Abstract
Foxp3+ regulatory T cells (Tregs) express both ectoenzymes CD39 and CD73, which in tandem hydrolyze pericellular ATP into adenosine, an immunoinhibitory molecule that contributes to Treg suppressive function. Using Foxp3GFP knockin mice, we noted that the mouse CD4+CD39+ T-cell pool contains two roughly equal size Foxp3+ and Foxp3− populations. While Foxp3+CD39+ cells are CD73bright and are the bone fide Tregs, Foxp3−CD39+ cells do not have suppressive activity and are CD44+CD62L−CD25−CD73dim/−, exhibiting memory cell phenotype. Functionally, CD39 expression on memory and Treg cells confers protection against ATP-induced apoptosis. Compared with Foxp3−CD39− naïve T cells, Foxp3−CD39+ cells freshly isolated from non-immunized mice express at rest significantly higher levels of mRNA for T-helper lineage-specific cytokines IFN-γ (Th1), IL-4/IL-10 (Th2), IL-17A/F (Th17), as well as pro-inflammatory cytokines, and rapidly secrete these cytokines upon stimulation. Moreover, the presence of Foxp3−CD39+ cells inhibits TGF-β induction of Foxp3 in Foxp3−CD39− cells. Furthermore, when transferred in vivo, Foxp3−CD39+ cells rejected MHC-mismatched skin allografts in a much faster tempo than Foxp3−CD39− cells. Thus, besides Tregs, CD39 is also expressed on pre-existing memory T cells of Th1-, Th2- and Th17-types with heightened alloreactivity.
Keywords: Cell-surface molecules, mouse, memory, regulatory, T cells
Introduction
Regulatory T cells (Tregs) suppress immune responses against self- and foreign antigens, and are extensively studied for their roles in autoimmunity, infectious disease, transplant rejection and cancer immunology. The intracellular transcription factor forkhead box protein P3 (Foxp3) has been identified as the lineage-specification marker of the conventional CD4+CD25+ Tregs. However, for various applications, it is highly desirable to define cell surface molecule(s) specific for Tregs that distinguish them from other resting or activated T cells. By this criterion, CD25, CTLA-4, GITR, PD-1 and CD127 cannot effectively differentiate Tregs from activated naïve T cells (1), and hence their use is limited. By exploiting Foxp3GFP reporter mice (2), we have recently demonstrated that CD39 (ENTPD1 [ectonucleoside triphosphate diphosphohydrolase-1]; EC 3.6.1.5) is expressed on Tregs (3). Compared with CD25 that is expressed on about 50–60% of CD4+Foxp3+ Treg cells, CD39 can be detected on over 70–80% of Tregs. CD39 is an ectoenzyme that hydrolyzes ATP and ADP into AMP. Because Tregs also express CD73 ([EC 3.1.3.5]), an ecto-5′-nucleotidase that converts AMP into adenosine (4), these suppressor cells are therefore able to catalyze ATP/ADP eventually into immunosuppressive nucleoside adenosine (3,5,6). Thus, dual expression of CD39 and CD73 not only serves as a phenotypic marker for Tregs, but also contributes to their functional suppressive activity by catalyzing the generation of pericellular adenosine.
In our previous study, we noted that a subpopulation of CD4+Foxp3− cells is CD39+. These cells do not have detectable CD73 transcripts, unable to generate adenosine, and do not exhibit suppressive function (3). In this report, we further show that the Foxp3−CD39+CD73dim/− population in naïve animals encompasses pre-existing Th1-, Th2-and Th17-type memory cells and may actively contribute to transplant rejection.
Materials and Methods
Mice
Foxp3GFP knockin mice that express a Foxp3-IRES-EGFP cassette under control of the endogenous FOXP3 promoter were reported previously (2). CD39 knockout mice have been generated and characterized in depth (7). C57BL/6 (H-2b), C57BL/6 RAG−/− and BDF1 (F1 of C57BL/6 and DBA/2, H-2b,d) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animal studies were approved by IACUC at Harvard Medical School.
Antibodies and flow cytometry
PE-Cy5-anti-CD4 (GK1.5), PE-Cy5-anti-CD44 (IM7), PE-anti-CD25 (PC61), APC-anti-CD62L (MEL-14), anti-CD3 (145–2C11) and anti-CD28 (37.51) were purchased from eBioscience (San Diego, CA). An anti-mouse CD39 polyclonal antibody was prepared by immunizing rabbits with mCD39-expressing plasmids (8). Anti-CD73 (BD Biosciences, San Diego, CA) was used at 1:400. Spleen and lymph node cells isolated from 6- to 8-week-old animals were stained with polyclonal anti-CD39 (1:200), followed by PE-conjugated goat F(ab′)2 anti-rabbit IgG(H+L) (Southern Biotech, Birmingham, AL). Cells were sorted on a FACSAria cell sorter with purity typically >98%.
Real-time PCR
Total RNA was extracted from cells using the RNeasy Mini Kit (Qiagen), and real-time PCR was performed as described (2), using TaqMan primer-probe sets directly purchased from Applied Biosystems. The CT value of gene of interest (GOI) was normalized with the formula ΔCT = CT GOI − CT GAPDH. Relative expression of GOI was calculated with the formula 2−ΔCT.
T-cell stimulation and ELISA
FACS-sorted CD4+GFP−CD39+ and CD4+GFP−CD39− cells were seeded at 2 × 105 cells per 48-well coated with anti-CD3 (0.3 μg/mL) and cultured in an RPMI-1640 medium with 10% FBS and soluble anti-CD28 (1 μg/mL) at 37°C for 3 days. At 48 hours, aliquots of activated cells were collected for real-time PCR analysis as above. To induce Foxp3 in CD4+GFP−CD39− cells, cells were activated by plate-bound anti-CD3 (10 μg/mL) and soluble anti-CD28 (1 μg/mL) in the presence of TGF-β (1 ng/mL, R&D Systems) for 3 days. For co-culture suppression assay, FACS-sorted GFP−CD39− cells (1 × 105) were stimulated with soluble anti-CD3 (2 μg/mL) in the presence of mitomycin C-treated CD4-depleted syngeneic splenocytes (1 × 105) in 96-well U-bottom plates. Some cultures were also added with 0.5 × 105 or 1 × 105 GFP−CD39+, GFP+CD39+ or GFP+CD39− cells. Culture supernatants were collected after 3 days for ELISA (SearchLight service, Pierce Biotechnology, Inc., Woburn, MA).
Apoptosis assay
To assay induction of apoptosis, freshly isolated splenocytes from WT or CD39 knockout mice (both in Foxp3GFP knockin background) were incubated at 37°C with 30 μM ATP (Sigma-Aldrich) in RPMI 1640 for 5, 15 and 30 min, and assayed for Annexin V staining.
Skin transplantation
C57BL/6 RAG−/− mice were transplanted with semi-allogeneic tail skin grafts from BDF1 mice. The grafts were covered with Vaseline gauze and an adhesive bandage for 7–10 days, at which time the bandage was removed. FACS-sorted CD4+GFP−CD39+ and CD4+GFP−CD39− cells (1 × 105, from 6- to 8-week-old naïve Foxp3GFP knockin mice) were transferred by tail vein injection, at least >1 month after skin transplantation to ensure that the surgery-caused inflammatory insult has waned down. Each graft was examined daily beginning at day 7 postadoptive transfer and was considered rejected when ~ 80% or more of the graft tissue was destroyed and scabbed as assessed by visual examination. Difference of graft survival times was assessed by Kaplan–Meier survival analysis with StatView software. P < 0.01 is considered statistically significant.
Results
CD39 can be detected on CD4+Foxp3− cells
When freshly prepared spleen and lymph node cells from naïve Foxp3GFP knockin mice were stained with anti-CD39, a distinctive population of CD4+Foxp3(GFP)− cells was found to be CD39+. The size of this population is similar to that of CD4+Foxp3(GFP)+ Tregs, which are also CD39+ (Figure 1A, right panel). These two dimorphic CD39+ populations were not detected when cells were from Foxp3GFP knockin crossed onto CD39 knockout background (Supporting Figure S1), indicating the specificity of the antibody. We also confirmed the similar staining pattern with our newly developed mouse anti-mCD39 mAb (5F2), and another mAb (24DMS1) against mCD39 from eBioscience (data not shown).
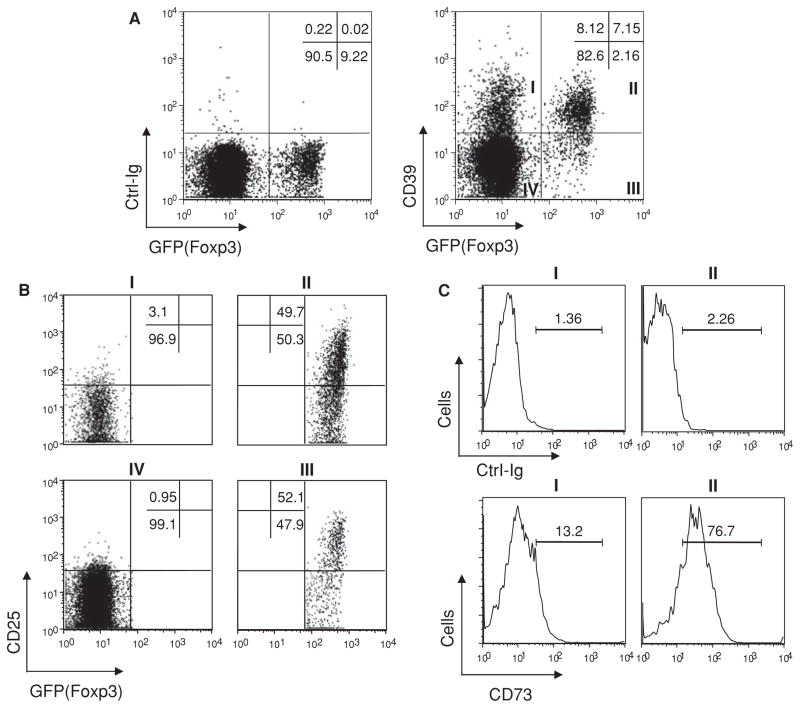
Figure 1. CD39 is expressed on CD4+Foxp3− cells that exhibit memory phenotype.
Spleen cells from 6- to 8-week-old naïve Foxp3GFP knockin mice were stained with a rabbit anti-mouse CD39 polyclonal antibody, and live CD4+ T cells were gated for multi-color FACS analysis. (A) Compared with control Ig staining (left panel), anti-CD39 stained positive for subpopulations of cells in Foxp3+ and Foxp3− gates (right panel). (B) Fractions I–IV of (A) were further analyzed for CD25 expression. About 50% of Foxp3+ cells (fractions II and III) are CD25+. Almost all the Foxp3−CD39+ cells (fraction I) are CD25−. (C) Foxp3+CD39+ cells are CD73bright, whereas Foxp3−CD39+ cells are CD73dim/−. Histograms of control Ig (upper panel) or anti-mCD73 (lower panel) staining of fractions I and II were plotted. Data represent more than three independent experiments.
Because CD25 has been used as a conventional albeit imperfect marker for Tregs, we next compared CD39 and CD25 expression on CD4+ T cells. While about 80% of GFP+ cells are CD39+, only 50% are CD25+. Expression of CD39 seems unassociated with that of CD25. The minor GFP+CD39− Treg population (fraction III in Figure 1A right panel) has the similar proportion of CD25+ cells as the major GFP+CD39+ Treg population (fraction II). Moreover, the GFP−CD39+ population (fraction I) is CD25− (Figure 1B), suggesting that CD39 expression on this population is not due to cell activation. Careful titration of CD73 staining showed that the GFP+CD39+ cells are mostly CD73bright, whereas the GFP−CD39+ cells are CD73dim/−(Figure 1C). The full titration data of CD73 staining are provided as Supporting Figure S2. Overall, the expression profile of CD39 and CD73 supports the previous observation that GFP+CD39+ Tregs are able to generate adenosine whereas the majority of GFP−CD39+ cells cannot (3).
To compare their suppressive activities, we FACS-sorted GFP−CD39+ (fraction I), GFP+CD39+ (fraction II) and GFP+CD39− (fraction III) cells as suppressors and used GFP−CD39− cells (fraction IV) as effectors in co-culture assay. Both GFP+CD39+ and GFP+CD39− cells suppressed effector cell proliferation, whereas GFP−CD39+ cells did not (see [3]). Moreover, GFP+CD39+ and GFP+CD39− cells suppressed the secretion of multiple effector cytokines (e.g. IFN-γ, IL-2, IL-4, TNF-α) from activated GFP−CD39− cells (Supporting Figure S3). In contrast, the co-cultures containing GFP−CD39+ cells not only had higher levels of cytokines than those in GFP−CD39− Teff culture alone, but also showed a GFP−CD39+ cell number-dependent increase in cytokine production (Supporting Figure S3). Therefore, CD39 labels CD4+ populations mixed with regulatory (Foxp3+) and non-regulatory (Foxp3−) T cells, the latter of which readily secrete cytokines upon stimulation.
CD4+Foxp3−CD39+ cells rapidly produce multiple cytokines upon stimulation
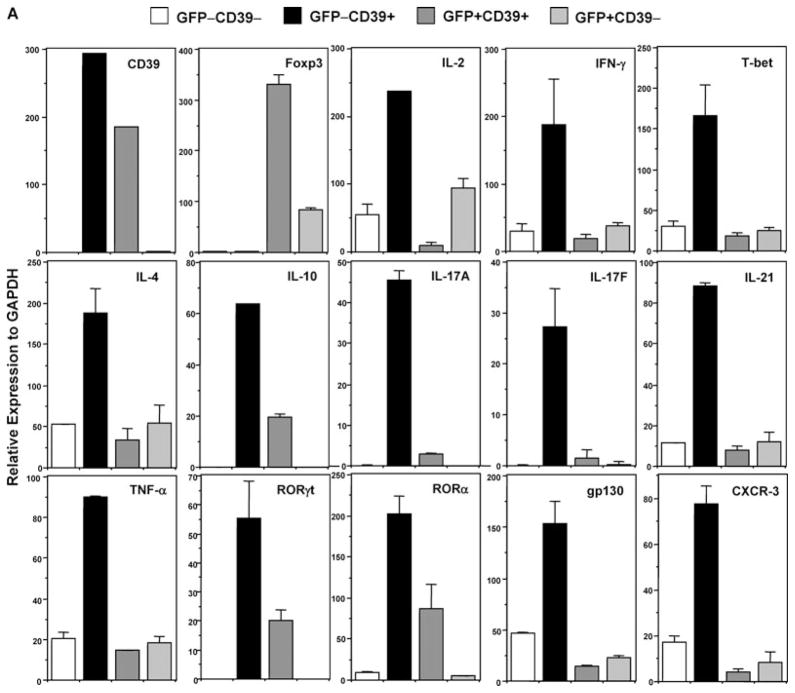
To confirm that GFP−CD39+ cells express high levels of cytokine transcripts at rest (one of the features of CD4+ memory T cells), we FACS-sorted all four populations of GFP−CD39−, GFP−CD39+, GFP+CD39+ and GFP+CD39− cells from unimmunized Foxp3GFP knockin mice and performed real-time PCR analysis. The purity of the sorted populations is confirmed by the absence and presence of CD39 and Foxp3 transcripts in respective populations (Figure 2A). Strikingly, GFP−CD39+ cells are highly enriched with transcripts associated with Th1 (IL-2, IFN-γ, T-bet), Th2 (IL-4, IL-10) and Th17 cells (IL-17A, IL-17F, IL-21, TNF-α, RORγ t, RORα). They also highly express gp130 and activated/memory T cell-associated chemokine receptor CXCR-3 (9)(Figure 2A).
Figure 2. CD4+Foxp3−CD39+ cells rapidly produce Th1-, Th2- and Th17-type cytokines.
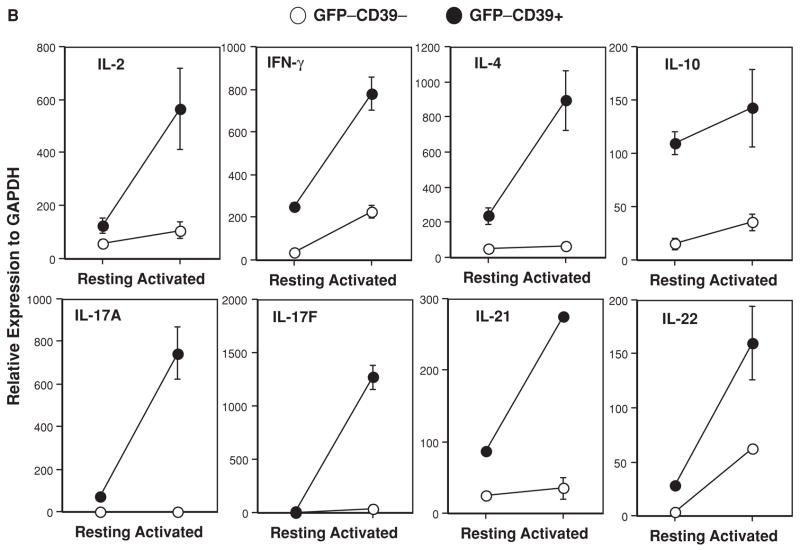
(A) Live CD4+ T cells from unimmunized 6- to 8-week old Foxp3GFP knockin mice were FACS-sorted into fractions I, II, III and IV based on the GFP(Foxp3) versus CD39 staining profile depicted in Figure 1A (right panel). Each sorted fraction was immediately lysed and mRNA extracted for real-time PCR analysis of multiple transcripts. Data represent more than three independent experiments. (B) Rapid production of cytokines by GFP−CD39+ Tmem cells. FACS-sorted naïve (GFP−CD39−) and memory (GFP−CD39+) T cells were activated by anti-CD3 and anti-CD28. At 48 hours, aliquots of activated naïve (GFP−CD39−, empty circle) and memory (GFP−CD39+, filled circle) T cells were analyzed by real-time PCR for cytokine messages. Data represent two independent experiments.
Next, we activated equal number of sorted GFP−CD39− and GFP−CD39+ cells with plate-bound low dose anti-CD3 (0.3 μg/mL) and soluble anti-CD28 (1.0 μg/mL). The GFP−CD39+ cells proliferated far more rapidly than GFP−CD39− cells (data not shown). At 48 hours after stimulation, gene expression for IL-2, IFN-γ, IL-4, IL-10, IL-17A, IL-17F, IL-21 and IL-22 is much elevated in GFP−CD39+ cells, as compared to GFP−CD39− cells (Figure 2B). Expression of T-bet, RORγ t, RORα, CXCR-3, gp130 and IL-6R is also increased in GFP−CD39+ cells although with various patterns (Supporting Figure S4). In line with this, GFP−CD39+ cells secreted much more T-helper lineage-specific cytokines IFN-γ, IL-4, IL-10, IL-17A, as well as pro-inflammatory cytokines IL-6, TNF-α and MCP-1 than GFP−CD39− cells after 3 days of activation (Supporting Figure S5).
CD4+Foxp3− cells exhibit memory phenotype
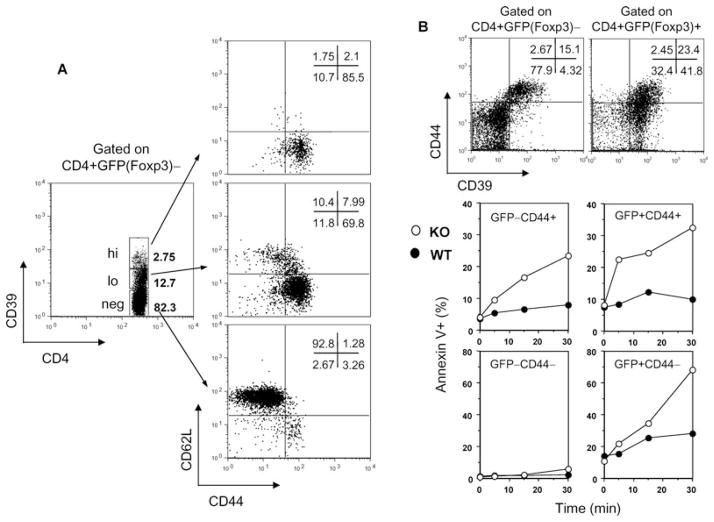
To further characterize the phenotype of the GFP−CD39+ population, we stained the mouse spleen cells with CD44 and CD62L to differentiate naïve T cells from memory T cells (Tmem). Depending on their surface phenotype, function and in vivo migration, memory T cells (Tmem) are divided into effector memory (TEM) and central memory (TCM) cells (10). Almost all the GFP−CD39hi cells exhibit the TEM phenotype (CD44+CD62L−). The GFP−CD39lo population contains a mixture of 70% TEM and 8% TCM cells (CD44+CD62L+), whereas the GFP−CD39neg population is mostly naïve T cells (CD44−CD62L+) (Figure 3A).
Figure 3. CD39 is also a memory T-cell marker and protects against ATP-induced apoptosis.
(A) Spleen cells from unimmunized 6- to 8-week-old Foxp3GFP knockin mice were stained and live CD4+Foxp3− T cells were gated into CD39hi, CD39lo and CD39neg populations for analysis of CD44 and CD62L expression. The Foxp3−CD39hi population is TEM cells (CD44+CD62L−), and the Foxp3−CD39lo population contains 70% TEM cells and 8% TCM cells (CD44+CD62L+), whereas the Foxp3−CD39neg population is mainly Tnaive cells (CD44−CD62L+). (B) CD39 confers protection against ATP-induced apoptosis to CD4+GFP−CD44+ Tmem and CD4+GFP+ Treg cells. CD39 knockout mice were bred with Foxp3GFP knockin mice. Freshly isolated splenocytes from WT (filled circle) and CD39 knockout mice (open circle) were incubated with 30 μM ATP for various times at 37°C. Apoptosis of each gated population (upper panel) was measured by Annexin V staining (lower panel). Similar findings were noted when ATP was added at 100 μM. Data represent two independent experiments.
What is the function of CD39 on Tmem and Tregs? CD39 has been implicated in resisting ATP-induced apoptosis (11,12). To study the role of CD39 on the survival of T-cell subsets with more precision, we bred CD39 knockout mice onto Foxp3GFP knockin background (both in C57BL/6)(2). Using multi-color FACS, we found that the majority of CD39+ cells in the CD4+GFP(Foxp3)− fraction are CD44+. Hence, CD44 was used as a surrogate marker to compare memory cells with or without CD39 expression. To compare Tregs from WT and CD39 knockout mice, GFP was used as a marker. Note that while one-third of CD39+ CD4+GFP(Foxp3)+ Treg cells are CD44+, the rest of the CD39+ Tregs are CD44− (Figure 3B, upper panel). We then incubated the freshly isolated splenocytes from WT and CD39 knockout mice with 30 μM ATP for various times at 37°C and measured apoptosis by Annexin V staining. In the absence of CD39, CD4+GFP−CD44+ Tmem are much more susceptible to ATP-induced apoptosis over time. Treg cells, regardless of CD44 expression, are also more susceptible to ATP-induced apoptosis when CD39 is absent. In contrast, Tnaive cells are more resistant to this kind of insult, probably due to the low expression of ATP receptors (Figure 3B, lower panel) (11,12). Thus, CD39 may offer survival advantage to Tmem and Tregs, especially during ATP influx into the milieu caused by local inflammation.
The presence of Foxp3−CD39+ cells inhibits TGF-β-induced conversion of Foxp3−CD39− cells into iTregs
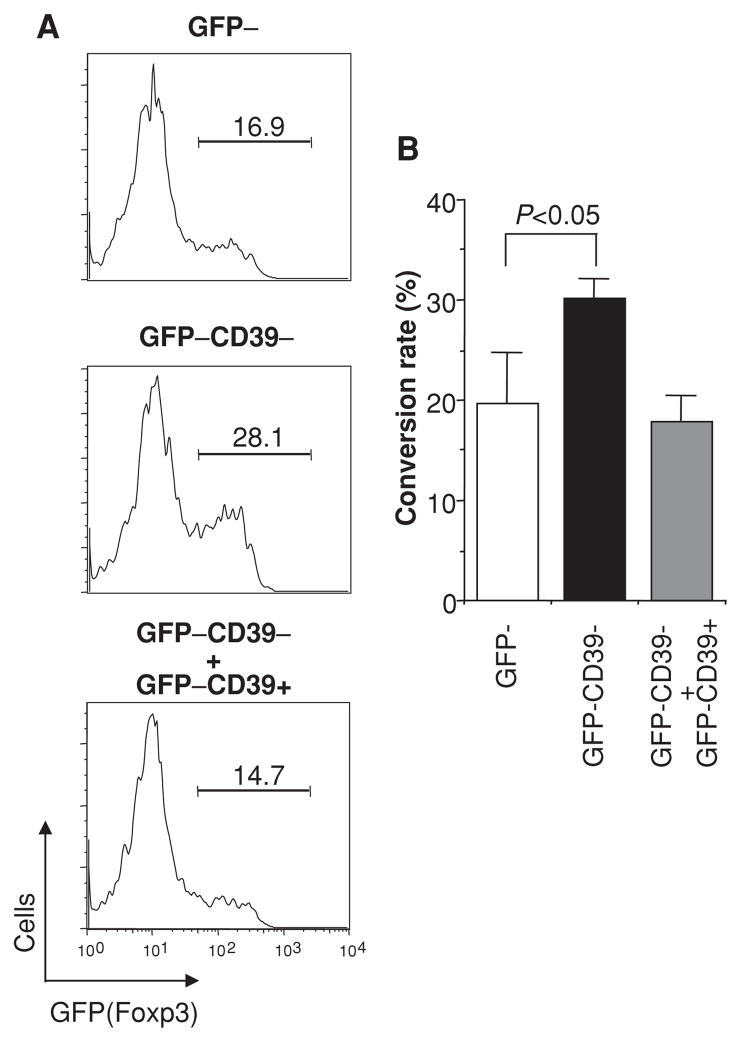
There is great interest in exploiting ex vivo cultivated regulatory T cells for therapy. These induced Tregs (iTregs) are usually derived from CD4+CD25− cells upon TGF-β induction of Foxp3. However, if the starting CD4+CD25− or CD4+GFP− cells are contaminated with the CD4+Foxp3−CD39+ Tmem cells, cytokines such as IFN-γ, IL-4 and IL-6 secreted from memory cells would suppress TGF-β induction of Foxp3 (2, 13–15). Indeed, under the same conditions, TGF-β induced 16.9% of FACS-sorted total CD4+GFP(Foxp3)− T cells into Foxp3+ phenotype, whereas the efficiency increased to 28.1% if GFP−CD39+ Tmem cells were separated from the GFP−CD39− cells. Conversely, when GFP−CD39+ Tmem cells were added back to the GFP−CD39− culture, the iTreg induction efficiency is again reduced to 14.7% (Figure 4A). Figure 4B summarizes the data from three different experiments. In the latter condition, the efficiency can be partially restored by anti-IFN-γ mAb (data not shown). This is in accordance with our recent report that IFN-γ secreted by CD44+CD62L− memory cells inhibits iTreg generation (15).
Figure 4. CD4+Foxp3−CD39+ cells inhibit the conversion of CD4+Foxp3−CD39− cells into iTregs.
(A) FACS-sorted CD4+GFP− cells (containing CD39+ and CD39− fractions I and IV as in Figure 1A) (upper panel) and CD4+GFP−CD39− (fraction IV) (middle panel) were stimulated with anti-CD3 and anti-CD28 in the presence of TGF-β for 3 days. In parallel, sorted CD4+GFP−CD39+ cells were added back to the CD4+GFP−CD39− culture at the physiological ratio determined during FACS sorting (lower panel). (B) Bar graph with standard deviations from three different experiments.
Foxp3−CD39+ cells rejected MHC-mismatched skin grafts in a much faster tempo than Foxp3−CD39− cells
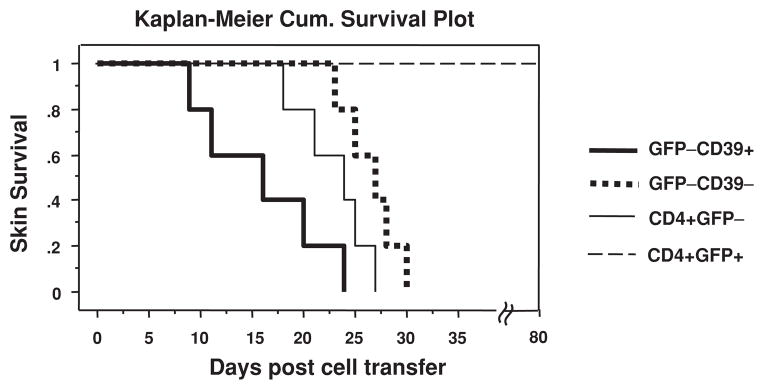
What are the antigen-specificities of Foxp3−CD39+ Tmem cells? To determine if Foxp3−CD39+ Tmem cells possess alloreactivity, we compared unfractionated CD4+GFP− cells, GFP−CD39+ Tmem cells and GFP−CD39− Tnaive cells from Foxp3GFP mice (H-2b) by transferring the equal number of these cells into C57BL/6 RAG−/− mice (H-2b) bearing semi-MHC mismatched BDF1 (H-2b,d) skin grafts. Strikingly, Foxp3−CD39+ Tmem cells rejected skin grafts in a significantly faster tempo (MST = 16.0 days, n = 5) than Foxp3−CD39− Tnaive cells (MST = 26.6 days, n = 5) (p = 0.0064). Unfractionated CD4+Foxp3− cells rejected in a kinetics that is in between (MST = 23.0 days, n = 5). In contrast, CD4+Foxp3+ Tregs never rejected skin grafts during the observation period up to 80 days (Figure 5). This experiment clearly shows that unlike Foxp3+CD39+ Tregs that have suppressive activities, Foxp3−CD39+ Tmem cells have heightened effector functions. Nevertheless, the data cannot distinguish between enrichment in alloreactivity (i.e. increased percentage of T cells that recognize alloantigens) versus more rapid effector function of the same number of alloreactive cells in Tmem versus Tnaive. The data also do not exclude the possibility for other antigen specificities encoded in this population.
Figure 5. CD4+Foxp3−CD39+ cells reject MHC-mismatched skin grafts at a significantly faster tempo than CD4+Foxp3−CD39− cells.
FACS-sorted GFP−CD39+ Tmem cells (heavy solid line), GFP−CD39− Tnaive cells (dotted line), CD4+GFP− T cells (light solid line) or CD4+GFP+ Tregs (broken line) from Foxp3GFP mice (H-2b) were adoptively transferred into C57BL/6 RAG−/− mice (H-2b) bearing semi-MHC mismatched BDF1 (H-2b,d) skin grafts. The skin MST of Foxp3−CD39+ Tmem cell group is 16.0 days, whereas that of Foxp3−CD39−Tnaive cell group is 26.6 days (p = 0.0064, n = 5). Unfractionated CD4+Foxp3− cells rejected in between (MST = 23.0 days, n = 5). CD4+Foxp3+ Tregs never rejected skin grafts during the observation period up to 80 days.
Discussion
We report here that mouse CD39 is expressed on both Foxp3+ and Foxp3− CD4+ T cells. The novel CD4+Foxp3−CD39+ population is not suppressive but exhibits typical memory markers, rapidly produces large amounts of Th1, Th2 and Th17 cytokines, and promptly rejects skin allografts. Our staining profile of CD39 versus Foxp3(GFP) is also confirmed by the data on their web-sites provided by eBioscience and Miltenyi Biotec when introducing their own mAbs against mouse and human CD39. Therefore, CD39 as a sole marker cannot differentiate Tregs from Tmem cells. Instead, CD39/CD73 dual positivity might be a better marker for murine Tregs.
It is noteworthy, however, that the staining of CD4+Foxp3−CD39+ cells with anti-CD73 always shows a minor shoulder, indicating that there may be a small subset of CD73+ cells in this population (Figure 1C and Supporting Figure S2). These cells could be the uncommitted primed precursor Th (Thpp) cells that are CD25−Foxp3−CD44+CD73+Ly-6A/E− (6). The capacity for Thpp cells to generate adenosine and become suppressive is highly conditional, i.e. only when exogenous AMP is provided and cells are cultured in the serum-free medium. Yet, these cells have the potential to develop into effector cells under Th1 or Th2 polarization conditions. Our finding that under the neutral stimulation condition, Foxp3−CD39+ cells rapidly produce large amounts of Th1-, Th2- and Th17-type cytokines indicates that this population contains already committed memory cells.
It is relatively easy to induce transplant tolerance in naïve rodent animals with various immune modulatory protocols, such as co-stimulation blockade. However, there seems to be great difficulty to achieve tolerance in animals with memory cells. This is particularly true when memory-like cells are generated from lymphopenia-induced homeostatic proliferation following peritransplant pan-T cell depletion (16). The Foxp3−CD39+ Tmem cells we have identified differ from memory-like cells after homeostatic proliferation in that they pre-exist in naïve immunocompetent mice. They might arise from recognizing self-antigens, innocuous food antigens or microbial antigens of the gut flora. Yet surprisingly, they are also enriched with memory cells that are highly alloreactive. A number of studies have shown that memory T cells against certain pathogens also crossreact to MHC alloantigens (17). The identification of such pre-existing alloreactive memory population could be useful for developing more effective tolerance-inducing protocols by means to specifically target this population in the transplant host. But the price of such protocol on affecting anti-microbial immunity needs to be further studied.
While the tandem action of CD39 and CD73 on Tregs contributes to their suppressive function by catalyzing ATP/ADP into adenosine, it is not clear why Tmem cells express CD39. It might be possible that by expressing CD39, Tmem cells can effectively reduce the pericellular concentration of ATP. ATP released from damaged or apoptotic cells at inflamed sites is a potent “danger signal” that activates the innate immune system (18,19) and drives Th17 cell differentiation (20). Ongoing experiments with CD39 knockout mice in Foxp3GFP knockin background will address questions regarding the cross-regulation of Tregs, Th17 and Tmem cells. On the other hand, both Tregs and CD4+CD62Llow Tmem cells but not CD4+CD62Lhi Tnaive cells are highly susceptible to ATP-induced apoptosis through purinergic P2X7 receptors (11,12). Our data suggest that CD39 expression may rescue Tregs and Tmem cells from ATP-induced apoptosis, a feature that could be relevant during inflammation and may be important for maintaining immune regulation and memory.
Supplementary Material
Acknowledgments
This work was supported by the Juvenile Diabetes Research Foundation grants 5-2007-690, 1-2007-551 (W.G.) and 7-2005-1329 (T.B.S.), and NIH grants HL076540 and HL63972 (S.C.R.).
Footnotes
Conflict of Interest
The authors have declared that no conflict of interest exists.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Aerts NE, Dombrecht EJ, Ebo DG, et al. Activated T cells complicate the identification of regulatory T cells in rheumatoid arthritis. Cell Immunol. 2008;251:109–115. doi: 10.1016/j.cellimm.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 3.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmermann H. 5′-Nucleotidase: Molecular structure and functional aspects. Biochem J. 1992;285(Pt 2):345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 6.Kobie JJ, Shah PR, Yang L, et al. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 7.Enjyoji K, Sevigny J, Lin Y, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 8.Sevigny J, Sundberg C, Braun N, et al. Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTPDase2 have implications for thromboregulation. Blood. 2002;99:2801–2809. doi: 10.1182/blood.v99.8.2801. [DOI] [PubMed] [Google Scholar]
- 9.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 11.Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: A role for P2X7 receptors. J Immunol. 2005;175:3075–3083. doi: 10.4049/jimmunol.175.5.3075. [DOI] [PubMed] [Google Scholar]
- 12.Aswad F, Dennert G. P2X7 receptor expression levels determine lethal effects of a purine based danger signal in T lymphocytes. Cell Immunol. 2006;243:58–65. doi: 10.1016/j.cellimm.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantel PY, Kuipers H, Boyman O, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei J, Duramad O, Perng OA, et al. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao X, Kroemer A, Gao W, et al. OX40/OX40L costimulation affects induction of Foxp3+ regulatory T cells in part by expanding memory T cells in vivo. J Immunol. 2008;181:3193–3201. doi: 10.4049/jimmunol.181.5.3193. [DOI] [PubMed] [Google Scholar]
- 16.Wu Z, Bensinger SJ, Zhang J, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Li XC. Transplant tolerance: Is it really free of concerns? Trends Immunol. 2007;28:376–377. doi: 10.1016/j.it.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Wilkin F, Duhant X, Bruyns C, et al. The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol. 2001;166:7172–7177. doi: 10.4049/jimmunol.166.12.7172. [DOI] [PubMed] [Google Scholar]
- 19.Hanley PJ, Musset B, Renigunta V, et al. Extracellular ATP induces oscillations of intracellular Ca2+ and membrane potential and promotes transcription of IL-6 in macrophages. Proc Natl Acad Sci USA. 2004;101:9479–9484. doi: 10.1073/pnas.0400733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.