Abstract
Aim: This functional magnetic resonance imaging (fMRI) study examined reactivity to alcohol, polydrug, marijuana and emotional picture cues in students who were referred to a college alcohol and drug assistance program. Methods: The fMRI data of 10 participants (5 females; 5 males) were collected while they viewed standardized emotional and appetitive cues. Results: Positive and negative emotional cues produced greater activity than neutral cues in the expected brain areas. Compared with neutral cues, alcohol cues produced greater brain activation in the right insula, left anterior cingulate, left caudate and left prefrontal cortex (Z = 2.01, 1.86, 1.82, 1.81, respectively; P < 0.05). Drug cues produced significantly greater left prefrontal activity compared with neutral cues, with polydrug cues activating the right insula and marijuana cues activating left anterior cingulate. Conclusions: Students at-risk for alcohol abuse showed neural reactivity to alcohol cues in four brain regions, which is consistent with their greater use of alcohol. Insula activation to appetitive cues may be an early marker of risk for progression to alcohol/drug abuse.
INTRODUCTION
Cue reactivity is defined as an observable, classically conditioned response to alcohol and drugs (McDonough and Warren, 2001) that correlates modestly with self-reported craving (Tiffany and Conklin, 2000). Craving is typically described as one component of alcohol and drug dependence, which is characterized by intense desire to self-administer alcohol or a drug, leading to a continued use of substance and relapse after treatment or during periods of abstinence (American Psychiatric Association, 2000). Cue reactivity and craving have been demonstrated in alcohol-dependent adults (Monti et al., 2000; Rohsenow et al., 1992) in the form of physiological changes, such as heart rate elevations or salivation in response to alcohol-related words, pictures, scents, tactile cues or imagined stimuli (McCusker and Brown, 1995; Stormark et al., 2000). These cue-exposure paradigms are widely used to study craving and have been used recently with brain imaging techniques to characterize the neural correlates underlying this experience.
Alcohol-dependent adults, who had been given small amounts of alcohol, showed increased blood oxygenation in the right caudate that was positively correlated with self-reports of alcohol craving (Modell and Mountz, 1995). The caudate has also been associated with craving in cocaine-dependent individuals (Garavan et al., 2000). In addition to caudate, other brain areas activating in response to alcohol-specific cues include the prefrontal cortex (Breiter et al., 1997; George et al., 2001; Grant et al., 1996; Maas et al., 1998) and orbitofrontal cortices (Childress et al., 1999; Garavan et al., 2000; Grant et al., 1996; Volkow et al., 1999; Wang et al., 1999). Further, activation of amygdala (Breiter et al., 1997; Childress et al., 1999; Grant et al., 1996) and anterior cingulate has been associated with self-reported craving (Breiter et al., 1997; Childress et al., 1999; Garavan et al., 2000; Grant et al., 1996; Kilts et al., 2001; Maas et al., 1998; Volkow et al., 1999). These studies compared activation of brain of adults with substance dependence with that of social drinkers or drug-naïve individuals, in response to alcohol/drug-related cues, or to alcohol/drug-related and neutral cues.
Tapert et al. (2004) found that young, alcohol-dependent women produced significantly larger blood oxygenation-level dependent (BOLD) signal changes than did matched social drinkers in response to alcohol words compared with neutral words in the prefrontal cortex, anterior cingulate and insula. Myrick et al. (2004) further showed that after having a sip of alcohol, alcohol-dependent individuals, but not social drinkers, reported higher craving ratings and demonstrated increased activation in the prefrontal cortex and anterior limbic regions for alcohol-related picture cues compared with neutral picture cues. As earlier suggested by Weinstein et al. (1998), this evidence supports the idea that individuals with a substance use disorder display selective, atypical brain responses to stimuli associated with their addiction (Tapert et al., 2003, 2004).
Recently, there has been increased interest in the role that insula may play in addiction, and specifically, its involvement in conscious urges. For example, Naqvi et al. (2007) reported that smokers with brain damage involving insula were more likely to undergo a disruption of smoking addiction characterized by their ability to quit smoking easily and immediately and to remain abstinent without persistent urges to smoke than smokers with brain damage not involving the insula. Paulus et al. (2005) further found that activity in the right insula predicted relapse in methamphetamine-dependent subjects. The insula may prove important for understanding the subjective experience of visceral components of craving, given evidence that this brain region mediates conscious feelings through its role in the representation of bodily states or urges (see Damasio et al., 2000).
The present study examined alcohol, polydrug, marijuana and emotional picture cue-induced brain changes through the use of functional magnetic resonance imaging (fMRI) in college students who had been referred for intervention to a university Alcohol and Drug Assistance Program for Students (ADAPS) following violation of alcohol and drug policy. College students who are mandated to such programs have been shown to be at heightened risk for the development of a substance use disorder (Barnett and Read, 2005). We previously found that a subgroup of high-risk college drinkers (e.g. high-quantity alcohol consumption, emotional suppression reasons for alcohol use) without a significant history of other drug use showed heightened cardiovascular response to alcohol, marijuana and other drug cues, as measured by an index of increased heart rate variability (HRV; Mun et al., 2008). This suggests that heightened physiological reactivity to drug cues may predate significant drug exposure in populations at risk for substance use. Accordingly, the present high-risk sample was expected to show an increased BOLD signal–response to alcohol- and other drug-related picture cues when compared with neutral picture cues, with activation localized in anterior cingulate, caudate and prefrontal regions. Because of the potential role of insula in reactivity to appetitive cues, we further explored insula as a brain region of interest in this study.
We compared brain responses during exposure to emotionally salient picture cues with that during exposure to neutral picture cues to validate our cue-reactivity-imaging protocol by replicating the results of previous fMRI studies of reactivity to emotional picture stimuli. Amygdala, prefrontal cortex and occipito-temporal areas have been linked to the aversive system (Meseguer et al., 2007), and are thus expected to be active when negative emotional cues are presented. The prefrontal cortex has been linked to the appetitive system (Meseguer et al., 2007), and we hypothesized that this area should be responsive to positive emotional cues and to the substance-related cues.
Finally, this study explored whether reactivity to substance-related and emotional cues measured as BOLD signal–response parallels cue reactivity as measured by cardiovascular response. We examined the HRV data, collected as part of a parent project (see Methods) from which the present sample was drawn, as a convergent index of physiological reactivity to picture cues.
METHODS
Participants
Ten (five females) university undergraduate student volunteers who were referred to the ADAPS program of a large northeastern US university took part in the experiment. Of the 10 participants, 3 were referred to the ADAPS for serious infractions (e.g. hospitalization for alcohol poisoning or by emergency medical services) and 7 were referred for more minor infractions (e.g. getting caught drinking or smoking marijuana in a dorm room by a residence hall advisor). The participants ranged in age from 19 to 21 years.
Participants were excluded if they reported a history of childhood learning disability, special education, or psychiatric or neurological disorder or treatment, serious medical conditions, a lifetime diagnosis of any substance use disorder on the part of the prospective participant's biological mother to rule out prenatal exposure effects, English not primary language, MRI contraindications, and for women, pregnancy or lactating. Participants were compensated $50 for their time spent in the study. All participants provided written informed consent approved by the Rutgers and the UMDNJ Institutional Review Boards for the Protection of Human Subjects Involved in Research.
Participants were recruited from an ongoing project investigating the manner in which memory, learning and modulation of emotional response contribute to the regulation of substance use behavior (Mun et al., 2008; Vaschillo et al., 2008). They took part in the fMRI study ∼1 month after their participation in the parent project. As part of the parent project, their medical, family (Family History Assessment Module; Cloninger and Reich, 1991), and alcohol and other drug use histories were assessed. Participants’ age of drinking onset ranged between 13 and 18 years, and none met criteria for alcohol dependence. Four participants had a positive family history of alcohol use disorder. On average, participants drank 5.2 standard alcohol drinks per occasion, one to two times per week in the month preceding their participation in the parent project (one standard alcohol drink = 12 oz. of 5% beer or malt beverage, 5 oz. of 12–17% wine, or 1(1/2) oz. of 80% proof-hard liquor). This quantity of alcohol consumed per drinking occasion exceeds the binge drinking criterion set by the National Institute on Alcohol Abuse and Alcoholism (2004). Four of the participants met criteria for ‘alcoholism’ based on the criterion of the Brief Michigan Alcohol Screening Test (Chan et al., 1994), two participants fell within the alcohol abuse category of the alcohol dependence scale (Skinner and Horn, 1984) and two participants reported previous treatment by a professional in connection with their alcohol use. Three participants used marijuana in the preceding month, but other drug use was infrequent. Thus, although not entirely homogeneous with respect to alcohol and drug use, on average, the sample's alcohol use behavior and problems exceeded that which would be expected in low-risk social drinkers.
Materials
Alcohol, marijuana and polydrug (club drugs and cocaine) picture cues were from the Normative Appetitive Picture System (Mun et al., 2008; Stritzke et al., 2004; Tapert et al., 2003). Emotionally valenced and neutral picture cues were from the International Affective Picture System (Lang et al., 2001). Positively and negatively valenced emotional cues were matched on standardized arousal ratings, but varied systematically in valence. Subsets of the appetitive cues used in the present study were successfully used to induce cue reactivity and/or craving responses in previous studies (Mun et al., 2008; Stritzke et al., 2004; Tapert et al., 2003). The average ratings for the negative, positive and neutral picture cue sets used in this study were 6.4 (SD = 2.2), 5.9 (SD = 2.2) and 3.0 (SD = 1.9), respectively, for arousal, and 2.1 (SD = 1.5), 7.3 (SD = 1.6) and 4.9 (SD = 1.3), respectively, for valence, based on the published norms (Lang et al., 2001). We conducted a rating study with 100 unselected college students to obtain normative arousal and valence ratings for the alcohol and drug cue sets used in the parent study, following the methods of Lang et al. (2001). The average ratings for alcohol, marijuana and polydrug picture cue sets were 4.0 (SD = 2.2), 3.3 (SD = 2.2) and 3.2 (SD = 2.2), respectively, for arousal, and 5.1 (SD = 1.7), 3.7 (SD = 1.9) and 3.5 (SD = 1.9), respectively, for valence.
Procedures
Upon arrival at the imaging lab, participants completed a standard screening to ensure that they could be scanned safely (e.g. no metal implants, metallic tatoos). Participants who passed the screening test were then asked to provide written informed consent. Participants were asked to refrain from alcohol and drug use for 24 h prior to scan. A zero blood alcohol concentration was confirmed by breath analysis using a hand-held intoxilyzer (Intoxilyzer S-D2; CMI, Inc., KY, USA). Women completed a urine test to rule out the possibility of pregnancy.
fMRI acquisition
Imaging was performed using a Siemens Allegra 3T (head-only model) magnet. Participants were scanned in a prone position and a standard quadrature head coil was used. Foam cushioning was used to stabilize head position and minimize head movement.
The stimuli were presented using E-prime software under the Windows XP operating system projected onto a back-projection screen placed at the rear of the scanner bore. Participants viewed the screen by looking in a mirror attached to the head coil. The mirror was adjusted individually to maximize viewing comfort. An MRI compatible two-button mouse was used for responses. Functional scanning was synchronized with the beginning of each block of experimental trials through a trigger pulse sent by the magnet to the E-prime software.
T1-weighted axial anatomical scans (TR = 2000 ms, TE = 4.38 ms, 204 × 256 matrix, field-of-view (FOV) = 22 cm, slice thickness 2 mm, 0 mm gap, 80 slices) were obtained prior to the experimental trial sequence. These anatomical scans were used to register the functional imaging data. Functional imaging was done using an echo planar gradient echo imaging sequence and axial orientation. These scans were obtained using the following parameters: TR = 2000 ms, TE = 30 ms, 64 × 64 matrix, FOV = 22 cm, slice thickness 4 mm, 0 mm gap, 32 axial slices covering the whole brain.
Experimental task
Functional scans were obtained while participants viewed 90 picture cues from six categories. The 90 stimuli included 15 alcohol-related, 15 club drug/cocaine-related, 15 marijuana-related, 15 positive emotional, 15 negative emotional and 15 neutral picture cues. The stimulus presentation was blocked by category so that each of the six blocks consisted of picture cues from the same category. The presentation of experimental blocks was counterbalanced across participants. As the present study was at an early, exploratory research stage in terms of examining cue reactivity, using fMRI, in at-risk college students, and due to a possibility of small effect size differences across picture cue types, we chose a block design rather than an event-related design (Amaro and Barker, 2006; Lashkari and Golland, 2009; Smith et al., 2007). Participants were given a 30 s break after the first three blocks. The scans collected during this break were used as baseline data in subsequent analyses.
Each picture cue was presented sequentially for 4 s. To ensure the attention of participants, they were asked to rate how well they liked the picture cue and the degree to which they found it arousing by pressing an MRI compatible two-button mouse. These mouse button press responses were not recorded. The total experimental task duration was ∼ 10 min. After completion of the task, participants took part in an fMRI study of implicit memory processing of the picture cues (Ray et al., 2010).
Parent project physiological data (ECG) recording and analysis
ECG data were recorded during a baseline condition and during each of the six picture cue presentation blocks. Each block included 30 picture cues and lasted for 5 min, a time epoch useful for HRV analysis (Task Force, 1996). Each block consisted of 15 picture cues from a particular category presented twice, in a different order each time. These picture cue blocks were equivalent to those used in the present study in terms of standardized valence and arousal ratings, but contained no individual cues in common. To enhance the sensitivity of cardiovascular assessment, the picture cues were presented sequentially at 0.1-Hz frequency rate (i.e. one cue every 10 s), corresponding to a resonance property of the cardiovascular system at about this frequency (Vaschillo et al., 2004). Each cue appeared for 5 s, and then the participant viewed a blank screen for 5 s. The task duration was ∼35 min. The ECG record was collected with a sampling rate of 1000 per second by a Powerlab Acquisition System (ADInstruments, Colorado Springs, CO, USA). WinCPRS software (Absolut Aliens Oy, Turku, Finland) was used to measure the beat-to-beat RR interval (RRI) of ECG, segment succession of RRI into 5-min blocks and calculate RRI Fourier spectra and the 0.1-Hz HRV index. Cubic interpolation of the non-equidistant waveform of the RRI sequence was completed, and RRIs were re-sampled at 4 Hz (Cooke et al., 1999). The 0.1-Hz HRV index, measured as the power of the RRI spectrum at 0.1 Hz (Vaschillo et al., 2006), was calculated for each picture cue block. Prior to analysis, the HRV data were subjected to natural log transformation to normalize the distributions (Ponikowski et al., 1996; Singh et al., 1998). Finally, reactivity to each picture cue category was calculated by subtracting the value of the 0.1-Hz HRV index at baseline from its value in response to that picture cue category. The 0.1-Hz HRV index evaluates moment-to-moment autonomic nervous system reaction to various challenges (Nickel and Nachreiner, 2003), including emotional picture cues (Vaschillo et al., 2008).
RESULTS
fMRI analysis
The fMRI data were analyzed using the FEAT (FMRI Expert Analysis) tool, Version 5.63, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). The BOLD scans were motion corrected and spatial smoothing (Gaussian kernel FWHM 5 mm) was applied. BOLD scans for each participant were registered first to that participant's anatomical (mprage) scan, and then registered to standard space using the FSL's MNI template.
The analysis of the fMRI data involved two stages. In the first stage of analysis, individual activation patterns for each participant in each experimental condition (cue block) were obtained by subtracting baseline activity from the activity recorded during a given block. Contrasts between experimental conditions were then computed by subtracting the above baseline activation of all participants in one condition (e.g. neutral picture cues) from that of the same participants in a second condition (e.g. alcohol-related picture cues). Contrasts between experimental conditions were obtained using FSL's OLS (ordinary least squares) simple mixed effects model (Worsley et al., 1992). fMRI analysis included the whole brain and significance levels used were 0.001, 0.01 and 0.05.
Alcohol- and polydrug-related picture cue blocks produced significantly greater brain activation, that is, increased BOLD signal in right superior frontal and middle temporal gyrus (left for alcohol cues and right for polydrug cues) compared with the neutral picture cue block (Table 1). Alcohol and polydrug cues also produced greater activity in the right insula (Figs. 1, 2, and 3). Alcohol and marijuana, but not polydrug cues, activated left anterior cingulate. Only alcohol cues produced greater activity in the left caudate and left middle frontal gyrus. All drug-related picture cues, but not alcohol cues, produced greater activity in left superior temporal gyrus and left inferior temporal gyrus regions compared with neutral picture cues. Compared with neutral cues, all other picture cue types produced greater brain activation in the right cingulate gyrus and the left medial frontal gyrus.
Table 1.
Significant increased BOLD response to alcohol, polydrug and marijuana picture cues compared to neutral picture cues.
| Region | MNI co-ordinates (x, y, z) | Number of voxels | Z |
|---|---|---|---|
| Alcohol > neutrala | |||
| Right insula | 34, 16, 6 | 45 | 2.01 |
| Left anterior cingulate | −8, 30, −14 | 15 | 1.86 |
| Left medial frontal gyrus | −8, 50, −6 | 53 | 1.81 |
| Left middle frontal gyrus | −24, 48, −16 | 23 | 2.19 |
| Right medial frontal gyrus | 20, 50, 0 | 14 | 1.68 |
| Left caudate | −4, 12, 2 | 34 | 1.82 |
| Right superior frontal gyrus | 26, 46, 18 | 49 | 1.74 |
| Right cingulate gyrus | 18, −2, 28 | 33 | 1.91 |
| Left middle temporal gyrus | −54, −60, −10 | 6 | 1.92 |
| Polydrug > neutrala | |||
| Right insula | 36, 14, 8 | 130 | 1.71 |
| Left cingulate gyrus | −16, −20, 28 | 23 | 1.77 |
| Right cingulate gyrus | 18, −22, 28 | 16 | 1.91 |
| Left superior temporal gyrus | −46, −36, 18 | 109 | 1.94 |
| Right superior temporal gyrus | 56, 4, −8 | 17 | 1.98 |
| Left inferior temporal gyrus | −54, −52, −12 | 5 | 1.98 |
| Right middle temporal gyrus | 62, −10, −16 | 12 | 1.91 |
| Right superior frontal gyrus | 44, 26, 48 | 75 | 1.71 |
| Left medial frontal gyrus | −16, −10, 50 | 15 | 1.72 |
| Marijuana > neutrala | |||
| Left anterior cingulate | −2, 28, −12 | 15 | 1.71 |
| Left superior temporal gyrus | −52, 6, −18 | 41 | 1.94 |
| Right superior temporal gyrus | 58, 4, −8 | 7 | 1.92 |
| Left inferior temporal gyrus | −62, −60, −8 | 57 | 1.76 |
| Left middle temporal gyrus | −68, −28, −6 | 53 | 1.72 |
| Right cingulate gyrus | 16, 2, 40 | 32 | 2.31 |
| Left medial frontal gyrus | −16, 54, −4 | 8 | 1.68 |
| Left superior frontal gyrus | −12, 54, −8 | 9 | 1.72 |
aP < 0.05.
Fig. 1.
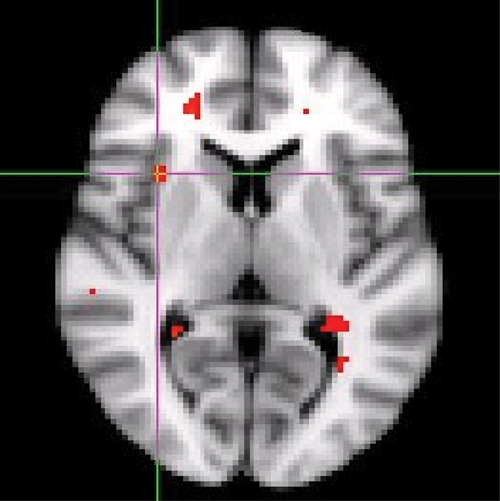
Increased right anterior insula activation in response to alcohol-related picture cues compared to neutral picture cues in the axial view.
Fig. 2.
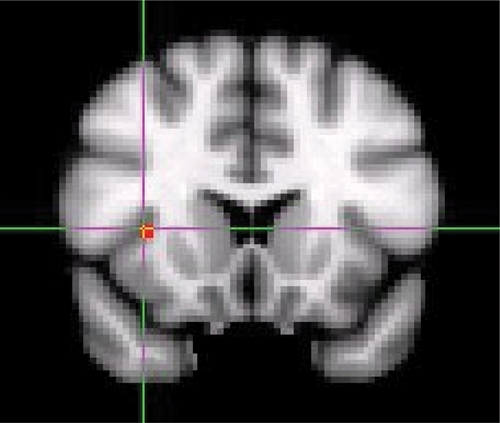
Increased right anterior insula activation in response to alcohol-related picture cues compared to neutral picture cues in the coronal view.
Fig. 3.
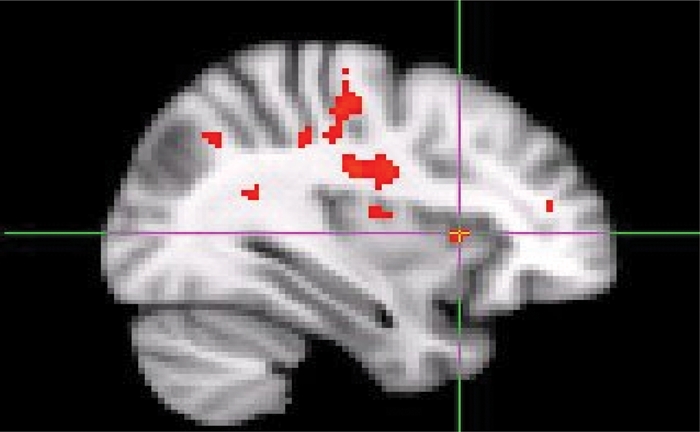
Increased right anterior insula activation in response to alcohol-related picture cues compared to neutral picture cues in the saggital view.
Compared with neutral picture cues, both positive and negative emotional picture cues triggered greater activity in the right middle frontal gyrus and right medial frontal gyrus, right cingulate gyrus, left caudate nucleus, right anterior cingulate, right middle and right superior temporal gyrus, and right middle occipital gyrus (Table 2). Negative emotional picture cues, additionally increased activation, compared with neutral cues, in right amygdala, left orbitofrontal cortex, left parahippocampal gyrus and right anterior insula. Positive emotional picture cues activated left hippocampus, whereas negative emotional picture cues activated right hippocampus.
Table 2.
Significant increased BOLD response to positive- and negative-emotional picture cues compared with neutral picture cues.
| Region | MNI co-ordinates (x, y, z) | Number of voxels | Z |
|---|---|---|---|
| Positive emotional > neutrala | |||
| Right anterior cingulate | 18, 42, 4 | 46 | 1.73 |
| Left caudate | −8, 20, −2 | 11 | 1.80 |
| Right cingulate gyrus | 16, −2, 38 | 46 | 1.71 |
| Left hippocampus | −32, −32, −8 | 2 | 1.71 |
| Right medial frontal gyrus | 14, 44, 12 | 15 | 1.89 |
| Right middle frontal gyrus | 54, 40, 10 | 5 | 1.76 |
| Right middle occipital gyrus | 50, −68, −4 | 12 | 2.13 |
| Right middle temporal gyrus | 54, −58, 2 | 171 | 1.77 |
| Right superior temporal gyrus | 64, 0, −4 | 25 | 1.83 |
| Left superior frontal gyrus | −26, 48, 30 | 2 | 1.77 |
| Negative emotional > neutrala | |||
| Right amygdala | 26, −10, −14 | 8 | 1.78 |
| Right anterior cingulate | 18, 40, 16 | 141 | 1.66 |
| Left caudate | −6, 16, 10 | 84 | 1.94 |
| Right cingulate gyrus | 18, −14, 28 | 52 | 2.35 |
| Right hippocampas | 34, −38, −2 | 97 | 2.10 |
| Left inferior frontal gyrus | −38, 22, 4 | 15 | 1.90 |
| Right insula | 34, 14, 10 | 233 | 2.23 |
| Left parahippocampal gyrus | −26, −22, −20 | 45 | 2.48 |
| Right medial frontal gyrus | 18, 42, 16 | 33 | 1.88 |
| Right middle frontal gyrus | 44, 52, 22 | 145 | 1.87 |
| Right middle occipital gyrus | 48, −68, −2 | 22 | 1.74 |
| Right middle temporal gyrus | 66, −8, −18 | 335 | 2.20 |
| Right superior frontal gyrus | 30, 52, 36 | 153 | 1.65 |
| Right superior temporal gyrus | 52, 6, −10 | 65 | 1.73 |
aP < 0.05.
Physiological data
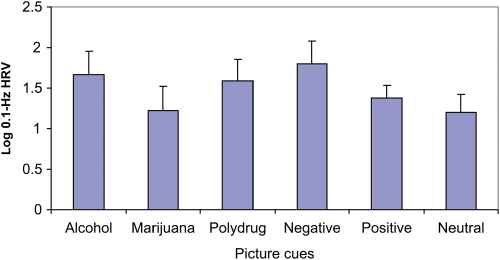
Planned contrast analysis between substance-related and neutral cues revealed significantly higher cardiovascular reactivity to alcohol and polydrug picture cues compared with neutral cues (M = 1.7 for alcohol, M = 1.6 for polydrug, M = 1.2 for neutral; t (9) = 2.51 and t (9) = 2.32, respectively, P < 0.05; Fig. 4). The marijuana and neutral contrast failed to reach significance, P = 0.47. Planned contrasts between emotional and neutral cues revealed significantly enhanced cardiovascular reactivity to negative emotional picture cues compared with neutral picture cues (M = 1.8 for negative emotional, M = 1.2 for neutral; t (9) = 2.55, P < 0.05). The positive emotional and neutral contrast failed to reach significance, P = 0.18 (see Fig. 4).
Fig. 4.
Physiological reactivity measured by a 0.1 Hz Heart Rate Variability (HRV) index in response to alcohol, marijuana, polydrug, negative-emotional, positive-emotional and neutral picture cues.
DISCUSSION
We examined the ability of alcohol-related, drug-related and emotionally valenced picture cues to induce brain response in college students at increased risk for hazardous alcohol use. The resemblance of the present participants’ brain response to that of individuals with alcohol use disorders suggests that some indicants of neural reactivity to alcohol and drug cues may exist prior to, or very early in the course of, problem use. Left prefrontal, anterior cingulate and right insula activation in response to substance-related cues suggests neural processing of cues in a healthy, but high-risk, college group that parallels neural ‘craving’ responses previously found in persons with substance use disorders, compared with controls without substance use disorders (Paulus et al., 2005; Tapert et al., 2004). These preliminary results tentatively suggest that neural responses may be early markers of progression to drug abuse, or perhaps represent neural signatures of risk. This interpretation of the present findings is tentative, given that the present at-risk college students’ brain response was not compared with a control group that comprised alcohol naïve or low-quantity–frequency drinkers. Nevertheless, the present results suggest that larger, comparative studies may be useful for identifying neural reactivity patterns that signal risk in young binge drinkers.
In particular, the higher brain activation found in right insula, left anterior cingulate, left caudate and left prefrontal regions in response to alcohol-related stimuli relative to neutral stimuli is consistent with the findings of significantly higher amplitude in event-related potentials to alcohol-related words in alcohol-dependent patients compared with controls (Herrmann et al., 2000), and in heavy social drinkers when compared with light drinkers (Herrmann et al., 2001). The present findings extend the results of George et al. (2001), Myrick et al. (2004) and Tapert et al. (2004) in adolescents and adults with alcohol use disorders, to college students who are at high risk for alcohol abuse (Barnett and Read, 2005). At-risk college students’ brain response resembled those with alcohol use disorders who, after a sip of alcohol, showed an enhanced activation in anterior cingulate, right insula and the left prefrontal cortex in response to alcohol beverage pictures compared with neutral pictures (George et al., 2001; Myrick et al., 2004; Tapert et al., 2004). Tapert et al. (2004) and Myrick et al. (2004) found an enhanced activation in bilateral insula regions, whereas in the present study activation was localized only in the right insula region. Two studies found that social drinkers showed no activation difference between alcohol beverage pictures and neutral pictures in anterior cingulate and the left prefrontal brain areas (George et al., 2001; Myrick et al., 2004). The previous cocaine cue reactivity literature showed that chronic users of cocaine exhibited activation in right insula while viewing a cocaine film, but not during viewing of a neutral film, whereas the no cocaine history group did not show any activation in this area during presentation of either cocaine or neutral film (Garavan et al., 2000). Taken together, the present and these previous results suggest that the current ADAPS-referred college students may have displayed an at-risk neural response to alcohol-related stimuli, consistent with their risky alcohol use behavior (e.g. binge drinking, and scores on standardized alcohol problem assessments) and their higher cardiovascular response to alcohol cues compared with neutral cues.
Club drug- and cocaine-related picture cues also produced an increased BOLD signal in the right insula and left prefrontal regions and, in the parent study, participants also showed heightened cardiovascular reactivity to these cue types. Marijuana-related picture cues produced an increased BOLD signal in the left anterior cingulate and left prefrontal regions. These results generally parallel the findings of Brody et al. (2002) and Naqvi et al. (2007) in nicotine-dependent individuals, Garavan et al. (2000), Childress et al. (1999), Grant et al. (1996), Maas et al. (1998) and Wexler et al. (2001) in cocaine-dependent individuals, and Paulus et al. (2005) in methamphetamine dependent individuals. In some cases, these studies found the same laterality patterns as did the present study, while in others bilateral activation was found in dependent persons.
Our finding of enhanced right anterior insula activation for alcohol- and polydrug-related stimuli and enhanced cardiovascular reactivity to these cue types is consistent with literature linking anterior insula to limbic, olfactory, gustatory and visceral-autonomic function (Naidicha et al., 2004). Naqvi et al. (2007) previously found that cigarette smokers who suffer damage to the insular region no longer exhibit addiction to nicotine. Naqvi's findings suggest a significant role for the insular cortex in both addiction and the maintenance of addiction. The present results suggest that insula activation to alcohol and drug cues may begin to appear early in the course of at-risk substance use.
In the present study, only negatively valenced picture cues activated amygdala. In addition to right amygdala activation, negatively valenced picture cues compared with neutral picture cues produced an enhanced activation in left parahippocampal gyrus, and right insula, whereas both negatively and positively valenced picture cues activated right prefrontal, right middle temporal and right superior temporal and right middle occipital regions. Thus, our results suggest that the prefrontal cortex is sensitive to arousal, that is, to cues eliciting positive and negative emotions. Consistent with our hypothesis, prefrontal (right) activation was observed for positive emotional picture cues as well as for alcohol- and polydrug-related picture cues. The present findings are consistent with theories that highlight the importance of circuitry-linking subcortical and cortical structures in the processing of emotion laden information (Lee et al., 2004). Thus, the present results support the utility of IAPS pictures in studying neurophysiological correlates of emotionally valenced stimuli as these cues elicited brain response in the aversive systems such as in the prefrontal, amygdala and occipito-temporal regions, and also in the appetitive system such as in the prefrontal cortex, consistent with other works (Meseguer et al., 2007). Participants showed significantly higher cardiovascular reactivity to negative, but not positive, cue types compared with neutral. We previously found increased levels of the 0.1 Hz index in response to both types of emotional picture cues (Vaschillo et al., 2008). In the present study, it is likely that the small sample size compromised power to detect a statistically significant difference between positive and neutral cue reactivities.
Overall, this at-risk college sample appeared to demonstrate neural activity related to cue reactivity in response to alcohol-related cues in four brain areas (insula, anterior cingulate, caudate and prefrontal), whereas cue reactivity responses for marijuana and polydrug cues were observed in two brain areas (anterior cingulate and prefrontal for marijuana; insula and prefrontal for polydrug). This pattern appears to be consistent with participants’ greater use of alcohol than other drugs. Four of the 10 participants had a positive family history of alcohol use disorder. Owing to power limitations, we were not able to examine the role of family history in cue reactivity and brain response. However, given that individuals with a positive family history of alcohol or drug use disorders are at a greater risk of developing a substance use disorder in their lifetime (Eng et al., 2005), the influence of family history on cue reactivity merits further study to determine the role of family history in mediating brain response and cue reactivity to alcohol- and drug-related cues.
Although the present findings regarding neural signatures of risk are provocative, several methodological limitations need to be considered. First, a low-risk control group was not included, so it was not possible to directly compare at-risk and low-risk participants’ BOLD responses to the cues used in this study. Nonetheless, our single group design is consistent with past addiction neuroimaging studies that used a single group (Janse Van et al., 2009; Li et al., 2000; McClernon et al., 2005) and a number of participants ranging between 7 and 13. Second, no subjective report of craving was obtained from the participants. An important goal for future research is to compare self-reported arousal, valence/liking and craving to brain activation patterns. Similarly, simultaneous assessment of cardiovascular and neural response to emotional and appetitive cues would be more informative about the integrated operation of neurophysiological system responsivity. Finally, we verified zero blood alcohol concentration prior to the scanning session; however, a urine drug screen was not employed. Although participants self-reported no recent drug use, we cannot unequivocally rule out the contribution of acute drug effects. The likelihood of acute drug effects in this study was not high, given the present sample characteristics, although this may be an important concern in heavier drug use and clinical populations.
Funding
This work was supported in part by the National Institute on Alcohol Abuse and Alcoholism [R01 AA015248, K02 AA00325 and HHSN275201000003C] and the National Institute on Drug Abuse [P20 DA017552].
REFERENCES
- Amaro E, Barker GJ. Study design in fMRI: basic principle. Brain Cognition. 2006;60:220–232. doi: 10.1016/j.bandc.2005.11.009. doi:10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnosis and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Barnett NP, Read JP. Mandatory alcohol intervention for alcohol-abusing college students: a systematic view. J Subst Abuse Treat. 2005;29:147–58. doi: 10.1016/j.jsat.2005.05.007. doi:10.1016/j.jsat.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. doi:10.1016/S0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. doi:10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Chan KWA, Pristach AE, Welte WJ. Detection of alcoholism in three populations by the brief-MAST. Alcohol Clin Exp Res. 1994;18:695–701. doi: 10.1111/j.1530-0277.1994.tb00933.x. doi:10.1111/j.1530-0277.1994.tb00933.x. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger R, Reich T. Family History Assessment Module. Based on HELPER Family Data Interview. St. Louis, MO: Washington University School of Medicine; 1991. [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, et al. Human response to upright tilt: a window on central automatic integration. J Physiology. 1999;517:617–628. doi: 10.1111/j.1469-7793.1999.0617t.x. doi:10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. doi:10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Eng MY, Schuckit MA, Smith TL. The level of response to alcohol in daughters of alcoholics and controls. Drug Alcohol Depend. 2005;79:83–93. doi: 10.1016/j.drugalcdep.2005.01.002. doi:10.1016/j.drugalcdep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- FEAT. (FMRI Expert Analysis Tool) Version 5.63, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl. )
- Garavan H, Pankiewicz J, Bloom A, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. doi:10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. doi:10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. doi:10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Weijers HG, Wiesbeck GA, et al. Event-related potentials and cue-reactivity in alcoholism. Alcohol Clin Exp Res. 2000;24:1724–1729. doi:10.1111/j.1530-0277.2000.tb01974.x. [PubMed] [Google Scholar]
- Herrmann MJ, Weijers HG, Wiesbeck GA, et al. Alcohol cue-reactivity in heavy and light social drinkers as revealed by event-related potentials. Alcohol Alcohol. 2001;36:588–593. doi: 10.1093/alcalc/36.6.588. [DOI] [PubMed] [Google Scholar]
- Janse Van Rensburg K, Taylor A, Hodgson T, et al. Acute exercise modulates cigarette cravings and brain activation in response to smoking-related images: an fMRI study. Psychopharmacology. 2009;203:589–598. doi: 10.1007/s00213-008-1405-3. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. doi:10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 2001. International Affective Picture System (IAPS): instruction manual and affective ratings. (Technical Report A-4) [Google Scholar]
- Lashkari D, Golland P. Exploratory fMRI analysis without spatial normalization. Inf Process Med Imaging. 2009;5636:398–410. doi: 10.1007/978-3-642-02498-6_33. doi:10.1007/978-3-642-02498-6_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GP, Meador KJ, Loring DW, et al. Neural substrates of emotion as revealed by functional magnetic resonance imaging. Cogn Behav Neurol. 2004;17:9–17. doi: 10.1097/00146965-200403000-00002. doi:10.1097/00146965-200403000-00002. [DOI] [PubMed] [Google Scholar]
- Li S, Biswal B, Li Z, et al. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med. 2000;43:45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, et al. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, et al. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker CG, Brown K. Cue-exposure to alcohol-associated stimuli reduces autonomic reactivity, but not craving and anxiety, in dependent drinkers. Alcohol Alcohol. 1995;30:319–327. [PubMed] [Google Scholar]
- McDonough BE, Warren CA. Effects of 12-h tobacco deprivation on event-related potentials elicited by visual smoking cues. Psychopharmacology. 2001;154:282–291. doi: 10.1007/s002130000647. doi:10.1007/s002130000647. [DOI] [PubMed] [Google Scholar]
- Meseguer V, Romero MJ, Barros-Loscertales A, et al. Mapping the appetitive and aversive systems with emotional pictures using block-design fMRI procedure. Psicothema. 2007;19:483–488. [PubMed] [Google Scholar]
- Modell JG, Mountz JM. Focal cerebral blood flow change during craving for alcohol measured by SPECT. J Neuropsychiatry Clin Neurosci. 1995;7:15–22. doi: 10.1176/jnp.7.1.15. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE. Toward bridging the gap between biological, psychobiological and psychosocial models of alcohol craving. Addiction. 2000;95(Suppl. 2):229–236. doi: 10.1080/09652140050111799. [DOI] [PubMed] [Google Scholar]
- Mun EY, von Eye A, Bates ME, et al. Finding groups using model-based cluster analysis: heterogeneous emotional self-regulatory processes and heavy alcohol use risk. Dev Psychol. 2008;44:481–495. doi: 10.1037/0012-1649.44.2.481. doi:10.1037/0012-1649.44.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. doi:10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Naidicha TP, Kang E, Fatterpeka GM, et al. The insula: Anatomic study and MR imaging display at 1.5 T. Am J Neuroradiol. 2004;25:222–232. [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, et al. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. doi:10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol and Alcoholism. NIAAA Council Approves Definition of Binge Drinking. Bethesda, MD: NIAAA; 2004. [Google Scholar]
- Nickel P, Nachreiner F. Sensitivity and diagnosticity of the 0.1-Hz component of heart rate variability as an indicator of mental workload. Hum Factors, 2003;45:575–590. doi: 10.1518/hfes.45.4.575.27094. doi:10.1518/hfes.45.4.575.27094. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. doi:10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Ponikowski P, Piepoli M, Amadi AA, et al. Reproducibility of heart rate variability measures in patients with chronic heart failure. Clin Sci. 1996;4:391–398. doi: 10.1042/cs0910391. [DOI] [PubMed] [Google Scholar]
- Powerlab Acquisition System. ADInstruments. Colorado Springs, CO; [Google Scholar]
- Ray S, Hanson C, Hanson SJ, Rahman RM, Bates ME. fMRI BOLD response in high-risk college students (part 2): during memory priming of alcohol, marijuana and polydrug picture cues. Alcohol Alcohol, 2010;45:444–8. doi: 10.1093/alcalc/agq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Abrams DB, et al. Cue elicited urge to drink and salivation in alcoholics: relationship to individual differences. Adv Behav Res Ther. 1992;14:195–210. doi:10.1016/0146-6402(92)90008-C. [Google Scholar]
- Singh PJ, Larson GM, Evans CJ, et al. Reduced heart rate variability and new onset hypertension: insights into pathogenesis of hypertension: the Framingham Study. Hypertension. 1998;32:293–297. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Horn JL. Alcohol Dependence Scale: Users Guide. Toronto, Canada: Addiction Research Foundation; 1984. [Google Scholar]
- Smith S, Jenkinson M, Beckmann C, et al. Meaningful design and contrast estimability in fMRI. NeuroImage. 2007;34:127–136. doi: 10.1016/j.neuroimage.2006.09.019. doi:10.1016/j.neuroimage.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Stormark KM, Laberg JC, Nordby H, et al. Alcoholics’ selective attention to alcohol stimuli: Automated processing? J Stud Alcohol. 2000;61:18–23. doi: 10.15288/jsa.2000.61.18. [DOI] [PubMed] [Google Scholar]
- Stritzke WGK, Breiner M, Curtin JJ, et al. Assessment of substance cue reactivity: advances in reliability, specificity, and validity. Psychol Addict Behav. 2004;18:148–159. doi: 10.1037/0893-164X.18.2.148. doi:10.1037/0893-164X.18.2.148. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, et al. Neural response to alcohol stimuli in adolescents with alcohol use disorders. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. doi:10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, et al. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. doi:10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95:145–153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Vaschillo E, Bates ME, Vaschillo B, et al. Heart rate variability response to alcohol, placebo, and emotional picture cue challenges: effects of 0.1 Hz stimulation. Psychophysiology. 2008;45:847–858. doi: 10.1111/j.1469-8986.2008.00673.x. doi:10.1111/j.1469-8986.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaschillo EG, Vaschillo B, Lehrer P. Heartbeat synchronizes with respiratory rhythm only under specific circumstances. Chest. 2004;126:1385–1386. doi: 10.1378/chest.126.4.1385-a. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Vaschillo B, Lehrer P. Characteristics of resonance in heart variability stimulated by biofeedback. Appl Psychophys Biof. 2006;31:129–142. doi: 10.1007/s10484-006-9009-3. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, et al. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experience. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. doi:10.1016/S0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Feldtkeller B, Malizia A, et al. Integrating the cognitive and physiological aspects of craving. J Psychophysiol. 1998;12:31–38. doi: 10.1177/026988119801200105. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, et al. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. doi:10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, et al. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;13:1040–1042. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]