Abstract
Purpose of the Review
Current EMS protocols rely on provider directed care for evaluation, management and triage of injured patients from the field to a trauma center. New methods to quickly diagnose, support and coordinate the movement of trauma patients from the field to the most appropriate trauma center are in development. These methods will enhance trauma care and promote trauma system development.
Recent Findings
Recent advances in machine learning, statistical methods, device integration and wireless communication are giving rise to new methods for vital sign data analysis and a new generation of transport monitors. These monitors will collect and synchronize exponentially growing amounts of vital sign data with electronic patient care information. The application of advanced statistical methods to these complex clinical data sets has the potential to reveal many important physiological relationships and treatment effects.
Summary
Several emerging technologies are converging to yield a new generation of smart sensors and tightly integrated transport monitors. These technologies will assist pre-hospital providers in quickly identifying and triaging the most severely injured children and adults to the most appropriate trauma centers. They will enable the development of real-time clinical support systems of increasing complexity, able to provide timelier, more cost-effective, autonomous care.
Keywords: Trauma, triage, machine learning, feature extraction, transport monitoring
Introduction
Emergency Medical Service (EMS) providers are responsible for pre-hospital care of injured patients and patient transport from the field to definitive care. When they arrive on-scene they quickly identify those who are at greatest risk of death due to injury and stratify these patients into expectant (expected to die) and immediate categories. The immediate needs of severely injured patients are addressed first and include measures such as establishing an airway, providing ventilatory support or dressing a wound to halt external bleeding. Individuals who are less severely injured are evaluated and treated in order of injury severity. As EMS providers identify and treat persons with varying types and degrees of injury severity, they are simultaneously determining the number of casualties requiring transport and the most appropriate facility to which to transport each patient. This decision process is known as “field triage” and the most commonly used method is based on a practice algorithm developed by the American College of Surgeons, called a “Decision Scheme” [1]. The current Field Triage Decision Scheme guides EMS providers through four decision steps [1]. These four steps take into account the patient’s physiology, anatomic injuries, mechanism of injury, and special considerations. They are designed to assist the medic in determining the most appropriate destination facility within a local or regional trauma system.
While the current Decision Scheme is the best method we have for identifying and prioritizing injured patients in the field, it has its limitations. There are very few scientific studies evaluating the Decision Scheme and its component criteria. Rather, the component criteria are based on individual studies of specific criteria, expert opinion, consensus and policy statements from specialties and disciplines involved in injury prevention and control [2]. Proper use of the Decision Scheme requires education and experience. Implementation is time consuming and the output is discontinuous; it represents a single point in time. We know from experience, however, that the physiology of an injured patient is constantly changing and triage should be a continuous process; casualties should move from one category of injury severity to another, based on their response to therapy. In critical trauma and especially triage situations there is a profound need for improved, physiology-based algorithms, which utilize noninvasive sensor technologies to provide real-time insight into the early compensatory changes that are triggered by injury. Once we know the injury severity of all active patients in a trauma system, we can actively plan how and where to transport those patients.
Recent developments in active machine learning, device integration and wireless communications are spawning a new era in pre-hospital patient care. This new era is founded on the premise that our organ systems are physiologically linked, that perturbation or injury to one or more organ systems affects many others, and most importantly, these perturbations can be measured, statistically analyzed and used to predict present and future clinical states in real-time. The following is an overview of how several new technologies are coalescing and building upon each other to yield a new paradigm in pre-hospital trauma care. This paradigm begins with noninvasive, real-time resuscitation monitoring in the field. It seeks to identify and automate the management of severely injured patients, while offering the potential for centralized monitoring with triage decision support.
Emergency Medical Services
EMS providers are regulated at the state level. They operate under the license and direction of a physician, who helps develop operational policies and medical care protocols. Medical direction is designated as offline, when an EMS provider delivers clinical care based on these protocols. Conversely, online medical direction involves direct (radio or telephone) communication between an EMS provider in the field and an emergency department physician. Most EMS systems operate using a combination of offline and online medical direction.
Trauma Centers and Systems
Where to transport an injured patient is an important decision, because where a trauma patient is cared for can have a significant impact on subsequent morbidity and mortality. A national study in 2006 identified a 25% reduction in mortality for severely injured patients who received care at a Level I trauma center, versus a nontrauma center [3]. Trauma centers are classified as Level I, II, III, IV or V (Table 1). This classification scheme dates back to 1976, when the ACS-Committee on Trauma developed guidelines for the verification of trauma centers, including standards for personnel, facility, and processes for the optimal care of injured persons [1]. Level I trauma centers provide the highest level of trauma care. They are further classified as adult, adult and pediatric, or pediatric Level 1 trauma centers. Level 1 trauma centers are tertiary referral centers, able to provide specialty and subspecialty trauma care for the most severely injured patients. In addition to complex patient care, these centers provide leadership in trauma education, research and prevention. Level II trauma centers provide a level of care similar to Level I trauma centers, but may not offer continuous availability of certain surgical subspecialties, or have well established prevention or research programs. Level III trauma centers provide initial evaluation and management of trauma patients, including emergency surgery, if necessary; however, once the patient is stabilized, transfer to a Level I or II facility may occur according to pre-existing agreements. Level IV trauma centers provide 24 hour physician coverage for the initial evaluation and resuscitation of injured patients. Level V centers are typically located in rural or frontier areas and are staffed by advanced practice nurses or physician assistants, who are trained in trauma resuscitation protocols. Once the patient is stabilized, transfer to a higher level facility is undertaken.
Table 1.
|
SOURCE: Adapted from (1,2).
Injured patients should be managed at the closest, most appropriate trauma center. In some cases this will be a Level II, III, IV or V trauma center. Transporting all patients to a Level I center, regardless of the severity of their injuries, would burden those facilities unnecessarily and make them less available for the most severely injured patients. Similarly, not all injured children should be transported to a Level 1 pediatric trauma center. Doing so would overwhelm our patient transport systems, burden these facilities and be a hardship on families, as most pediatric trauma centers are regional resource centers, primarily located in free-standing children’s hospitals around the country.
Regionalized trauma systems with formalized trauma protocols for adult and pediatric trauma patients can improve patient outcomes [4,5,6,7,8,9,10]. A comparison of injury mortality rates in states with regional or statewide trauma systems to those without such systems found that crude injury-related mortality rates were 9% lower in the 22 states with trauma systems [11]. Several reports have documented reductions in pediatric injury mortality, ranging from 3.8% to 9.7%, together with improved functional outcomes when moderate to severely injured children (aged < 16 years) are treated at pediatric trauma centers or at adult trauma centers with added qualifications to treat children [12,13]. Thus, children and adults who meet triage criteria should be transported to the closest and most appropriate trauma center.
Undertriage occurs when an injured patient is transported to a trauma center that cannot provide the specialized trauma care that is required to treat that patient. Overtriage occurs when a less severely injured patient is transported to a higher level trauma center than necessary to treat that patient’s injuries. Overtriage consumes valuable trauma resources and raises the overall cost of trauma care. However, because undertriage can result in excess morbidity and mortality, most trauma systems err on the side of minimizing undertriage. Target levels for undertriage in a trauma system range from 0 to 5%, whereas target levels for overtriage range from 25% to 50% [1].
Step One of the Field Triage Decision Scheme requires an EMS provider to measure a patient’s vital signs and determine the patient’s level of consciousness. Any of the following criteria would trigger transport of an adult trauma patient to a trauma center: systolic blood pressure < 90, respiratory rate < 10 or > 29, Glasgow Coma Score < 14, or a revised trauma score < 11. The sensitivity of these physiologic criteria to identify severely injured trauma patients has been reported to range from 55.6% to 64.8%, with a positive predictive value of 41.8% and a specificity of 85.7% [14,15]. Studies have shown, however, that humans can loose as much as 40–50% of their central blood volume without exhibiting clinically meaningful changes in what many would consider to be standard or “legacy” vital signs: mental status [16], pulse character [16], systolic and mean blood pressures [16,17,18,19,20], arterial oxygen saturation [18,21], end-tidal CO2 [22], respiratory rate [23], blood pH or base deficit [24].
A child’s physiological response to injury is especially rapid and compensatory. The duration of compensation, however, is typically shorter in children than adults due to the small size of the pediatric heart and its limited capacity to increase stroke volume. Cardiac output (stroke volume × heart rate) in a young child is almost entirely dependent on heart rate. A child will become extremely tachycardic before the onset of hypotension. And when hypotension occurs, it happens very quickly and with little warning. New methods and automated algorithms are needed to assist EMS providers in identifying and triaging potentially unstable children and adults, well before the onset of hypotension and hemodynamic instability. Minimizing under and overtriage would increase trauma system efficiency, reduce morbidity and mortality and lower the overall cost of trauma care.
Feature Extraction and Active Machine Learning
Feature extraction is a useful tool for identifying important phenomena in a noisy or confounded signal. Task or process related knowledge and classic signal processing techniques are used to interrogate large, complex datasets, in order to identify salient events and signals (features). These features are then subjected to powerful modeling methods to find important co-occurrences. For example, high dimensional non-parametric statistical modeling techniques can be applied to complex, multi-input sequences to not only recognize, but predict temporal phenomena. The utility of these feature extraction methods has been successfully demonstrated on data from such diverse fields as Robotics [25], Criminology [26] and WiFi Localization [27]. We are exploring the application of these tools in the fields of physiology and medicine.
Machine learning is concerned with the design and development of algorithms that can be used to automatically extract information from large volumes of data. In 2008, we began using feature extraction and machine learning methods to analyze complex physiological waveform data, in order to identify subtle changes in human physiology that are predictive of acute blood loss. The physiological waveform data that we analyzed was generated at the U.S. Army Institute of Surgical Research (USAISR), where a human model of severe, acute hemorrhage has been developed. This model uses lower body negative pressure (LBNP) as a method for investigating cardiovascular changes under conditions of controlled, experimentally induced hypovolemic hypotension. For every 15 mm Hg of negative pressure that is applied to the lower body, approximately 500 cc’s of blood are pulled away from the head and torso to be redistributed to the pelvis and lower extremities. LBNP, as a surrogate for acute blood loss, reduces central blood volume and cardiac output [18,19], increases sympathetic nerve activity [28], reduces tissue oxygen content and pH [24,25,29], and eventually leads to severe hypotension and decompensation [18,19,20].
Continuous, noninvasively measured physiological signals from 104 human LBNP experiments were analyzed by Grudic and Mulligan using feature extraction and advanced statistical methods to build predictive models of acute blood loss and CV collapse. The algorithms they developed use continuous, noninvasive blood pressure waveform data, generated by a Finometer blood pressure monitor. The first prediction occurs at 30 beats of the heart and a new prediction is made with each subsequent beat of the heart. Statistically unbiased accuracy analysis on all 104 subjects showed a correlation of 0.95 for: 1) predicted LBNP level, or predicted volume of blood loss in cc’s, and 2) predicted level at which the test subject will experience hemodynamic decompensation, or cardiovascular collapse.
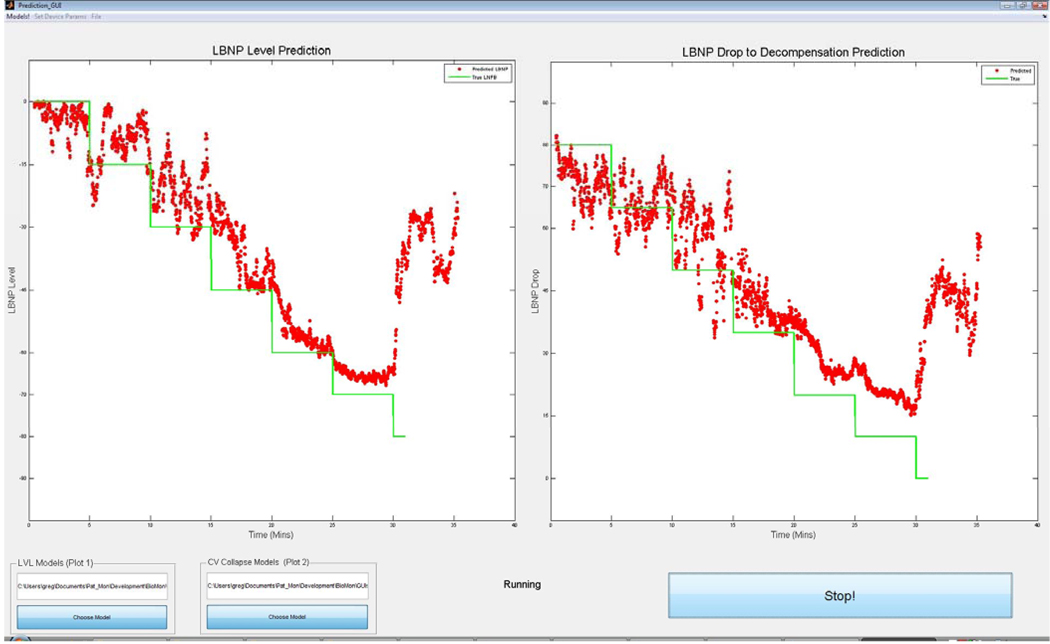
This technology quickly, accurately and noninvasively predicts LBNP, which correlates with how much central blood volume has been redistributed to the pelvis and lower extremities; i.e. how much blood a patient has “lost” (Figure 1, red dots). More importantly, it provides real-time information on the predicted level of “blood loss” (LBNP) that will cause CV collapse for that specific individual. Humans who are exposed to LBNP will collapse at different times and at different levels of LBNP. The early identification of trauma patients who are more likely to experience hemodynamic decompensation with less blood loss could prove critical to early intervention, establishing a triage order and better outcomes, since these patients are at greatest risk for rapid development of hemorrhage shock.
Figure 1.
The figure on the left shows actual LBNP (solid line) versus beat-to-beat predicted LBNP level (dots) during a live test. The figure on the right figure shows actual LBNP (line) versus predicted LBNP level (dots) at which the subject will decompensate.
The mechanisms that underlie greater tolerance to LBNP are under investigation. Preliminary results indicate that high tolerant (HT) individuals have a significantly greater elevation in heart rate than low tolerant subjects [30]. The greater heart rate response of high tolerant individuals may be due to greater cardiac vagal withdrawal and higher sympathetic nerve activity, the latter enhancing vasoconstriction to support blood pressure. Additionally, high tolerant subjects have significantly greater oscillations in cerebral blood flow velocity and arterial blood pressure than low tolerant subjects [31]. These are metrics that no human can detect or decipher in a beat-to-beat fashion. Their incorporation into future algorithms may, however, allow greater precision in the prediction of hemodynamic decompensation and the early identification of patients at greatest risk for CV collapse.
Transport Monitoring and Autonomous Care
Civilian pre-hospital providers (e.g. paramedics and emergency medical technicians) collect and act upon a wide variety of complex visual clues, while, at the same time, monitoring and adjusting to continually changing sets of vital signs. A patient’s vital signs are typically displayed on a transport monitor and periodically noted in the record. In the vast majority of cases, vital sign information is not automatically integrated into the patient care record, so how the vital signs change in response to specific treatment measures is largely undocumented and poorly understood. As a consequence, the care that is provided to a patient in the field is anticipatory and reactive. It is not very time sensitive and the accompanying patient medical record is poorly contextual. Healthcare providers do not give medications or adjust the ventilator and IV fluids as often or as accurately as a smart, vigilant system might and, as a result, patients do not respond or recover as quickly as they could.
An integrated approach is needed to assist pre-hospital providers in managing and triaging the most severely injured patients to the most appropriate trauma centers. The ideal solution will be an integrated platform composed of physiological monitoring and therapeutic hardware devices, linked by a suite of software applications. Software will oversee a growing number of autonomous care applications [32], which will reduce the need for constant attention by an EMS provider or crew medical officer. The components integral to the next generation of transport monitors include: a ventilator; 3/5-lead ECG; pulse oximeter; noninvasive blood pressure (NIBP); end-tidal carbon dioxide (CO2); patient temperature; invasive arterial and intracranial pressure capabilities; Ethernet communications; closed-loop control of oxygenation [33], ventilation [34] and IV fluid control [35]; an integrated electronic medical record (data storage/export); alarming and smart help. The system should support several external intravenous (IV) pumps and other to be developed noninvasive monitors, all connected via powered-USB ports. Other potential modules that could be included are an oxygen concentrator, patient warming and an anesthesia control module.
The U.S. military is currently the major driver for developing the next generation of transport monitors; however, space flight requirements are being considered in the design process. These monitors will need to be lightweight, rugged, and have low power needs. They should have facility for remote calibration and maintenance, incorporate redundant systems, and be capable of fully supporting a patient from the site of injury to a definitive care facility aboard various transport vehicles.
Real-time Communication and Network Centric Patient Care
The future of communication lies in internet protocol based and wireless broadband technologies. With the support of the Federal Communications Commission (FCC) and the Association of Public-Safety Communications Officials (APCO) these technologies are being targeted for critical public safety communications, including EMS Telemedicine. Next generation Wi-Fi networks, called WiMAX mesh networks, will be able to cover large areas with a radius of 2 – 6 miles. More importantly, WiMAX networks are being designed to handle moving objects, like a vehicle or ambulance. These network capabilities will make it possible for ambulances to communicate with a base station or hospital in real-time. Full voice, patient vital sign and patient care record (PCR) information, as well as live video will be available from the back of a ground ambulance or transport helicopter. The ability to forward this information to a receiving hospital, well ahead of a patient, will enable individuals at a centralized communication or trauma center to assist in the diagnosis and management of acutely ill and injured patients. Such a system would allow hospital-based providers the ability to anticipate patient care needs, well ahead of the patient’s arrival. If transport times were prolonged or a problem found to be more acute, a central monitor or physician at the destination hospital could locate an alternative trauma center, check on surgeon and OR availability, and recommend the patient be diverted there. A recent review article [36] evaluating the role of telemedicine in accident and emergency work found that a variety of communications equipment has been tried, including radio links, telephone and mobile wireless videoconferencing devices. These communications technologies were found to transfer information effectively, but success was sometimes limited by technical failure and by staff lacking confidence in using the systems. Larger trials and cost-effectiveness studies are required in this area.
The value of consolidating patient monitoring and patient support into a single system, capable of transmitting real-time patient care data, cannot be overemphasized. This platform technology has many advantages, not only in providing real-time information display at the destination trauma center, but in the areas of triage and autonomous care. If real-time transport data was made available at a central location, this data could be used to direct patients to the closest, most qualified trauma center. More importantly, the physiologic and electronic patient care data that will be captured by these fully integrated communications and information management systems will be able to be queried. New machine learning, feature extraction and advanced statistical methods will be used to analyze these complex data sets, in order to reveal many important, previously hidden physiological relationships and treatment effects. As these relationships are defined and understood, our models of health and disease will become increasingly more complex and more accurate. They will provide reliable, real-time insight into the current and predicted future status of a patient.
Conclusion
The next generation of transport monitors will be tightly integrated, clinical support and communication systems. They will utilize closed loop control for basic patient support functions, such as ventilation and IV fluid management. State-of-the art algorithms will constantly monitor each patient’s physiological waveform and patient care data, looking for signs of abnormal or perturbed physiological change. Early identification of physiological changes will alert healthcare providers to intervene when the physiology is less complex and more likely to respond to therapy. These algorithms will assist pre-hospital providers in quickly identifying and triaging the most severely injured patients to the most appropriate trauma centers. Real-time scene and transport information will be coordinated at the trauma system level and shared with destination hospitals. In time, machine-based comprehension of semantic clinical information together with real-time physiological data will lead to the development of structured clinical knowledge bases, upon which next generation clinical decision support systems will be built. These systems will increase the safety, lower the cost and contribute to the possibility of autonomous patient care.
Acknowledgment
The authors would like to thank Greg Grudic for sharing unpublished data and Kristine Hansen for reviewing the manuscript.
Supported in part by: This work was supported by funding under NIH/NCRR Colorado CTSI Grant Number UL1 RR025780, Department of Defense STTR award W81XWH-09-C-0160 and the Department of Surgery, University of Colorado, School of Medicine. Its contents are the authors’ sole responsibility and do not necessarily represent official views of the NIH, DOD or the University of Colorado.
Abbreviations
- ACS
American College of Surgeons
- ECG
Electrocardiogram
- EMS
Emergency Medical Services
- LBNP
Lower body negative pressure
- PCR
Patient care record
- TC
Trauma Center
- USAISR
United States Army Institute of Surgical Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Steven L. Moulton, Director, Pediatric Trauma and Burns, The Children’s Hospital, Professor of Surgery, University of Colorado, School of Medicine.
Stephanie Haley-Andrews, EMS Coordinator, Director, EMS Outreach & Education Program, The Children's Hospital.
Jane Mulligan, Assistant Professor, Computer Science, University of Colorado, Boulder.
References
- 1.American College of Surgeons. Resources for Optimal Care of the Injured Patient: 2006. Chicago, IL: American College of Surgeons; 2006. [PubMed] [Google Scholar]
- 2. Sasser SM, Hunt RC, Sullivent EE, Wald MM, Mitchko J, Jurkovich GJ, Henry MC, Salomone JP, Wang SC, Galli RL, Cooper A, Brown LH, Sattin RW National Expert Panel on Field Triage, Centers for Disease Control and Prevention (CDC) Guidelines for field triage of injured patients. Recommendations of the National Expert Panel on Field Triage. MMWR Recomm Rep. 2009 Jan 23;58(RR-1):1–35. * This is a recent review of the Field Triage Criteria developed by the ACS.
- 3.MacKenzie EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of the effect of trauma center care on mortality. N Engl J Med. 2006;354:366–378. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- 4.West JG, Trunkey DD, Lim RC. Systems of trauma care. A study of two counties. Arch Surg. 1979;114:455–460. doi: 10.1001/archsurg.1979.01370280109016. [DOI] [PubMed] [Google Scholar]
- 5.Cales RH. Trauma mortality in Orange County: the effect of implementation of a regional trauma system. Ann Emerg Med. 1984;13:1–10. doi: 10.1016/s0196-0644(84)80375-3. [DOI] [PubMed] [Google Scholar]
- 6.Shackford SR, Mackersie RC, Hoyt DB, et al. Impact of a trauma system on outcome of severely injured patients. Arch Surg. 1987;122:523–527. doi: 10.1001/archsurg.1987.01400170029003. [DOI] [PubMed] [Google Scholar]
- 7.Mullins RJ. A historical perspective of trauma system development in the United States. J Trauma. 1999;47 Suppl 3:S8–S14. doi: 10.1097/00005373-199909001-00004. [DOI] [PubMed] [Google Scholar]
- 8.MacKenzie EJ. Review of evidence regarding trauma system effectiveness resulting from panel studies. J Trauma. 1999;47 Suppl 3:S34–S41. doi: 10.1097/00005373-199909001-00008. [DOI] [PubMed] [Google Scholar]
- 9.Jurkovich GJ, Mock C. Systematic review of trauma system effectiveness based on registry comparisons. J Trauma. 1999;47 Suppl 3:S46–S55. doi: 10.1097/00005373-199909001-00011. [DOI] [PubMed] [Google Scholar]
- 10.Hulka F, Mullins RJ, Mann NC, et al. Influence of a statewide trauma system on pediatric hospitalization and outcome. J Trauma. 1997;42:514–519. doi: 10.1097/00005373-199703000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Nathens AB, Jurkovich GJ, Rivara FP, Maier RV. Effectiveness of state trauma systems in reducing injury-related mortality: a national evaluation. J Trauma. 2000;48:25–31. doi: 10.1097/00005373-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Potoka DA, Schall LC, Gardner MJ, Stafford PW, Peitzman AB, Ford HR. Impact of pediatric trauma centers on mortality in a statewide system. J Trauma. 2000;49:237–245. doi: 10.1097/00005373-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Potoka DA, Schall LC, Ford HR. Improved functional outcome for severely injured children treated at pediatric trauma centers. J Trauma. 2001;51:824–834. doi: 10.1097/00005373-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Wuerz R, Taylor J, Smith JS. Accuracy of trauma triage in patients transported by helicopter. Air Med J. 1996;15:168–170. doi: 10.1016/s1067-991x(96)90025-5. [DOI] [PubMed] [Google Scholar]
- 15.Norcross ED, Ford DW, Cooper ME, Zone-Smith L, Byrne TK, Yarbrough DR. Application of American College of Surgeons’ field triage guidelines by pre-hospital personnel. J Am Coll Surg. 1995;181:539–544. [PubMed] [Google Scholar]
- 16.Ryan KL, Batchinsky A, McManus JG, Rickards CA, Convertino VA. Changes in pulse character and mental status are late responses to central hypovolemia. Prehosp Emerg Care. 2008;12:192–198. doi: 10.1080/10903120801907562. [DOI] [PubMed] [Google Scholar]
- 17.Cooke WH, Ryan KL, Convertino VA. Lower body negative pressure as a model to study progression to acute hemorrhagic shock in humans. J Appl Physiol. 2004;96:1249–1261. doi: 10.1152/japplphysiol.01155.2003. [DOI] [PubMed] [Google Scholar]
- 18.Convertino VA, Ryan KL, Rickards CA, Salinas J, McManus JG, cooke WH, Holcomb JB. Physiological and medical monitoring for en route combat casualties. J Trauma. 2008;64:S342–S353. doi: 10.1097/TA.0b013e31816c82f4. [DOI] [PubMed] [Google Scholar]
- 19.Ryan KL, Rickards CA, Ludwig DA, Convertino VA. Tracking central hypovolemia with ECG in humans: Cautions for the use of heart period variability in patient monitoring. Shock. 2010 doi: 10.1097/SHK.0b013e3181cd8cbe. (in press) [DOI] [PubMed] [Google Scholar]
- 20.McManus J, Yershov AL, Ludwig DA, Holcomb JB, Salinaas J, Dubick MA, Convertino VA, Hinds D, David D, Flanagan T, Duke JH. Radial pulse character relationships to systolic blood pressure and trauma outcomes. Prehosp Emerg Care. 2005;9:423–428. doi: 10.1080/10903120500255891. [DOI] [PubMed] [Google Scholar]
- 21.Convertino VA, Ryan KL. Identifying physiological measurements for medical monitoring: implications for autonomous health in austere environments. J Gravit Physiol. 2007;14:P39–P42. [PubMed] [Google Scholar]
- 22.McManus JG, Ryan KL, Morton MJ, Rickards CA, Cooke WH, Convertino VA. Limitations of end-tidal CO2 as an early indicator of central hypovolemia in humans. Prehosp Emerg Care. 2008;12:199–205. doi: 10.1080/10903120801907182. [DOI] [PubMed] [Google Scholar]
- 23.Convertino VA, Rickards CA, Lurie KG, Ryan KL. Hyperventilation in response to progressive reduction in central blood volume to near syncope. Aviat Space Environ Med. 2009;80(12):1012–1017. doi: 10.3357/asem.2598.2009. [DOI] [PubMed] [Google Scholar]
- 24.Soller BR, Soyemi OO, Yang Y, Ryan KL, Rickards CA, Walz JM, Heard SO, Convertino VA. Noninvasively measured muscle oxygen saturation is an early indicator of central hypovolemia in humans. J Appl Physiol. 2008;104:475–481. doi: 10.1152/japplphysiol.00600.2007. [DOI] [PubMed] [Google Scholar]
- 25.Grudic G, Mulligan J. Proceedings of Robotics: Science and Systems. Philadelphia, USA: 2006. Aug, Outdoor path labeling using polynomial mahalanobis distance. [Google Scholar]
- 26.Breitenbach M, Brennan T, Dieterich W, Grudic G. ECML PKDD 2006 Workshop on Practical Data Mining. Berlin, Germany: 2006. Sep, Clustering of psychological personality tests of criminal offenders; pp. 15–22. [Google Scholar]
- 27.Bauer K, McCoy D, Anderson E, Breitenbach M, Grudic G, Grunwald D, Sicker D. Attacking WiFi Localization with Directional Antennas. IEEE Globecom. 2009 [Google Scholar]
- 28.Cooke WH, Rickards CA, Ryan KL, Kuusela TA, Convertino VA. Muscle sympathetic nerve activity during intense lower body negative pressure to syncope in humans. J Physiol. 2009;587:4987–4999. doi: 10.1113/jphysiol.2009.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soller BR, Soyemi OO, Yang Y, Ryan KL, Rickards CA, Walz JM, Heard SO, Cooke WH, Crookes BA, Convertino VA. Oxygen saturation determined from deep muscle, not thenar tissue, is an early indicator of central hypovolemia in humans. Crit Care Med. 2008;36:176–182. doi: 10.1097/01.CCM.0000295586.83787.7E. [DOI] [PubMed] [Google Scholar]
- 30.Convertino VA, Rickards CA, Ryan KL. Evidence for a heart rate mechanism associated with tolerance to progressive central hypovolemia. FASEB J. 2009;23 1019.6. [Google Scholar]
- 31.Rickards CA, Ryan KL, Convertino VA. Tolerance to central hypovolemia: The influence of cerebral blood flow velocity oscillations. FASEB J. 2009;23:613–617. doi: 10.1152/japplphysiol.00231.2011. [DOI] [PubMed] [Google Scholar]
- 32. Pauldine R, Beck G, Salinas J, Kaczka DW. Closed-loop strategies for patient care systems. J Trauma. 2008;64:S289–S294. doi: 10.1097/TA.0b013e31816bce43. * Overview of closed-loop strategies for patient care
- 33. Johannigman JA, Muskat P, Barnes S, Davis K, Beck G. Autonomous control of oxygenation. J Trauma. 2008;64:S295–S301. doi: 10.1097/TA.0b013e31816bce54. ** Seminal article on the application of a closed-loop strategy for oxygenation.
- 34. Johannigman JA, Muskat P, Barnes S, Davis K, Branson RD. Autonomous control of ventilation. J Trauma. 2008;64:S302–S320. doi: 10.1097/TA.0b013e31816bf4e2. ** Seminal article on the application of a closed-loop strategy for ventilation.
- 35.Salinas J, Drew G, Gallagher J, Cancio LC, Solf SE, Wade CE, Holcomb JB, Herndon DN, Kramer GC. Closed-loop and decision-assist resuscitation of burn patients. J Trauma. 2008;64:S321–S332. doi: 10.1097/TA.0b013e31816bf4f7. [DOI] [PubMed] [Google Scholar]
- 36.Keane MG. A review of the role of telemedicine in the accident and emergency department. Journal of Telemedicine & Telecare. 2009;15(3):132–134. doi: 10.1258/jtt.2009.003008. [DOI] [PubMed] [Google Scholar]