Abstract
Aims: This study examined brain activity using functional magnetic resonance imaging (fMRI) and reaction time (RT) during an implicit repetition priming memory task involving alcohol, polydrug, marijuana and emotional picture cues. Methods: Participants were 5 male and 5 female high-risk college students who had just participated in a cue exposure study (Ray et al., this issue). fMRI and RT data were collected while participants made decisions about previously seen and new picture cues. Results: Both behavioral RT and brain imaging data revealed strong memory priming for drug and alcohol cues. Neurologically, a repetition priming effect (suppression in neural activity for repeated cues) was observed in response to alcohol cues in the left prefrontal, bilateral occipital, and bilateral occipitotemporal regions, as well as right insula and right precuneus (Z ranged from 3.03 to 3.31 P < 0.05). Polydrug cues elicited priming in the occipital and temporal areas, and marijuana cues in the occipital area. Conclusions: Prefrontal and insular cortex involvement both in reactivity to alcohol cues (Ray et al., this issue) and subsequent implicit memory processing of these cues, as found in this study, suggests their potential role in the maintenance of high-risk alcohol use behaviors.
INTRODUCTION
Much research attention in cognitive neuroscience has been focused on the search for neuronal networks that underlie implicit memory processing (Meister et al., 2005; Schacter et al., 2004). One of the most commonly studied phenomena in implicit memory is repetition priming. Repetition priming denotes a facilitative change in the ability to identify a particular stimulus because of a prior encounter with that stimulus (Tulving and Schacter, 1992). Such repetition effects have been shown to arise from as little as one stimulus exposure, yet can be long-lasting (Cave, 1997). Repetition priming has been studied using a number of paradigms, and it usually manifests as a decrease in reaction time or biased response selection when there has been a prior encounter with the stimulus (Buckner et al., 1998; Miller et al., 1996). This facilitation in reaction indicates that priming allows for a faster, more efficient neural processing of previously experienced stimuli (Badgaiyan, 2008; Schacter and Buckner, 1998; Van Turennout et al., 2000).
Although behavioral paradigms often show a reduction in reaction time (RT), functional magnetic resonance imaging (fMRI) has been used to understand the specific neural underpinnings of the repetition priming phenomenon (Buckner et al., 1998; Van Turennout et al., 2000). In the brain, suppression effects during repetition priming have been indicated by a reduction in cortical activity elicited by repeated or previously studied stimuli (Meister et al., 2005; Vuilleumier et al., 2002). The brain areas most often associated with repetition suppression effects are in the extrastriate cortex, and frontal and occipitotemporal areas (Buckner et al., 1998; Henson et al., 2000; Vuilleumier et al., 2002). Suppression of neural activity has been robustly demonstrated during repeated exposure to various classes of stimuli, including objects, words and faces (Ishai et al., 2004).
How the emotional valence of the stimuli affects memory priming is less clear. Ishai et al. (2004) demonstrated that fearful faces elicited repetition suppression effects in occipital and temporal areas and amygdala, using event-related fMRI. Ishai et al. (2006) also reported suppression effects evidenced by reduced amplitude of the magnetoencephalography (MEG) signal to repeated fearful faces in the extrastriate cortex. While these studies suggested a facilitative effect of emotional valence on priming, other studies have reported inconsistent results. Using a matching task that involved neutral and fearful faces, Bentley et al. (2003) found behavioral evidence of repetition priming for neutral, but not for fearful faces. At a neural level, they further found attenuated decreases in orbitofrontal cortex activation in response to fearful faces, relative to neutral faces, consistent with the behavioral results. Using a stimulus detection task with negative and neutral emotional pictures (International Affective Picture System [IAPS]; Lang et al., 2001) and negative and neutral emotional words, Marchewka and Nowicka (2007) found that the emotional valence of the stimuli affected RT such that only neutral stimuli showed behavioral priming effects. Thus, it is not currently certain how and under what circumstances the emotional value of a stimulus affects memory priming (c.f., Phan et al., 2002).
The question of whether negative emotional or appetitive stimuli differentially facilitate memory priming is particularly germane to understanding alcohol and drug cue reactivity. It is important to examine implicit repetition priming of substance-related cues, because maintenance of alcohol and drug use, as well as the development of substance use disorders, is believed to be influenced by implicit memory processes that lie outside of conscious awareness and effortful cognitive control (Robinson and Berridge, 1993). Accordingly, unintentional memory processes may play a key role in the promoting substance use behaviors. The present study used RT and fMRI to examine behavioral facilitation and suppression of brain activity related to repetition priming of alcohol, polydrug, marijuana and emotional picture cues in the high-risk college students who were initially exposed to some, but not all of these pictures as a part of the cue exposure study reported in Ray et al. (this issue, pp. XX). To our knowledge, behavioral and neural repetition priming responses to substance-related cues have not previously been studied. Because college students who violate university's alcohol and drug policy are at high risk to develop a substance use disorder (Barnett and Read, 2005), we hypothesized that they would demonstrate a decreased RT and a decreased brain activation, that is, reduced blood oxygenation-level-dependent (BOLD) response, in reaction to previously studied alcohol-, polydrug- and marijuana-related cues compared with cues from these classes that had not previously been seen (Ray et al., this issue, pp. XX).
We further predicted that the repetition priming effect would be localized in brain areas, including frontal, occipital and occipitotemporal cortices (Buckner et al., 1998; Henson et al., 2000; Vuilleumier et al., 2002). Because of the potential role of insula in reactivity to appetitive cues (Myrick et al., 2004; Naqvi et al., 2007; Tapert et al., 2004), we further included, in this study, insula as a region of interest and tentatively hypothesized repetition priming effects (i.e. suppression of neural activity in response to previously seen picture cues) would also be found in this area. Owing to the lack of consistency in the previous literature regarding the influence of emotional valence of stimuli on repetition priming, we hypothesized that neutral picture cues would demonstrate a repetition priming effect, whereas investigation of priming effects involving negative and positive emotional pictures cues was exploratory.
METHODS
Participants completed this memory study immediately following their participation in the cue exposure study described in Ray et al. (2010, this issue, pp. XX). See this accompanying main study for a description of the selection of participants, their alcohol and drug use characteristics, materials, general procedures and fMRI acquisition parameters.
Experimental task
The experimental task was a repetition priming paradigm that included a study phase and a test phase. The study phase comprised the cue exposure paradigm that participants completed as a part of the accompanying main study (see Ray et al., this issue, pp. XX for a detailed description), and the test phase comprised the picture/non-picture decision task described below.
In between the study and test phases, participants completed a 2-min distractor task that involved counting backwards from 100 by intervals of 7. The distractor task ensured that participants were not thinking about the subset of picture cues that had been presented during the study phase. Then during the test phase, participants completed a picture/non-picture decision task, wherein they viewed a total of 360 stimuli including the 90 studied (previously viewed in Ray et al., this issue) picture cues from 6 categories, 90 non-studied (new) picture cues from the same 6 categories and 180 non-picture cues. “Non-picture” cues were created by electronically distorting pictures that were not used in the experiment. The non-pictures were partially distorted to retain some of their original picture's characteristics using a previously validated technique (Ray et al., in review). Retention of some of the original picture's characteristics in the non-pictures’ helped ensure that participants cognitively processed each stimulus cue while they made decisions about whether it was a picture or non-picture. Non-pictures were described to participants as pictures that did not represent an object, person or scene that they might potentially see in real life. Several examples of non-picture stimuli were shown as examples to ensure that participants understood the decision task. Each stimulus cue was presented sequentially and the order of presentation was randomized. We utilized an event-related design for the repetition priming paradigm, because it is more powerful than the block design for examining activation in response to single, specific trial types (i.e. priming effects) (Buckner et al., 1998; Wagner et al., 1998). Each stimulus was presented for 2.5 s and participants were asked to press the left mouse button if they decided the stimulus was a picture, and the right mouse button if they decided the stimulus was a non-picture. Both RT and accuracy data were collected. The total experimental task duration was ∼30 min.
RESULTS
Behavioral data analysis
For each participant, the amount of behavioral repetition priming for each picture cue type was calculated by subtracting the mean RT (in ms) to the studied picture cues from that to the non-studied picture cues (see Table 1; Buckner et al., 1998; Ray and Bates, 2006). Only RT data for correct picture/non-picture decisions were included in the priming analyses. Errors were defined when participants did not respond within the duration limit for a particular item or made an incorrect response (pressed an incorrect response key). Response errors were rare, suggesting that participants could reliably make picture versus non-picture decisions (5.6%). Paired t-test results revealed that participants showed significant repetition priming for alcohol-related picture cues (t = 6.31, 9 df, P = 0.000, η2 = 0.82) and polydrug-related picture cues (t = 3.80, 9 df, P = 0.004, η2 = 0.62). There was a trend-level priming effect for marijuana-related picture cues (t = 2.17, 9 df, P = 0.052, η2 = 0.34). Thus, participants demonstrated large effect sizes for behavioral repetition priming involving alcohol and polydrug cues (Cohen, 1988). There were no significant repetition priming effects for positive emotional, negative emotional and neutral picture cues.
Table 1.
Amount of repetition priming by picture cue type
| Picture cue type | Mean (ms) | SD (ms) |
|---|---|---|
| Alcohol | 146a,** | 73 |
| Polydrug | 175b,* | 146 |
| Marijuana | 91c,† | 133 |
| Positive emotional | 55d | 113 |
| Negative emotional | 125e | 222 |
| Neutral | 50f | 148 |
Note: a1119–973 ms.
b1203–1028 ms.
c1148–1057 ms.
d1120–1065 ms.
e1258–1133 ms.
f1095–1045 ms.
†P = 0.052.
*P < 0.05.
**P < 0.001.
fMRI data analysis
The fMRI data were analyzed using FEAT (FMRI Expert Analysis) Tool, Version 5.90, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). The BOLD scans were motion corrected and spatial smoothing (Gaussian kernel FWHM 10 mm) was applied. BOLD scans for each participant were registered first to that participant's anatomical (T1; mprage) scans, and then registered to standard space using the FSL's MNI (Montreal Neurologic Institute) template.
The analysis of the fMRI data involved two stages. In the first stage of analysis, individual activation patterns for each participant in each experimental condition were obtained by subtracting baseline activity from the activity recorded during a given experimental condition. There were a total of 12 experimental conditions, including the studied and non-studied picture cues for alcohol, polydrug, marijuana, positive emotional, negative emotional, and neutral stimulus conditions. Contrasts between conditions (e.g. alcohol non-studied versus alcohol studied) were then computed by subtracting the above baseline activation of all participants in one condition (e.g. alcohol cues that were studied) from that of the same participants in a second condition (e.g. alcohol cues that were non-studied). fMRI analyses included the whole brain. Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z > 3.01 and a (corrected) cluster significance threshold of P = 0.05 (Worsley, 2001).
Participants demonstrated a neural repetition priming effect for alcohol-, polydrug- and marijuana-related picture cues, but not for emotional and neutral picture cues. During the test phase of the repetition priming paradigm, non-studied alcohol-related picture cues produced significantly greater brain activation, that is, an increased BOLD signal in right insula (Fig. 1), right precuneus, left middle frontal gyrus, left inferior frontal gyrus, left occipital fusiform gyrus (Fig. 2), right temporal occipital fusiform cortex, right occipital pole (Fig. 2) and bilateral lingual gyrus regions compared with the studied alcohol-related cues (Table 2). Non-studied polydrug-related pictures cues elicited significantly greater activation in bilateral occipital cortex and left inferior temporal gyrus compared with studied polydrug-related picture cues, whereas non-studied marijuana-related picture cues showed significantly greater activation only in the left occipital pole compared with studied marijuana-related picture cues (Table 2).
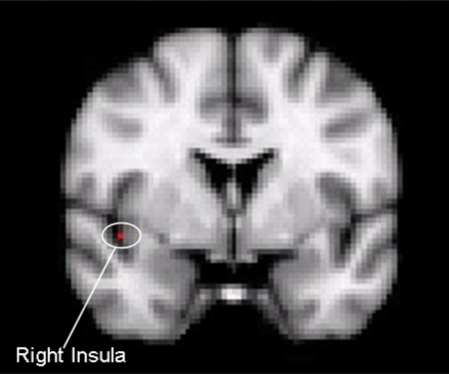
Fig. 1.
Involvement of right insula during repetition priming of alcohol-related picture cues in this coronal section of brain.
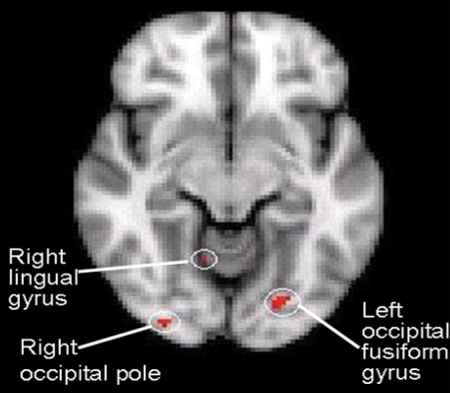
Fig. 2.
A repetition priming effect involving alcohol-related picture cues in the left occipital fusiform gyrus, right occipital pole and right lingual gyrus in this axial section of brain.
Table 2.
Significant increased BOLD response to non-studied alcohol-, polydrug- and marijuana-related picture cues compared with studied alcohol-, polydrug- and marijuana-related picture cues
| Brain region | NMI co-ordinates (x, y, z) |
Number of voxels |
Z-score |
|---|---|---|---|
| Alcohol non-studied > alcohol studieda | |||
| Left middle frontal gyrus | −50, 2, 42 | 50 | 3.08 |
| Left lingual gyrus | −8, −62, −4 | 30 | 3.24 |
| Right lingual gyrus | 20, −68, −4 | 21 | 3.31 |
| Right insula | 44, 0, −8 | 18 | 3.03 |
| Left inferior frontal gyrus | −42, 8, 20 | 16 | 3.23 |
| Left occipital fusiform gyrus | −22, −80, −10 | 46 | 3.31 |
| Right occipital pole | 30, −90, −10 | 29 | 3.28 |
| Right precuneus | 6, −66, 14 | 55 | 3.15 |
| Right temporal occipital fusiform cortex | 34, −52, −16 | 70 | 3.15 |
| Polydrug non-studied > polydrug studieda | |||
| Left lateral occipital cortex | −28, −88, 16 | 19 | 3.22 |
| Left inferior temporal gyrus | −46, −60, −8 | 20 | 3.18 |
| Right lateral occipital cortex | 32, −76, 22 | 17 | 3.11 |
| Marijuana non-studied > marijuana studieda | |||
| Left occipital pole | −6, −94, 2 | 23 | 3.10 |
aP < 0.05.
DISCUSSION
The present study used RT and fMRI to examine behavioral facilitation and brain activity related to implicit memory processing of alcohol, polydrug, marijuana and emotional picture cues in college students who binge drink and are at increased risk for developing a substance use disorder. To our knowledge, this is the first study that examined the repetition priming effects involving alcohol- and drug-related cues. The primary finding was that participants revealed strong behavioral priming and neural suppression effects for alcohol- and drug-related picture cues to which they had previously been exposed. That is, they responded in the decision task more quickly and exhibited a decreased BOLD signal in response to the previously studied (repeated) alcohol and drug cues compared with non-studied (new) alcohol and drug cues in multiple brain areas. The neural suppression results were particularly noteworthy in response to alcohol cues. We found that implicit memory processing of alcohol-related picture cues not only involved brain areas such as prefrontal, occipital and occipitotemporal that have previously been found for implicit memory processing of neutral picture and word stimuli (Buckner et al., 1998; Henson et al., 2000; Meister et al., 2005; Schacter et al., 2004; Vuilleumier et al., 2002), but also insula which is believed to play an important role in the development and maintenance of addictive behaviors and alcohol and other drug craving (Myrick et al., 2004; Naqvi et al., 2007; Park et al., 2007; Tapert et al., 2004). Similarly, we found neural suppression in the precuneus during repetition priming of alcohol cues. A recent study showed that activation in response to alcohol-related cues in the precuneus area was correlated with the level of craving among participants with alcohol use disorders (Park et al., 2007).
Considering together the results of the cue exposure study (see Ray et al., 2010, this issue, pp. XX) and the present implicit memory study, the prefrontal and insular cortices showed significantly high activation during the processing of alcohol-related cues when they were presented for the first time, indicating suppression of neural activity that occurred along with reduced RT when these same alcohol cues were processed a second time, consistent with facilitated implicit information processing during repetition priming. Given the role of prefrontal cortex in attention allocation and action planning, and the role of the insula in determining the value of a stimulus as it may affect bodily state (Paulus and Stein, 2006), one way in which implicit memory for alcohol stimuli may promote future alcohol use is through fast and effortless attention capture and valuation of remembered visceral state.
In contrast, participants did not show significant involvement of craving-related brain areas during repetition priming of polydrug and marijuana picture cues. Yet, consistent with the behavioral evidence for faster processing of drug cues, their functional imaging data did show neural evidence of priming in the occipital (marijuana and polydrug cues) and temporal (polydrug cues) areas. This pattern of neural suppression is consistent with previous priming research that used non-appetitive picture and word stimuli (Buckner et al., 1998; Henson et al., 2000; Meister et al., 2005; Schacter et al., 2004; Vuilleumier et al., 2002). The absence of suppression in insula and precuneous to drug cues appears to be consistent with participants’ much greater use of alcohol than other drugs.
Despite the finding that mandated college students showed repetition priming involving alcohol- and drug-related picture cues, they failed to show behavioral or neural priming effects in response to positive emotional, negative emotional and neutral picture cues. Several previous studies also failed to demonstrate a repetition priming effect using negative picture and word stimuli (Bentley et al., 2003; Marchewka and Nowicka, 2007). It has been suggested that a lack of priming for emotionally negative stimuli may be explained by the operation of an attentional bias mechanism, wherein attention is always preferentially directed toward emotionally negative stimuli. Thus, it may be that the level of attention remained unchanged during an initial and subsequent presentation of negative stimuli, resulting in similarly fast detection of those stimuli and, as a consequence, an absence of the priming effect (Marchewka and Nowicka, 2007). This idea, however, does not explain the present study's failure to find priming for neutral cues that was found in two previous studies (Bentley et al., 2003; Marchewka and Nowicka, 2007). It is possible that a difference in experimental methodology (matching and stimulus detection task in the previous studies versus picture/non-picture decision task in this study) contributed to this lack of consistency. Perhaps more importantly, the large RT variances in priming effects for the emotional and neutral stimuli (see Table 1) suggest that there were substantial individual differences in behavioral facilitation due to prior exposure. Six of 10 participants showed priming for the negative emotional, positive emotional and/or neutral stimuli, whereas 4 failed to show priming. The six participants who did exhibit priming did not show it consistently across the three categories, suggesting that emotional and neutral cues were differentially meaningful to different individuals (individual-level analyses are not shown but are available from the authors by request). It should also be noted that the present study involved six different categories of picture cues, so that the experimental memory processing context was more complex than in previous studies. This complexity may have contributed to preferential processing of the most individually salient cue types. In the present study of high-risk college students, alcohol-, polydrug- and marijuana-related cues appeared to be more uniformly salient in that they were successful in producing a significant priming effect as measured both behaviorally and neurologically at the group level.
This study involves the same limitations as described for the main study (Ray et al., 2010; this issue, pp. XX), including the modest N, and the absence of a low-risk control group and urine drug screen. The present study examined only exposure effects and memory for cues while participants were in the sober state. It is also important for future research to consider how these processing operations may serve to promote alcohol and drug use behaviors during the time course of drinking episodes. The literature consistently shows that repetition priming, as well as other automatic and implicit memory processes, proceed efficiently during acute intoxication at alcohol doses that significantly impair effortful memory (Fillmore et al., 1999; Ray et al., 2004; Ray and Bates, 2006; Tracy and Bates, 1999). It may be speculated that enhanced behavioral priming and neural suppression in response to alcohol cues during acute episodes of intoxication may also serve to further interfere with effortful control of alcohol use behaviors once drinking has been initiated.
Funding
This work was supported in part by the National Institute on Alcohol Abuse and Alcoholism [R01 AA015248, K02 AA00325 and HHSN275201000003C] and the National Institute on Drug Abuse [P20 DA017552].
REFERENCES
- Badgaiyan RD. Neuroanatomical organization of perceptual memory: an fMRI study of picture priming. Hum Brain Mapp. 2008;10:197–203. doi: 10.1002/1097-0193(200008)10:4<197::AID-HBM50>3.0.CO;2-B. doi:10.1002/1097-0193(200008)10:4<197::AID-HBM50>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NP, Read JP. Mandatory alcohol intervention for alcohol-abusing college students: a systematic view. J Subst Abuse Treat. 2005;29:147–158. doi: 10.1016/j.jsat.2005.05.007. doi:10.1016/j.jsat.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Bentley P, Vuilleumier P, Thiel C, et al. Effects of attention and emotion on repetition priming and their modulation by cholinergic enhancement. J Neurophysiol. 2003;90:1171–1181. doi: 10.1152/jn.00776.2002. doi:10.1152/jn.00776.2002. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, et al. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:258–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Cave CB. Very long-lasting picture priming in picture naming. Psychol Sci. 1997;8:322–325. doi:10.1111/j.1467-9280.1997.tb00446.x. [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavior Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- FEAT (FMRI Expert Analysis Tool) Version 5.90, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl. )
- Fillmore MT, Vogel-Sprott M, Gavrilescu D. Alcohol effects on intentional behavior: dissociating controlled and automatic influences. Exp Clin Psychopharmacol. 1999;7:372–378. doi: 10.1037//1064-1297.7.4.372. doi:10.1037/1064-1297.7.4.372. [DOI] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. doi:10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Ishai A, Pessoa L, Bikle P, et al. Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci USA. 2004;101:9827–9832. doi: 10.1073/pnas.0403559101. doi:10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Bikle PC, Ungerleider LG. Temporal dynamics of face repetition suppression. Brain Res Bull. 2006;70:289–295. doi: 10.1016/j.brainresbull.2006.06.002. doi:10.1016/j.brainresbull.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 2001. (Tech. Rep. No. A-4) [Google Scholar]
- Marchewka A, Nowicka A. Emotionally negative stimuli are resistant to repetition priming. Acta Neurobiol Exp. 2007;67:83–92. doi: 10.55782/ane-2007-1635. [DOI] [PubMed] [Google Scholar]
- Meister IG, Weidemann J, Foltys H, et al. The neural correlate of very-long-term picture priming. Eur J Neurosci. 2005;21:1101–1106. doi: 10.1111/j.1460-9568.2005.03941.x. doi:10.1111/j.1460-9568.2005.03941.x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the Macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. doi:10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, et al. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. doi:10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Sohn J, Suk J, et al. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol Alcohol. 2007;42:17–422. doi: 10.1093/alcalc/agl117. doi:10.1093/alcalc/agm091. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. doi:10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, et al. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimaging. 2002;2:331–348. doi: 10.1006/nimg.2002.1087. doi:10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Ray S, Bates ME. Acute alcohol effects on repetition priming and word recognition memory with equivalent memory cues. Brain Cognition. 2006;60:118–127. doi: 10.1016/j.bandc.2005.07.009. doi:10.1016/j.bandc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Ray S, Bates ME, Bly BM. Alcohol's dissociation of implicit and explicit memory processes: implications of a parallel distributed processing model of semantic priming. Exp. Clin. Psychopharmacol. 2004;12:118–125. doi: 10.1037/1064-1297.12.2.118. doi:10.1037/1064-1297.12.2.118. [DOI] [PubMed] [Google Scholar]
- Ray S, Hanson C, Hanson SJ, et al. fMRI BOLD response in high risk college students (Part 1): during exposure to alcohol, marijuana, polydrug and emotional picture cues. Alcohol Alcohol. 2010;45:437–43. doi: 10.1093/alcalc/agq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T, Berridge K. The neural basis of craving: an incentive sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. doi:10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. doi:10.1016/S0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Dobbins IG, Schyner DM. Specificity of Priming: a cognitive neuroscience perspective. Nature Rev Neurosci. 2004;5:853–862. doi: 10.1038/nrn1534. doi:10.1038/nrn1534. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, et al. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. doi:10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Bates ME. Selective effects of alcohol on automatic and effortful memory process. Neuropsychol. 1999;13:282–290. doi: 10.1037//0894-4105.13.2.282. doi:10.1037/0894-4105.13.2.282. [DOI] [PubMed] [Google Scholar]
- Tulving E, Schacter DL. Priming and human memory systems. Science. 1992;247:301–306. doi: 10.1126/science.2296719. doi:10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- Van Turennout M, Ellmore T, Martin A. Long-lasting cortical plasticity in the object naming system. Nat Neurosci. 2000;3:1329–1334. doi: 10.1038/81873. doi:10.1038/81873. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Henson RN, Driver J, et al. Multiple levels of visual object constancy revealed by event-related fMRI of repetition priming. Nat Neurosci. 2002;5:491–499. doi: 10.1038/nn839. doi:10.1038/nn839. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. doi:10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: an Introduction to Methods. New York, NY, Oxford University Press; 2001. pp. 251–270. [Google Scholar]