and Schwann cell differentiation. Science. 1996;273(5274):507–510. doi: 10.1126/science.273.5274.507. [DOI] [PubMed] [Google Scholar]
- Jaegle M, Meijer D. Role of Oct-6 in Schwann cell differentiation. Microsc Res Tech. 1998;41(5):372–378. doi: 10.1002/(SICI)1097-0029(19980601)41:5<372::AID-JEMT4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Jang SW, LeBlanc SE, Roopra A, Wrabetz L, Svaren J. In vivo detection of Egr2 binding to target genes during peripheral nerve myelination. J Neurochem. 2006;98(5):1678–1687. doi: 10.1111/j.1471-4159.2006.04069.x. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6(9):671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Jones EA, Jang S-W, Mager GM, Chang L-W, Srinivasan R, Gokey NG, Ward RM, Nagarajan R, Svaren J. Interactions of Sox10 and Egr2 in myelin gene regulation. Neuron Glia Biology. 2007;3(04):377–387. doi: 10.1017/S1740925X08000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurevics HA, Morell P. Sources of cholesterol for kidney and nerve during development. J Lipid Res. 1994;35(1):112–120. [PubMed] [Google Scholar]
- Kellerer S, Schreiner S, Stolt CC, Scholz S, Bosl MR, Wegner M. Replacement of the Sox10 transcription factor by Sox8 reveals incomplete functional equivalence. Development. 2006;133(15):2875–2886. doi: 10.1242/dev.02477. [DOI] [PubMed] [Google Scholar]
- Koenig HL, Schumacher M, Ferzaz B, Thi AN, Ressouches A, Guennoun R, Jung-Testas I, Robel P, Akwa Y, Baulieu EE. Progesterone synthesis and myelin formation by Schwann cells. Science. 1995;268(5216):1500–1503. doi: 10.1126/science.7770777. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Enderich J, Hermans-Borgmeyer I, Wegner M. Cooperative function of POU proteins and SOX proteins in glial cells. J Biol Chem. 1998a;273(26):16050–16057. doi: 10.1074/jbc.273.26.16050. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998b;18(1):237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci U S A. 2005a;102(7):2596–2601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Svaren J, LaPash C, Araki T, Schmidt RE, Milbrandt J. Nab proteins are essential for peripheral nervous system myelination. Nat Neurosci. 2005b;8(7):932–940. doi: 10.1038/nn1490. [DOI] [PubMed] [Google Scholar]
- LeBlanc SE, Jang SW, Ward RM, Wrabetz L, Svaren J. Direct regulation of myelin protein zero expression by the Egr2 transactivator. J Biol Chem. 2006;281(9):5453–5460. doi: 10.1074/jbc.M512159200. [DOI] [PubMed] [Google Scholar]
- Leblanc SE, Srinivasan R, Ferri C, Mager GM, Gillian-Daniel AL, Wrabetz L, Svaren J. Regulation of cholesterol/lipid biosynthetic genes by Egr2/Krox20 during peripheral nerve myelination. J Neurochem. 2005;93(3):737–748. doi: 10.1111/j.1471-4159.2005.03056.x. [DOI] [PubMed] [Google Scholar]
- LeBlanc SE, Ward RM, Svaren J. Neuropathy-associated Egr2 mutants disrupt cooperative activation of myelin protein zero by Egr2 and Sox10. Mol Cell Biol. 2007;27(9):3521–3529. doi: 10.1128/MCB.01689-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, Badache A, DeVries GH. Phosphorylation of CREB in axon-induced Schwann cell proliferation. J Neurosci Res. 1999;55(6):702–712. doi: 10.1002/(SICI)1097-4547(19990315)55:6<702::AID-JNR5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Lee MM, Sato-Bigbee C, De Vries GH. Schwann cells stimulated by axolemma-enriched fractions express cyclic AMP responsive element binding protein. J Neurosci Res. 1996;46(2):204–210. doi: 10.1002/(SICI)1097-4547(19961015)46:2<204::AID-JNR8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Leimeroth R, Lobsiger C, Lussi A, Taylor V, Suter U, Sommer L. Membrane-bound neuregulin1 type III actively promotes Schwann cell differentiation of multipotent Progenitor cells. Dev Biol. 2002;246(2):245–258. doi: 10.1006/dbio.2002.0670. [DOI] [PubMed] [Google Scholar]
- Luciano RL, Wilson AC. HCF-1 functions as a coactivator for the zinc finger protein Krox20. J Biol Chem. 2003;278(51):51116–51124. doi: 10.1074/jbc.M303470200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager GM, Ward RM, Srinivasan R, Jang SW, Wrabetz L, Svaren J. Active Gene Repression by the Egr2-NAB Complex during Peripheral Nerve Myelination. J Biol Chem. 2008;283(26):18187–18197. doi: 10.1074/jbc.M803330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnaghi V, Ballabio M, Roglio I, Melcangi RC. Progesterone derivatives increase expression of Krox-20 and Sox-10 in rat Schwann cells. J Mol Neurosci. 2007;31(2):149–157. doi: 10.1385/jmn/31:02:149. [DOI] [PubMed] [Google Scholar]
- Mandemakers W, Zwart R, Jaegle M, Walbeehm E, Visser P, Grosveld F, Meijer D. A distal Schwann cell-specific enhancer mediates axonal regulation of the Oct-6 transcription factor during peripheral nerve development and regeneration. Embo J. 2000;19(12):2992–3003. doi: 10.1093/emboj/19.12.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcangi RC, Azcoitia I, Ballabio M, Cavarretta I, Gonzalez LC, Leonelli E, Magnaghi V, Veiga S, Garcia-Segura LM. Neuroactive steroids influence peripheral myelination: a promising opportunity for preventing or treating age-dependent dysfunctions of peripheral nerves. Prog Neurobiol. 2003;71(1):57–66. doi: 10.1016/j.pneurobio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Magnaghi V, Cavarretta I, Zucchi I, Bovolin P, D'Urso D, Martini L. Progesterone derivatives are able to influence peripheral myelin protein 22 and P0 gene expression: possible mechanisms of action. J Neurosci Res. 1999;56(4):349–357. doi: 10.1002/(SICI)1097-4547(19990515)56:4<349::AID-JNR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304(5671):700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Mikesova E, Huhne K, Rautenstrauss B, Mazanec R, Barankova L, Vyhnalek M, Horacek O, Seeman P. Novel EGR2 mutation R359Q is associated with CMT type 1 and progressive scoliosis. Neuromuscul Disord. 2005;15(11):764–767. doi: 10.1016/j.nmd.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Kuhn R, Lemke G. Repression of the myelin P0 gene by the POU transcription factor SCIP. Mech Dev. 1993;42(1–2):15–32. doi: 10.1016/0925-4773(93)90095-f. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Kuhn R, Weinmaster G, Trapp BD, Lemke G. Expression and activity of the POU transcription factor SCIP. Science. 1990;249(4974):1300–1303. doi: 10.1126/science.1975954. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Weinmaster G, Kuhn R, Lemke G. SCIP: a glial POU domain gene regulated by cyclic AMP. Neuron. 1989;3(6):783–793. doi: 10.1016/0896-6273(89)90247-x. [DOI] [PubMed] [Google Scholar]
- Morgan L, Jessen KR, Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J Cell Biol. 1991;112(3):457–467. doi: 10.1083/jcb.112.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso M, Balestra P, Bellone E, Cassandrini D, Di Maria E, Doria LL, Grandis M, Mancardi GL, Schenone A, Levi G, et al. The D355V mutation decreases EGR2 binding to an element within the Cx32 promoter. Neurobiol Dis. 2001;8(4):700–706. doi: 10.1006/nbdi.2001.0397. [DOI] [PubMed] [Google Scholar]
- Musso M, Balestra P, Taroni F, Bellone E, Mandich P. Different consequences of EGR2 mutants on the transactivation of human Cx32 promoter. Neurobiol Dis. 2003;12(1):89–95. doi: 10.1016/s0969-9961(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Nagarajan R, Le N, Mahoney H, Araki T, Milbrandt J. Deciphering peripheral nerve myelination by using Schwann cell expression profiling. Proc Natl Acad Sci U S A. 2002;99(13):8998–9003. doi: 10.1073/pnas.132080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan R, Svaren J, Le N, Araki T, Watson M, Milbrandt J. EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron. 2001;30(2):355–368. doi: 10.1016/s0896-6273(01)00282-3. [DOI] [PubMed] [Google Scholar]
- Nickols JC, Valentine W, Kanwal S, Carter BD. Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nat Neurosci. 2003;6(2):161–167. doi: 10.1038/nn995. [DOI] [PubMed] [Google Scholar]
- Nikam SS, Tennekoon GI, Christy BA, Yoshino JE, Rutkowski JL. The zinc finger transcription factor Zif268/Egr-1 is essential for Schwann cell expression of the p75 NGF receptor. Mol Cell Neurosci. 1995;6(4):337–348. doi: 10.1006/mcne.1995.1026. [DOI] [PubMed] [Google Scholar]
- Pareyson D, Taroni F, Botti S, Morbin M, Baratta S, Lauria G, Ciano C, Sghirlanzoni A. Cranial nerve involvement in CMT disease type 1 due to early growth response 2 gene mutation. Neurology. 2000;54(8):1696–1698. doi: 10.1212/wnl.54.8.1696. [DOI] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon L, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, et al. c-Jun is a negative regulator of myelination. J Cell. Biol. 2008;181:625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D'Antonio M, Mirsky R, Jessen KR. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol. 2004;164(3):385–394. doi: 10.1083/jcb.200307132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Dickinson S, Bhaskaran A, Kinsella MT, Brophy PJ, Sherman DL, Sharghi-Namini S, Duran Alonso MB, Mirsky R, Jessen KR. Regulation of the myelin gene periaxin provides evidence for Krox-20-independent myelin-related signalling in Schwann cells. Mol Cell Neurosci. 2003;23(1):13–27. doi: 10.1016/s1044-7431(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Peirano RI, Goerich DE, Riethmacher D, Wegner M. Protein zero gene expression is regulated by the glial transcription factor Sox10. Mol Cell Biol. 2000;20(9):3198–3209. doi: 10.1128/mcb.20.9.3198-3209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirano RI, Wegner M. The glial transcription factor Sox10 binds to DNA both as monomer and dimer with different functional consequences. Nucleic Acids Res. 2000;28(16):3047–3055. doi: 10.1093/nar/28.16.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennuto M, Tinelli E, Malaguti M, Del Carro U, D'Antonio M, Ron D, Quattrini A, Feltri ML, Wrabetz L. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron. 2008;57(3):393–405. doi: 10.1016/j.neuron.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MW, Sevetson BR, Milbrandt J. Identification of NAB1, a repressor of NGFI-A- and Krox20-mediated transcription. Proc Natl Acad Sci U S A. 1995;92(15):6873–6877. doi: 10.1073/pnas.92.15.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu EJ, Wang JY, Le N, Baloh RH, Gustin JA, Schmidt RE, Milbrandt J. Misexpression of Pou3f1 results in peripheral nerve hypomyelination and axonal loss. J Neurosci. 2007;27(43):11552–11559. doi: 10.1523/JNEUROSCI.5497-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifi GM, Szigeti K, Snipes GJ, Garcia CA, Lupski JR. Molecular mechanisms, diagnosis, and rational approaches to management of and therapy for Charcot-Marie-Tooth disease and related peripheral neuropathies. J Investig Med. 2003;51(5):261–283. doi: 10.1136/jim-51-05-14. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Wang DY, Kuhn R, Lemke G, Wrabetz L, Kamholz J. Axons regulate Schwann cell expression of the POU transcription factor SCIP. J Neurosci. 1994;14(4):1930–1942. doi: 10.1523/JNEUROSCI.14-04-01930.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlierf B, Ludwig A, Klenovsek K, Wegner M. Cooperative binding of Sox10 to DNA: requirements and consequences. Nucleic Acids Res. 2002;30(24):5509–5516. doi: 10.1093/nar/gkf690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner S, Cossais F, Fischer K, Scholz S, Bosl MR, Holtmann B, Sendtner M, Wegner M. Hypomorphic Sox10 alleles reveal novel protein functions and unravel developmental differences in glial lineages. Development. 2007;134(18):3271–3281. doi: 10.1242/dev.003350. [DOI] [PubMed] [Google Scholar]
- Sereda MW, Meyer zu Horste G, Suter U, Uzma N, Nave KA. Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT-1A) Nat Med. 2003;9(12):1533–1537. doi: 10.1038/nm957. [DOI] [PubMed] [Google Scholar]
- Sevetson BR, Svaren J, Milbrandt J. A novel activation function for NAB proteins in EGR-dependent transcription of the luteinizing hormone beta gene. J Biol Chem. 2000;275(13):9749–9757. doi: 10.1074/jbc.275.13.9749. [DOI] [PubMed] [Google Scholar]
- Shen-Orr SS, Milo R, Mangan S, Alon U. Network motifs in the transcriptional regulation network of Escherichia coli. Nat Genet. 2002;31(1):64–68. doi: 10.1038/ng881. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Li WW, Lakatos A, Franklin RJ. Expression of the POU-domain transcription factors SCIP/Oct-6 and Brn-2 is associated with Schwann cell but not oligodendrocyte remyelination of the CNS. Mol Cell Neurosci. 2002;20(4):669–682. doi: 10.1006/mcne.2002.1145. [DOI] [PubMed] [Google Scholar]
- Slutsky SG, Kamaraju AK, Levy AM, Chebath J, Revel M. Activation of myelin genes during transdifferentiation from melanoma to glial cell phenotype. J Biol Chem. 2003;278(11):8960–8968. doi: 10.1074/jbc.m210569200. [DOI] [PubMed] [Google Scholar]
- Sobue G, Shuman S, Pleasure D. Schwann cell responses to cyclic AMP: proliferation, change in shape, and appearance of surface galactocerebroside. Brain Res. 1986;362(1):23–32. doi: 10.1016/0006-8993(86)91394-6. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Jang SW, Ward RM, Sachdev S, Ezashi T, Svaren J. Differential regulation of NAB corepressor genes in Schwann cells. BMC Mol Biol. 2007;8:117. doi: 10.1186/1471-2199-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Mager GM, Ward RM, Mayer J, Svaren J. NAB2 represses transcription by interacting with the CHD4 subunit of the nucleosome remodeling and deacetylase (NuRD) complex. J Biol Chem. 2006;281(22):15129–15137. doi: 10.1074/jbc.M600775200. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Friedrich RP, Wegner M. Transcription factors Sox8 and Sox10 perform non-equivalent roles during oligodendrocyte development despite functional redundancy. Development. 2004;131(10):2349–2358. doi: 10.1242/dev.01114. [DOI] [PubMed] [Google Scholar]
- Svaren J, Sevetson BR, Apel ED, Zimonjic DB, Popescu NC, Milbrandt J. NAB2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and differentiative stimuli. Mol Cell Biol. 1996;16(7):3545–3553. doi: 10.1128/mcb.16.7.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaren J, Sevetson BR, Golda T, Stanton JJ, Swirnoff AH, Milbrandt J. Novel mutants of NAB corepressors enhance activation by Egr transactivators. Embo J. 1998;17(20):6010–6019. doi: 10.1093/emboj/17.20.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiers G, Patient R, Loose M. Genetic regulatory networks programming hematopoietic stem cells and erythroid lineage specification. Dev Biol. 2006;294(2):525–540. doi: 10.1016/j.ydbio.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Swirnoff AH, Apel ED, Svaren J, Sevetson BR, Zimonjic DB, Popescu NC, Milbrandt J. Nab1, a corepressor of NGFI-A (Egr-1), contains an active transcriptional repression domain. Mol Cell Biol. 1998;18(1):512–524. doi: 10.1128/mcb.18.1.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szigeti K, Garcia CA, Lupski JR. Charcot-Marie-Tooth disease and related hereditary polyneuropathies: molecular diagnostics determine aspects of medical management. Genet Med. 2006;8(2):86–92. doi: 10.1097/01.gim.0000200160.29385.73. [DOI] [PubMed] [Google Scholar]
- Szigeti K, Wiszniewski W, Saifi GM, Sherman DL, Sule N, Adesina AM, Mancias P, Papasozomenos S, Miller G, Keppen L, et al. Functional, histopathologic and natural history study of neuropathy associated with EGR2 mutations. Neurogenetics. 2007;8(4):257–262. doi: 10.1007/s10048-007-0094-0. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Pizzagalli A, Fagiani E, Messing A, Feltri ML, Wrabetz L. Characterization of a Schwann cell enhancer in the myelin basic protein gene. J Neurochem. 2004;91(4):813–824. doi: 10.1111/j.1471-4159.2004.02745.x. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47(5):681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman V, De Jonghe P, Ceuterick C, De Vriendt E, Lofgren A, Nelis E, Warner LE, Lupski JR, Martin JJ, Van Broeckhoven C. Novel missense mutation in the early growth response 2 gene associated with Dejerine-Sottas syndrome phenotype. Neurology. 1999;52(9):1827–1832. doi: 10.1212/wnl.52.9.1827. [DOI] [PubMed] [Google Scholar]
- Topilko P, Levi G, Merlo G, Mantero S, Desmarquet C, Mancardi G, Charnay P. Differential regulation of the zinc finger genes Krox-20 and Krox-24 (Egr-1) suggests antagonistic roles in Schwann cells. J Neurosci Res. 1997;50(5):702–712. doi: 10.1002/(SICI)1097-4547(19971201)50:5<702::AID-JNR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371(6500):796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Trembleau A, Gourdji D, Driancourt MA, Rao CV, Charnay P. Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, Egr-1)-targeted mice. Mol Endocrinol. 1998;12(1):107–122. doi: 10.1210/mend.12.1.0049. [DOI] [PubMed] [Google Scholar]
- Verheijen MH, Chrast R, Burrola P, Lemke G. Local regulation of fat metabolism in peripheral nerves. Genes Dev. 2003;17(19):2450–2464. doi: 10.1101/gad.1116203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner LE, Mancias P, Butler IJ, McDonald CM, Keppen L, Koob KG, Lupski JR. Mutations in the early growth response 2 (EGR2) gene are associated with hereditary myelinopathies. Nat Genet. 1998;18(4):382–384. doi: 10.1038/ng0498-382. [DOI] [PubMed] [Google Scholar]
- Warner LE, Svaren J, Milbrandt J, Lupski JR. Functional consequences of mutations in the early growth response 2 gene (EGR2) correlate with severity of human myelinopathies. Hum Mol Genet. 1999;8(7):1245–1251. doi: 10.1093/hmg/8.7.1245. [DOI] [PubMed] [Google Scholar]
- Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 2005;28(11):583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Wissmuller S, Kosian T, Wolf M, Finzsch M, Wegner M. The high-mobility-group domain of Sox proteins interacts with DNA-binding domains of many transcription factors. Nucleic Acids Res. 2006;34(6):1735–1744. doi: 10.1093/nar/gkl105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrabetz L, D'Antonio M, Pennuto M, Dati G, Tinelli E, Fratta P, Previtali S, Imperiale D, Zielasek J, Toyka K, et al. Different intracellular pathomechanisms produce diverse Myelin Protein Zero neuropathies in transgenic mice. J Neurosci. 2006;26(8):2358–2368. doi: 10.1523/JNEUROSCI.3819-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Wang Z, Wysocka J, Sanyal M, Aufiero DJ, Kitabayashi I, Herr W, Cleary ML. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;24(13):5639–5649. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C, Korade Z, Carter BD. Protein Kinase A-Induced Phosphorylation of the p65 Subunit of Nuclear Factor-{kappa}B Promotes Schwann Cell Differentiation into a Myelinating Phenotype. J Neurosci. 2008;28(14):3738–3746. doi: 10.1523/JNEUROSCI.4439-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1(5):661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- Zorick TS, Syroid DE, Brown A, Gridley T, Lemke G. Krox-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development. 1999;126(7):1397–1406. doi: 10.1242/dev.126.7.1397. [DOI] [PubMed] [Google Scholar]