Abstract
Presentation of peptides by class I or class II major histocompatibility complex (MHC) molecules is required for the initiation and propagation of a T cell-mediated immune response. Peptides from the Wilms Tumor 1 transcription factor (WT1), upregulated in many hematopoetic and solid tumors, can be recognized by T cells and numerous efforts are underway to engineer WT1-based cancer vaccines. Here we determined the structures of the class I MHC molecule HLA-A*0201 bound to the native 126–134 epitope of the WT1 peptide and a recently described variant (R1Y) with improved MHC binding. The R1Y variant, a potential vaccine candidate, alters the positions of MHC charged side chains near the peptide N-terminus and significantly reduces the peptide/MHC electrostatic surface potential. These alterations indicate that the R1Y variant is an imperfect mimic of the native WT1 peptide, and suggest caution in its use as a therapeutic vaccine. Stability measurements revealed how the R1Y substitution enhances MHC binding affinity, and together with the structures suggest a strategy for engineering WT1 variants with improved MHC binding that retain the structural features of the native peptide/MHC complex.
Keywords: peptide/MHC, structure, WT1, cancer vaccines, electrostatics
Introduction
Recognition of a class I MHC-presented antigenic peptide by a T cell receptor (TCR) of a cytotoxic T lymphocyte (CTL) is essential for lymphocyte activation and the initiation of an antigen-specific immune response. Since the discovery of class I MHC-presented tumor associated antigens (TAA), there have been numerous efforts to engineer peptide-based cancer vaccines, with the hope that exogenous peptides will activate TAA-specific CTLs leading to tumor destruction. However, TAA are typically poorly immunogenic, a result that in some cases is attributable to the weak binding of TAA to the MHC protein (Yu et al., 2004). Modification of TAA to improve MHC binding has in some cases led to improved antigenicity (e.g., (Parkhurst et al., 1996; Valmori et al., 1998), and many cancer vaccine trials employ such modified antigens, either alone or in concert with other strategies such as adoptive T cell transfer.
The Wilms Tumor 1 transcription factor (WT1) is overexpressed in many cancers, including both hematopoetic and solid tumors. The epitope spanning residues 126–134 (RMFPNAPYL) is presented by the class I MHC HLA-A*0201 (HLA-A2) and is considered a promising lead for therapeutic vaccine development (Oka et al., 2008). Recently, it was found that substitution of Arg1 of the peptide with tyrosine enhances the affinity of the peptide for HLA-A2 as well as its immunogenicity with T cell populations that recognize the native peptide (Al Qudaihi et al., 2009; Pinilla-Ibarz et al., 2006). This R1Y variant of WT1126 has been discussed as a potentially improved WT1126-based cancer vaccine (Kline, 2009), and recently evaluated as a component of a polyvalent vaccine for acute myeloid leukemia (Maslak et al.).
It is notable though that unlike other modified TAA considered for use in immunotherapy, the R1Y substitution in WT1126 does not alter a primary anchor residue. In the engineering of peptides to improve MHC binding affinity and thus antigenicity, sub-optimal primary anchors are typically replaced with their optimal counterparts, which are fully buried upon peptide binding to HLA-A2. Examples of this “anchor fixing” strategy are the T2M variant of the gp100209 antigen and the A2L variant of the MART-1/Melan-A26-35 antigen, both which introduce optimal primary anchors for HLA-A2 and do not discernibly alter any peptide or MHC structural features (Borbulevych et al., 2005; Borbulevych et al., 2007). With WT1126, however, the R1Y substitution alters a side chain that is typically exposed for TCR in peptide/HLA-A2 structures. The nonconservative modification could thus alter conformational or surface properties, potentially influencing T cell receptor binding. In addition to negatively impacting recognition by WT1126-specific T cells, this alteration of antigenic identity could lead to activation of different sets of T cells than those stimulated by the native peptide, potentially limiting therapeutic efficiency and in the worst case inducing autoimmunity.
Here, we solved the crystallographic structures of the native and R1Y WT1126 peptides bound to HLA-A2 and studied the mechanism of how the R1Y substitution improves HLA-A2 binding. We show that although the R1Y substitution does not alter the conformation of the peptide in the HLA-A2 peptide binding groove, it does alter the positions of charged side chains that in other studies have been implicated in T cell receptor recognition of peptide/HLA-A2 complexes. The loss of a surface charge also alters the electrostatic potential of the peptide/HLA-A2 surface in a manner that in other cases has been shown to alter TCR recognition. Together, these results suggest caution in the exploration of the R1Y variant as a therapeutic vaccine. The mechanism of affinity enhancement with the R1Y modification was attributed to electrostatic interactions, which along with the structures suggests a route for developing WT1 variants with improved HLA-A2 affinity that do not alter structural or surface properties.
Materials and Methods
Proteins and peptides
Soluble peptide/HLA-A2 was produced and refolded from E. coli inclusion bodies as previously described (Davis-Harrison et al., 2005). Peptides were synthesized using an Applied Biosystems 433A synthesizer and verified using LC-MS.
Crystallography and structural analyses
The peptide/HLA-A2 complexes were crystallized at 4 °C from 24% PEG3350, 0.1 M NaCl (native WT1) or 0.1 M KCl (R1Y) buffered with 25 mM MES at pH 6.5 using sitting drop/vapor diffusion. Streak seeding was used to obtain higher quality crystals. For data collection, crystals were transferred to 30% PEG3350, 20% glycerol and flash-frozen in liquid nitrogen. Diffraction data were collected at the indicated beamlines. Frames were integrated and scaled with HKL2000 (Otwinowski and Minor, 1997). The structures were solved by molecular replacement with MOLREP (Vagin and Teplyakov, 2010), using the 1TVB structure as a search model, with the coordinates for the peptide and waters excluded. Rigid body refinement and TLS refinement followed by multiple steps of restrained refinement were performed with Refmac5 (Murshudov et al., 1997). TLS groups for refinement were chosen as previously described (Gagnon et al., 2006). Anisotropic and bulk solvent corrections were performed throughout the refinement. After TLS refinement, it became possible to unambiguously position the peptide against 2Fo-Fc maps. Waters were added using ARP/wARP (Perrakis et al., 1997). Graphical evaluation of the models and fitting to maps was performed using Coot (Emsley and Cowtan, 2004) and XtalView (McRee, 1999). Structure quality was monitored and enforced with Coot as well as Procheck (Laskowski et al., 1993). Side chain orientations were validated using MolProbity (Lovell et al., 2003). Surface potentials were calculated with the program Delphi as implemented in the Discovery Studio package from Accelrys.
Thermal stability
Thermal stability measurements were performed with CD spectroscopy as previously described (Borbulevych et al., 2009), using a Jasco J-815 spectrometer monitoring a wavelength of 218 nm. Solution conditions were 20 mM phosphate pH 7.4 with the indicated NaCl concentration. Protein concentrations were 10 μM. A temperature increment of approximately 1 °C/min was used. Because unfolding of HLA-A2 is irreversible, the data were fit to a six order polynomial and the apparent Tm taken from the first derivative of the fitted curve.
Results
Structures of the WT1126 and WT1126 R1Y peptides bound to HLA-A2: alteration of surfaces charges and electrostatic surface potential
Crystals of the WT1126 and WT1126 R1Y peptides bound to HLA-A2 were grown from recombinant, refolded peptide/HLA-A2. Structures were solved by molecular replacement, using the structure of the gp100209/HLA-A2 complex with peptide and solvent excluded as a search model (Borbulevych et al., 2005). Crystallization and refinement statistics are given in Table 1. Electron density images for the two structures are available in the Supplementary Material.
Table 1.
X-ray data and refinement statistics
| Protein | WT1126/HLA-A2 | WT1126(R1Y)/HLA-A2 |
| PDB code | 3HPJ | 3MYJ |
| Radiation Source | APS 19BM | APS 19ID |
| Space group | P21 | P1 |
| a [Å] | 63.6 | 50.2 |
| b [Å] | 86.8 | 62.8 |
| c [Å] | 79.1 | 74.8 |
| α [°] | 90.0 | 81.9 |
| b [°] | 90.1 | 75.9 |
| γ [°] | 90.0 | 77.9 |
| Molecules/a.u. | 2 | 2 |
| Resolution [Å] | 20 – 2.0 | 20 – 1.9 |
| Number of unique reflections | 56716 | 66006 |
| Mosaicity [°] | 0.32 | 0.69 |
| Completeness a [%] | 97.6 (86.2) | 96.9 (95.9) |
| I/σ | 14.1 (2.0) | 14.5 (1.9) |
| Rmerge b [%] | 8.6 (46.8) | 6.3 (41.9) |
| Redundancy | 3.6 (3.0) | 1.9 (1.9) |
| Rwork c [%] (no. reflections) | 18.1 (53808) | 18.6 (62657) |
| Rfree d [%] (no. reflections) | 23.6 (2871) | 23.0 (3333) |
| Average B factor [Å2] | ||
| Main-chain atoms | 16.90 | 18.80 |
| Side-chain atoms | 19.52 | 21.91 |
| Water/glycerol molecules | 25.11 | 25.78 |
| All atoms | 18.72 | 20.8 |
| Ramachandran plot | ||
| Most favored [%] | 92.5 | 91.0 |
| Allowed [%] | 7.2 | 8.7 |
| Generously allowed [%] | 0.3 | 0.3 |
| RMS deviations from ideality | ||
| Bonds [Å] | 0.018 | 0.020 |
| Angles [°] | 1.741 | 1.878 |
| Coordinate error e [Å] | 0.13 | 0.11 |
Numbers in parenthesis refer to the highest resolution shell
Rmerge = Σhkl Σi |Ii(hkl) −〈I(hkl)rang; |/Σhkl Σi Ii(hkl), where Ii(hkl) is the observed intensity of reflection i and 〈I(hkl)rang; is the average intensity of multiple observations
Rcryst = Σ | |Fo|−|Fc| |/Σ|Fo|, where Fo and Fc are the observed and calculated structure-factor amplitudes, respectively
Rfree is calculated over a randomly selected 5% subset of reflections excluded from refinement
Mean estimate based on maximum likelihood methods
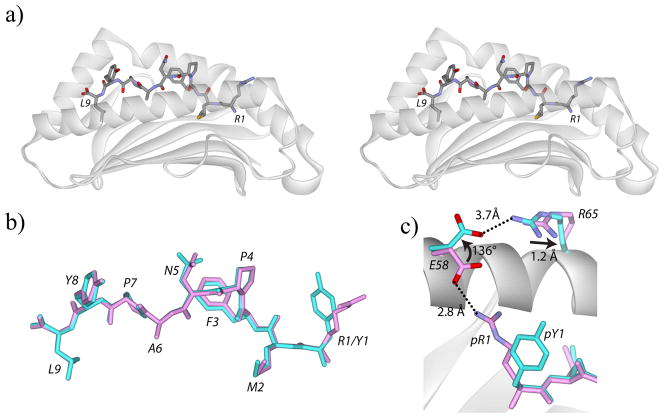
The structure of the native WT1126/HLA-A2 complex revealed the usual architecture of class I MHC/peptide complexes, with the peptide adopting an extended conformation characterized by a bulge at Pro4 and Asn5 (Figure 1A). The two molecules in the asymmetric unit were essentially the same, with the backbones and all atoms of the two peptides superimposing with RMSDs of 0.10 Å and 0.42 Å, respectively. The conformation of the R1Y variant in the peptide binding groove was nearly identical to that of the native peptide, with the backbones of the first and second molecules in the asymmetric units superimposing with RMSDs of 0.15 Å and 0.17 Å, respectively (Figure 1B). There was no indication of side chain or backbone conformational heterogeneity in either structure as has been seen in some other peptide/HLA-A2 structures (e.g., (Borbulevych et al., 2005; Kuhns et al., 1999).
Figure 1.
Overview of the WT1126/HLA-A2 and R1Y/HLA-A2 pMHC complexes. A) Cross-eyed stereo view of the native WT1126 peptide in the HLA-A2 peptide binding groove. The peptide N-terminus is to the right. B) The native (pink) and R1Y (cyan) WT1126 peptides have identical conformations in the HLA-A2 peptide binding groove. C) The R1Y substitution alters the conformation of Glu58 of HLA-A2 and shifts Arg65, resulting in a loss of the salt-bridge between pArg1 and Glu58 and the formation of a new salt-bridge with Glu58 and Arg65. All superimpositions are through the backbones of residues 1-180 of the HLA-A2 peptide binding domain.
Differences between the native and R1Y WT1126/HLA-A2 structures were clearly seen in the region of the Arg-to-Tyr substitution. In the native complex, the arginine at position 1 extends away from the binding groove and forms a salt-bridge with Glu58 of the HLA-A2 heavy chain. In the R1Y complex, the shorter tyrosine does not interact with Glu58, which instead is rotated approximately 140° away. This allows it to form a salt-bridge with Arg65 of the heavy chain, which itself is displaced by 1.3 Å relative to its position in the native complex (Figure 1C) (the first copy of the molecule in the asymmetric unit of the R1Y structure shows Glu58 occupying both this altered position as well as a position close to that seen in the native structure; the second shows only the altered conformation).
The structure with the native WT1126 peptide indicated that the arginine at position 1 is almost fully solvent exposed. Replacing this with tyrosine thus removes a surface charge. Together with the alterations in Glu58 and Arg65, this would be expected to alter the electrostatic surface potential in the vicinity of the peptide N-terminus. To confirm this, we calculated surface potentials and mapped them to the solvent accessible surface of the two peptide/HLA-A2 complexes. As shown in Figure 2, this analysis revealed a large reduction in surface positive charge density in the vicinity of the peptide N-terminus.
Figure 2.
The R1Y substitution alters the electrostatic surface potential of the peptide/MHC complex in the vicinity of the peptide N-terminus. A) Electrostatic surface potential of the native WT1126/HLA-A2 complex, with Arg1 of the peptide and Glu58 and Arg65 of HLA-A2 highlighted. The surface potential from these three side chains is circled and the view magnified in the lower panel. B) Same as panel A, but shows the electrostatic surface potential of the R1Y/HLA-A2 complex. The potential of the circled region is substantially reduced. The scale, from red to blue, is from -25 kT to +25 kT.
Mechanism of affinity enhancement with the R1Y substitution
Enhancement of peptide binding affinity for class I MHC peptides has traditionally been achieved through substitution of sub-optimal anchor residues with more optimal counterparts (Valmori et al., 1998). For HLA-A2, good examples are substitution of alanine with leucine at P2 for the Melan-A/MART-126-35 antigen (Valmori et al., 1998) or methionine for threonine with the gp100209 antigen (Parkhurst et al., 1996). In the case of the R1Y substitution with WT1126, although the enhanced affinity was predicted using models based on earlier peptide binding studies (Ruppert et al., 1993), the underlying mechanism is not apparent from these data.
Examination of the crystallographic structures revealed multiple contributions to the affinity enhancement. The aromatic side chains of Tyr1 of the R1Y peptide and Trp167 of the HLA-A2 heavy chain are 4–5 Å of each other, forming a classic π-π interaction (McGaughey et al., 1998). The other face of Tyr1 is within 3–4 Å of the charged nitrogen of Lys66 of the HLA-A2 heavy chain, forming a classic cation-π interaction (Dougherty, 1996) (Figure 3A). Both interactions should be expected to enhance binding of the R1Y variant to HLA-A2.
Figure 3.
The R1Y peptide binds more tightly to HLA-A2 through optimization of electrostatic interactions. A) pTyr1 in the R1Y/HLA-A2 complex forms a π-π stacking interaction with Trp167 and a cation-π interaction with Lys66 of HLA-A2. B) pArg1 in the native WT1126/HLA-A2 complex experiences electrostatic repulsion from Lys66 of HLA-A2. C) The R1Y variant binds more tightly to HLA-A2 than the native peptide as shown by the greater thermal stability of the R1Y/HLA-A2 complex. The apparent Tm of each complex is indicated on the curves. The NaCl concentration in this experiment was 75 mM. D) The difference in thermal stability between the R1Y and native complexes diminishes as the ionic strength of the solution is increased, revealing the role that electrostatics plays in enhancing peptide binding.
As noted above, the R1Y substitution results in the loss of a salt-bridge between Arg1 of the native peptide and Glu58 of the heavy chain. At first consideration, this might be expected to at least partially offset the stabilizing contributions from the π-π and cation-π interactions. However, the strength of salt-bridges in protein structures is debated, as salt-bridge formation requires an unfavorable desolvation penalty that is not always offset by columbic interactions, and for surface side chains, a potential entropic penalty from fixing what might otherwise be mobile side chains (Sheinerman et al., 2000). Thus the Arg1 – Glu58 salt-bridge in the structure with the native WT1126 peptide may be only weakly stabilizing and its loss more than compensated for by the π-π and cation-π interactions formed when tyrosine is at position 1. Further, in the structure with the native peptide, the charged nitrogen of Arg1 is within 4 Å of the charged nitrogen of Lys66 of the heavy chain, introducing a degree of electrostatic repulsion in the native complex that could offset the Arg1 – Glu58 salt-bridge (Figure 3B).
The structural features discussed above imply an electrostatic mechanism to the R1Y affinity enhancement. To confirm the role of electrostatics, we measured the thermal stability of the native and R1Y peptide/HLA-A2 complexes via CD spectroscopy as a function of salt concentration; increased ionic strength should screen both favorable and unfavorable electrostatic effects, reducing their effects on peptide binding. At a concentration of 75 mM NaCl, the concentration used in other assays of pMHC stability (Borbulevych et al., 2009; Khan et al., 2000), the complex with the native WT1126 peptide had a Tm of 54 °C. As expected, the stability of the R1Y complex was higher, with a Tm of 61 °C (Figure 3C). However, although the stability of both complexes increased somewhat as a function of salt as reported previously (Batalia et al., 2000), the difference in stability diminished, ultimately yielding a difference of only 2 °C at 1 M NaCl (Figure 3D). These experiments confirm the role electrostatics plays in the enhanced affinity of the R1Y variant for HLA-A2.
Discussion
Most tumor associated antigens are poorly immunogenic. In some cases, this can be attributed to weak binding of the peptide to the restricting MHC protein (Yu et al., 2004). Alteration of the peptide to strengthen MHC binding affinity can thus improve antigenicity (Borbulevych et al., 2005; Parkhurst et al., 1996; Valmori et al., 1998; Yu et al., 2004), and such modified peptides have been explored as cancer vaccine candidates. A requirement for this strategy is that peptide modification does not alter structural features of the pMHC complex in a manner that either negatively impacts T cell receptor binding or permits recognition by T cells with specificities different from those that recognize the native antigen. The latter requirement is especially important given the level of cross-reactivity inherent in the T cell repertoire (Mason, 1998) (peptides which achieve these goals are sometimes referred to as “heteroclitic”). The degree of structural variation that can be tolerated without impacting TCR recognition is not yet predictable and, importantly, will differ with different TCRs. For example, the A6 and B7 TCRs both recognize the Tax11-19 antigen presented by HLA-A2, but respond differently to various modifications within the TCR-pMHC interface (Hausmann et al., 1999; Piepenbrink et al., 2009). As the T cell response to most antigens is clonally diverse, the ideal modified antigen is thus one that conserves pMHC structural features as much as possible. The best examples of this reported to-date include the T2M variant of the gp100209 peptide (Borbulevych et al., 2005), the A2L variant of the Melan-A/MART-126-35 decamer (Borbulevych et al., 2007), and a variant of NY-ESO-1 in which the C-terminal cysteine is replaced with 2-aminoisobutyric acid (Webb et al., 2004). Notably though, even close structural mimics can still result in subtle variations in the expanded T cell repertoires (Wieckowski et al., 2009) (although this result could be attributable at least in part to the stronger TCR affinity necessary to respond to peptides that bind weakly to the restricting MHC).
Here we demonstrated that the R1Y modification to WT1126 alters the conformations of HLA-A2 charged side chains as well as the electrostatic surface potential of the peptide/HLA-A2 complex. Are these changes significant enough to alter T cell receptor recognition? In two studies, the R1Y variant showed improved potency with CD8+ T cell lines from healthy HLA-A2+ donors and triggered the lysis of leukemic cells presenting the native WT1126 peptide in culture (Al Qudaihi et al., 2009; Pinilla-Ibarz et al., 2006). In a recent pilot trial evaluating a polyvalent vaccine for acute myeloid leukemia, the R1Y variant activated T cells that were cross-reactive with the native peptide (Maslak et al., 2010). The T cells used in these studies are thus tolerant of the differences between the native and R1Y WT1126/HLA-A2 complexes. However, other studies have shown that alterations of peptide/HLA-A2 electrostatic properties can strongly influence TCR recognition of peptide/HLA-A2 complexes, particularly in the region dominated by Arg65 and Lys66 of HLA-A2, i.e., the region impacted by the R1Y substitution in WT1126 (Gagnon et al., 2005; Miller et al., 2007; Wang et al., 2002). Still other studies have shown that altering MHC surface electrostatics can alter receptor specificity (Huseby et al., 2006).
As at least one study has shown clonal diversity in the CD8+ T cell response to WT1126 antigen (Rezvani et al., 2005), the structural differences observed here suggest caution in the use of the R1Y variant of the WT1126 antigen as a therapeutic vaccine. Expanded studies are likely to reveal differences in TCR binding properties and thus the sets of T cells that respond to the two peptides. Indeed, in the recent pilot trial where the R1Y WT1126 peptide was a vaccine component, two of three HLA-A2+ patients developed hypersensitivity and were removed from the trial (Maslak et al., 2010), an outcome consistent with the activation of T cells with overlapping but still variant specificities.
If a better-binding WT1126 variant that does not alter pMHC structural features is desirable, do the structures determined here suggest a route for achieving it? Traditionally, modifications to improve peptide binding to the MHC molecule have used standard amino acids, substituting a sub-optimal primary anchor residue with an optimal counterpart. Yet the structure of WT1126 bound to HLA-A2 shows optimal use of the primary anchors and normal hydrogen bonding at the termini. Further, the packing within the peptide binding groove in the WT1126/HLA-A2 structure is efficient. We suggest that an improved WT1126 variant could be engineered through the use of non-natural amino acids and/or synthetic chemistry, a strategy that has been discussed but not seen widespread applicability in peptide engineering. The mechanism of affinity enhancement observed with the R1Y variant of WT1126 hints at a possibility: an aromatic ring capable of forming π-π and cation-π interactions with Trp167 and Lys66, respectively, could be derivatized with a positively charged group (e.g., phenylguanidine or benzylguanidine), enhancing binding via electrostatic means yet retaining the surface potential and positions of charged HLA-A2 side chains. Such structure-guided engineering may help overcome limitations commonly encountered with the 20 standard amino acids.
Supplementary Material
Acknowledgments
We thank Cynthia Piepenbrink for outstanding technical assistance. Supported by grants RSG-05-202-01-GMC from the American Cancer Society and R01GM067079 from the National Institute of General Medical Sciences, National Institutes of Health. Results shown in this report are derived from work performed at Argonne National Laboratory, Structural Biology Center at the Advanced Photon Source. Argonne is operated by UChicago Argonne, LLC, for the U.S. Department of Energy, Office of Biological and Environmental Research under contract DE-AC02-06CH11357.
Footnotes
Accession numbers
Structure factors and coordinates have been deposited in the Protein Data Bank as entries 3HPJ (native complex) and 3MYJ (R1Y complex).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Qudaihi G, Lehe C, Negash M, Al-Alwan M, Ghebeh H, Mohamed SY, Saleh AJM, Al-Humaidan H, Tbakhi A, Dickinson A, Aljurf M, Dermime S. Enhancement of lytic activity of leukemic cells by CD8+ cytotoxic T lymphocytes generated against a WT1 peptide analogue. Leukemia & Lymphoma. 2009;50:260–269. doi: 10.1080/10428190802578478. [DOI] [PubMed] [Google Scholar]
- Batalia MA, Kirksey TJ, Sharma A, Jiang L, Abastado JP, Yan S, Zhao R, Collins EJ. Class I MHC is stabilized against thermal denaturation by physiological concentrations of NaCl. Biochemistry. 2000;39:9030–8. doi: 10.1021/bi000442n. [DOI] [PubMed] [Google Scholar]
- Borbulevych OY, Baxter TK, Yu Z, Restifo NP, Baker BM. Increased Immunogenicity of an Anchor-Modified Tumor-Associated Antigen Is Due to the Enhanced Stability of the Peptide/MHC Complex: Implications for Vaccine Design. J Immunol. 2005;174:4812–4820. doi: 10.4049/jimmunol.174.8.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbulevych OY, Insaidoo FK, Baxter TK, Powell DJ, Jr, Johnson LA, Restifo NP, Baker BM. Structures of MART-1(26/27-35) Peptide/HLA-A2 Complexes Reveal a Remarkable Disconnect between Antigen Structural Homology and T Cell Recognition. J Mol Biol. 2007;372:1123–36. doi: 10.1016/j.jmb.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbulevych OY, Piepenbrink KH, Gloor BE, Scott DR, Sommese RF, Cole DK, Sewell AK, Baker BM. T cell receptor cross-reactivity directed by antigen-dependent tuning of peptide-MHC molecular flexibility. Immunity. 2009;31:885–96. doi: 10.1016/j.immuni.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Harrison RL, Armstrong KM, Baker BM. Two Different T Cell Receptors use Different Thermodynamic Strategies to Recognize the Same Peptide/MHC Ligand. Journal of Molecular Biology. 2005;346:533–550. doi: 10.1016/j.jmb.2004.11.063. [DOI] [PubMed] [Google Scholar]
- Dougherty DA. Cation-pi Interactions in Chemistry and Biology: A New View of Benzene, Phe, Tyr, and Trp. Science. 1996;271:163–168. doi: 10.1126/science.271.5246.163. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Gagnon SJ, Borbulevych OY, Davis-Harrison RL, Baxter TK, Clemens JR, Armstrong KM, Turner RV, Damirjian M, Biddison WE, Baker BM. Unraveling a Hotspot for TCR Recognition on HLA-A2: Evidence Against the Existence of Peptide-independent TCR Binding Determinants. Journal of Molecular Biology. 2005;353:556. doi: 10.1016/j.jmb.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Gagnon SJ, Borbulevych OY, Davis-Harrison RL, Turner RV, Damirjian M, Wojnarowicz A, Biddison WE, Baker BM. T Cell Receptor Recognition via Cooperative Conformational Plasticity. Journal of Molecular Biology. 2006;363:228–243. doi: 10.1016/j.jmb.2006.08.045. [DOI] [PubMed] [Google Scholar]
- Hausmann S, Biddison WE, Smith KJ, Ding YH, Garboczi DN, Utz U, Wiley DC, Wucherpfennig KW. Peptide recognition by two HLA-A2/Tax11-19-specific T cell clones in relationship to their MHC/peptide/TCR crystal structures. J Immunol. 1999;162:5389–97. [PubMed] [Google Scholar]
- Huseby ES, Crawford F, White J, Marrack P, Kappler JW. Interface-disrupting amino acids establish specificity between T cell receptors and complexes of major histocompatibility complex and peptide. Nat Immunol. 2006;7:1191–1199. doi: 10.1038/ni1401. [DOI] [PubMed] [Google Scholar]
- Khan AR, Baker BM, Ghosh P, Biddison WE, Wiley DC. The structure and stability of an HLA-A*0201/octameric tax peptide complex with an empty conserved peptide-N-terminal binding site. J Immunol. 2000;164:6398–405. doi: 10.4049/jimmunol.164.12.6398. [DOI] [PubMed] [Google Scholar]
- Kline J. Will changing the face of WT1 make it more attractive to T cells? Leukemia & Lymphoma. 2009;50:156–157. doi: 10.1080/10428190802699365. [DOI] [PubMed] [Google Scholar]
- Kuhns JJ, Batalia MA, Yan S, Collins EJ. Poor binding of a HER-2/neu epitope (GP2) to HLA-A2.1 is due to a lack of interactions with the center of the peptide. J Biol Chem. 1999;274:36422–7. doi: 10.1074/jbc.274.51.36422. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst. 1993;26:283–291. [Google Scholar]
- Lovell SC, Davis IW, Arendall WB, 3rd, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC. Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins. 2003;50:437–50. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- Maslak PG, Dao T, Krug LM, Chanel S, Korontsvit T, Zakhaleva V, Zhang R, Wolchok JD, Yuan J, Pinilla-Ibarz J, Berman E, Weiss M, Jurcic J, Frattini MG, Scheinberg DA. Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T cell responses in patients with complete remission from acute myeloid leukemia (AML) Blood. 2010 doi: 10.1182/blood-2009-10-250993. blood-2009-10-250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunology Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- McGaughey GB, Gagne M, Rappe AK. Pi-Stacking Interactions: alive and well in proteins. Journal of Biological Chemistry. 1998;273:15458–15463. doi: 10.1074/jbc.273.25.15458. [DOI] [PubMed] [Google Scholar]
- McRee DE. XtalView/Xfit--A versatile program for manipulating atomic coordinates and electron density. J Struct Biol. 1999;125:156–65. doi: 10.1006/jsbi.1999.4094. [DOI] [PubMed] [Google Scholar]
- Miller PJ, Pazy Y, Conti B, Riddle D, Appella E, Collins EJ. Single MHC mutation eliminates enthalpy associated with T cell receptor binding. J Mol Biol. 2007;373:315–27. doi: 10.1016/j.jmb.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–55. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Oka Y, Tsuboi A, Oji Y, Kawase I, Sugiyama H. WT1 peptide vaccine for the treatment of cancer. Current Opinion in Immunology. 2008;20:211–220. doi: 10.1016/j.coi.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Parkhurst M, Salgaller M, Southwood S, Robbins P, Sette A, Rosenberg S, Kawakami Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- Perrakis A, Sixma TK, Wilson KS, Lamzin VS. wARP: improvement and extension of crystallographic phases by weighted averaging of multiple-refined dummy atomic models. Acta Crystallogr D Biol Crystallogr. 1997;53:448–55. doi: 10.1107/S0907444997005696. [DOI] [PubMed] [Google Scholar]
- Piepenbrink KH, Borbulevych OY, Sommese RF, Clemens J, Armstrong KM, Desmond C, Do P, Baker BM. Fluorine substitutions in an antigenic peptide selectively modulate T-cell receptor binding in a minimally perturbing manner. Biochemical Journal. 2009;423:353–361. doi: 10.1042/BJ20090732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinilla-Ibarz J, May RJ, Korontsvit T, Gomez M, Kappel B, Zakhaleva V, Zhang RH, Scheinberg DA. Improved human T-cell responses against synthetic HLA-0201 analog peptides derived from the WT1 oncoprotein. Leukemia. 2006;20:2025–33. doi: 10.1038/sj.leu.2404380. [DOI] [PubMed] [Google Scholar]
- Rezvani K, Brenchley JM, Price DA, Kilical Y, Gostick E, Sewell AK, Li J, Mielke S, Douek DC, Barrett AJ. T-Cell Responses Directed against Multiple HLA-A*0201-Restricted Epitopes Derived from Wilms' Tumor 1 Protein in Patients with Leukemia and Healthy Donors: Identification, Quantification, and Characterization. Clinical Cancer Research. 2005;11:8799–8807. doi: 10.1158/1078-0432.CCR-05-1314. [DOI] [PubMed] [Google Scholar]
- Ruppert J, Sidney J, Celis E, Kubo RT, Grey HM, Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993;74:929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- Sheinerman FB, Norel R, Honig B. Electrostatic aspects of protein-protein interactions. Curr Opin Struct Biol. 2000;10:153–9. doi: 10.1016/s0959-440x(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Vagin A, Teplyakov A. Molecular replacement with MOLREP. Acta Crystallogr D Biol Crystallogr. 2010;66:22–5. doi: 10.1107/S0907444909042589. [DOI] [PubMed] [Google Scholar]
- Valmori D, Fonteneau JF, Lizana CM, Gervois N, Lienard D, Rimoldi D, Jongeneel V, Jotereau F, Cerottini JC, Romero P. Enhanced Generation of Specific Tumor-Reactive CTL In Vitro by Selected Melan-A/MART-1 Immunodominant Peptide Analogues. J Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]
- Wang Z, Turner R, Baker BM, Biddison WE. MHC Allele-Specific Molecular Features Determine Peptide/HLA-A2 Conformations That Are Recognized by HLA-A2-Restricted T Cell Receptors. J Immunol. 2002;169:3146–3154. doi: 10.4049/jimmunol.169.6.3146. [DOI] [PubMed] [Google Scholar]
- Webb AI, Dunstone MA, Chen W, Aguilar M-I, Chen Q, Jackson H, Chang L, Kjer-Nielsen L, Beddoe T, McCluskey J, Rossjohn J, Purcell AW. Functional and Structural Characteristics of NY-ESO-1-related HLA A2-restricted Epitopes and the Design of a Novel Immunogenic Analogue. J Biol Chem. 2004;279:23438–23446. doi: 10.1074/jbc.M314066200. [DOI] [PubMed] [Google Scholar]
- Wieckowski S, Baumgaertner P, Corthesy P, Voelter V, Romero P, Speiser DE, Rufer N. Fine Structural Variations of {alpha}{beta}TCRs Selected by Vaccination with Natural versus Altered Self-Antigen in Melanoma Patients. J Immunol. 2009;183:5397–5406. doi: 10.4049/jimmunol.0901460. [DOI] [PubMed] [Google Scholar]
- Yu Z, Theoret MR, Touloukian CE, Surman DR, Garman SC, Feigenbaum L, Baxter TK, Baker BM, Restifo NP. Poor immunogenicity of a self/tumor antigen derives from peptide/MHC-I instability and is independent of tolerance. Journal of Clinical Investigation. 2004;114:551–559. doi: 10.1172/JCI21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.